Abstract
Background:
Patients with obstructive sleep apnea syndrome (OSAS) are always exposed to intermittent hypoxia and reoxygenation. The metabolic syndrome (MetS) and OSAS are also known to accelerate atherosclerosis, diabetes, and dyslipidemia. Therefore, nasal continuous positive airway pressure (CPAP) therapy may have beneficial effects in patients with the MetS and OSAS.
Hypothesis:
This study in patients with the MetS and OSAS tested the validity of the hypothesis that chronic CPAP therapy improves factors involved in atherosclerosis, including impaired endothelial function.
Methods:
Thirty‐two patients (19 males and 13 females, mean age 54 ± 9 y) diagnosed with the MetS and OSAS were enrolled in the study and received CPAP therapy for 3 months. Vascular function was investigated by measuring forearm blood flow (FBF) responses to reactive hyperemia (RH) using venous occlusion strain‐gauge plethysmography. Biochemical markers were also measured before and after this procedure.
Results:
Basal apnea‐hypopnea index was statistically correlated with FBF response to RH. The FBF response to RH was increased significantly after 3 months of CPAP therapy. A significant increase in plasma nitric oxide levels and a decrease in the levels of asymmetrical dimethylarginine, thiobarbituric acid reactive substance, soluble Fas ligand, and soluble CD40 ligand were detected after CPAP therapy. The plasma concentrations of tumor necrosis factor‐α, interleukin (IL)‐6, and IL‐8 also decreased significantly with CPAP therapy, whereas IL‐1β levels remained unchanged.
Conclusions:
Continuous positive airway pressure therapy has beneficial effects on vascular function and inflammatory and oxidative stress in patients with the MetS and OSAS.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
The metabolic syndrome (MetS) is characterized by a clustering of metabolic abnormalities, including obesity, hyperglycemia, dyslipidemia, and hypertension. The syndrome has been identified as a common precursor to the development of cardiovascular (CV) disease.1 The prevalence of the MetS is increasing in Japan as a result of changes in diet and physical activity during recent decades.2 This has resulted in an urgent need to obtain appropriate evidence for treatment of individuals with the MetS who have a high risk of developing CV disease, thereby preventing a further increase in the incidence of the syndrome.
Obstructive sleep apnea syndrome (OSAS) is also a highly prevalent disorder, affecting approximately 17% of adults,3 and is associated with repetitive episodes of transient oxygen desaturation that cause apneas and hypopneas during sleep. The syndrome is regarded as an independent risk factor for a number of CV diseases, including systemic arterial hypertension,4., 5. coronary artery disease, congestive cardiac failure, and cerebral vascular events.4., 6. Treatment with nasal continuous positive airway pressure (CPAP) ameliorates oxygen desaturation and decreases CV morbidity7 and mortality.8., 9. Although the pathophysiological basis of CV complications in OSAS is multifactorial, involving sympathetic excitation, endothelial dysfunction, inflammation, and insulin resistance,10 it is likely that the intermittent episodes of hypoxia, particularly the associated episodes of intermittent reoxygenation, are important mediators of these complications. Therefore, patients who suffer from not only the MetS, but also OSAS, must be treated appropriately. However, the effects of CPAP therapy on endothelial dysfunction and atherosclerotic biomarkers are not fully understood.
Methods
Study Design and Protocol
The Institutional Review Board of Human Research at Kyushu University approved this study, and written, informed consent was obtained from all participants. The study was a single‐arm prospective design that examined the impact of CPAP treatment on endothelial function and biochemical alterations, including inflammation and oxidative stress, in patients with the MetS and stable OSAS (apnea‐hypopnea index [AHI] >20 events/h).
Patients with the MetS suspected of having OSAS were recruited. Patients with a history of heart failure, severe coronary artery disease with residual cardiac ischemia or arrhythmia, significant pulmonary disease, or subjects taking β‐blockers or other negative chronotropic drugs were excluded. Patients already diagnosed as having hyperglycemia, dyslipidemia, and/or hypertension and who were receiving drug treatment and satisfied the criteria of the MetS were included the study.
