Abstract
Patients with type 2 diabetes mellitus are at 2 to 4 times increased risk of cardiovascular events compared with those without diabetes, both among patients with multiple risk factors only and those with established atherothrombosis. In this review, we provide recommendations for the use of statins and aspirin for the prevention of cardiovascular events in high‐risk patients with diabetes mellitus. Clin. Cardiol. 2012 doi: 10.1002/clc.22032
The SAVOR‐TIMI 53 trial is sponsored by AstraZeneca and Bristol‐Myers Squibb. Dr. Udell is a recipient of a Postdoctoral Research Fellowship from the Canadian Institutes for Health Research (CIHR) and Canadian Foundation for Women's Health. Dr. Scirica receives research grants from AstraZeneca, Bristol‐Myers Squibb, Merck, Johnson & Johnson, Bayer Healthcare, and Gilead Sciences and consultancy fees from Gilead Sciences, Lexicon, and Arena Pharmaceuticals. Dr. Braunwald receives research grants from AstraZeneca and Bristol‐Myers Squibb. Dr. Raz has received honoraria/expenses for advisory board participation from AstraZeneca, Bristol‐Myers Squibb, Novo Nordisk, Merck, Sharp & Dohme, and Eli Lilly; has served as a consultant for Andromea, AstraZeneca, Bristol‐Myers Squibb, Eli Lilly, Johnson & Johnson, HealOr, Insuline, Teva, and TransPharma; and has participated in speakers bureaus for AstraZeneca, Bristol‐Myers Squibb, Eli Lilly, Novo Nordisk, Johnson & Johnson, and Roche. Dr. Steg receives research grants from Servier. Dr. Davidson participated on advisory panels for AstraZeneca, Bristol‐Myers Squibb, Merck, Johnson & Johnson, Boehringer Ingelheim, Eli Lilly, Generex Biotechnology, Novo Nordisk A/S, Roche Diagnostics, and Takeda Pharmaceutical Company and participated in speaker's bureaus for Eli Lilly and Takeda Pharmaceutical Company. Dr. Hirshberg is an employee of and holds stock in AstraZeneca. Dr. Bhatt discloses the following relationships—Advisory Board: Medscape Cardiology; Board of Directors: Boston VA Research Institute, Society of Chest Pain Centers; Chair: American Heart Association Get With The Guidelines Science Subcommittee; Honoraria: American College of Cardiology (Editor, Clinical Trials, Cardiosource), Duke Clinical Research Institute (clinical trial steering committees), Slack Publications (Chief Medical Editor, Cardiology Today Intervention), WebMD (CME steering committees); Research Grants: Amarin, AstraZeneca, Bristol‐Myers Squibb, Eisai, Ethicon, Medtronic, Sanofi Aventis, The Medicines Company; Unfunded Research: FlowCo, PLx Pharma, Takeda. The design and conduct of the SAVOR‐TIMI 53 study are being done by the academic executive committee in collaboration with the sponsors.
Introduction
Patients with type 2 diabetes mellitus (T2DM) have an increased risk of incident and recurrent cardiovascular events including coronary, cerebrovascular, and peripheral arterial ischemia. For over 25 years, there has been rigorous study and provocative demonstration that lowering low‐density lipoprotein cholesterol with a potent statin, and platelet inhibition with aspirin therapy, can reduce recurrent cardiovascular risk in these patients. T2DM patients with additional cardiovascular risk factors are at high risk for incident ischemic events and statin therapy is also efficacious in this setting. As a result, there has been widespread clinical implementation of statin therapy in patients with T2DM. In contrast, the additional benefit of aspirin therapy in patients without established cardiovascular disease, even those with T2DM, awaits definitive results. One important arena in which decisions and strategies for implementation of these therapies are crucial is within cardiovascular outcomes trials studying new therapies in patients with T2DM. A tested novel cardioprotective therapy is likely to be judged in the context of the trial patients' background treatment. In this review, we provide the data in support of, and our recommendations for, the use of statins and aspirin for the prevention of cardiovascular (CV) events in high‐risk patients with T2DM. Current guidelines separate patients into those with either established cardiovascular disease (secondary prevention) and those patients at high‐risk for cardiovascular disease as a result of multiple risk factors (primary prevention) when discussing the evidence for these cardiovascular preventive therapies, and we discuss these therapies in that context. In particular, we review the role of statins and aspirin for primary prevention therapy in high‐risk patients with diabetes, considering recent results, potential controversy, and misconceptions of the benefits and risks of these medications in diabetic patients without a prior cardiovascular event.1., 2., 3. We conclude with a summary of the landscape of background therapy in contemporary cardiovascular outcomes trials of patients with T2DM, including trial recommendations for statin and aspirin treatment, highlighting the large ongoing SAVOR‐TIMI 53 trial as an example.
Why Is Cardiovascular Risk Reduction Important in Diabetes?
