Abstract
Vitamin K is required for the activity of various biologically active proteins in our body. Apart from clotting factors, vitamin K–dependent proteins include regulatory proteins like protein C, protein S, protein Z, osteocalcin, growth arrest‐specific gene 6 protein, and matrix Gla protein. Glutamic acid residues in matrix Gla protein are γ‐carboxylated by vitamin K–dependent γ‐carboxylase, which enables it to inhibit calcification. Warfarin, being a vitamin K antagonist, inhibits this process, and has been associated with calcification in various animal and human studies. Though no specific guidelines are currently available to prevent or treat this less‐recognized side effect, discontinuing warfarin and using an alternative anticoagulant seems to be a reasonable option. Newer anticoagulants such as dabigatran and rivaroxaban offer promise as future therapeutic options in such cases. Drugs including statins, alendronate, osteoprotegerin, and vitamin K are currently under study as therapies to prevent or treat warfarin‐associated calcification. Copyright © 2011 Wiley Periodicals, Inc.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
Warfarin, a vitamin K antagonist, has been the mainstay of oral anticoagulant therapy for many years. In the United States alone, about 2.5 million patients are estimated to be on long‐term treatment with warfarin, principally for thromboprophylaxis in the presence of atrial fibrillation (AF) or a mechanical heart valve.1 Warfarin exerts its effect by inhibiting vitamin K epoxide reductase in the liver, which is needed for the synthesis of functional clotting factors II, VII, IX, and X. Warfarin also affects the synthesis and function of the matrix Gla protein (MGP), a vitamin K–dependent protein, which is a potent inhibitor of tissue calcification. Animal studies have demonstrated that warfarin can cause calcification in vivo and in isolated organ preparations incubated with serum. Recently, human studies have implicated warfarin as one of the factors associated with calcification. In this review, we summarize the current data on association of warfarin use with vascular and valvular calcification.
Vitamin K–Dependent Proteins
There are many vitamin K–dependent proteins that need γ‐carboxylation for their physiologic activity (Table 1). The vitamin K–dependent carboxylase is an integral membrane glycoprotein that uses vitamin K to modify glutamyl residues to γ‐carboxylated glutamyl residues post‐translationally in vitamin K–dependent proteins as they pass through the endoplasmic reticulum. While this carboxylase enzyme is directly vitamin K dependent for its activity, vitamin K–dependent proteins depend on the carboxylase enzyme to be metabolically active. The clotting factors II, VII, IX and X are γ‐carboxylated in the liver to be functionally active.2 Anticoagulant factors—protein C, protein S, and protein Z—are γ‐carboxylated predominantly in the liver and to some extent in extrahepatic tissues. The other proteins are bone γ‐carboxyglutamate (Gla)‐ protein osteocalcin, the calcification‐inhibiting matrix γ‐carboxyglutamate protein (MGP), growth arrest‐specific gene 6 (GAS6) protein, and transmembrane γ‐carboxyglutamate (Gla) proteins.3 The precursors of these factors require carboxylation of their glutamic acid residues to allow the coagulation factors to bind to phospholipid surfaces. This carboxylation is linked to oxidation of vitamin K to form vitamin K epoxide, which is in turn recycled back to the reduced form by the enzyme vitamin K epoxide reductase. Warfarin inhibits the epoxide reductase (specifically the C1 subunit), thereby diminishing available vitamin K stores and inhibiting production of functioning coagulation factors.
Table 1.
Vitamin K–Dependent Proteins
| Coagulation factors: II, VII, IX, X |
|---|
| Anticoagulation factors: proteins C, S, Z |
| Others: |
| Matrix Gla protein |
| Osteocalcin |
| GAS6 protein |
| TGF‐β–inducible protein |
| Periostin |
| Proline‐rich Gla proteins 1–4 |
Abbreviations: GAS6, growth arrest‐specific gene 6; Gla, γ‐carboxyglutamate; TGF‐β, transforming growth factor β.