At the start of the study, 53 patients with the MetS agreed to participate and underwent full polysomnography, and 40 newly diagnosed candidates for CPAP therapy who had an AHI >20 events per hour were enrolled. All the patients were requested to fast and underwent polysomnography, followed by blood sampling and endothelial function tests between 9 and 11 a.m. They were assessed using the Epworth Sleepiness Scale (ESS) to investigate changes in subjective daytime sleepiness.11 Five patients were excluded because they withdrew from participation, 1 patient stopped CPAP therapy, and 2 patients were excluded due to poor compliance (<60%). The remaining 32 patients with the MetS and OSAS who received CPAP therapy were evaluated throughout the study. No patient changed their medications during the study. All the experimental evaluations were repeated 3 months after CPAP intervention in all the patients.
Definition of MetS
The MetS was defined according to the Japanese criteria as the presence of ≥2 abnormalities in addition to a waist circumference >85 cm in males and >90 cm in females. Other abnormalities included in the definition were (1) dyslipidemia, indicated by hypertriglyceridemia (serum triglyceride concentration ≥150 mg/dL) and/or low high‐density lipoprotein cholesterol (serum concentration ≤40 mg/dL); (2) hypertension, indicated by systolic blood pressure ≥130 mm Hg and/or diastolic blood pressure ≥85 mm Hg; and (3) high fasting glucose, indicated by serum glucose concentration ≥110 mg/dL.
Polysomnography and Definition of Obstructive Sleep Apnea Syndrome
Full polysomnography was performed using the Somno Track Pro system (Fukuda Denshi Co, Ltd, Tokyo, Japan). An electroencephalograph (EEG), electro‐oculography, electromyography, and electrocardiogram were performed simultaneously. Surface electrodes were used to record 2 channels of the EEG (CA2, C4A1), right and left electro‐oculography, and submental electromyography. Respiratory movements of the chest and abdomen were monitored by inductive plethysmography bands, and nasal pressure cannulas were used to record airflow. Arterial oxygen saturation (SatO2) was measured using fingertip pulse oximeters. Apnea was defined as complete cessation of airflow for ≥10 seconds, and hypopnea as a reduction in airflow of ≥50%, accompanied by ≥4% oxygen desaturation, or an EEG arousal from sleep. Apnea‐hypopnea index was defined as the total number of apneas and hypopneas per hour of sleep. The patients who had an AHI >20 were enrolled in this study as candidates for CPAP therapy.
CPAP Therapy
Patients with an AHI >20 slept while attached to the automatic titration device (ResMed S8; ResMed Ltd, Sydney, Australia). After using the CPAP device for ≥3 months, data of CPAP usage and mean AHI were obtained from data cards inside the CPAP machine. Compliance with CPAP was defined as percent of days with CPAP usage for ≥4 hours among the total days of the study. Subjects who showed good compliance (≥60%) with the CPAP device were included in the analysis. Sleep stages and respiratory parameters were scored according to the standard criteria of the American Academy of Sleep Medicine.12 At the end of study protocol, the patients had a repeat polysomnography while using CPAP devices to evaluate the exact evaluation of the efficacy of CPAP therapy.
Measurement of Forearm Blood Flow
The measurement of FBF has been described previously.13 Briefly, all the studies were performed in a temperature‐controlled room, with the subjects in the resting and supine states. Forearm blood flow (mL · min−1 · 100 mL−1 of forearm volume) was measured by the venous‐occlusion technique using a mercury‐filled silastic strain‐gauge plethysmograph (model EC‐5R; D.E. Hokanson Inc, Bellevue, WA). The subjects were requested to rest for 20 minutes to obtain stable baseline measurements. After the baseline condition was established, the basal FBF measurement was obtained from the rate of the increase in forearm volume, with venous return from the forearm being prevented by inflation of a cuff on the upper arm using a venous occlusion pressure of 50 mm Hg. The flow measurements were recorded for 7 seconds every 15 seconds, and the mean of 4 measurements was used in the analyses. To evaluate FBF induced by reactive hyperemia (RH), FBF was occluded by inflation of a cuff placed over the left upper arm to a pressure of 200 mm Hg for 5 minutes. After the ischemic cuff occlusion was released, FBF was measured every 15 seconds for 3 minutes, according to the procedures described above.