Patients with T2DM are at 2‐ to 4‐ times increased risk of CV events compared with those without T2DM.4., 5. In patients older than 65 years, the primary cause of death in 2 out of 3 diabetic patients is coronary heart disease, followed by stroke in 1 out of 6.6 The risk of major vascular events may be lowered with lifestyle changes and medical therapy, but nevertheless the emphasis on prevention is important because a diabetic patient's first vascular event may be fatal.7
Patients with established atherothrombosis (occlusive vascular disease) have a high risk of recurrent major vascular events (myocardial infarction [MI], stroke, or CV death).5 The highest risk group is those with prior ischemic events, followed by those with stable atherosclerosis, and those with T2DM and multiple risk factors without established cardiovascular disease (CVD). Patients with T2DM are at higher risk within all of these disease state categories (Figure 1).5
Figure 1.
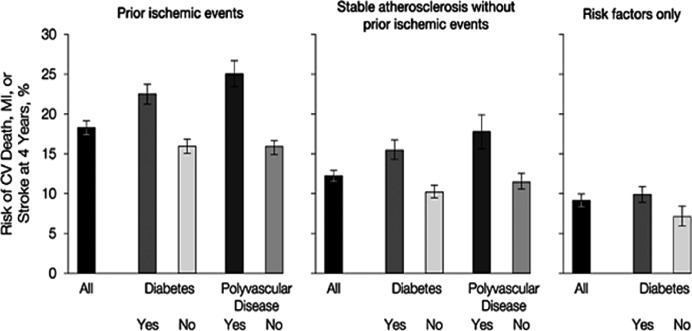
Risk of major vascular events with or without diabetes mellitus in patients with prior ischemic events, stable atherosclerosis, and multiple risk factors only in the REACH registry. Prior ischemic events were defined as prior myocardial infarction or stroke. Error bars indicate 95% confidence intervals. Abbreviations: CV, cardiovascular; REACH, REduction of Atherothrombosis for Continued Health. Reprinted from Bhatt DL et al.5 Comparative determinants of 4‐year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis, JAMA. 2010;304:1350–1357 © (2010), with permission from the American Medical Association. All rights reserved.
Statin Therapy
Secondary Prevention With Statins
Diabetic patients commonly have dyslipidemia.8 Characteristic abnormalities in the lipid profile in T2DM include elevated triglycerides and apolipoprotein B, increased low‐density lipoprotein (LDL) cholesterol, very low‐density lipoprotein cholesterol (VLDL), and free fatty acids, as well as decreased atheroprotective high‐density lipoprotein (HDL) cholesterol levels.4., 8., 9., 10. The lipid abnormalities that develop in T2DM promote atherogenesis and are strongly related to CVD risk.4., 11. Therefore, dyslipidemia is an important therapeutic target.
Metabolic and lipid abnormalities improve with weight loss, exercise, smoking cessation, and dietary modification. We emphasize counseling of patients toward achieving these therapeutic lifestyle modifications (see What Do the Guidelines Say?). Just as important is pharmacologic treatment with statins that have proven benefit in decreasing CVD risk in T2DM, especially in those patients with established atherosclerosis11 (Figure 2). Statins are safe, very well tolerated, and highly effective in reducing LDL cholesterol by increasing LDL clearance and decreasing VLDL secretion.12., 13.
Figure 2.
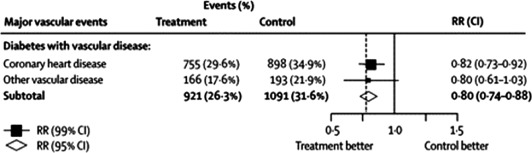
CTT Collaboration meta‐analysis of the efficacy of statin therapy for secondary prevention in patients with diabetes mellitus between 1994 and 2004. This figure demonstrates the weighted RR per 40 mg/dL (1 mmol/L) reduction in LDL cholesterol at 1 year, comparing outcomes in patients treated with statin compared to placebo. The area of each square is proportional to the amount of statistical information in that particular category; a larger square reflects more patient years and events of study. Diamonds represent the synthesis of data. Diamonds and squares to the left of the solid line of unity indicate benefit with statins. The treatment effect is statistically significant if the diamond or horizontal lines (CIs) do not cross the solid line (square horizontal lines represent a 99% CI, P < 0.01; and diamonds a 95% CI, P < 0.05). Vascular disease corresponds to a previous history of coronary artery disease, cerebrovascular disease, or peripheral arterial disease. Abbreviations: CI, confidence interval; CTT, The Cholesterol Treatment Trialists'; LDL, low‐density lipoprotein; RR, relative risk. Reprinted from Cholesterol Treatment Trialists' (CTT) Collaborators.11 Efficacy of cholesterol‐lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta‐analysis. Lancet. 2008;371:117–125 © (2008), with permission from Elsevier.
The Cholesterol Treatment Trialists' (CTT) Collaboration meta‐analysis of all secondary prevention trials among patients with T2DM has shown that the use of statins leads to a highly significant 20% reduction in the incidence of major vascular events (Figure 2: events [%] in control arm, 31.6%, vs events in statin arm, 26.3%; relative risk [RR] 0.80, 99% confidence interval [CI], 0.74–0.88; treating 1000 patients for 5 years will result in 57 [95% CI 34–80] fewer major vascular events per 40 mg/dL [1 mmol/L] reduction in LDL cholesterol) regardless of baseline lipid levels and an overall 9% reduction in all‐cause mortality per 40 mg/dL (1 mmol/L) reduction in LDL cholesterol with statin therapy.11
What Do the Guidelines Say?