Figure 1 illustrates the promoters and inhibitors of vascular calcification.4 Vascular smooth muscle cells (VSMCs) are induced toward the osteoblastic phenotype by bone morphogenetic proteins (BMP) type 2 and 4. Matrix γ‐carboxyglutamate protein prevents interaction of BMP‐2 with its receptor, thereby inhibiting calcification. Binding of BMP‐2/4 to its receptor results in up‐regulation of key osteogenic transcription factors: core‐binding factor α‐1 (Cbfα1), Msx2, and osterix. Cbfα1 expression is also up‐regulated by oxidant stress, leptin, vitamin D, and high phosphate levels. Smad 6 and BMP7 are inhibitors of intracellular BMP receptor signaling. Other inhibitors include osteoprotegerin, which inhibits osteoblastic phenotype in VSMCs, and osteopontin, which inhibits hydroxyapatite formation.
Figure 1.
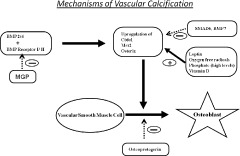
In mechanisms of vascular calcification, VSMCs are induced toward the osteoblastic phenotype by BMP type 2 and 4. MGP prevents interaction of BMP‐2 with its receptor, thereby inhibiting calcification. Binding of BMP‐2/4 to its receptor results in up‐regulation of key osteogenic transcription factors: Cbfα1, homeobox gene Msx2, and osterix. Cbfα1 expression is also up‐regulated by oxidant stress, leptin, vitamin D, and high phosphate levels. Smad6 and BMP‐7 are inhibitors of intracellular BMP receptor signaling. Other inhibitors include osteoprotegerin, which inhibits osteoblastic phenotype in VSMCs, and osteopontin, which inhibits hydroxyapatite formation. Abbreviations: BMP, bone morphogenetic protein; Cbfα1, core‐binding factor α‐1; MGP, matrix Gla protein; VSMCs, vascular smooth muscle cells.
Animal Studies on Warfarin‐Associated Calcification
Animal experimentation has demonstrated that either the lack of MGP or the use of warfarin has effects on cardiovascular calcification (Table 2). An initial study done on mice lacking MGP showed that they developed spontaneous calcification of arteries and cartilage and died of vascular rupture 2 months later. This identified MGP as the first recognized inhibitor of calcification in vivo.5 Howe and Webster designed a study to cause extrahepatic vitamin K deficiency with a warfarin treatment regimen.6 Rats were treated with daily doses of warfarin and concurrent vitamin K1 from birth for 5–12 weeks. This treatment was presumed to cause extrahepatic vitamin K deficiency without affecting the vitamin K–dependent coagulation factors. At the end of treatment, the examination of the vascular system of these rats revealed extensive arterial calcification. A study by Price et al showed that warfarin causes focal calcification of the elastic lamellae in the tunica media of major arteries and in aortic valves in mice.7 These investigators found that the calcification of arteries induced by warfarin was similar to that seen in MGP‐deficient mice, and suggested that warfarin induces arterial calcification by inhibiting carboxylation of MGP, thereby inactivating the putative calcification‐inhibitory activity of the protein. Later, they also showed that concurrent warfarin administration increased the extent of calcification in the media of vitamin D–treated mice.8 It was hypothesized that living arteries secrete the calcification inhibitor MGP; inactivation of MGP with warfarin causes living arteries to calcify, and that addition of MGP to medium containing warfarin prevents this calcification. In a subsequent study, they demonstrated that addition of warfarin to culture medium caused extensive Alizarin red staining for calcification in the living carotid artery segment, whereas no staining could be detected in living carotid arteries incubated in the same medium without warfarin.9 No calcification could be detected if the living arteries were incubated in a culture medium containing warfarin with no serum, which confirms the role of serum in arterial calcification in this system. Purified bovine MGP also prevented warfarin‐induced calcification of devitalized arteries in the same medium.
Table 2.
