Abstract
Phidippides was a Greek messenger who experienced sudden death after running more than 175 miles in two days. In today's world, marathon running and other endurance sports are becoming more popular and raising concern about sudden deaths at these events. Once etiologies such has hypertrophic cardiomyopathy, anomalous coronary arteries, and coronary atherosclerosis have been excluded, there is now an additional consideration termed Phidippides cardiomyopathy. Because endurance sports call for a sustained increase in cardiac output for several hours, the heart is put into a state of volume overload. It has been shown that approximately one‐third of marathon runners experience dilation of the right atrium and ventricle, have elevations of cardiac troponin and natriuretic peptides, and in a smaller fraction later develop small patches of cardiac fibrosis that are the likely substrate for ventricular tachyarrhythmias and sudden death. Cardiac magnetic resonance imaging is emerging as the diagnostic test of choice for this condition. This review and case report summarizes the key features of this newly appreciated disorder. © 2012 Wiley Periodicals, Inc.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Phidippides and Sudden Cardiac Death
In 490 BC, during the Greco‐Persian War, Persian King Darius I commanded his army to attack the Greeks, whom they greatly outnumbered. In the face of this attack, a 40‐year‐old Athenian herald, Phidippides, was ordered to run nearly 75 miles through mountainous terrain to Sparta to request military support. Although the Spartans agreed to assist the Greeks, they could not help immediately due to certain religious obligations, so Phidippides began his journey back to Marathon, completing 150 miles in less than 2 days. Upon his arrival in Marathon, to Phidippides' disbelief, the Greeks had defeated the Persians. Phidippides was then sent to Athens, 26.2 miles from Marathon, to spread the news of this impressive victory with the other Greeks. Upon his arrival in Athens, after running the 26.2 mile journey, Phidippides stretched out his arms and exclaimed, “We are victorious!” He then collapsed and died. His death was the first report of sudden cardiac death in a long‐distance runner. Since his demise, numerous accounts of endurance athletes suffering cardiac related complications have been documented.1
Sudden Death After Extreme Exertion
There has been considerable confusion surrounding the etiology of sudden death after extreme exertion.2, 3, 4 The focus has been on hypertrophic cardiomyopathy, anomalous coronary arteries, premature coronary artery disease, and acute myocardial ischemia; however, there is now a new understanding that repetitive and sustained cardiac exertion may actually cause a form of cardiomyopathy.5 It has been known that endurance athletes have cardiac chamber enlargement, left ventricular hypertrophy, and increased rates of atrial and ventricular arrhythmias.6, 7 It has been recently shown that focal areas of cardiac fibrosis seen on delayed gadolinium cardiac magnetic resonance imaging and at autopsy appear to be the implicated substrate for increased risk of sudden cardiac death when other etiologies have been excluded.8 Sustained cardiac output leading to transitory chamber dilation and the induction of patchy cardiac fibrosis in persons predisposed is now termed in this article as Phidippides cardiomyopathy. We present a case of an amateur endurance athlete who presented with sudden cardiac death as a result of this syndrome.
Long‐Distance Training and Running
In 1975, 25 000 runners completed a marathon in the United States. Distance running has enjoyed increasing popularity over the last 2 decades. In 2010, there were an estimated 468 000 marathon finishers. With increasing popularity, there has been a rising occurrence of sudden death. The risk of dying suddenly while participating in a marathon is 0.8 per 100 000.9 Although the risk has not changed, absolute mortality has increased with more participants.10 For the development of multiple cardiac abnormalities (fibrosis, atrial arrhythmias, ventricular arrhythmias, and sudden death) associated with endurance training and competition, such as marathon running, we propose the term Phidippides cardiomyopathy (Figure 1).
Figure 1.
