Abstract
Background:
The Myocardial Infarction Network Essen was initiated in order to establish a standardized procedure with immediate reopening of the infarcted vessel for patients with ST‐elevation myocardial infarction (STEMI) in the city of Essen, Germany. The present study aims to evaluate gender‐related differences in presentation of disease and clinical outcome.
Hypothesis:
Gender is associated with differences in presentation and outcome of STEMI.
Methods:
All patients with STEMI were included without exception. Parameters such as risk profile, mortality, and relevant time intervals were documented. The follow‐up period was 1 year.
Results:
For this study, 1365 patients (72.1% male) were recruited. Women were significantly older, with higher prevalence of diabetes (28.1% vs 20.3%, P = 0.004) and hypertension (76.5% vs 64.8%, P<0.0005). Analysis of time intervals between symptoms to actions showed no significant differences. However, women tended to wait longer before calling for medical assistance (358 vs 331 min, P = 0.091). In‐hospital mortality was comparable with respect to gender, whereas women had higher 1‐year mortality (18.6% vs 13.2%). Age and diabetes were associated with a higher mortality. Adjusted for age, gender is no longer an independent risk factor. In the follow‐up period, significantly more women were readmitted to the hospital without a difference in the frequency of reangiography, surgery, or target‐vessel revascularization.
Conclusions:
The present data display a successful implementation of a standardized procedure in patients with STEMI. Although differences between genders are not as obvious as expected, efforts should be taken to perform a gender‐specific risk analysis as well as to promote education about proper behavior in case of new onset of angina. © 2011 Wiley Periodicals, Inc.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
Although gender‐related differences in treatment and prognosis of acute myocardial infarction (MI) have been documented for more than a decade, the results are still conflicting. Some investigations have described a generally consistent pattern of less‐intensive treatment in acute MI in women as compared with men,1, 2, 3 whereas more recent studies have shown differences in clinical characteristics and risk profile, leading to a poorer prognosis of ST‐elevation myocardial infarction (STEMI) in women.4
In general, there is a decreasing mortality in STEMI due to a more effective therapeutic management based on immediate acute interventional revascularization instead of primary thrombolytic therapy.5., 6 However, the implementation of a direct transfer of all STEMI patients within an urban environment to a catheterization laboratory with 24‐hour standby requires special logistical conditions.
The Myocardial Infarction Network Essen was initiated in September 2004 to establish a standardized strategy and therapy for patients with STEMI in the city of Essen, Germany. The primary goal is the immediate reopening of the infarcted vessel. The logistics of the emergency system were reorganized so that all patients with documented STEMI (12‐lead electrocardiogram [ECG] was recorded in the emergency ambulance) were immediately transferred to a hospital with a 24‐hour standby catheterization laboratory. If the patient presented in a hospital without the standby option, or at any outpatient physician, he was directly transferred to a cooperating invasive center. Compared with previously established networks, the present project ensures a complete acquisition of all STEMI patients with the initiation of identical logistical procedures depending on the first medical point of contact of the patient. This approach permits the comprehensive and realistic description of the behavior and therapy of all these patients in a city with a population >500 000. Furthermore, there is a consistent acquisition of all relevant time intervals (from symptoms onset through call for medical assistance to the duration of the invasive procedure). Patient handling including medical therapy is totally standardized and documented in a field manual. In collaboration with several health insurance companies, innovative therapy strategies were applied according to guidelines, including implementation of drug‐eluting stents (DES) and the application of magnetic resonance imaging. Additionally, patients were enrolled in rehabilitation following their hospital discharge.
As all patients with STEMI are included without exception with the same therapeutic approach regardless of age, gender, or comorbidities, the project offers the possibility to analyze gender‐related differences in the outcome of acute MI patients with special consideration of the severity of the disease, variations in patient behavior in the context of typical symptoms, as well as the influence of risk factors of coronary artery disease, such as diabetes or hypertension. All patients are followed up for 1 year. The present study summarizes the gender‐related differences after 4 years of the study, with follow‐up completed in August 2008.