Animal Studies (on Mice) on Association of Warfarin Use With Calcification
| Study No. | Authors | Duration of Warfarin Therapy | Modality Used to Assess Calcification | Results |
|---|---|---|---|---|
| 1 | Howe AM, Webster WH6 (2000) | 5–12 wk | Alizarin red technique | Extensive arterial calcification; cerebral arteries, veins, and capillaries not calcified. |
| 2 | Price et al7 (1998) | 1–5 wk | Von Kossa staining, radiographs, visual inspection of the artery, northern blot analysis of MGP mRNA levels | Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Warfarin treatment markedly increased the levels of MGP mRNA and protein in calcifying arteries and decreased the level of MGP in serum. |
| 3 | Price et al8 (2000) | Group aged 20–42 d: 2 wk Group aged 10 mo: 4 wk | Von Kossa staining | Warfarin‐induced artery calcification is accelerated by growth and vitamin D. |
| 4 | Koos et al18 (2009) | 4 wk | PCR analysis for MGP mRNA | Warfarin may decrease MGP serum levels and increased aortic valve calcifications. |
| 5 | Liu et al26 (2008) | 4 wk | Von Kossa staining | Warfarin treatment led to elevation of SBP and aortic medial calcification. Chronic treatment also increased collagen, but decreased elastin, in the aorta. |
| 6 | Price et al9 (2006) | 6 d | Staining of whole arteries with Alizarin red, Von Kossa staining of histological sections | Addition of warfarin to culture medium caused extensive Alizarin‐red staining for calcification in the living carotid artery segment, whereas no staining could be detected in living carotid arteries incubated in the same medium without warfarin. |
| 7 | Price et al33 (2001) | 1 wk | Alizarin red staining | Doses of osteoprotegerin that inhibit bone resorption are able to potently inhibit the calcification of arteries that is induced by warfarin treatment. |
Abbreviations: MGP, matrix γ‐carboxyglutamate protein; PCR, polymerase chain reaction; SBP, systolic blood pressure.
Sweatt et al elucidated the interaction of MGP and BMP‐2.10 Using immunohistochemistry, these investigators showed that calcified lesions in the aortic wall of aging rats contained elevated concentrations of MGP that was poorly γ‐carboxylated and did not bind BMP‐2. They demonstrated the existence of BMP‐2/MGP complex in vivo, consistent with a role for MGP as a BMP‐2 inhibitor. They postulated that age‐related arterial calcification may be a consequence of under–γ‐carboxylation of MGP, allowing unopposed BMP‐2 activity.
Human Studies on Warfarin‐Associated Calcification
In humans, various studies have shown the association of warfarin use with vascular and cardiac valvular calcification (Table 3).
Table 3.
Human Studies on Association of Warfarin Use With Calcification
| Study No. | Author | Type of Study | No. of Patients | Modality Used to Assess Calcification | Characteristic of Patients | Follow‐Up | Results |
|---|---|---|---|---|---|---|---|
| 1 | Yamamoto et al20 (2010) | Retrospective observational study | 556 | Echocardiogram | Age >50 years, calcification in any aortic valve leaflet or peak aortic jet velocity >2 ms−1 | Mean follow‐up: 2.8 years | Warfarin use was also associated with early‐stage CAVD progression (P = 0.03). |
| 2 | Villines et al15 (2009) | Cross‐sectional analysis | 70 | Electron beam CT | Patients with mean age 68 ± 13 y on warfarin | 3 groups of warfarin use duration: <6 m (n = 31), 6–24 m (n = 11), >24 m (n = 28) | Bivariate analysis revealed no correlation between warfarin duration and CAC score. |
| 3 | Lerner RG et al21 (2009) | Cross‐sectional analysis | 1155 | Echocardiogram | Nonvalvular AF: 725 (63%) patients and 430 (37%) controls | Unspecified | Valvular calcification present in 473 of 725 patients (65%) vs 225 of 430 controls (52%) (P<0.0001). Significant association between warfarin use and risk of calcification (OR: 1.71, 95% CI: 1.34–2.18). |
| 4 | Koos et al18 (2009) | Cross‐sectional analysis | 226 | Echocardiogram, multislice spiral CT, circulating ucMGP levels | 191 patients with CAVD vs 35 control subjects | Unspecified | Serum ucMGP levels were significantly lower in the study group (348.6 ± 123.1 nM vs 571.6 ± 153.9 nM, p<0.001). Patients on long‐term warfarin (n = 27) demonstrated lower serum ucMGP levels compared with patients without anticoagulation (n = 142). |
| 5 | Verdalles Guzmán et al14 (2008) | Retrospective observational study | 8 | Biopsy | Female patients on hemodialysis with calciphylaxis | Unspecified | Warfarin therapy is a risk factor for developing calciphylaxis. |
| 6 | Holden et al19 (2007) | Retrospective cohort study | 108 | Echocardiogram | Hemodialysis patients | 36.7 ± 19.7 mo | 18 subjects with long‐term warfarin exposure were more likely to have severe aortic valve calcification (P = 0.04). OR: 3.77, 95% CI: 0.97–14.70, P = 0.055). |
| 7 | Koos et al17 (2005) | Cross‐sectional analysis | 86 | Multislice spiral CT | CAVD; 23 patients vs 63 controls | 88 ± 113 mo | Patients on warfarin therapy had increased coronary calcium score (1561 ± 1141 vs 738 ± 978; P = 0.024) and valvular calcium score (2410 ± 1759 vs 1070 ± 1085, respectively; P = 0.002). |
| 8 | Schori and Stungis13 (2004) | Case report | 1 | Cardiac MRI | Otherwise healthy man found to have severe CAC on warfarin for 11 y | Unspecified | Long‐term warfarin treatment may have induced arterial calcification. |
Abbreviations: AF, atrial fibrillation; CAC, coronary artery calcification; CAVD, calcific aortic valve disease; CI, confidence interval; CT, computed tomography; MGP, matrix γ‐carboxyglutamate protein; MRI, magnetic resonance imaging; OR, odds ratio; ucMGP, inactive MGP.