Current guidelines14., 15., 16., 17. recommend lifestyle modification to lower lipid levels by focusing on:
Reducing saturated fat, trans fat, and cholesterol intake;
Increasing omega‐3 fatty acid, viscous fiber, and plant oil intake;
Weight loss if indicated;
Increased physical activity.
The guidelines also recommend statin therapy for secondary prevention of CVD in diabetic patients with established atherosclerosis regardless of baseline lipid levels.14., 15., 16., 17. The primary goal of therapy is to achieve an LDL cholesterol (LDL‐C) <100 mg/dL (<2.6 mmol/L) and ideally <70 mg/dL (<1.8 mmol/L) using higher‐dose statins (Table 1).
Table 1.
Summary Guideline Recommendations for Primary and Secondary Prevention of Cardiovascular Disease With Statin and Aspirin Therapy in Patients With Type 2 Diabetes Mellitus
| Guidelines recommend | Aspirin | Statins | Qualification | Ideal LDL‐C goal | Other lipid goals |
|---|---|---|---|---|---|
| T2DM + established CVD | Yes | Yes | Everyone without contraindication regardless of baseline lipid values | <70 mg/dL | ↓ LDL‐C 30%–40% below baseline; |
| (<1.8 mmol/L) | Triglycerides: <150 mg/dL (<1.7 mmol/l); | ||||
| HDL‐C men: >40 mg/dL (>1 mmol/L); | |||||
| T2DM >40 years + multiple risk factors | Yes | Yes | ADA/AHA/ACC/ESC: >40 years + ≥ 1 risk factor regardless of baseline lipid values; low risk of bleeding | <100 mg/dL | HDL‐C women: >50 mg/dL (>1.3 mmol/L); |
| (<2.6 mmol/l) | Apo‐B: <80 mg/dL (<0.8 g/L) |
Abbreviations: ACC, American College of Cardiology; ADA, American Diabetes Association; AHA, American Heart Association; Apo‐B, apolipoprotein‐B; T2DM, type 2 diabetes mellitus; ESC, European Society of Cardiology; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol.
When lipid levels do not meet these targets on maximally tolerated statin therapy, the guidelines provide an alternative acceptable goal for a reduction in LDL‐C of at least 30% below baseline. In addition, desirable non–HDL‐C levels in patients with T2DM are <130 mg/dL (<3.3 mmol/L) and ideally <100 mg/dL (<2.6 mmol/L) (or triglyceride levels <150 mg/dL (<1.7 mmol/L) and HDL cholesterol >40 mg/dL (>1 mmol/L) in men or >50mg/dL (>1.3 mmol/L) in women) and apolipoprotein‐B (Apo‐B) <100 mg/dL (1.0 g/L) and ideally <80 mg/dL (0.80 g/L). Nevertheless, LDL values remain the preferred lipid‐lowering targets with statins.
Primary Prevention With Statins
The benefits of statin therapy to prevent vascular events or vascular death in high‐risk diabetic men and women without established CVD are also clear. There have been 14 major trials involving 18,686 T2DM patients (including the Heart Protection Study, Collaborative Atorvastatin Diabetes Study [CARDS], Anglo‐Scandinavian Cardiac Outcomes Trial [ASCOT], and Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non‐Insulin‐Dependent Diabetes Mellitus [ASPEN])18., 19., 20., 21. that studied statins for primary prevention of CVD incorporated into the CTT meta‐analysis.11 Many trials enrolled a combination of diabetic patients with and without established atherosclerosis, but CARDS was one of the few trials that enrolled exclusively diabetic patients without established atherosclerosis (Figure 3). Overall, the CTT meta‐analysis found that the onset of a first major vascular event was reduced by 27% (Figure 4: events [%] in control arm, 11.8%, vs events in statin arm, 9.2%, RR 0.73, [99% CI 0.66–0.82]; treating 1000 patients for 5 years will result in 36 [95% CI, 23–49] fewer major vascular events) per 40 mg/dL (1 mmol/L) reduction in LDL cholesterol by statin therapy in patients with T2DM without established atherosclerosis.
Figure 3.
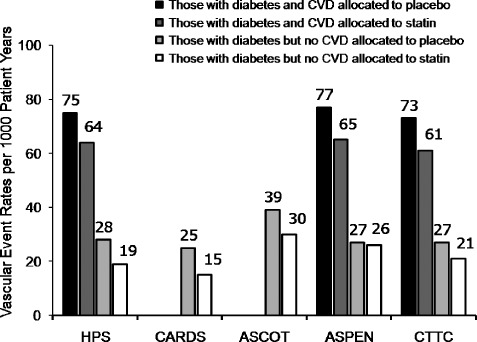
Vascular event rates in major primary and secondary prevention randomized controlled trials of statin therapy in patients with type 2 diabetes mellitus. First major vascular event in HPS was defined as nonfatal MI, coronary death, stroke, or coronary and noncoronary revascularization; in CARDS as nonfatal or silent MI, unstable angina, coronary death, resuscitated cardiac arrest, stroke or coronary revascularization; in ASCOT‐LLA as CV death, nonfatal or silent MI, unstable angina, chronic stable angina, life‐threatening arrhythmias, nonfatal heart failure, nonfatal stroke, peripheral arterial disease, retinal vascular thrombosis, revascularization procedures, transient ischemic attacks, and reversible ischemic neurological deficits; in ASPEN as CV death, nonfatal or silent MI, nonfatal stroke, coronary revascularization, coronary artery bypass grafting, resuscitated cardiac arrest, or worsening or unstable angina requiring hospitalization; in CTTC as MI or coronary death, stroke, or coronary revascularization. Abbreviations: ASCOT‐LLA, Anglo‐Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm; ASPEN, Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non‐Insulin‐Dependent Diabetes Mellitus; CARDS, Collaborative Atorvastatin Diabetes Study; CTTC, Cholesterol Treatment Trialists' Collaboration; CV, cardiovascular; CVD, cardiovascular disease; MI, myocardial infarction; T2DM, type 2 diabetes mellitus. Adapted from Tonkin AM, Chen L. Effects of combination lipid therapy in the management of patients with type 2 diabetes mellitus in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Circulation. 2010;122(8):850–852. © (2010), with permission from Wolters Kluwer Health, LWW.