Methods
Patients with STEMI were included immediately at admission; or, in case of emergency transport to the 24‐hour standby catheterization laboratory, if the diagnosis of STEMI could be documented by ECG, whether they survived to hospital admission or not. Patients were only excluded in the case of resuscitation for unknown reasons and no ECG was available.
Data acquired from all patients with STEMI were systematically documented. In addition to risk profile, blood test results, localization of MI, and coronary status, relevant time intervals from symptoms onset to the reopening of the target vessel are registered. Further data include ejection fraction (EF), shock or hemodynamic instability at time of admission (defined as a systolic blood pressure <90 mm Hg, heart rate >100/bpm, or cardiac index <1.8 L/min/m2 body surface and left ventricular end diastolic pressure >20 mm Hg), TIMI (Thrombolysis In Myocardial Infarction Trial) flow prior to and after intervention, type of stent, the concomitant medical therapy, as well as bleeding complications in the course of the interventional therapy (defined as need for blood transfusion or vascular intervention). Most important, the in‐hospital mortality and survival after 6 and 12 months following hospital discharge were documented. All patients gave their written informed consent for evaluation of data.
Within 3 days after discharge from the hospital or rehabilitation center, the patients presented to a cardiologist or a general practitioner who is part of the network. Further follow‐ups were carried out by outpatient cardiologists.
In the 1‐year follow‐up period subsequent to hospital discharge, a structured interview with the patient and general practitioner was performed after 6 and 12 months. The questions asked included, among others, the incidence of cardiovascular events, rehospitalization, re‐angiography with need for interventional therapy, and medication. Inconsistencies in the comments of the patient and the general practitioner were evaluated according to plausibility. In case of readmission to hospital, the medical report was viewed with the patients' approval.
Statistical Analysis
The association between gender and categorical or ordinal variables was analyzed using contingency tables and the Fisher exact test. The distribution of the examined time intervals of men and women were compared using the Mann‐Whitney U test. Logistic regression was used to examine the influence of gender on mortality, adjusted for age and the presence of diabetes. In the tables, the numbers of subjects in some subcategories may not add up to the total number of subjects in the study due to missing data.
Results
The first 1‐year follow‐up was completed on August 31, 2006, and the third on August 31, 2008, with 1355 out of 1365 patients with a completed follow‐up period. More male than female patients were recruited (men 72.1%, women 27.9%); women were significantly older (67.9 ± 13.4 y vs 61.2 ± 12.8 y, P<0.0001) and had a lower body mass index (27.4 ± 5.3 kg/m2 vs 27.9 ± 4.3 kg/m2, P<0.002). Baseline characteristics of all subjects are given in Table 1. With respect to risk factors (see also Table 2), significantly more women suffered from diabetes (28.1% vs 20.3%, P = 0.004) and hypertension (76.5% vs 64.8%, P<0.0005), whereas more male patients were smokers (47.1% vs 30.8%, P<0.0005). Furthermore, bleeding complications occurred more often in the women. No significant difference between gender could be found in EF, infarct localization, and coronary status related to the number of vessels with relevant stenosis ≥75%. Gender did not play a role with respect to renal dysfunction. The frequency of shock or cardiac resuscitation at time of admission was similar in both genders.
Table 1.