Biochemical Analyses
Blood samples were collected from the patients before the FBF measurements, centrifuged at 4°C within 20 minutes, and then stored at −80°C until assayed. The plasma levels of the nitric oxide compounds (NOx; NO2−+ NO3−) and asymmetrical dimethylarginine (ADMA) were measured by high‐performance liquid chromatography using methods described previously.14 The plasma levels of tumor necrosis factor‐α (TNF‐α), interleukin 1β (IL‐1β), IL‐6, IL‐8 (BioSource International, Inc, Camarillo, CA), thiobarbituric acid reactive substance (TBARS; Cayman Chemical Co, Ann Arbor, MI), soluble CD40 ligand (sCD40L; BioSource International), and soluble Fas ligand (sFasL; Medical Biological Laboratories Co, Ltd, Nagoya, Japan) were measured by enzyme‐linked immunosorbent assay.13., 14. The biochemical parameters were measured in duplicate and the mean value used in the analyses.
Statistical Analysis
The data were expressed as mean ± SD. Paired t tests were performed to compare endothelial responses or biochemical changes before and after CPAP intervention. A univariate regression analysis was carried out to evaluate the correlations of AHI and various parameters, including FBF response to RH at baseline. To assess independent determinants that showed significant correlations with improvement of FBF to RH, multivariate analysis based on stepwise regression analysis used after a univariate regression analysis was carried out as the independent variable. The odds ratios and 95% confidence intervals were calculated. A P value of <0.05 was considered statistically significant.
Results
Patient Characteristics and Effect of Continuous Positive Airway Pressure on Physiological Factors and Sleep Status
The baseline characteristics of the 32 patients with OSAS are presented in Table 1. Mean AHI before treatment was 56.2 ± 21.6, and mean ESS score at baseline was 17.4 ± 5.3. Continuous positive airway pressure therapy markedly improved AHI and ESS score. Waist circumference, body weight, body mass index, and arterial blood pressure were decreased significantly after treatment with CPAP, in association with a slight improvement in hyperglycemia and dyslipidemia. However, glycated hemoglobin (HbA1c) did not alter after CPAP therapy; HbA1c reflects the glycemic profile over a period of a few months prior to the measurement. Therefore, longer observation may be needed.
Table 1.
Baseline Characteristics and Changes in Physiological Parameters of the Patients Following CPAP Therapy
| Before CPAP Therapy | After CPAP Therapy | P Value | |
|---|---|---|---|
| Age, y | 53.9 ± 8.6 | ||
| Gender, M/F | 19/13 | ||
| Height, cm | 164.3 ± 8.6 | ||
| Weight, kg | 72.6 ± 13.0 | 71.1 ± 11.5 | <0.01 |
| BMI, kg/m2 | 26.7 ± 3.6 | 26.2 ± 3.3 | <0.01 |
| Waist circumference, cm | 90.7 ± 6.5 | 90.2 ± 6.5 | <0.01 |
| ESS | 17.4 ± 5.3 | 7.2 ± 3.9 | <0.01 |
| AHI, events/h | 56.2 ± 21.6 | 3.5 ± 2.3 | <0.01 |
| Hypertension, n (%) | 26 (81.3%) | ||
| Receiving drug, n (%) | 11 (42.3%) | ||
| SBP, mm Hg | 142.5 ± 11.0 | 137.7 ± 7.9 | <0.01 |
| DBP, mm Hg | 80.2 ± 8.3 | 76.3 ± 6.0 | <0.01 |
| mBP, mm Hg | 101.0 ± 8.1 | 96.8 ± 5.7 | <0.01 |
| HR, bpm | 73.9 ± 8.1 | 73.3 ± 7.6 | 0.71 |
| DM, n (%) | 22 (71.0) | ||
| Receiving drug, n (%) | 11 (50.0) | ||
| FBG, mg/dL | 113.9 ± 6.5 | 110.7 ± 7.1 | 0.032 |
| HbA1c, % | 5.9 ± 0.4 | 5.9 ± 0.4 | 0.28 |
| Dyslipidemia, n (%) | 26 (81.3) | ||
| Receiving drug, n (%) | 8 (30.8) | ||
| LDL‐C, mg/dL | 128.4 ± 25.6 | 123.3 ± 16.1 | 0.090 |
| HDL‐C, mg/dL | 45.0 ± 9.4 | 47.1 ± 7.0 | 0.070 |
| Triglycerides, mg/dL | 220.4 ± 36.9 | 215.1 ± 36.8 | 0.305 |
Abbreviations: AHI, apnea‐hypopnea index; BMI, body mass index; CPAP, continuous positive airway pressure; DBP, diastolic blood pressure; DM, diabetes mellitus; ESS, Epworth Sleepiness Scale; F, female; FBG, fasting blood glucose; HDL‐C, high‐density lipoprotein cholesterol; HR, heart rate; LDL‐C, low‐density lipoprotein cholesterol; M, male; mBP, mean blood pressure; SBP, systolic blood pressure.