Vascular Calcification
A series of 16 patients with cutaneous necrosis from calcific uremic arteriolopathy identified warfarin as a risk factor.11 In 2 of these patients, substitution of warfarin with low‐molecular‐weight heparin resulted in clinical improvement. Spronk et al12 found that MGP accumulated at the borders of vascular calcification in human tissue specimens. These investigators suggested that undercarboxylated MGP is biologically inactive, and that poor vascular vitamin K status may be a risk factor for vascular calcification. Schori and Stungis reported a case of arterial calcification in a patient with long‐term warfarin use.13 In a retrospective analysis by Verdalles Guzmán et al of 8 patients that developed calciphylaxis, 6 patients were on anticoagulation therapy with warfarin.14 All patients were obese women, with metabolic syndrome and poorly controlled hypertension. These patients did not have significant alterations of calcium metabolism to explain calciphylaxis; all had a calcium‐phosphorus product <55. Of these 8 patients, calciphylaxis was seen in proximal regions such as the abdomen and thighs in 7 patients. Histopathologic examination of these lesions revealed calcium deposits in arterioles with vascular thrombosis. These authors concluded that anticoagulant therapy was one of the risk factors to develop calciphylaxis, in the absence of severe disorders of calcium metabolism.
In a cross‐sectional analysis of 70 patients (46 men, mean age 68 ± 13 years) on warfarin therapy without known coronary artery disease, after adjustment for cardiovascular risk factors, no correlation between warfarin duration and coronary artery calcification (CAC) score was observed on multivariate analysis. They concluded that warfarin exposure does not appear to play a significant role in potentiating arterial calcification as measured by electron beam computed tomography (CT) in a middle‐aged to older screening population.15
Valvular Calcification
Schurgers et al investigated whether long‐term oral anticoagulant treatment may induce calcification in humans.16 These investigators measured the grade of aortic valve calcification in valves removed from patients undergoing surgical valve replacement. Calcifications in valves from patients receiving preoperative oral anticoagulant treatment were significantly larger than those in patients not receiving preoperative oral anticoagulants. These investigators concluded that oral anticoagulants may induce cardiovascular calcification as an adverse side effect.
Koos et al17 reported the association of warfarin use with aortic valve calcification (AVC) and CAC, assessed by multislice spiral CT. They studied 86 patients (53 men, mean age 71 ± 8 years) with calcific aortic valve disease, 23 patients on long‐term warfarin therapy (mean duration 88 ± 113 months), and 66 patients without anticoagulation. Patients on warfarin therapy had increased CAC score (coronary Agatston score 1561 ± 1141 vs 738 ± 978, P = 0.024) and AVC (valvular Agatston score 2410 ± 1759 vs 1070 ± 1085, P = 0.002), compared with patients without anticoagulation treatment. Subsequently, they investigated the effect of long‐term warfarin treatment on circulating MGP levels in humans and on MGP expression in mice and the association between circulating inactive MGP (ucMGP) levels and the presence and severity of AVC in patients with calcific aortic valve disease (CAVD).18 They analyzed the circulating ucMGP levels in 191 patients with echocardiographically proven CAVD and 35 control subjects. They found that MGP levels were significantly lower in patients with CAVD (348.6 ± 123.1 nM) compared with the control group (571.6 ± 153.9 nM, P<0.001). In mice on warfarin, mRNA expression of MGP in the aorta was down‐regulated. They concluded that patients with CAVD had significantly lower levels of circulating ucMGP as compared with a reference population free of coronary and valvular calcifications. In addition, warfarin treatment may decrease local expression of MGP, resulting in decreased circulating MGP levels and subsequently increased aortic valve calcifications as an adverse side effect.