Baseline Characteristics of All Patients
| Male (n = 984) | Female (n = 381) | P Value | |
|---|---|---|---|
| Age (y) | 61.2 ± 12.8 | 67.9 ± 13.4 | <0.000a,b |
| BMI (kg/m2) | 27.9 ± 4.3 | 27.4 ± 5.3 | 0.002a,b |
| Infarct localization (%) | |||
| AMI | 42.9 | 43.8 | 0.761 |
| IMI | 50.2 | 48.8 | 0.673 |
| LMI | 12.5 | 12.6 | 1 |
| Hypertension (%) | 64.8 | 76.5 | <0.0005b |
| Diabetes (%) | 20.3 | 28.1 | 0.004b |
| Hypercholesterol (%) | 55.2 | 56.7 | 0.659 |
| Smoking (%) | 47.1 | 30.8 | <0.0005b |
| Serum creatinine >2 mg/dL (%) | 1.19 ± 0.7 | 1.15 ± 0.84 | 0.410 |
| 3.3 | 4.4 | ||
| Coronary status (%) | 0.563 | ||
| 0 VD | 4.4 | 5.0 | |
| 1 VD | 41.0 | 41.8 | |
| 2 VD | 28.7 | 27.9 | |
| 3 VD | 20.5 | 21.8 | |
| Center main | 5.5 | 3.4 | |
| EF (%) | 0.831 | ||
| >55% | 41.4 | 37.5 | |
| 41%–55% | 33.7 | 35.7 | |
| 31%–40% | 17.0 | 17.9 | |
| ≤30% | 7.8 | 8.9 | |
| Stents (%) | 0.728 | ||
| No stent | 14.3 | 13.6 | |
| BMS | 28.8 | 32.0 | |
| DES | 53.8 | 51.3 | |
| BMS + DES | 3.1 | 3.1 | |
| Shock/CPR at time of admission (%) | 16.8 | 20.3 | 0.133 |
| TIMI before intervention (%) | 0.063 | ||
| ≤2 | 91.9 | 88.4 | |
| 3 | 8.1 | 11.6 | |
| TIMI after intervention (%) | 0.893 | ||
| ≤2 | 7.4 | 8.5 | |
| 3 | 92.6 | 91.5 | |
| Bleeding complications including blood transfusion (%) | 0.9 | 3.8 | 0.001b |
Abbreviations: AMI, anterior myocardial infarction; BMI, body mass index; BMS, bare‐metal stent; CPR, cardiopulmonary resuscitation; DES, drug‐eluting stent; EF, ejection fraction; IMI, inferior myocardial infarction; LMI, lateral myocardial infarction; TIMI, Thrombolysis In Myocardial Infarction; VD, vessel disease.
P values are from Fisher exact test. Percent values are separate for males and females.
Mann‐Whitney U test is given for comparison between groups.
P<0.05.
Table 2.
Analysis of Risk Factors and Disease Parameters With Respect to Dependency on Mortality
| Male | Female | |||||
|---|---|---|---|---|---|---|
| Survivor (n) | Nonsurvivor (n) | P Value | Survivor (n) | Nonsurvivor (n) | P Value | |
| Hypercholesterol | 0.089 | 0.270 | ||||
| Yes | 457 | 55 | 166 | 33 | ||
| No | 355 | 61 | 119 | 33 | ||
| Smoking | 0.005a | 0.001a | ||||
| Yes | 401 | 40 | 101 | 9 | ||
| No | 420 | 75 | 191 | 56 | ||
| Diabetes | 0.013a | 0.014a | ||||
| Yes | 158 | 34 | 73 | 27 | ||
| No | 673 | 81 | 218 | 38 | ||
| Hypertension | 0.356 | 0.522 | ||||
| Yes | 531 | 82 | 228 | 52 | ||
| No | 296 | 37 | 73 | 13 | ||
| BMI >30 kg/m2 | 0.834 | 0.557 | ||||
| Yes | 239 | 38 | 86 | 17 | ||
| No | 612 | 92 | 224 | 54 | ||
| EF | <0.0005a | <0.0005a | ||||
| >55% | 369 | 13 | 122 | 8 | ||
| 41%–55% | 287 | 24 | 109 | 15 | ||
| 31%–40% | 113 | 44 | 46 | 16 | ||
| ≤30% | 34 | 38 | 9 | 22 | ||
| Shock/CPR at time of admission | <0.0005a | <0.0005a | ||||
| No | 748 | 67 | 267 | 36 | ||
| Yes | 102 | 62 | 43 | 34 | ||
| Antiplatelet therapy after 1 year | <0.0005a | <0.0005a | ||||
| No ASS/C | 30 | 31 | 13 | 21 | ||
| ASS or C | 498 | 2 | 184 | 3 | ||
| ASS + C | 247 | 5 | 82 | 2 | ||
| Bleeding complications including blood transfusion | 0.096 | 0.007a | ||||
| Yes | 6 | 3 | 7 | 7 | ||
| No | 833 | 120 | 292 | 63 | ||
| Serum creatinine >2 mg/dL (%) | <0.0005a | <0.0005a | ||||
| Yes | 18 | 14 | 6 | 10 | ||
| No | 819 | 108 | 293 | 55 | ||
| Cardiac rehabilitationb | 0.149 | 0.012a | ||||
| Yes | 631 | 24 | 216 | 15 | ||
| No | 124 | 9 | 43 | 10 | ||
Abbreviations: BMI, body mass index; C, clopidogrel; CPR, cardiopulmonary resuscitation; EF, ejection fraction; TIMI, Thrombolysis In Myocardial Infarction.