Association Between Apnea‐Hypopnea Index and Forearm Blood Flow Responses to Reactive Hyperemia Before Treatment
There was a significant inverse correlation between the level of basal AHI and the FBF responses to RH (Figure 1; r = 0.569, P = 0.0007). The basal AHI also correlated with the levels of NOx (r = 0.628, P = 0.0001), ADMA (r = 0.494, P = 0.004), TBARS (r = 0.462, P = 0.008), CD40L (r = 0.356, P = 0.046), and TNF‐α (r = 0.420, P = 0.017). The FBF response to RH has significant associations with the levels of NOx (r = 0.559, P = 0.0009), ADMA (r = 0.436, P = 0.0127), and TBARS (r = 0.435, P = 0.0128).
Figure 1.
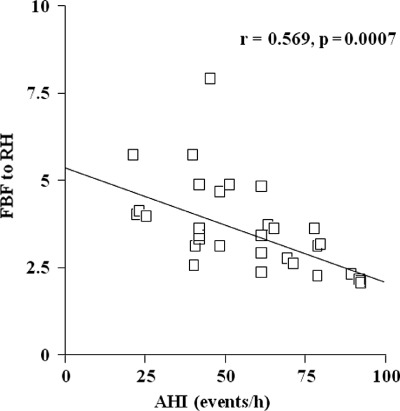
The correlation between AHI and FBF response to RH. Abbreviations: AHI, apnea‐hypopnea index; FBF, forearm blood flow; h, hour; RH, reactive hyperemia.
Effect of Continuous Positive Airway Pressure on Forearm Blood Flow Responses to Reactive Hyperemia
Baseline FBFs were almost the same before and after CPAP treatment. As shown in Figure 2, the peak FBF RH‐induced response was increased significantly after CPAP treatment.
Figure 2.
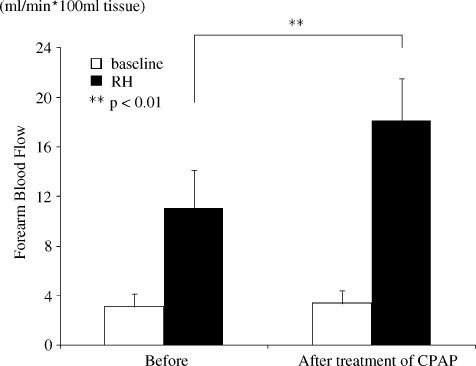
The effects of CPAP therapy on endothelial function in patients with MetS and OSAS for 3 months. Abbreviations: CPAP, continuous positive airway pressure; MetS, metabolic syndrome; OSAS, obstructive sleep apnea syndrome; RH, reactive hyperemia. **P < 0.01.
Assessment of Biochemical Changes Associated With the Use of Continuous Positive Airway Pressure
After CPAP therapy, the level of NOx increased, whereas the levels of TBARS, ADMA, sFasL, and sCD40L decreased (Figure 3). Decreased plasma levels of TNF‐α, IL‐6, and IL‐8 were also observed after CPAP treatment, although the level of IL‐1β remained unchanged (Figure 4). Table 2 shows the results of multiple stepwise regression analysis to find the determinants of improvement of FBF response to RH. The independent variables were increment of NOx, decrement of ADMA, IL‐6, and TNF‐α.
Figure 3.
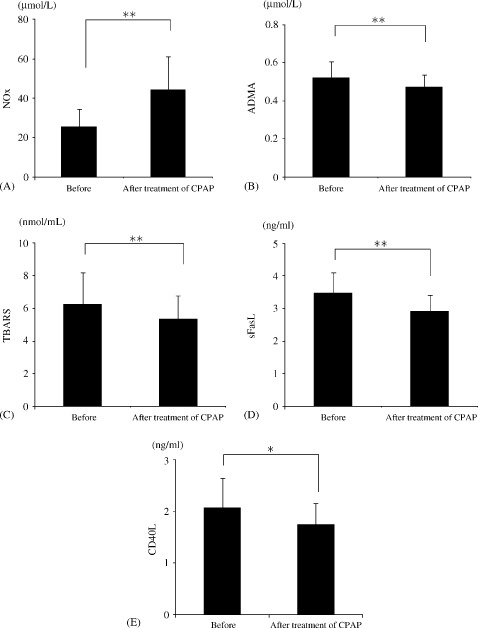
Alterations in the plasma levels of NOx (A), ADMA (B), TBARS (C), sFasL (D), and CD40L (E) in each group. Abbreviations: ADMA, asymmetrical dimethylarginine; CD40L, soluble CD40 ligand; CPAP, continuous positive airway pressure; NOx, nitric oxide compounds; sFasL, soluble Fas ligand; TBARS, thiobarbituric acid reactive substance. *P < 0.05, **P < 0.01.