In a retrospective cohort study among hemodialysis patients by Holden et al, the odds ratio (OR) of falling into a higher category of AVC following 18 months of warfarin use was not statistically significant (P = 0.055).19 However, there was an association between lifetime months of warfarin exposure and severity of AVC (P = 0.004) that was independent of dialysis use, calcium, and calcitriol intake. These investigators suggested that warfarin use may be associated with severity of AVC in hemodialysis patients.
In the Japanese Aortic Stenosis Study,20 a retrospective observational study of 556 subjects aged ≥50 years and with calcification in any aortic valve leaflet or peak aortic jet velocity ≥2 m/sec, use of warfarin was identified to be a prognostic factor in early‐stage disease (peak aortic jet velocity of ≥2m/sec) and not for late‐stage disease (peak aortic jet velocity of ≥3 m/sec). These investigators concluded that we should be vigilant about progression of CAVD in patients treated with warfarin.
We had previously reported the incidences of mitral valve calcification (MVC), mitral annular calcification (MAC) and AVC with 2‐dimensional echocardiograms in 1155 patients with nonvalvular AF. Of these 1155 patients, mean age 74 years, 725 (63%) were treated with warfarin and 430 (37%) without warfarin. Mitral valve calcification, MAC, or AVC was present in 473 of 725 patients (65%) on warfarin vs 225 of 430 patients (52%) not on warfarin (P<0.0001). On stepwise logistic regression analysis, there was a significant association between the use of warfarin and the risk of calcification (unadjusted OR: 1.71, 95% CI: 1.34–2.18) after adjustment for confounding risk factors. We concluded that use of warfarin in patients with nonvalvular AF is associated with an increased prevalence of MVC, MAC, or AVC.21 The distribution of vascular involvement in warfarin‐treated animals seems to favor medium‐sized vessels, with relative sparing of capillaries and veins.6 Given the complexity and the notable differences between hepatic and peripheral carboxylation, it seems plausible that warfarin might affect the vitamin K–dependent mechanisms in the liver and blood vessels in different ways. Recent studies show that low doses of warfarin inhibit peripheral carboxylation without affecting hepatic carboxylation, suggesting that vascular effects may occur at lower doses than that used for anticoagulation.22 Presently, there are no human studies to support this finding.
Treatment
Until recently, calcification was thought to be an irreversible phenomenon. Current understanding, however, dictates that calcification is an active process, with inhibitors and stimulators of calcification. Being a recently recognized phenomenon, current guidelines do not discuss the screening, prevention, or treatment of warfarin‐associated calcification. Stopping warfarin and using an alternative anticoagulant can help. This has been shown in patients with cutaneous necrosis from calcific uremic arteriolopathy.11 In another case report, a patient with biopsy‐proven calciphylaxis thought to be attributable to warfarin was treated with a therapeutic substitution of enoxaparin, and hyperbaric oxygen therapy leading to resolution of cutaneous lesions.23 The mechanism by which hyperbaric therapy might benefit patients with calciphylaxis is unclear.
