Abstract
Background:
Obesity has become an important health problem throughout the world. Early detection of cardiovascular abnormalities may be useful in the future for patient management. This study aimed to identify subclinical ventricular dysfunction in obese patients.
Hypothesis:
Morbid obesity is associated with ventricular dysfunction.
Methods:
Doppler echocardiogram was performed in 92 morbidly obese and in 31 healthy controls. Conventional echocardiography and tissue Doppler‐based strain imaging were used to analyze ventricular function. Intra‐ and interobserver strain imaging variabilities were tested on 15 randomly selected cases.
Results:
Left ventricular (LV) global strain (22.5% ± 3.5 vs 24.4% ± 2.5, P<0.005) and right ventricular (RV) strain (25.8% ± 5.2 vs 28.2% ± 5.2, P<0.029) were lower in obese patients when compared with healthy controls. Echocardiographic parameters of diastolic function were also different from controls. LV strain correlated with LV mass, E/e′ ratio, left atrial volume, and RV strain. At multivariate analysis, morbid obesity remained a significant determinant of global LV strain, independently of associated comorbidities.
Conclusions:
These findings suggest that incipient biventricular dysfunction is present in morbidly obese patients when new echocardiographic indices are used to investigate ventricular function. In addition, strain imaging may provide a more accurate assessment of the ventricular function in obese patients. © 2011 Wiley Periodicals, Inc.
This work was supported by grants from CNPq and FAPEMIG.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Introduction
Obesity has become a modern epidemic, with statistics showing that more than 60 million adults are affected in the United States.1 Severe obesity (body mass index [BMI] ≥40 kg/m2), often referred as morbid obesity, is estimated to exist in 5 to 10 million individuals,1 and is associated with a very high risk of comorbidities and mortality. Because it is an independent risk factor for the development of heart failure, the expected increasing burden of this epidemic will have important public health implications.2
Cardiac involvement in obesity has been frequently reported in the literature.3, 4, 5 Indeed, obesity has been linked to a spectrum of minor cardiovascular abnormalities, ranging from a hyperdynamic circulation to subclinical structural changes. Early detection of cardiovascular abnormalities is important because treatment to reverse the process is most likely to be effective earlier in the disease.
The potential impact of morbid obesity on biventricular function has not been fully established. New echocardiographic techniques provide a more accurate assessment of ventricular function, especially in obese patients. Tissue Doppler imaging (TDI), myocardial strain, and strain rate have been introduced to better quantify segmental and global myocardial dysfunction. Previous studies have demonstrated incipient dysfunction in obesity detected by strain and strain rate imaging.6, 7, 8, 9
The aim of the present study was to investigate subclinical ventricular abnormalities assessed by conventional echocardiographic, TDI, strain, and strain rate indexes in morbidly obese subjects. We also aimed to identify the determinants of left ventricular (LV) strain changes.
Methods
Study Group
Patients from 2 obesity clinics (Hospital das Clínicas of the Universidade Federal de Minas Gerais–UFMG and the Center of Medical Specialties of Santa Casa de Misericórdia, both in Belo Horizonte, Minas Gerais, Brazil) who were candidates for bariatric surgery were enrolled. Patients were selected if they had had a BMI of 40 kg/m2 or higher for more than 5 years and no history of bariatric surgery. Exclusion criteria were the presence of renal failure, defined as serum creatinine higher than 2.0 mg/dL. Except for controlled hypertension or diabetes, patients with clinical evidence of cardiovascular diseases, such as heart valve diseases, congenital heart diseases, atrial fibrillation, or coronary artery disease were also excluded. The study protocol was approved by the ethics committee of both institutions, and written informed consent was obtained from all participants.
Patients were weighed wearing light clothing without shoes using a Welmy (capacity up to 300 kg) scale, with an accuracy of 100 g. Height was measured using a stadiometer with accuracy of 0.5 cm. BMI was calculated using the conventional formula of weight in kilograms divided by the square of height in meters.
Blood pressure was measured using an appropriate sized cuff, after at least a 30‐minute interval from caffeine or cigarette consumption. Glucose and lipids were collected following a 12‐hour fasting recommendation.
In addition, 31 healthy individuals were selected as a control group. The control group comprised asymptomatic subjects referred for echocardiography who had normal echocardiograms.
Doppler Echocardiogram
A comprehensive Doppler echocardiogram with color flow mapping and tissue Doppler imaging was performed in all patients using commercially available hardware and software with an electronic high‐resolution multifrequency transducer (Vivid 7 and Echopac software; GE Vingmed Ultrasound AS, Horten, Norway). Measurements were performed by 2 experienced cardiologists who were blinded to other data from the patients. LV measurements were obtained according to the American Society of Echocardiography (ASE) standards.10 Left atrial volume was assessed by the biplane area‐length method from apical 4‐ and 2‐chamber views and indexed to body surface area.11 LV mass, calculated by the ASE method, was normalized for height to the power of 2.7 to obtain the LV mass index.12
Pulmonary artery pressure was estimated using the modified Bernoulli equation ([tricuspid regurgitation jet velocity]2 × 4) and adding 10 mm Hg as a clinical estimation of right atrial pressure,13 because inferior vena cava respiratory variation could not be analyzed in these patients.