Figure 4.
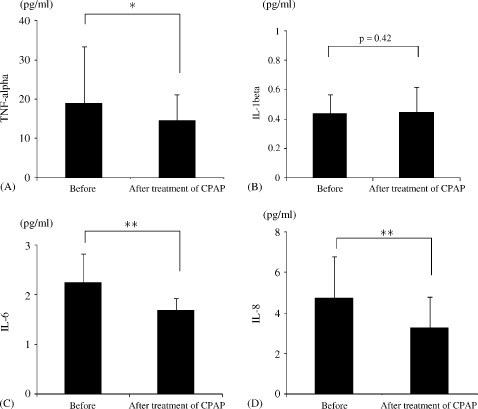
Alterations in the plasma levels of TNF‐α (A), IL‐1β (B), IL‐6 (C), and IL‐8 (D) in each group. Abbreviations: CPAP, continuous positive airway pressure; IL, interleukin; TNF, tumor necrosis factor. *P < 0.05, **P < 0.01.
Table 2.
Multivariate Stepwise Regression Analysis of Improvement of Forearm Blood Flow Responses to Reactive Hyperemia
| Odds Ratio | P Value | 95% CI | |
|---|---|---|---|
| ΔNO | 0.04 | 0.002 | 0.01–0.06 |
| ΔADMA | −7.41 | 0.007 | −12.63 to −2.20 |
| ΔIL‐6 | −0.49 | 0.052 | −1.00 to 0.01 |
| ΔTNF‐α | −0.07 | 0.006 | −0.12 to −0.02 |
Abbreviations: ADMA, asymmetrical dimethylarginine; CI, confidence interval; IL, interleukin; NO, nitric oxide; TNF, tumor necrosis factor. Δ represents increment.
Discussion
The present study demonstrated that medium‐term CPAP treatment ameliorated RH‐induced FBF responses in patients with the MetS and OSAS. We also showed that CPAP therapy markedly reduced the plasma levels of TBARS, TNF‐α, IL‐6, IL‐8, sFas L, and sCD40L. Continuous positive airway pressure therapy resulted in an improvement in the forearm vascular function, with the patients appearing to develop potent anti‐inflammatory and antiapoptotic activities.
The Metabolic Syndrome, Obstructive Sleep Apnea Syndrome, and Endothelial Function
Obstructive sleep apnea syndrome and the MetS are well‐established CV risk factors for the development of atherosclerosis. The prevalence of OSAS is also a risk factor for the development of hypertension and diabetes mellitus.15 Previous studies have also shown the presence of endothelial dysfunction in patients with the MetS16., 17. or OSAS.18 However, little is known regarding the impact of these 2 diseases on vascular function and biochemical changes and their influence on the pathogenesis of future CV risk. It has been proposed that endothelial dysfunction plays a pathogenic role in the early manifestation of atherosclerotic vascular disease and may predict future CV events. This supports the concept that abnormalities in the endothelium are one of the earliest manifestations of vasculopathies. The response of FBF to RH is considered to be a marker of endothelial function.13., 19., 20.
Effect of Continuous Positive Airway Pressure on Endothelial Dysfunction, Oxidative Stress, and Biomarkers
Continuous positive airway pressure therapy prevents apneas and associated oxygen desaturation, and there is growing evidence that the treatment has long‐term benefits on CV morbidity and mortality. A 7‐year follow‐up study carried out by Peker et al7 showed an increased incidence of CV disease in OSAS patients whose treatment was incomplete compared with those who were treated efficiently. Furthermore, a reduction in CV deaths was observed in OSAS patients treated with CPAP compared with untreated patients over an average follow‐up of 7.5 years.8 Similarly, in a large CV outcome study with a 10‐year follow‐up period, severe untreated OSAS significantly increased the risk of fatal and nonfatal CV events.9 These data suggest there is a selective and dose‐dependent activation of inflammatory pathways by intermittent hypoxia/reoxygenation and support a specific role for this event in the pathophysiology of CV complications in OSAS.