P value (Fisher exact test) is given for comparison between groups.
P<0.05.
Only outpatient data.
Detailed analysis of logistical procedures expressed as time intervals from symptoms onset to balloon inflation time ruled out significant differences between men and women, except for the fact that women tended to wait longer from beginning of symptoms before calling for medical assistance (median value: 226 min vs 208 min, P = 0.091) (Figure 1).
Figure 1.
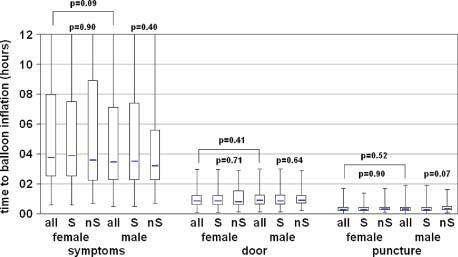
Boxplots of time to balloon inflation with respect to gender and mortality. P value is given for the comparison between groups (Mann‐Whitney U test). Abbreviations: nS, nonsurvivors; S, survivors.
Considering the angiographic results, the success rate of revascularization as well as the TIMI flow before intervention was comparable between genders. No difference could be found in choice of stent; in both genders, DES were used in >50%.
With respect to mortality, there was no significant difference between genders regarding in‐hospital mortality, although women showed a trend to a higher mortality (8.5% vs 11.0%, P = 0.175). This trend turned statistically significant after 1 year, mainly due to a higher mortality in women within the first 6 months after hospital discharge (13.2% vs 18.6%, P = 0.013) (Figure 2).
Figure 2.
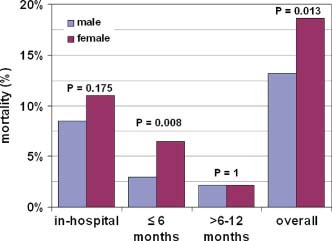
Mortality with respect to gender. P value is given for the comparison between groups (Fisher exact test, P < 0.05 in bold).
The analysis of variables with possible influence on mortality revealed that increasing age is associated with a higher mortality in both genders. This was also true for diabetes, whereas the presence of hypertension had no influence on mortality. Paradoxically, nonsmoking was unfavorable for both genders.
Adjusted for age, gender was no longer an independent risk factor for mortality (odds ratio [OR]: 1.079, P = 0.676), contrary to the presence of diabetes and age (OR: 1.662, P = 0.005 and OR: 1.061, P<0.001, respectively), as both were independent risk factors for mortality.
Furthermore, bleeding complications not only occurred more often in women, but were associated with a significantly higher mortality. On the other hand, renal dysfunction expressed as serum creatinine >2 mg/dL was associated with a higher mortality in both genders (Table 2). With regard to the characteristics of disease, infarct localization did not affect mortality except in the case of anterior MI, which was associated with a higher mortality in men. Shock or resuscitation at time of admission resulted in higher mortality for both men and women (Table 2).
Furthermore, in the present data there was no dependency on mortality on any time interval from the onset of symptoms to balloon inflation, except for the time from puncture to balloon, which showed a trend to a decreasing mortality in men in the case of shorter time intervals (Figure 1).
Concerning the choice of stent—bare‐metal stent (BMS) compared with DES—the data displayed a lower mortality only in men with implanted DES.
As expected, the mortality was substantially higher in the case of discontinuation of dual platelet therapy (aspirin and clopidogrel) in both genders.
About 80% in both genders took part in rehabilitation, but this was associated with a lower mortality only in women (Table 2).