Figure 4.
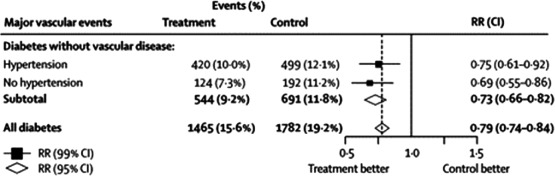
The CTT Collaboration meta‐analysis of the efficacy of statin therapy for primary prevention in patients with diabetes mellitus between 1994 and 2004. See the legend of Figure 2 for statistical definitions. Abbreviation: CTT, Cholesterol Treatment Trialists'. Reprinted from Cholesterol Treatment Trialists' (CTT) Collaborators.11 Efficacy of cholesterol‐lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta‐analysis. Lancet. 2008;371:117–125. © (2008), with permission from Elsevier.
A potential remaining controversy in the use of statin therapy for primary prevention in T2DM, is whether there is any additional benefit derived from a statin if a diabetic patient has already reached recommended target lipid levels (ie, <100 mg/dL or <2.6 mmol/L) without administration of a statin. The CTT Collaboration meta‐analysis included over 2700 patients with T2DM whose baseline LDL cholesterol concentrations were already <100 mg/dL (<2.6 mmol/l).11 In diabetic patients who started with an LDL <100 mg/dL (<2.6 mmol/L), a 32% reduction of serious vascular events for every 40 mg/dL (1 mmol/L) reduction in LDL achieved with statins (Figure 5: events [%] in control arm, 12.2%, vs events in statin arm, 9.8%; RR 0.68, 99% CI 0.45–1.03; P = 0.02) was observed. In other words, CTT demonstrated a decrease from 12.2% events per year to 9.8% per year with statins; a number needed to treat of 42 patients over 5 years to prevent 1 additional event. The CTT investigators concluded that a statin should be recommended for all patients with T2DM at risk of CVD, regardless of baseline lipid levels.
Figure 5.
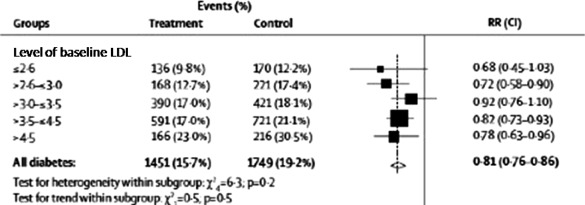
Proportional effect of cardiovascular risk reduction with statin therapy stratified by baseline LDL‐cholesterol in patients with diabetes mellitus between 1994 and 2004. See the legend of Figure 2 for statistical definitions. The CTT Collaborators observed no significant difference in statin effect regardless of baseline LDL level based on the statistical tests for heterogeneity or trend within these subgroups. Reprinted from Cholesterol Treatment Trialists' (CTT) Collaborators.11 Efficacy of cholesterol‐lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta‐analysis. Lancet. 2008;371:117–125. © (2008), with permission from Elsevier.
What Do the Guidelines Say?
Current guidelines recommend lifestyle modification and then statin therapy for primary prevention of a first cardiovascular event in diabetic patients older than 40 years of age with multiple risk factors regardless of baseline lipid levels (Table 1).14., 15., 16., 17. The primary goal of therapy is to achieve an LDL cholesterol (LDL‐C) <100 mg/dL (<2.6 mmol/L) and ideally <70 mg/dL (<1.8 mmol/L) using higher dose statins. When lipid levels do not meet these targets on maximally tolerated statin therapy, the guidelines again provide an alternative acceptable goal for an LDL‐C reduction of at least 30%.14., 15., 16., 17., 22., 23., 24. The health benefit translates into 36 fewer (95% CI, 23–49) serious vascular events for every 1000 diabetic patients without established CVD who achieve an LDL cholesterol reduction of 40 mg/dL (1 mmol/L) with statin therapy over 5 years; additional reductions achieved in LDL cholesterol would translate into even more prevented events.
High‐risk individuals, originally determined from results of the Framingham Heart Study,25 are those without established CVD with an estimated 10% risk of developing an incident CV event over the ensuing 10 years due to the presence of multiple risk factors. This group includes diabetic men ≥50 years or women ≥60 years with at least 1 additional CVD risk factor (smoking, hypertension, hyperlipidemia, albuminuria, or family history of CVD). Therefore, we recommend these high‐risk diabetic patients receive statin therapy for primary prevention of CV events regardless of baseline lipid levels.