In vitro investigations have shown sustained hypoxia activates hypoxia‐inducible factor‐1–dependent transcription, whereas intermittent hypoxia selectively activates nuclear factor κB (NFκB)‐dependent transcription.21 In OSAS patients, the levels of C‐reactive protein, IL‐6, and 8‐hydroxydeoxyguanosine (8‐OHdG) are increased compared with control subjects and are proportional to the severity of AHI.22., 23. Moreover, the relationship between expression of endothelial nitric oxide synthase (eNOS), phosphorylated eNOS, nitrotyrosine, and NFκB and the severity of OSAS has been identified, with the expression of eNOS and phosphorylated eNOS increasing, whereas expression of nitrotyrosine and NFκB and the levels of C‐reactive protein and IL‐6 decrease in patients with OSAS using CPAP.23., 24. Measuring the level of TBARS is a method for monitoring lipid peroxidation, a major indicator of oxidative stress. Oxidative stress increases Fas ligand expression in endothelial cells,25 and it has been reported that the plasma level of sFasL correlates with the forearm RH in patients with coronary artery disease.26 Hypoxia and subsequent reoxygenation induce signal transducer and activator of transcription 1 (STAT1),27 which in turn stimulates expression of proapoptotic genes such as Fas and Fas L.28 The effects of CPAP may therefore alter its role against stress in the progressive stages of CV disease. The findings of the present study demonstrate that CPAP treatment for 3 months effectively improves endothelial function in patients with the MetS and OSAS who have an increased risk of developing deteriorative atherosclerotic diseases. The study also suggests that improving endothelial dysfunction using CPAP therapy may reduce CV events by suppressing oxidative stress, inflammation, and apoptosis. These mechanisms may also be associated with improved FBF responses and inflammation in patients with the MetS and OSAS using CPAP.
Study Limitations
This was a preliminary, single‐arm study carried out for only 3 months in a small number of subjects. A multicenter, large‐scale, comparative study over a longer period is therefore needed in the future, although it is difficult to subject patients to CPAP in a randomized placebo‐controlled study, as it is well established that the procedure is one of the most beneficial therapies for OSAS. However, we found significant effects of CPAP, not only on AHI, but also on changes in plasma levels of TBARS and inflammatory cytokines in patients with OSAS and the MetS.
Conclusion
We demonstrated that CPAP therapy improves endothelial dysfunction and decreases the levels of oxidative stress and inflammatory cytokines in patients with the MetS and OSAS. As endothelial function provides a prognostic marker of atherosclerotic CV disease, our data suggest that CPAP may be helpful for preventing the progressive development of atherogenic risk factors in patients with the MetS and OSAS.
Acknowledgements
The authors thank Keiko Tsuchida, Sachiyo Taguchi, and Yasuko Ueda for their expert technical assistance during the study.