Statins have numerous pleiotropic effects, resulting from their ability to block synthesis of isoprenoid intermediates and inhibit prenylation of Rho family guanosine triphosphatases.24 Cerivastatin and atorvastatin inhibit in vitro calcification of human VSMCs induced by inflammatory mediators in a dose‐dependent manner. Inhibition of Rho and its downstream target, Rho kinase may mediate this inhibitory effect of statins.25 In vivo administration of atorvastatin reduced vitamin D3 and warfarin‐induced arterial calcification, plasma calcium concentration, and alkaline phosphatase levels in rats.26., 27. Protective effective of atorvastatin on vascular remodeling in renovascular hypertensive rats is attributed to decreased expression of osteopontin.28
Etidronate, clodronate, and several other first‐generation bisphosphonates had been shown to inhibit vitamin D–induced artery calcification in rats.29., 30. This was thought to be due to inhibitory effect of bisphosphonates on formation of hydroxyapatite crystals. Later, alendronate and ibandronate were demonstrated to inhibit warfarin‐induced calcification of arteries and heart valves in rats at doses comparable to the doses that inhibit bone resorption.31 In a randomized controlled trial to determine the effect of bisphosphonates on vascular calcification in patients with stage 3/4 chronic kidney disease, 50 patients were randomly assigned to either alendronate 70 mg (n = 25) or matching placebo (n = 25), administered weekly. At 18 months, there was no difference in vascular calcification progression assessed by CT with alendronate compared with placebo.32
Osteoprotegerin, a secreted protein of the tumor necrosis factor family that inhibits osteoclast differentiation and activation, has been shown to protect against warfarin‐induced calcification in rats.33 Warfarin‐induced vascular calcification in rats was prevented, and in some cases reversed, by high vitamin K intake.34 Vascular smooth muscle cell–derived apoptotic vesicles were loaded with calcification inhibitors, including MGP, and these vesicles had promineralizing properties when MGP function was impaired.35 High vitamin K intake was associated with significantly less VSMC apoptosis and with significant regression of arterial calcification. Calcium deposits were removed by phagocytosis carried out by the surrounding VSMCs.36 Vitamin K is present in different forms. Vessel walls specifically accumulate vitamin K2, even when the diet exclusively contains vitamin K1.37 Spronk et al showed that K2 is more effective than K1 in preventing calcification during warfarin treatment.38 However, Schurgers et al proposed that in the high‐K1 group, K1 had been converted to K2 to such an extent that in the high‐K1 group, arterial K2 had comparable tissue concentrations as in the K2–treated group. They concluded that at very high intakes of K1 (200‐fold the daily requirement), both these forms of vitamin K might help decrease arterial calcification.34
Table 4 summarizes the therapies that have been studied for warfarin‐associated calcification.
Table 4.
Treatment of Warfarin‐Associated Calcification
| Discontinuation of warfarin |
|---|
| Hyperbaric oxygen therapy (for calciphylaxis) |
| Statins (atorvastatin) |
| Bisphosphonates (alendronate) |
| Osteoprotegerin |
| Vitamin K (high‐dose vitamin K1, vitamin K2) |
Conclusion
Although animal studies have proved that warfarin causes calcification, and human studies have shown this strong association, much of available data on warfarin‐associated calcification is derived from experimental and observational studies. These preliminary studies do suggest an association of warfarin use with calcification, but the potential relationship warrants larger prospective randomized studies to better evaluate this relationship. Based on the available data, we do not currently recommend screening for vascular calcification before initiation of warfarin therapy. It is still unclear whether warfarin‐associated calcification is clinically significant enough to affect the morbidity and mortality associated with vascular and valvular calcification. Warfarin still remains the least expensive and most widely available mode of anticoagulation with long‐term expertise in managing it, and inexpensive testing for monitoring the dose. It has a proven benefit in preventing strokes in AF patients, recurrent deep venous thrombosis and pulmonary embolism, and in reducing mortality in patient populations at risk for thromboembolic disease. Warfarin is also fairly quickly reversible in case of bleeding and can be safely used in patients with low creatinine clearance. However, the use of warfarin has always been difficult because of complex pharmacodynamics, a narrow therapeutic window, numerous drug‐drug and drug‐food interactions, and multiple adverse effects. New oral anticoagulants with different mechanisms of action than warfarin have been developed. The direct thrombin inhibitor dabigatran etexilate and the factor Xa inhibitor rivaroxaban have shown promise in early trials. With the advent of these newer anticoagulants, the association of warfarin and calcification needs to be examined urgently. Further studies are needed to determine the dose and duration of warfarin use that might lead to calcification, the target population that need to be screened, an appropriate and cost‐effective screening modality, prevention strategies, and treatment of warfarin‐associated calcification.