Aspirin Therapy
Secondary Prevention With Aspirin
The Antithrombotic Trialists' (ATT) Collaboration conducted a meta‐analysis of the 195 randomized clinical trials that studied 135,640 patients with a wide range of atherothrombosis (including acute MI, prior MI, prior stroke or transient ischemic attack [TIA], peripheral artery disease, angina, coronary artery bypass surgery, or angioplasty). Aspirin therapy significantly lowered the risk of major vascular events (a composite of CV death, MI, or stroke) by 22% (events [%] in control arm, 13.2%, vs events in antiplatelet arm, 10.7%; odds ratio [OR] 0.78 [95% CI, 0.75–0.81]; treating 1000 patients for 2 years will result in 25 [95% CI, 22–28] fewer major vascular events) regardless of prior type of atherothrombosis, in those with and without T2DM, and among both men and women (Figure 6).26
Figure 6.
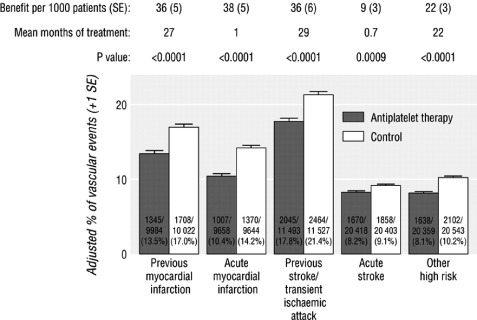
ATT Collaboration meta‐analysis of the absolute effects of antiplatelet therapy on major vascular events in 5 categories of established atherosclerosis prior to 1997. Major vascular events were defined as myocardial infarction, stroke, or vascular death. Error bars indicate 95% confidence intervals. Abbreviations: ATT, Antithrombotic Trialists'; CV, cardiovascular; SE, standard error. Reprinted from Antithrombotic Trialists' Collaboration.26 Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. © (2002), with permission from the BMJ Publishing Group Ltd.
What Do the Guidelines Say?
Current guidelines recommend aspirin therapy (75–162 mg/d) for secondary prevention of recurrent CV events in diabetic patients with established coronary artery disease, cerebrovascular disease, and symptomatic peripheral arterial disease (Table 1).14., 15., 16., 17.
Primary Prevention With Aspirin
Aspirin may also prevent a first vascular event in patients with T2DM and multiple risk factors without established atherosclerosis; however, definitive evidence of its benefit remains to be demonstrated. Among 4000 diabetic men and women without CVD studied in the ATT Collaboration, aspirin therapy lowered the risk of serious vascular events by 12% (events [%] in control arm, 0.57% per year, vs events in aspirin arm, 0.50% per year; RR 0.88, 95% CI, 0.82–0.94; P = 0.0001; treating 1429 patients for 1 year will result in 1 fewer major vascular event).27 Cardiovascular risk reduction was driven primarily by reduction in nonfatal MI. More recent findings from the Prevention of Progression of Arterial Disease and Diabetes (POPADAD) trial, a factorial randomized placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease (PAD), failed to demonstrate the efficacy of aspirin therapy for primary prevention of incident CV events.28 When the results of this study were incorporated into an updated meta‐analysis of the effect of aspirin for primary prevention of vascular and nonvascular outcomes, the totality of evidence did not support the routine use of aspirin prophylaxis in patients without established CVD for the prevention of CV death (events in control arm, 4.0 per 1000 person‐years, vs events in aspirin arm, 3.9 per 1000 person‐years; OR, 0.99; 95% CI, 0.85–1.15), despite important reductions in nonfatal MI (events in control arm, 5.1 per 1000 person‐years, vs events in aspirin arm, 4.1 per 1000 person‐years; OR, 0.80; 95% CI, 0.67–0.96; treating 162 patients for 1 year will result in 1 fewer nonfatal MI).2 In addition, an observed increased risk of major bleeding (defined as fatal bleeding, bleeding requiring hospitalization and/or transfusion, cerebrovascular, retinal, or major organ bleeding, or study‐defined major bleeding regardless of source) was found to be associated with aspirin use (events in control arm, 7.4 per 1000 person‐years, vs events in aspirin arm, 9.7 per 1000 person‐years; OR, 1.31; 95% CI, 1.14–1.50; treating 73 patients for 1 year will result in 1 additional major bleeding event). One hypothesis for why a diminished improvement in CV events was observed in more recent aspirin trials may be the observed higher rate of statin use in these primary prevention trials, underscoring again their importance in diminishing CV risk in these patients.