Diastolic function was assessed by pulsed Doppler of the mitral inflow and by TDI14 measurements obtained at the medial and lateral border of the mitral annulus in the apical 4‐chamber view. Systolic tissue Doppler velocity, early (e′), and late (A′) diastolic tissue velocities were acquired, and the ratio of the mitral E velocity and mean e′ were calculated (E/e′). Measurements were averaged from 3 beats.
Doppler‐derived strain and strain rates were obtained by placing a 10‐mm sample bar at the basal part of the 6 LV walls in the 3 different apical views.15 The image sector was narrowed to allow for the highest frame rate (>200 frames/second), and the imaging angle was kept as low as possible (usually below 30 degrees) to allow for a better parallel alignment to the wall of interest (Figure 1). Peak systolic strain and strain rate were measured, and global strain and strain rate were obtained dividing the sum of each longitudinal peak strain and strain rate by the number of walls. Strain and strain rate were also obtained for the right ventricle (RV) at its basal free wall in the apical 4‐chamber view.9
Figure 1.
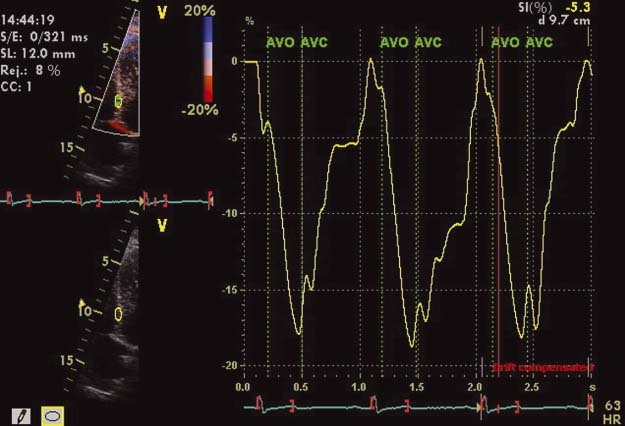
Peak systolic strain obtained at the basal segment of the interventricular septum. Narrowed sector enables maximum yield in frame rate (ideally >200/s), optimizing image clarity and data acquisition.
Intraobserver and interobserver variability of strain and strain rate imaging parameters were tested on 15 randomly selected obese patients. For the analysis of variability, we calculated an adjusted coefficient of variation, defined as the ratio of the standard deviation and the mean absolute readings for LV and RV strain and strain rate parameters.
Statistical Analysis
Categorical data were presented as numbers and percentages, and continuous data were expressed as means ± standard deviation. Echocardiographic variables of patients and controls were compared using unpaired Student t test.
Stepwise multivariate linear regression models were used to estimate the relative contributions of the clinical, demographic, and echocardiographic variables of LV mass. Multivariate models were constructed selecting variables that were significant in univariate analyses. A value of P<0.05 was considered significant. SPSS version 17 (SPSS Inc., Chicago, IL) was used for all analyses.
Results
Clinical Data
A total of 101 class III obese patients were recruited. Nine patients were excluded because of technically inadequate echocardiographic images, leaving the study sample composed of 92 subjects (group I). Thirty‐one healthy subjects were used as controls (group II). Clinical data from individuals in both groups are described in Table 1.
Table 1.