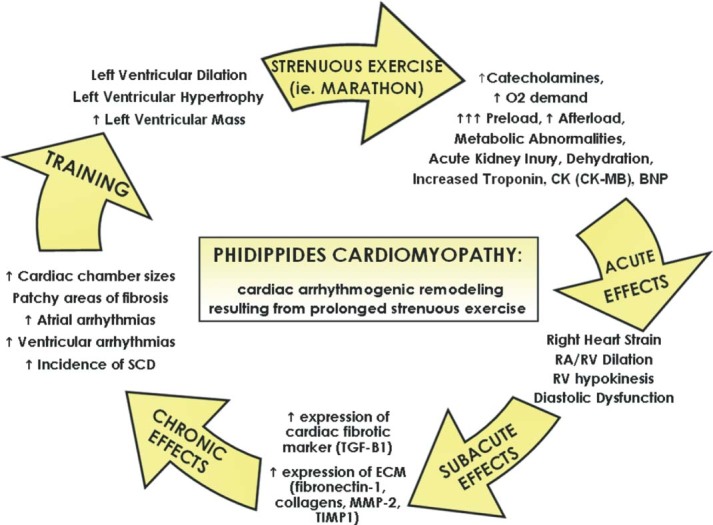
Proposed pathogenesis of Phidippides cardiomyopathy. Abbreviations: BNP, B‐type natriuretic peptide; CK, creatine kinase; CK‐MB, creatine kinase myocardial band; ECM, extracellular matrix; MMP‐2, matrix metalloproteinase‐2; RA, right arterial; RV, right ventricular; SCD, sudden cardiac death; TGF‐β1, transforming growth factor‐β1; TIMP1, tissue inhibitor of metalloproteinase‐1.
Development of Phidippides Cardiomyopathy
Some marathon runners train for years, whereas many others train for 18 to 20 weeks as advised on the Internet or in many widely available books. Marathon training differs drastically among participants in regard to days per week, miles per week, and recovery periods. As a result, marathon runners have different levels of fitness at baseline for the race. Despite different training regimens and fitness levels, in all cases the heart faces increased pressure and volume overload and responds by increasing left ventricular chamber size, thickness, and mass. Running a marathon initiates multiple events within one's body for cardiac injury, including increased release of catecholamines and resultant coronary vasoconstriction, increased heart rate leading to decreased diastolic filling time of the coronary arteries, increased demand for oxygen, changes in free fatty acid metabolism, lactic acidosis, and metabolic derangements.11 During the event, in susceptible individuals the heart is unable to keep up with the demands of marathon running and increasing right heart preload and afterload, and begins to dilate and stretch in response to these hemodynamic changes.12 These changes may be more pronounced in those with less training.13 Right heart dilation and hypokinesis after prolonged strenuous exercise has been observed using cardiac magnetic resonance imaging.8, 14 Diastolic dysfunction is frequently observed in both younger and older athletes.15 Multiple cardiac biomarkers are released, including myoglobin, cardiac troponin‐I, creatine kinase, and creatine kinase myocardial band, and B‐type natriuretic peptide.16 Volume depletion and reduced renal filtration occurs with elevated blood urea nitrogen, serum creatinine, and cystatin C.17 During the recovery period after the marathon, the cardiac geometric dimensions are restored, and many athletes continue this cycle with training, marathon running, cardiac disturbances, and again cardiac recovery. With this repetitive stretch of the chambers and restoration of the chamber geometry, we believe there are individuals prone to developing chronic structural changes, including chamber dilation and myocardial fibrosis, as a response to the repetitive volume overload and dynamic strain of the heart.18 Approximately one‐third of individuals after a marathon, irrespective of baseline factors, speed, or conditioning, will have a rise and fall of cardiac troponin and B‐type natriuretic peptide.8 It is unclear whether this group, in particular, will go on to develop patches of cardiac fibrosis. These changes are asymptomatic and probably occur over many years. However, in those who experience ventricular arrhythmias and/or sudden cardiac death there is this lethal disorder, Phidippides cardiomyopathy. Thus, it is rational to develop risk prediction and screening methods for this disorder to allow counseling for individuals who are considering endurance sports such as marathon running.