In the follow‐up period, significantly more women were readmitted to hospital but without a significant difference with respect to the frequency of reangiography, surgery, or target‐vessel revascularization (TVR). In case of drug therapy, the compliance in taking medication (statins, aspirin, clopidogrel, β‐blocker, angiotensin‐converting enzyme inhibitor) was comparable between genders except for statins, which were taken more often in men (Table 3).
Table 3.
1‐Year Follow‐Up
| Male (n = 984) | Female (n = 381) | P Value | |
|---|---|---|---|
| Reangiography (%) | 54.6 | 50.4 | 0.089 |
| CABG (%) | 6.3 | 3.0 | 0.155 |
| TVR (% of gender) | 7.5 | 4.2 | 0.196 |
| Rehabilitation (%) | 79.0 | 76.6 | 0.415 |
| Drug therapy (%) | |||
| β‐Blocker | 89.1 | 90.3 | 0.655 |
| ACE inhibitor | 77.4 | 68.8 | 0.075 |
| Statins | 85.9 | 80.5 | 0.036 |
| Antiplatelet therapy (%) | 0.617 | ||
| ASS or clopidogrel | 63.1 | 64.9 | |
| No ASS or clopidogrel | 5.7 | 6.6 | |
| ASS + clopidogrel | 31.2 | 28.5 |
Abbreviations: ACE, angiotensin‐converting enzyme; ASS, aspirin; CABG, coronary artery bypass grafting; TVR, target‐vessel revascularization. Fisher exact test is given for comparison between groups.
Percent values are separate for males and females.
Discussion
The present study gives an insight into the clinical presentation and outcome of gender‐specific differences in patients with STEMI. Contrary to most data available from registries or randomized clinical trials, the ongoing study represents the actual situation of medical healthcare in clinical practice, as all STEMI patients are included independent of age, comorbidities, or first contact with medical aid. This comprehensive inclusion of all patients explains the relatively high 1‐year mortality (male 13.2%, female 18.6%). Lower mortalities in previous studies are based on an exclusion of several patient groups, such as those with cardiogenic shock, low EF, prior MI, or hypertension difficult to treat.7, 8, 9 On the other hand, data from registries show higher 1‐year mortalities resulting from the lack of a standardized therapeutic approach.10 The results of the Danish Acute Myocardial Infarction (DANAMI) study, with a 3‐year mortality of 13.6% in the group of patients with interventional therapy, are more comparable with the STEMI network Essen.6
With respect to gender‐related differences, the clear predominance of males is in accordance with other multicenter trials in which about 25%–30% of women were recruited.5., 11 In our data, the in‐hospital mortality was comparable between genders. This contrasted to the results after 1 year, with a higher mortality in women, mainly due to an increased mortality within the first 6 months after discharge from hospital. This difference appears to be largely explained by age and the clinical differences at presentation. Significantly more women suffered from diabetes and hypertension, whereas more male patients were smokers. This is in accordance with existing data revealing a higher prevalence of these risk factors in women.1, 12 Adjusted for age, gender is no longer an independent risk factor for mortality. On the other hand, the presence of diabetes was confirmed as an independent risk factor for mortality. This is also true for data from the Austrian acute percutaneous coronary intervention (PCI) registry, which revealed higher in‐hospital mortality in women as a result of higher comorbidities and older age in women.1 The main difference to the present data can be explained by the lack of a standardized therapeutic approach, as more women were treated on the basis of a conservative strategy in the Austrian registry.
Further data evaluating gender differences for in‐hospital and out‐of‐hospital mortality could show that male patients died more frequently within the first few hours of MI, mostly due to malignant arrhythmias, whereas female patients died in the subacute stage due to heart failure and lower EF.2, 13., 14 One could assume that the higher mortality of women in the first 6 months after discharge from hospital in our data could be also due to heart failure, keeping in mind that success rate of PCI as well as TVR in the follow‐up period did not show any difference in frequency between genders. Furthermore, the total rate of bleeding complications was low, but it was higher in the women than in the men. It is known that such complications have a strong impact on prognosis,15 and this factor was associated with higher mortality in the women. To confirm this hypothesis, a more detailed analysis of causes of death is required to explain this increased mortality in women in the first months following hospital discharge.