Clinical Parameters in Obese (Group I) and Healthy Individuals (Group II)
| Parameters | Group I (n = 92) | Group II (n = 31) | P Value |
|---|---|---|---|
| Age (y) | 43.5 ± 11.3 | 40.0 ± 6.5 | 0.105 |
| Gender (M/F) | 18/74 | 10/21 | 0.239 |
| Weight (kg) | 138.4 ± 26.8 | 65.6 ± 11.1 | <0.001 |
| Height (m) | 1.61 ± 0.1 | 1.7 ± 0.1 | <0.001 |
| BSA (m2) | 2.32 ± 0.26 | 1.75 ± 0.17 | <0.001 |
| BMI (kg/m2) | 53.2 ± 8.0 | 22.9 ± 2.7 | <0.001 |
| HR (bpm) | 79.0 ± 11.5 | 69.9 ± 9.5 | <0.001 |
| SBP (mm Hg) | 140.9 ± 22.5 | 112.2 ± 10.1 | <0.001 |
| DBP (mm Hg) | 87.0 ± 16.3 | 74.1 ± 8.8 | <0.001 |
| Fasting glucose (mg/dL) | 107.3 ± 35.1 | 84.8 ± 9.5 | 0.003 |
| Cholesterol (mg/dL) | 192.1 ± 49.4 | 165.8 ± 32.1 | 0.017 |
| HDL‐C (mg/dL) | 45.8±12.1 | 57.7 ± 10.5 | <0.001 |
| LDL‐C (mg/dL) | 114.4 ± 45.1 | 94.1 ± 24.8 | 0.040 |
| Triglyceride (mg/dL) | 155.8 ± 89.2 | 68.7 ± 28.2 | <0.001 |
| Insulin (ng/mL) | 18.8 ± 14.1 | NA | |
| HOMA‐IR | 4.96 ± 4.7 | NA |
Abbreviations: BMI, body mass index; BSA, body surface area; DBP, diastolic blood pressure; F, female; HDL‐C, high‐density lipoprotein cholesterol; HR, heart rate; HOMA‐IR, homeostasis model assessment insulin resistance; LDL‐C, low‐density lipoprotein cholesterol; M, male; NA, not applicable; SBP, systolic blood pressure.
Groups were similar for age and gender. Although history of hypertension was present in 78% in group I, the mean systolic and diastolic blood pressure were 141 ± 22 mm Hg and 74 ± 9 mm Hg, respectively. Diabetes was reported in 35%, with mean glycosylated hemoglobin of 7.2% (range, 5–12%). History compatible with obstructive sleep apnea was reported in 27%, and metabolic syndrome was present in 78% of the patients. Mean waist‐to‐hip ratio was 0.94 (range, 0.73–1.15), and mean waist circumference was 138 cm (range, 107–170 cm). Laboratory data are shown in Table 1.
In group I, 72 patients (78%) were taking antihypertensive agents, including angiotensin‐converting enzyme inhibitors in 52 patients (57%); thiazide diuretics in 39 patients (42%); calcium channel blockers in 22 patients (24%); and β‐blockers in 22 patients (24%). Thirty‐four patients (37%) were taking oral hypoglycemic agents, and the use of hypolipidemic drugs was reported by 20 patients (22%). Individuals in the control group were not taking any medications.
Doppler Echocardiogram
Measurements comparing both groups are shown in Table 2. Left ventricular ejection fraction (LVEF) was not different between the 2 groups. As expected, all other M‐mode measurements were larger in group I patients.
Table 2.
Echocardiographic Parameters in Obese and Healthy Controls
| Parameters | Group I (n = 92) | Group II (n = 31) | P Value |
|---|---|---|---|
| LVd (mm) | 51.3 ± 4.7 | 47.3 ± 3.5 | <0.001 |
| LVs (mm) | 32.3 ± 3.8 | 28.9 ± 2.9 | <0.001 |
| EF (%) | 66.7 ± 5.7 | 68.9 ± 5.1 | 0.053 |
| Ao (mm) | 32.6 ± 3.5 | 30.4 ± 3.5 | 0.006 |
| LAd (mm) | 40.3 ± 4.3 | 33.7 ± 3.8 | <0.001 |
| LAV (mL) | 59.5 ± 16.7 | 43.7 ± 10.7 | <0.001 |
| LAVi (mL/m2)a | 26.1 ± 7.1 | 24.7 ± 5.0 | 0.340 |
| VS (mm) | 11.7 ± 1.9 | 9.2 ± 1.1 | <0.001 |
| PW (mm) | 11.6 ± 1.7 | 9.2 ± 1.2 | <0.001 |
| LV mass (gr) | 237.1 ± 62.7 | 150.7 ± 38.8 | <0.001 |
| LV mass/height2.7 b | 65.5 ± 15.6 | 36.5 ± 7.8 | <0.001 |
| LV mass /BSA (gr/m2) | 102.4 ± 23.7 | 85.4 ± 15.8 | <0.001 |
| RWT | 0.46 ± 0.08 | 0.39 ± 0.04 | <0.001 |
Abbreviations: Ao, ascending aortic diameter; BSA, body surface area; EF, ejection fraction; LAD, left atrial diameter; LAV, left atrial volume; LAVi, indexed left atrial volume; LVd, left ventricular end‐diastolic diameter; LVs, left ventricular end‐systolic diameter; PW, posterior wall end‐diastolic thickness; RWT, relative wall thickness; VS, ventricular septal end‐diastolic thickness.
Left atrial volume was indexed for body surface area.
LV mass was normalized for height to the power of 2.7.
As shown on Table 3, LV diastolic function parameters were different between groups. Deceleration time was more prolonged in obese patients. Both septal and lateral e′ were lower and E/e′ ratio was higher in obese patients.
Table 3.