Animal Studies
In rat models subjected to prolonged strenuous exercise, there is increased expression of transforming growth factor‐β1 in the right and left atria and right ventricle, which is believed to stimulate collagen‐producing cardiac myofibroblasts resulting in patchy areas of fibrosis.19 In addition, in animal models of extreme exertion, there is dysregulation of the extracellular matrix with increased expression of fibronection‐1, collagens, metalloproteinase‐2, and tissue inhibitor of metalloproteinase‐1, resulting in increased cardiac stiffness (Figure 1).20
Risk Stratification of Long‐Distance Runners
At this point in time, there have been no studies to evaluate screening methods for Phidippides cardiomyopathy. We anticipate that an approach using both cardiac biomarkers and advanced imaging will be developed to identify individuals at risk for and with subclinical Phidippides cardiomyopathy. For an individual who is considering long‐distance running or any other activity that elevates cardiac output for a sustained period of time (continuously over several hours), it is reasonable to perform a clinical evaluation for common cardiovascular disorders that are known to precipitate ischemia or arrhythmias with exercise.21 However, we now know that prolonged endurance exercise itself may be causative in the development of this cardiomyopathy, although the role of myocardial ischemia has been suggested but remains unclear.22 Magnetic resonance imaging with late gadolinium enhancement can readily identify areas of patchy fibrosis in an endurance athlete and may indeed be shown to be predictive of serious arrhythmias in the future.23 This imaging modality can also identify hypertrophic cardiomyopathy, anomalous coronary arteries, Marfan's aortopathy, and appears to have considerable advantages over echocardiography.24 It is also possible that the identification of elevated cardiac biomarkers with prolonged exertion is a harbinger for the future development of cardiac fibrosis. A particularly attractive marker is galectin‐3, which has been recently approved as a prognostic tool in patients with heart failure and is believed to be the product of cardiac macrophages.25 Galectin‐3 is known to stimulate fibroblasts to secrete procollagen I, which is then cross‐linked to become mature collagen, the basis of cardiac fibrosis.
An Illustrative Case
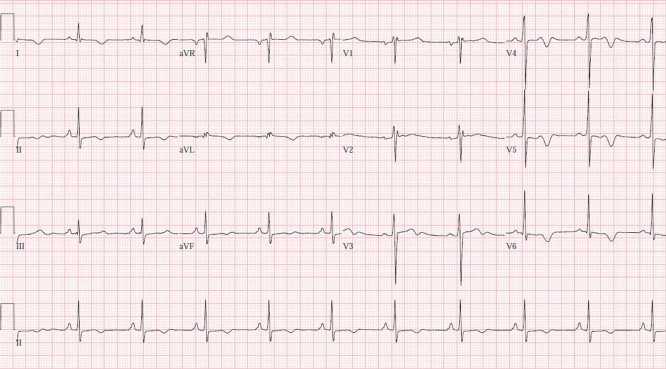
A 50‐year‐old previously healthy man, who participated in multiple half‐marathons and engaged in aerobic activity twice daily, 6 days per week, collapsed while driving. This occurred approximately 12 hours since his last workout. A rhythm strip (Figure 2) performed by emergency medical services revealed ventricular fibrillation, for which he was successfully treated with cardiopulmonary resuscitation and electrical cardioversion. He was intubated, mechanically ventilated, and upon presentation to the hospital underwent therapeutic hypothermia. His electrolytes and creatine kinase were normal. Cardiac troponin‐I was indeterminate (0.06 ng/mL). His electrocardiogram revealed normal sinus rhythm with diffuse non‐specific ST abnormalities (Figure 3). There was no prolongation of the QT interval noted at any time. Two‐dimensional echocardiography revealed normal left and right ventricular function, no pathological chamber enlargements, normal mitral valve apparatus, and no outflow tract obstruction. He underwent cardiac catheterization, which showed normal coronary arteries with diminutive RCA (posterior descending from left circumflex) (Figure 4) with left ventriculography showing left ventricular hypertrophy (large papillary muscles) with hyperdynamic function (Figure 5). Subsequently, cardiac magnetic resonance imaging was performed, which revealed mild left ventricular hypertrophy with a mass of 166.2 grams and normal cardiac function. Using delayed gadolinium imaging (Figure 6), a patchy focal area in the mid‐myocardial basal anterior septum, in a non‐coronary artery distribution, was identified as the likely substrate of this patient's ventricular arrhythmia and subsequent cardiac arrest. He had complete neurologic recovery and underwent implantation of a cardio defibrillator before discharge. This case represents the first documented patient with sudden death occurring at a time remote from the endurance event and raises important points concerning the predisposition to cardiac fibrosis and the occurrence of lethal arrhythmias with and without exercise as a precipitant. We recognize that this case and accompanying review present a hypoth‐ esis concerning pathophysiology and that considerable research is needed for this to mature into accepted understanding in clinical practice. Furthermore, we emphasize that the health benefits of exercise do not require the prolonged or severe exertion that has been mentioned in this paper.
Figure 2.
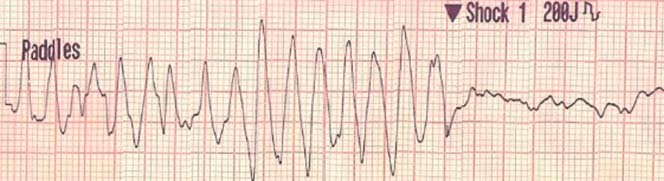
Presenting rhythm.