In most of the currently available studies, there is a general agreement that the increased mortality in women may be explained by their older age and higher prevalence of comorbidities, especially in the case of diabetes.1, 16, 17, 18 Nevertheless, the question of whether women have poorer results due to these comorbidities or because they are women has not yet been unambiguously answered. Earlier data with higher mortalities in women with acute coronary syndrome result mainly from differences in therapeutic strategies, with less‐invasive strategies in women and the limited amount of data due to the lack of randomized clinical studies including sufficient numbers of women. As the interventional approach in the STEMI network Essen is standardized, differences in therapeutic strategies for the gender subgroups can be ruled out. The significantly lower percentage of women in the present data remains a problem with regard to the analysis of gender‐related differences. Nevertheless, the obviously different risk profile in women highlights the importance of aggressive strategies to manage and prevent cardiovascular events in this high‐risk population.
Contrary to most registry data and randomized studies, the presentation of coronary artery disease in our data in terms of EF or hemodynamic status at time of admission was comparable between genders.11, 12, 19 The influence of these disease‐dependent parameters on mortality was similar in men and women. In agreement with other data, a low EF as well as hemodynamic instability were associated with a higher mortality.20 The poorer prognosis in case of nonsmoking for both genders was not anticipated, and may be due to the study size as well as to the veracity of the information given by the patient.
The comparison of certain time intervals between genders in the course of the total logistical procedure from symptoms' onset to the successful reopening of the infarcted vessel was of special interest to analyze gender‐specific differences in behavior, not only of the patient but also of the medical supply chain. Overall there were no specific differences between genders, except for the fact that women tended to wait longer before healthcare utilization. This trend is confirmed by data of Zimmermann et al showing longer prehospital delays before hospital admission.21 One of the reasons may be the greater diversity of symptoms in women, leading to more incertitude in the correct interpretation of symptoms.22, 23, 24 Therefore, efforts should be made to shed light on these aspects of different presentation of symptoms in women and the need for an immediate call for medical aid.
With respect to the type of stent used, mortality was lower in men if a DES was used, whereas no difference between DES und BMS could be found in women, contrary to previous data.25 As the present study is ongoing, the validity of the data with respect to differences in number between genders will improve over time. On the other hand, it should be kept in mind that the overall decreased mortality after MI results primarily from a more effective therapy, which has been applied consistently not only to men, but also to women.26, 27 The network in Essen, with its standardized therapy for both genders, therefore displays gender differences independent of different therapeutic approaches.
As mentioned above, there were no significant differences in rate of reangiography, need for surgery, or TVR in the 1‐year follow‐up as a possible explanation for the higher mortality in women in that period. In terms of medication, women were less likely to be on therapy with statins compared with men, a fact that again raises the need for a more differentiated risk analysis and aggressive therapy in women.
As rehabilitation has been part of the project, >80% of both genders made use of this offer. In general, about 50% of patients after MI take part in rehabilitation, and this has been associated with decreased mortality in long term follow‐up.28 In our data, prognosis after rehabilitation was improved only in women.
The higher mortality in case of discontinuation of antiplatelet therapy in both genders (76.5% vs 64.8%, P<0.0005) was not unexpected but dramatic. A very strict surveillance of medication and patient behavior is inevitable for both genders to stabilize the high success rate of primary interventional therapy.
Study Limitations
The main limitation of the present study is given by the differences in the number of males and females enrolled. These differences were expected, as the number of women with STEMI is lower in all available data. With further inclusion of patients the data improve validity with respect to gender‐related differences. Nevertheless, the present inclusion of >1300 patients permits a preliminary description of trends in the presentation and outcome for both genders.