POPADAD is currently the only trial to study aspirin for primary prevention exclusively in patients with diabetes in the current era; however, patients enrolled in this trial were relatively low‐risk patients with type 1 DM or T2DM over the age of 40 with asymptomatic PAD and no other risk factor enrichment criteria. In addition, the POPADAD trial was not powered to demonstrate a modest effect of aspirin therapy in this population because the investigators designed the study with an anticipated annual primary endpoint event rate of 8%, but observed an annual event rate of only 2.3%.28
Important trials are ongoing to determine the contemporary benefits of aspirin for primary prevention of cardiovascular events in diabetic patients.29 Until the results of these studies are reported, it is reasonable to consider aspirin for patients with T2DM and multiple risk factors who are not at high risk for bleeding. Although the absolute benefit may be small for aspirin in primary prevention, the effect is very meaningful on a population‐wide perspective because millions of patients with T2DM have multiple risk factors without established atherosclerosis. In addition, patients with diabetes who have more than 1 risk factor have an even higher baseline risk of incident CV events than the event rates reported in aspirin primary prevention studies and may derive even greater benefit from aspirin.25 If treating physicians and/or patients are concerned about the potential risk of gastrointestinal (GI) bleeding, then concurrent aspirin use with proton‐pump inhibitors has been shown to decrease the risk of first or recurrent aspirin‐related GI bleeding.30., 31.
In summary, available data suggest that there may not be a net clinical benefit in favor of routine aspirin for the prevention of a first major vascular event in T2DM patients, but studies are still ongoing to determine the benefit of aspirin in diabetic patients with multiple risk factors.
What Do the Guidelines Say?
Current guidelines encourage consideration of aspirin (75–162 mg/d) for the primary prevention of a first vascular event in high‐risk men and women with T2DM (Table 1). Specifically, aspirin is appropriate in diabetic men aged ≥50 years or women aged ≥60 years with at least 1 additional CVD risk factor who are at low risk for major bleeding (ie, no history of previous GI bleeding or peptic ulcer disease or concurrent use of other medications that increase bleeding risk, such as warfarin).14., 15., 16., 22., 23., 32. These criteria fit the description of the high‐risk patients with T2DM, and therefore we recommend that appropriately selected patients at low risk for bleeding be treated with low‐dose aspirin for the primary prevention of CV events.
What Are the Recommendations for, and Use of, Statin and Aspirin Therapy in Contemporary Cardiovascular Outcomes Trials Studying Patients With T2DM?
A review of published contemporary major cardiovascular outcomes trials, cohort studies, and design papers in patients with T2DM revealed that the vast majority of studies stipulated that background risk–factor preventive care be consistent with current guideline recommendations at the time of conduct.33., 34., 35., 36., 37., 38., 39., 40., 41., 42., 43., 44., 45., 46. The baseline use of statin and aspirin therapy, along with angiotensin‐converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) use, and the concomitant use of antidiabetic medications within these studies are presented in Table 2. Only 1 study reported in their main publication or methods paper that statin and aspirin therapy was expected to be prescribed to all participants unless contraindicated.38 Nevertheless, even under these ideal circumstances, contemporary cardiovascular outcomes trials studying secondary prevention in patients with T2DM enroll cohorts with approximately 75% statin use at baseline and approximately 60% use in mixed primary/secondary prevention cohorts, achieving only slight improvement over the course of the observation period. Regarding aspirin therapy, a similar ceiling is seen; with baseline use of 50% to 70% in most trials that enroll mixed primary/secondary prevention cohorts from diverse international regions with varied access to background care.
Table 2.
Summary of the Concomitant Use of Statin and Aspirin Therapy, in Addition to Other Medications, in Contemporary Cohorts of Patients With Type 2 Diabetes Mellitus
| EXAMINE44 | ORIGIN 201245., 46. | TREAT 200940 | RECORD 200939 | BARI 2D 200937 | VADT 200933,38 | ADVANCE 200836 | ACCORD 200835 | PROACTIVE 200834 | REACH Registry T2DM Cohort 200641–43 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Disease state | ||||||||||
| Established CV disease | 100 | 59 | ∼ 65 | ∼ 20 | 100 | 50 | 32 | 35 | 100 | ∼ 30 |
| Primary prevention | 0 | 41 | ∼ 35 | ∼ 80 | 0 | 60 | 68 | 65 | 0 | ∼ 60 |
| T2DM medications | ||||||||||
| Insulin | 33b | 50 | 49 | 0 | 28 | 50 | 2 | 33 | 42 | 27 |
| Metformin | 33b | 27 | 35 | 100 | 54 | 69 | 61 | 62 | 53 | 42 |
| Sulfonylurea | 33b | 30 | 17 | N/A | 53 | 3 | 71 | 52 | 57 | 45 |
| TZD | 33b | 0.5 | 24 | 0 | 19 | 13 | 4 | 18 | 50 | 18 |
| CV medications | ||||||||||
| Aspirin | 53 | 66 | 42 | 20 | 88 | 73 | 44 | 54 | 75 | 71 |
| Statin | 21 | 54 | 60 | 18 | 75 | 57 | 28 | 62 | 43 | 71 |
| ACEI and/or ARB | 26 | 69 | 80 | 43 | 77 | ∼ 70 | 75a | ∼ 65 | 70 | ∼ 75 |
Abbreviations: ACEI, angiotensin‐converting‐enzyme inhibitor; ARB, angiotensin II receptor blocker; CV, cardiovascular; N/A, not applicable; T2DM, type 2 diabetes mellitus; TZD, thiazolidinedione. All values are percentages.
Any blood‐pressure lowering drug.
All diabetic agents including metformin.