Figure 3.
Electrocardiogram after successful resuscitation.
Figure 4.
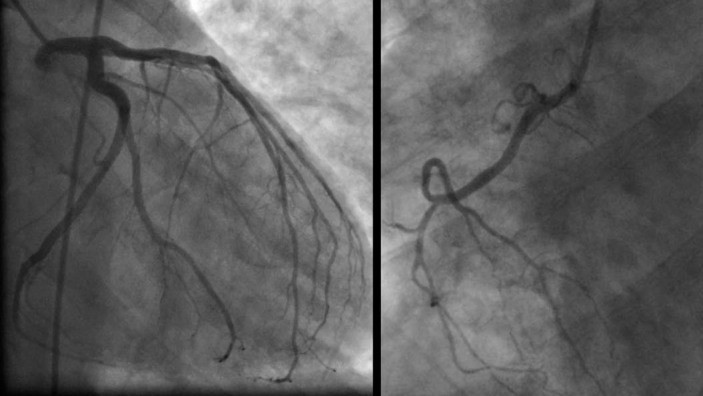
Coronary angiogram.
Figure 5.
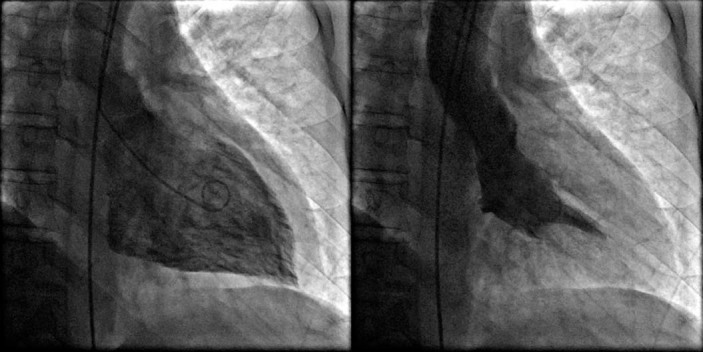
Left ventriculography showing a hyperdynamic left ventricle and ejection fraction of 75%.
Figure 6.

Cardiac magnetic resonance imaging with late gadolinium enhancement demonstrating a small patch of scar in the interventricular septum consistent with a focus of cardiac fibrosis.
Conclusion
We postulate that repetitive, sustained elevations of cardiac output for several hours, in predisposed individuals, causes recurrent dilation of cardiac chambers and stimulates resident macrophages, pericytes, and fibroblasts, resulting in the deposition of collagen causing patchy fibrosis. The fibrotic areas, in turn, become the substrate for re‐entrant ventricular tachycardia and degeneration to ventricular fibrillation. We believe that in the absence of a readily identifiable cardiac abnormality (eg, acute myocardial infarction, hypertrophic cardiomyopathy), Phidippides cardiomyopathy is a likely cause of sudden death in athletes, such as marathon runners, who endure repetitive sustained exertion.
References
- 1. Thompson PD, Venero HC. A history of medical reports on the Boston Marathon: 112 years and still running. Med Sci Sports Exerc. 2009;41:1341–1348. [DOI] [PubMed] [Google Scholar]
- 2. Douglas PS, O'Toole ML, Hiller WD, et al. Cardiac fatigue after prolonged exercise. Circulation. 1987;76:1206–1213. [DOI] [PubMed] [Google Scholar]
- 3. Westrol MS, Kapitanyan R, Marques‐Baptista A, et al. Causes of sudden cardiac arrest in young athletes. Postgrad Med. 2010;122:144–157. [DOI] [PubMed] [Google Scholar]
- 4. Giada F, Biffi A, Cannom DS, et al. Sports and arrhythmias: a report of the International Workshop Venice Arrhythmias 2009. Eur J Cardiovasc Prev Rehabil. 2010;17:607–612. [DOI] [PubMed] [Google Scholar]
- 5. Möhlenkamp S, Lehmann N, Breuckmann F, et al; Marathon Study Investigators; Heinz Nixdorf Recall Study Investigators. Running: the risk of coronary events: prevalence and prognostic relevance of coronary atherosclerosis in marathon runners. Eur Heart J. 2008;29:1903–1910. [DOI] [PubMed] [Google Scholar]
- 6. Heidbuchel H, Hoogsteen J, Fagard R, et al. High prevalence of right ventricular involvement in endurance athletes with ventricular arrhythmias: role of an electrophysiologic study in risk stratification. Eur Heart J. 2003;24:1473–1480. [DOI] [PubMed] [Google Scholar]
- 7. Karjalainen J, Kujala UM, Kapiro J, et al. Lone atrial fibrillation in vigorously exercising middle aged men: case‐control study. BMJ. 1998;316:1784–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Trivax JE, Franklin BA, Goldstein JA, et al. Acute cardiac effects of marathon running. J Appl Physiol. 2010;108:1148–1153. [DOI] [PubMed] [Google Scholar]
- 9. Redelmeier DA, Greenwald JA. Competing risks of mortality with marathons: retrospective analysis. BMJ. 2007;335:1275–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. USA marathoning : 2007. overview. http://www.marathonguide.com/features/Articles/2007RecapOverview.cfm. Accessed December 12, 2008.