Conclusion
The present data demonstrate a successful implementation of a standardized organizational structure and therapy of STEMI patients in an urban environment. Differences between genders are not as obvious as expected. The in‐hospital mortality is comparable between genders, whereas the 1‐year mortality is increased in women. Adjusted for age, gender is no longer an independent risk factor. The presence of diabetes is an independent risk factor for mortality in both genders. Success rate of PCI and reintervention rate are comparable between genders. There is no statistically significant difference in duration of time from beginning of symptoms to balloon inflation, except the fact that women tend to wait longer before calling for medical aid. Efforts should be made in optimizing the risk profile in women as well as in education about cardiac symptoms and proper behavior in case of new onset of angina.
Acknowledgements
The authors would like to thank Silke Lange for her helpful support.
References
- 1. Suessenbacher A, Doerler J, Alber H, et al. Gender‐related outcome following percutaneous coronary intervention for ST‐elevation myocardial infarction: data from the Austrian acute PCI registry. EuroIntervention. 2008;4:271–276. [DOI] [PubMed] [Google Scholar]
- 2. Srichaiveth B, Ruengsakulrach P, Visudharom K, et al. Impact of gender on treatment and clinical outcomes in acute ST elevation myocardial infarction patients in Thailand. J Med Assoc Thai. 2007;90(suppl 1): 65–73. [PubMed] [Google Scholar]
- 3. Hvelplund A, Galatius S, Madsen M, et al. Women with acute coronary syndrome are less invasively examined and subsequently less treated than men. Eur Heart J. 2010;31:684–690. [DOI] [PubMed] [Google Scholar]
- 4. Wake R, Yoshiyama M. Gender differences in ischemic heart disease. Recent Pat Cardiovasc Drug Discov. 2009;4:234–240. [DOI] [PubMed] [Google Scholar]
- 5. The GUSTO Investigators . An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993;329:673–682. [DOI] [PubMed] [Google Scholar]
- 6. Busk M, Maeng M, Rasmussen K, et al; DANAMI‐2 Investigators . The Danish multicentre randomized study of fibrinolytic therapy vs primary angioplasty in acute myocardial infarction (the DANAMI‐2 trial): outcome after 3 years follow‐up. Eur Heart J. 2008;29:1259–1266. [DOI] [PubMed] [Google Scholar]
- 7. Spaulding C, Henry P, Teiger E, et al; TYPHOON Investigators . Sirolimus‐eluting versus uncoated stents in acute myocardial infarction. N Engl J Med. 2006;355:1093–1104. [DOI] [PubMed] [Google Scholar]
- 8. Laarman GJ, Suttorp MJ, Dirksen MT, et al. Paclitaxel‐eluting versus uncoated stents in primary percutaneous coronary intervention. N Engl J Med. 2006;355:1105–1113. [DOI] [PubMed] [Google Scholar]
- 9. Montalescot G, Barragan P, Wittenberg O, et al; ADMIRAL Investigators. Abciximab before direct angioplasty and stenting in myocardial infarction regarding acute and long‐term follow‐up. N Engl J Med. 2001;344:1895–1903. [DOI] [PubMed] [Google Scholar]
- 10. Kostis WJ, Demissie K, Marcella SW, et al; Myocardial Infarction Data Acquisition System (MIDAS 10) Study Group . Weekend versus weekday admission and mortality from myocardial infarction. N Engl J Med. 2001;356:1099–1109. [DOI] [PubMed] [Google Scholar]
- 11. Sinnaeve P, Alexander J, Belmans A, et al. Randomized comparison of single‐bolus tenecteplase and front‐loaded alteplase in 16,949 patients with ST‐elevation acute myocardial infarction. Am Heart J. 2003;146:27–32. [DOI] [PubMed] [Google Scholar]
- 12. Berger JS, Elliott L, Gallup D, et al. Sex differences in mortality following acute coronary syndromes. JAMA. 2009;302:874–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miric̀; L , Miric̀; D , Duplancic̀; D , et al. Specific and gender differences between hospitalized and out of hospital mortality due to myocardial infarction. Coll Antropol. 2008;32:361–367. [PubMed] [Google Scholar]