References
- 1. Douketis JD, Berger PB, Dunn AS, et al; American College of Chest Physicians . The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(suppl 6):S299–S339. [DOI] [PubMed] [Google Scholar]
- 2. Shearer MJ, Newman P. Metabolism and cell biology of vitamin K. Thromb Haemost. 2008;100:530–547. [PubMed] [Google Scholar]
- 3. Cranenburg EC, Schurgers LJ, Vermeer C. Vitamin K: the coagulation vitamin that became omnipotent. Thromb Haemost. 2007;98:120–125. [PubMed] [Google Scholar]
- 4. Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications [published correction appears in Circ Res. 2009;105:e8]. Circ Res. 2006;99: 1044–1059. [DOI] [PubMed] [Google Scholar]
- 5. Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;385:78–81. [DOI] [PubMed] [Google Scholar]
- 6. Howe AM, Webster WS. Warfar in exposure and calcification of the arterial system in the rat. Int J Exp Pathol. 2000;81:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Price PA, Faus SA, Williamson MK. Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arterioscler Thromb Vasc Biol. 1998;18:1400–1407. [DOI] [PubMed] [Google Scholar]
- 8. Price PA, Faus SA, Williamson MK. Warfarin‐induced artery calcification is accelerated by growth and vitamin D. Arterioscler Thromb Vasc Biol. 2000;20:317–327. [DOI] [PubMed] [Google Scholar]
- 9. Price PA, Chan WS, Jolson DM, et al. The elastic lamellae of devitalized arteries calcify when incubated in serum: evidence for a serum calcification factor. Arterioscler Thromb Vasc Biol. 2006;26:1079–1085. [DOI] [PubMed] [Google Scholar]
- 10. Sweatt A, Sane DC, Hutson SM, et al. Matrix Gla protein (MGP) and bone morphogenetic protein‐2 in aortic calcified lesions of aging rats. J Thromb Haemost. 2003;1:178–185. [DOI] [PubMed] [Google Scholar]
- 11. Coates T, Kirkland GS, Dymock RB, et al. Cutaneous necrosis from calcific uremic arteriolopathy. Am J Kidney Dis. 1998;32:514–518. [DOI] [PubMed] [Google Scholar]
- 12. Spronk HM, Soute BA, Schurgers LJ, et al. Matrix Gla protein accumulates at the border of regions of calcification and normal tissue in the media of the arterial vessel wall. Biochem Biophys Res Commun. 2001;289:485–490. [DOI] [PubMed] [Google Scholar]
- 13. Schori TR, Stungis GE. Long‐term warfarin treatment may induce arterial calcification in humans: case report. Clin Invest Med. 2004;27:107–109. [PubMed] [Google Scholar]
- 14. Verdalles Guzmán U, de la Cueva P, Verde E, et al. Calciphylaxis: fatal complication of cardiometabolic syndrome in patients with end stage kidney disease [in Spanish]. Nefrologia. 2008;28: 32–36. [PubMed] [Google Scholar]
- 15. Villines TC, O'Malley PG, Feuerstein IM, et al. Does prolonged warfarin exposure potentiate coronary calcification in humans? Results of the warfarin and coronary calcification study. Calcif Tissue Int. 2009;85:494–500. [DOI] [PubMed] [Google Scholar]
- 16. Schurgers LJ, Aebert H, Vermeer C, et al. Oral anticoagulant treatment: friend or foe in cardiovascular disease? Blood. 2004;104:3231–3232. [DOI] [PubMed] [Google Scholar]
- 17. Koos R, Mahnken AH, Mühlenbruch G, et al. Relation of oral anticoagulation to cardiac valvular and coronary calcium assessed by multislice spiral computed tomography. Am J Cardiol. 2005;96:747–749. [DOI] [PubMed] [Google Scholar]
- 18. Koos R, Krueger T, Westenfeld R, et al. Relation of circulating Matrix Gla‐Protein and anticoagulation status in patients with aortic valve calcification. Thromb Haemost. 2009;101: 706–713. [PubMed] [Google Scholar]
- 19. Holden RM, Sanfilippo AS, Hopman WM, et al. Warfarin and aortic valve calcification in hemodialysis patients. J Nephrol. 2007;20:417–422. [PubMed] [Google Scholar]
- 20. Yamamoto K, Yamamoto H, Yoshida K, et al. Prognostic factors for progression of early‐and late‐stage calcific aortic valve disease in Japanese: the Japanese Aortic Stenosis Study (JASS) Retrospective Analysis. Hypertens Res. 2010;33:269–274. [DOI] [PubMed] [Google Scholar]