- 11. La Gerche A, Connelly KA, Mooney DJ. Biochemical and functional abnormalities of left and right ventricular function after ultra‐endurance exercise. Heart. 2008;94:860–866. [DOI] [PubMed] [Google Scholar]
- 12. Krip B, Gledhill N, Jamnik V, et al. Effect of alterations in blood volume on cardiac function during maximal exercise. Med Sci Sports Exerc. 1997;29:1469–1476. [DOI] [PubMed] [Google Scholar]
- 13. Neilan TG, Januzzi JL, Lee‐Lewandrowski E, et al. Myocardial injury and ventricular dysfunction related to training levels among nonelite participants in the Boston marathon. Circulation. 2006;114:2325–2333. [DOI] [PubMed] [Google Scholar]
- 14. Breuckmann F, Möhlenkamp S, Nassenstein K, et al. Myocardial late gadolinium enhancement: prevalence, pattern, and prognostic relevance in marathon runners. Radiology. 2009;251:50–57. [DOI] [PubMed] [Google Scholar]
- 15. Knebel F, Schimke I, Schroeckh S, et al. Myocardial function in older male amateur marathon runners: assessment by tissue Doppler echocardiography, speckle tracking, and cardiac biomarkers. J Am Soc Echocardiogr. 2009;22:803–809. [DOI] [PubMed] [Google Scholar]
- 16. Fortescue EB, Shin AY, Greenes DS, et al. Cardiac troponin increases among runners in the Boston Marathon. Ann Emerg Med. 2007;49:137–143. [DOI] [PubMed] [Google Scholar]
- 17. McCullough PA, Chinnaiyan KM, Gallagher MJ, et al. Changes in renal markers and acute kidney injury after marathon running. Nephrology (Carlton). 2011;16:194–199. [DOI] [PubMed] [Google Scholar]
- 18. Mousavi N, Czarneck A, Kumar K, et al. Relation of biomarkers and cardiac magnetic resonance imaging after marathon running. Am J Cardiol. 2009;103:1467–1472. [DOI] [PubMed] [Google Scholar]
- 19. Benito B, Gay‐Jordi G, Serrano‐Mollar A, et al. Cardiac arrhythmogenic remodeling in a rat model of long‐term intensive exercise training. Circulation. 2011;123:13–22. [DOI] [PubMed] [Google Scholar]
- 20. Kwak HB, Kim JH, Joshi K, et al. Exercise training reduces fibrosis and matrix metalloproteinase dysregulation in the aging rat heart. FASEB J. 2011;25:1106–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McCullough PA. Integration of the clinical laboratory in cardiovascular medicine. Rev Cardiovasc Med. 2010;11(suppl 2):S1–S2. [DOI] [PubMed] [Google Scholar]
- 22. Rowe WJ. A world record marathon runner with silent ischemia without coronary atherosclerosis. Chest. 1991;99:1306–1308. [DOI] [PubMed] [Google Scholar]
- 23. Drezner JA. Contemporary approaches to the identification of athletes at risk for sudden cardiac death. Curr Opin Cardiol. 2008;23:494–501. [DOI] [PubMed] [Google Scholar]
- 24. Prakken NH, Velthuis BK, Cramer MJ, et al. Advances in cardiac imaging: the role of magnetic resonance imaging and computed tomography in identifying athletes at risk. Br J Sports Med. 2009;43:677–684. [DOI] [PubMed] [Google Scholar]
- 25. de Boer RA, Yu L, van Veldhuisen DJ. Galectin‐3 in cardiac remodeling and heart failure. Curr Heart Fail Rep. 2010;7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]