- 14. Rasoul S, Ottervanger JP, de Boer MJ, et al; Zwolle Myocardial Infarction Study Group . Predictors of 30‐day and 1‐year mortality after primary percutaneous coronary intervention for ST‐elevation myocardial infarction. Coron Artery Dis. 2009;20:415–421. [DOI] [PubMed] [Google Scholar]
- 15. Rao SV, Eikelboom JW, Granger CB, et al. Bleeding and blood transfusion issues in patients with non‐ST segment elevation acute coronary syndromes. Eur Heart J. 2007;28:1193–1204. [DOI] [PubMed] [Google Scholar]
- 16. Donahoe SM, Stewart GC, McCabe CH, et al. Diabetes and mortality following acute coronary syndromes. JAMA. 2007;298:765–775. [DOI] [PubMed] [Google Scholar]
- 17. Tillmanns H, Waas W, Voss R, et al. Gender differences in the outcome of cardiac interventions. Herz. 2005;30:375–389. [DOI] [PubMed] [Google Scholar]
- 18. Halvorsen S, Eritsland J, Abdelnoor M, et al. Gender differences in management and outcome of acute myocardial infarctions treated in 2006‐2007. Cardiology. 2009;114:83–88. [DOI] [PubMed] [Google Scholar]
- 19. Koeth O, Zahn R, Heer T, et al. Gender differences in patients with acute ST‐elevation myocardial infarction complicated by cardiogenic shock. Clin Res Cardiol. 2009;98:781–786. [DOI] [PubMed] [Google Scholar]
- 20. Zeymer U, Vogt A, Zahn R, et al; Arbeitsgemeinschaft Leitende Kardiologische Krankenhausärzte (ALKK) . Predictors of in‐hospital mortality in 1333 patients with acute myocardial infarction complicated by cardiogenic shock treated with primary percutaneous coronary intervention (PCI): results of the primary PCI registry of the Arbeitsgemeinschaft Leitende Kardiologische Krankenhausärzte (ALKK). Eur Heart J. 2004;25:322–328. [DOI] [PubMed] [Google Scholar]
- 21. Zimmermann S, Ruthrof S, Nowak K, et al. Short‐term prognosis of contemporary interventional therapy of ST‐elevation myocardial infarction: does gender matter? Clin Res Cardiol. 2009;98:709–715. [DOI] [PubMed] [Google Scholar]
- 22. Berg J, Björck L, Dudas K, et al. Symptoms of a first acute myocardial infarction in women and men. Gend Med. 2009;6:454–462. [DOI] [PubMed] [Google Scholar]
- 23. Løvlien M, Johansson I, Hole T, et al. Early warning signs of an acute myocardial infarction and their influence on symptoms during the acute phase, with comparisons by gender. Gend Med. 2009;6:444–453. [DOI] [PubMed] [Google Scholar]
- 24. Nguyen JT, Berger AK, Duval S, et al. Gender disparity in cardiac procedures and medication use for acute myocardial infarction. Am Heart J. 2008;155:862–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Onuma Y, Kukreja N, Daemen J, et al; Interventional Cardiologists of Thoraxcenter . Impact of sex on 3‐year outcome after percutaneous coronary intervention using bare‐metal and drug‐eluting stents in previously untreated coronary artery disease: insights from the RESEARCH (Rapamycin‐Eluting Stent Evaluated at Rotterdam Cardiology Hospital) and T‐SEARCH (Taxus‐Stent Evaluated at Rotterdam Cardiology Hospital) Registries. JACC Cardiovasc Interv. 2009;2:603–610. [DOI] [PubMed] [Google Scholar]
- 26. Rogers WJ, Frederick PD, Stoehr E, et al. Trends in presenting characteristics and hospital mortality among patients with ST elevation and non‐ST elevation myocardial infarction in the National Registry of Myocardial Infarction from 1990 to 2006. Am Heart J. 2008;156:1026–1034. [DOI] [PubMed] [Google Scholar]
- 27. Vaccarino V, Parsons L, Peterson ED, et al. Sex differences in mortality after acute myocardial infarction: changes from 1994 to 2006. Arch Intern Med. 2009;169:1767–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brandi JW, Jacobsen SJ, Weston SA, et al. Cardiac rehabilitation after myocardial infarction in the community. Am J Cardiol. 2004;5:988–996. [DOI] [PubMed] [Google Scholar]