In ongoing trials, the guideline‐defined high‐risk diabetic patient groups who either have established CVD (secondary prevention) or multiple risk factors (primary prevention), parallel those of patients enrolled in the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR)‐TIMI 53 trial.47 SAVOR‐TIMI 53 is a phase 4, randomized, double‐blind, placebo‐controlled trial of 16,500 subjects from 26 countries, designed to evaluate the cardiovascular efficacy and safety of saxagliptin, a dipeptidyl peptidase 4 (DPP‐4) inhibitor, for the long‐term treatment of T2DM. Eligible patients who are either antidiabetic treatment–naive or on any other background therapy are randomized 1:1 to saxagliptin 5 mg daily or matching placebo, stratified by the presence or absence of a history of established CVD. The objectives of the trial are to demonstrate the CV efficacy of a novel antidiabetic therapy in conjunction with other cardioprotective therapies in patients with T2DM, such as statins, aspirin, and blood pressure control medications. The primary endpoint is the composite of CV death, nonfatal MI, or nonfatal ischemic stroke. Within the primary prevention cohort of SAVOR‐TIMI 53, all subjects with T2DM without established CVD had at least 2 additional risk factors present at enrollment, increasing their risk for an incident CV event. Therefore, the aforementioned recommendations for the use of statin and aspirin therapy in patients with T2DM were provided to the investigators of the SAVOR‐TIMI 53 trial, which enrolled 16,500 patients with T2DM with, or at risk of, atherothrombosis and who generally meet the threshold for these impactful preventive cardiovascular therapies.
Conclusion
Statin and aspirin therapy protect a wide range of patients with T2DM with established, or at high risk of developing, atherothrombosis. These therapies, along with diet, exercise, and blood pressure control with medications such as ACE inhibitors, are currently considered the cornerstones of secondary and, in appropriately selected patients, primary cardiovascular therapy in high‐risk diabetic patients. There remains considerable controversy regarding the balance of cardiovascular efficacy and potential bleeding with aspirin for primary prevention among younger or low‐risk patients with T2DM. The results of ongoing large randomized clinical trials are therefore eagerly awaited to provide insight into this group of patients. In appropriately selected diabetic patients with multiple risk factors, the risk for incident or recurrent CV events is considered high enough to justify the protective therapy statins and aspirin afford.
References
- 1. dummy “Should healthy people take cholesterol drugs to prevent heart disease?”. The Wall Street Journal. January 23, 2012:R3. [Google Scholar]
- 2. Seshasai SR, Wijesuriya S, Sivakumaran R, et al. Effect of aspirin on vascular and nonvascular outcomes: meta‐analysis of randomized controlled trials. Arch Intern Med. 2012;172:209–216. [DOI] [PubMed] [Google Scholar]
- 3. Mora S. Aspirin therapy in primary prevention: comment on “effect of aspirin on vascular and nonvascular outcomes”. Arch Intern Med. 2012;172:217–218. [DOI] [PubMed] [Google Scholar]
- 4. Stamler J, Vaccaro O, Neaton JD, et al.; The Multiple Risk Factor Intervention Trial Research Group. Diabetes, other risk factors, and 12‐yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. [DOI] [PubMed] [Google Scholar]
- 5. Bhatt DL, Eagle KA, Ohman EM, et al. Comparative determinants of 4‐year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304:1350–1357. [DOI] [PubMed] [Google Scholar]
- 6. dummy Centers for Disease Control and Prevention. Diabetes Public Health Resource. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011.. http://www.cdc.gov/diabetes/pubs/factsheet11.htm. Accessed June 4, 2012.
- 7. Braunwald E. Shattuck lecture–cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–1369. [DOI] [PubMed] [Google Scholar]
- 8. Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581. [DOI] [PubMed] [Google Scholar]
- 9. Sniderman AD, Scantlebury T, Cianflone K. Hypertriglyceridemic hyperapob: the unappreciated atherogenic dyslipoproteinemia in type 2 diabetes mellitus. Ann Intern Med. 2001;135:447–459. [DOI] [PubMed] [Google Scholar]
- 10. Prospective Diabetes U.K. Study Group. U.K. Prospective Diabetes Study 27: plasma lipids and lipoproteins at diagnosis of NIDDM by age and sex. Diabetes Care. 1997;20:1683–1687. [DOI] [PubMed] [Google Scholar]
- 11. Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol‐lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta‐analysis. Lancet. 2008;371:117–125. [DOI] [PubMed] [Google Scholar]
- 12. dummy Cholesterol Treatment Trialists Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heart Protection Study Collaborative Group . Effects on 11‐year mortality and morbidity of lowering LDL cholesterol with simvastatin for about 5 years in 20,536 high‐risk individuals: a randomised controlled trial. Lancet. 2011;378:2013–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. IDF Clinical Guidelines Task Force . Global guideline for type 2 diabetes. Brussels, Belgium: International Diabetes Federation; 2005.. http://www.idf.org/guidelines/type‐2‐diabetes. Accessed June 4, 2012. [Google Scholar]
- 15. American Diabetes Association . Executive Summary: Standards of Medical Care in Diabetes–2011. Diabetes Care. 2011;34(Supplement 1):S4–S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reiner Z, Catapano AL, De Backer G, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32:1769–1818. [DOI] [PubMed] [Google Scholar]