References
- 1. Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta‐analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–414. [DOI] [PubMed] [Google Scholar]
- 2. McCurry J. Japanese people warned to curb unhealthy lifestyles: health experts urge a return to dietary basics to prevent future health problems. Lancet. 2004;363:1126. [DOI] [PubMed] [Google Scholar]
- 3. Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep‐disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep‐disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. [DOI] [PubMed] [Google Scholar]
- 5. Nieto FJ, Young TB, Lind BK, et al. Association of sleep‐disordered breathing, sleep apnea, and hypertension in a large community‐based study: Sleep Heart Health Study. JAMA. 2000;283:1829–1836. [DOI] [PubMed] [Google Scholar]
- 6. Shahar E, Whitney CW, Redline S, et al. Sleep‐disordered breathing and cardiovascular disease: cross‐sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. [DOI] [PubMed] [Google Scholar]
- 7. Peker Y, Hedner J, Norum J, et al. Increased incidence of cardiovascular disease in middle‐aged men with obstructive sleep apnea: a 7‐year follow‐up. Am J Respir Crit Care Med. 2002;166:159–165. [DOI] [PubMed] [Google Scholar]
- 8. Doherty LS, Kiely JL, Swan V, et al. Long‐term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest. 2005;127:2076–2084. [DOI] [PubMed] [Google Scholar]
- 9. Marin JM, Carrizo SJ, Vicente E, et al. Long‐term cardiovascular outcomes in men with obstructive sleep apnoea‐hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. [DOI] [PubMed] [Google Scholar]
- 10. Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290:1906–1914. [DOI] [PubMed] [Google Scholar]
- 11. Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–545. [DOI] [PubMed] [Google Scholar]
- 12. American Academy of Sleep Medicine Task Force. Sleep‐related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;2:667–689. [PubMed] [Google Scholar]
- 13. Oyama J, Maeda T, Sasaki M, et al. Green tea catechins improve human forearm vascular function and have potent anti‐inflammatory and anti‐apoptotic effects in smokers. Intern Med. 2010;49:2553–2559. [DOI] [PubMed] [Google Scholar]
- 14. Oyama J, Maeda T, Kouzuma K, et al. Green tea catechins improve human forearm endothelial dysfunction and have antiatherosclerotic effects in smokers. Circ J. 2010;74:578–588. [DOI] [PubMed] [Google Scholar]
- 15. Parati G, Lombardi C, Narkiewicz K. Sleep apnea: epidemiology, pathophysiology, and relation to cardiovascular risk. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1671–R1683. [DOI] [PubMed] [Google Scholar]
- 16. Lind L. Endothelium‐dependent vasodilation, insulin resistance and the metabolic syndrome in an elderly cohort: the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Atherosclerosis. 2008;196:795–802. [DOI] [PubMed] [Google Scholar]
- 17. Title LM, Lonn E, Charbonneau F, et al. Relationship between brachial artery flow mediated dilatation, hyperemic shear stress, and the metabolic syndrome. Vasc Med. 2008;13:263–270. [DOI] [PubMed] [Google Scholar]
- 18. Chung S, Yoon IY, Shin YK, et al. Endothelial dysfunction and inflammatory reactions of elderly and middle‐aged men with obstructive sleep apnea syndrome. Sleep Breath. 2009; 13:11–17. [DOI] [PubMed] [Google Scholar]
- 19. Tagawa T, Imaizumi T, Endo T, et al. Role of nitric oxide in reactive hyperemia in human forearm vessels. Circulation. 1994;90:2285–2290. [DOI] [PubMed] [Google Scholar]
- 20. Higashi Y, Sasaki S, Nakagawa K, et al. Effect of the angiotensin‐converting enzyme inhibitor imidapril on reactive hyperemia in patients with essential hypertension: relationship between treatment periods and resistance artery endothelial function. J Am Coll Cardiol. 2001;37:863–870. [DOI] [PubMed] [Google Scholar]
- 21. Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–2667. [DOI] [PubMed] [Google Scholar]
- 22. Shamsuzzaman AS, Winnicki M, Lanfranchi P, et al. Elevated C‐reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105:2462–2464. [DOI] [PubMed] [Google Scholar]
- 23. Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of C‐reactive protein and interleukin‐6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107:1129–1134. [DOI] [PubMed] [Google Scholar]
- 24. Jelic S, Lederer DJ, Adams T, et al. Vascular inflammation in obesity and sleep apnea. Circulation. 2010;121:1014–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suzuki M, Aoshiba K, Nagai A. Oxidative stress increases Fas ligand expression in endothelial cells. J Inflamm (Lond). 2006; 3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blanco‐Colio LM, Martín‐Ventura JL, Tuñón J, et al. Soluble Fas ligand plasma levels are associated with forearm reactive hyperemia in subjects with coronary artery disease: a novel biomarker of endothelial function? Atherosclerosis. 2008;201:407–412. [DOI] [PubMed] [Google Scholar]
- 27. Terui K, Haga S, Enosawa S, et al. Hypoxia/re‐oxygenation‐induced, redox‐dependent activation of STAT1 (signal transducer and activator of transcription 1) confers resistance to apoptotic cell death via hsp70 induction. Biochem J. 2004;380(part 1):203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stephanou A, Scarabelli TM, Brar BK, et al. Induction of apoptosis and Fas receptor/Fas ligand expression by ischemia/reperfusion in cardiac myocytes requires serine 727 of the STAT‐1 transcription factor but not tyrosine 701. J Biol Chem. 2001;276: 28340–28347. [DOI] [PubMed] [Google Scholar]