- 21. Lerner RG, Aronow WS, Sekhri A, et al. Warfarin use and the risk of valvular calcification. J Thromb Haemost. 2009;7:2023–2027. [DOI] [PubMed] [Google Scholar]
- 22. Hara K, Kobayashi M, Akiyama Y. Comparison of inhibitory effects of warfarin on gamma‐carboxylation between bone and liver in rats. J Bone Miner Metab. 2005;23:366–372. [DOI] [PubMed] [Google Scholar]
- 23. Banerjee C, Woller SC, Holm JR, et al. Atypical calciphylaxis in a patient receiving warfarin then resolving with cessation of warfarin and application of hyperbaric oxygen therapy. Clin Appl Thromb Hemost. 2010;16:345–350. [DOI] [PubMed] [Google Scholar]
- 24. Palaniswamy C, Selvaraj DR, Selvaraj T, et al. Mechanisms underlying pleiotropic effects of statins. Am J Ther. 2010;17:75–78. [DOI] [PubMed] [Google Scholar]
- 25. Kizu A, Shioi A, Jono S, et al. Statins inhibit in vitro calcification of human vascular smooth muscle cells induced by inflammatory mediators. J Cell Biochem. 2004;93:1011–1019. [DOI] [PubMed] [Google Scholar]
- 26. Liu C, Wan J, Yang Q, et al. Effects of atorvastatin on warfarin‐induced aortic medial calcification and systolic blood pressure in rats. J Huazhong Univ Sci Technolog Med Sci. 2008;28: 535–538. [DOI] [PubMed] [Google Scholar]
- 27. Li H, Tao HR, Hu T, et al. Atorvastatin reduced calcification in rat arteries and vascular smooth muscle cells. Basic Clin Pharmacol Toxicol. 2010;107:798–802. [DOI] [PubMed] [Google Scholar]
- 28. Yang S, Zhou JZ, Chen YH. Effects of atorvastatin calcium on osteopontin in renovascular hypertensive rats [in Chinese]. Beijing Da Xue Xue Bao. 2010;42:147–150. [PubMed] [Google Scholar]
- 29. Francis MD, Russell RG, Fleisch H. Diphosphonates inhibit formation of calcium phosphate crystals in vitro and pathological calcification in vivo. Science. 1969;165:1264–1266. [DOI] [PubMed] [Google Scholar]
- 30. Fleisch HA, Russell RG, Bisaz S, et al. The inhibitory effect of phosphonates on the formation of calcium phosphate crystals in vitro and on aortic and kidney calcification in vivo. Eur J Clin Invest. 1970;1:12–18. [DOI] [PubMed] [Google Scholar]
- 31. Price PA, Faus SA, Williamson MK. Bisphosphonates alendronate and ibandronate inhibit artery calcification at doses comparable to those that inhibit bone resorption. Arterioscler Thromb Vasc Biol. 2001;21:817–824. [DOI] [PubMed] [Google Scholar]
- 32. Toussaint ND, Lau KK, Strauss BJ, et al. Effect of alendronate on vascular calcification in CKD stages 3 and 4: a pilot randomized controlled trial. Am J Kidney Dis. 2010;56:57–68. [DOI] [PubMed] [Google Scholar]
- 33. Price PA, June HH, Buckley JR, et al. Osteoprotegerin inhibits artery calcification induced by warfarin and by vitamin D. Arterioscler Thromb Vasc Biol. 2001;21:1610–1616. [DOI] [PubMed] [Google Scholar]
- 34. Schurgers LJ, Spronk HM, Soute BA, et al. Regression of warfarin‐induced medial elastocalcinosis by high intake of vitamin K in rats. Blood. 2007;109:2823–2831. [DOI] [PubMed] [Google Scholar]
- 35. Reynolds JL, Joannides AJ, Skepper JN, et al. Human vascular smooth muscle cells undergo vesicle‐mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol. 2004;15:2857–2867. [DOI] [PubMed] [Google Scholar]
- 36. Proudfoot D, Davies JD, Skepper JN, et al. Acetylated low‐density lipoprotein stimulates human vascular smooth muscle cell calcification by promoting osteoblastic differentiation and inhibiting phagocytosis. Circulation. 2002;106:3044–3050. [DOI] [PubMed] [Google Scholar]
- 37. Ronden JE, Drittij‐Reijnders MJ, Vermeer C, et al. Intestinal flora is not an intermediate in the phylloquinone‐menaquinone‐4 conversion in the rat. Biochim Biophys Acta. 1998;1379:69–75. [DOI] [PubMed] [Google Scholar]
- 38. Spronk HM, Soute BA, Schurgers LJ, et al. Tissue‐specific utilization of menaquinone‐4 results in the prevention of arterial calcification in warfarin‐treated rats. J Vasc Res. 2003;40: 531–537. [DOI] [PubMed] [Google Scholar]