- 17. Smith SC Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124: 2458–2473. [DOI] [PubMed] [Google Scholar]
- 18. Collins R, Armitage J, Parish S, et al. MRC/BHF Heart Protection Study of cholesterol‐lowering with simvastatin in 5963 people with diabetes: a randomised placebo‐controlled trial. Lancet. 2003;361: 2005–2016. [DOI] [PubMed] [Google Scholar]
- 19. Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo‐controlled trial. Lancet. 2004;364: 685–696. [DOI] [PubMed] [Google Scholar]
- 20. Knopp RH, d'Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non‐insulin‐dependent diabetes mellitus (ASPEN). Diabetes Care. 2006;29:1478–1485. [DOI] [PubMed] [Google Scholar]
- 21. Sever PS, Poulter NR, Dahlof B, et al. Reduction in cardiovascular events with atorvastatin in 2,532 patients with type 2 diabetes: Anglo‐Scandinavian Cardiac Outcomes Trial–lipid‐lowering arm (ASCOT‐LLA). Diabetes Care. 2005;28:1151–1157. [DOI] [PubMed] [Google Scholar]
- 22. Buse JB, Ginsberg HN, Bakris GL, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2007;115:114–126. [DOI] [PubMed] [Google Scholar]
- 23. Mosca L, Benjamin EJ, Berra K, et al. Effectiveness‐based guidelines for the prevention of cardiovascular disease in women–2011 update: a guideline from the American Heart Association. Circulation. 2011;123:1243–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Graham I, Atar D, Borch‐Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary: Fourth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by representatives of nine societies and by invited experts). Eur Heart J. 2007;28: 2375–2414. [DOI] [PubMed] [Google Scholar]
- 25. Wilson PW, D'Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. [DOI] [PubMed] [Google Scholar]
- 26. Antithrombotic Trialists' Collaboration . Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta‐analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337:a1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. dummy A Study of Cardiovascular Events in Diabetes: A Randomized 2×2 Factorial Study of Aspirin Versus Placebo, and of Omega‐3 Fatty Acid Supplementation Versus Placebo, for Primary Prevention of Cardiovascular Events in People With Diabetes. Last updated August 10, 2011.. http://clinicaltrials.gov/ct2/show/NCT00135226. Accessed June 4, 2012.
- 30. Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363: 1909–1917. [DOI] [PubMed] [Google Scholar]
- 31. Lai KC, Lam SK, Chu KM, et al. Lansoprazole for the prevention of recurrences of ulcer complications from long‐term low‐dose aspirin use. N Engl J Med. 2002;346:2033–2038. [DOI] [PubMed] [Google Scholar]
- 32. Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Circulation. 2010;121:2694–2701. [DOI] [PubMed] [Google Scholar]
- 33. Abraira C, Duckworth W, McCarren M, et al. Design of the cooperative study on glycemic control and complications in diabetes mellitus type 2: Veterans Affairs Diabetes Trial. J Diabetes Complications. 2003;17314–322. [DOI] [PubMed] [Google Scholar]
- 34. Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. [DOI] [PubMed] [Google Scholar]
- 35. Action to Control Cardiovascular Risk in Diabetes Study Group , Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Advance Collaborative Group , Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. [DOI] [PubMed] [Google Scholar]
- 37. The BARI 2D Study Group , Frye RL, August P, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. [DOI] [PubMed] [Google Scholar]
- 39. Home PD, Pocock SJ, Beck‐Nielsen H, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open‐label trial. Lancet. 2009;373:2125–2135. [DOI] [PubMed] [Google Scholar]
- 40. Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. [DOI] [PubMed] [Google Scholar]
- 41. Roussel R, Travert F, Pasquet B, et al. Metformin use and mortality among patients with diabetes and atherothrombosis. Arch Intern Med. 2010;170:1892–1899. [DOI] [PubMed] [Google Scholar]
- 42. Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295: 180–189. [DOI] [PubMed] [Google Scholar]
- 43. Ohman EM, Bhatt DL, Steg PG, et al. The REduction of Atherothrombosis for Continued Health (REACH) Registry: an international, prospective, observational investigation in subjects at risk for atherothrombotic events‐study design. Am Heart J. 2006;151:786.e1–786.e10. [DOI] [PubMed] [Google Scholar]
- 44. White WB, Bakris GL, Bergenstal RM, et al. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J. 2011;162: 620–626.e1. [DOI] [PubMed] [Google Scholar]
- 45. Origin Trial Investigators , Gerstein H, Yusuf S, et al. Rationale, design, and baseline characteristics for a large international trial of cardiovascular disease prevention in people with dysglycemia: the ORIGIN Trial (Outcome Reduction with an Initial Glargine Intervention). Am Heart J. 2008;155:26–32, 32.e1–32.e6. [DOI] [PubMed] [Google Scholar]
- 46. The ORIGIN Trial Investigators . Basal Insulin and Cardiovascular and Other Outcomes in Dysglycemia. N Engl J Med. 2012. DOI: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 47. Scirica BM, Bhatt DL, Braunwald E, et al. The design and rationale of the saxagliptin assessment of vascular outcomes recorded in patients with diabetes mellitus‐thrombolysis in myocardial infarction (SAVOR‐TIMI) 53 study. Am Heart J. 2011;162: 818–825. [DOI] [PubMed] [Google Scholar]