Conventional Doppler and Tissue Doppler‐Derived Parameters in Obese and Healthy Individuals
| Parameters | Group I (n = 92) | Group II (n = 31) | P Value |
|---|---|---|---|
| E (cm/s) | 90.2 ± 22.9 | 91.9 ± 17.1 | 0.694 |
| A (cm/s) | 81.4 ± 20.3 | 56.7 ± 17.1 | <0.001 |
| E/A | 1.1 ± 0.4 | 2.3 ± 3.1 | 0.042 |
| DT (ms) | 242.4 ± 50.2 | 226.1 ± 28.9 | 0.032 |
| e′ septal (cm/s) | 10.1 ± 2.6 | 12.8 ± 2.2 | <0.001 |
| A′ septal (cm/s) | 10.3 ± 3.4 | 8.2 ± 1.8 | <0.001 |
| S septal (cm/s) | 8.6 ± 1.8 | 8.4 ± 0.9 | 0.700 |
| e′ lateral (cm/s) | 11.8 ± 3.4 | 15.3 ± 3.6 | <0.001 |
| A′ lateral (cm/s) | 10.2 ± 3.4 | 8.3 ± 2.5 | 0.002 |
| S lateral (cm/s) | 8.7 ± 2.1 | 9.8 ± 1.9 | 0.020 |
| E/e′m ratio | 8.6 ± 2.7 | 6.7 ± 1.5 | <0.001 |
| LV global strain (%) | 22.5 ± 3.5 | 24.4 ± 2.5 | 0.005 |
| LV global strain rate (1/s) | 1.5 ± 0.3 | 1.6 ± 0.3 | 0.879 |
| RV e′ (cm/s) | 14.1 ± 3.8 | 15.2 ± 2.9 | 0.112 |
| RV A′ (cm/s) | 16.6 ± 5.0 | 13.6 ± 2.9 | <0.001 |
| RV e′/A ratio | 0.9 ± 0.4 | 1.2 ± 0.3 | 0.007 |
| RV S (cm/s) | 13.8 ± 3.1 | 14.1 ± 1.8 | 0.545 |
| RV strain (%) | 25.8 ± 5.2 | 28.2 ± 4.3 | 0.029 |
| RV strain rate (1/s) | 1.8 ± 0.6 | 1.9 ± 3.2 | 0.246 |
| SPAP (mm Hg) | 37.8 ± 7.5 | 29.9 ± 4.0 | <0.001 |
Abbreviations: A, A wave of the mitral inflow; A′ septal, Tissue Doppler A′ at the septal mitral annulus; A′ lateral, tissue Doppler A′ at the lateral mitral annulus; DT, deceleration time of the E wave; e′ septal, tissue Doppler e′ at the septal mitral annulus; e′ lateral, tissue Doppler e′ at the lateral mitral annulus; E, E wave of the mitral inflow; E/A, ratio of the E wave of the mitral inflow by the A wave of the mitral inflow; E/e′, ratio of the E wave mitral inflow by the mean e′; RV, right ventricular; RV A′, tissue Doppler A′ at the RV free wall; RV e′, tissue Doppler e′ at the RV free wall; RV e′/A′, RV e′ to A′ ratio; RV S, systolic tissue Doppler velocity at the tricuspid lateral annulus; RV strain, right ventricular Doppler based strain; RV strain rate, Doppler‐based RV strain rate; S lateral, systolic tissue Doppler velocity at the mitral lateral annulus; SPAP, systolic pulmonary artery pressure.
Systolic tissue Doppler velocity at the LV lateral wall and global LV strain, indices of systolic function, were lower in obese patients. Likewise, RV strain was lower and pulmonary systolic pressure was higher in obese patients. Neither RV TDI systolic velocity nor RV strain rate were different between obese and controls. When RV diastolic function was analyzed by TDI, RV A′ was higher in Group I patients and e′/A′ was lower.
Because associated hypertension and/or diabetes could explain a lower strain in obese patients, we compared strain values between obese patients with and without diabetes (22.6% vs 21.7%; P = 0.246) and between obese patients with and without hypertension (22.6% vs 22.3%; P = 0.755); no difference was found.
Simple linear regression analysis revealed that LV strain was significantly correlated with LV mass indexed to height2.7, E/e′ratio, indexed left atrial volume, and RV strain (Table 4). Traditional measurements of systolic and diastolic function, such as LVEF and deceleration time of mitral inflow, did not correlate with LV strain.
Table 4.
Correlation Between Demographic, Clinical, and Echocardiographic Variables With Left Ventricular Global Strain
| Parameters | r | P Value |
|---|---|---|
| BMI (kg/m2) | 0.082 | 0.236 |
| HR (bpm) | −0.224 | 0.024 |
| SBP (mm Hg) | −0.208 | 0.033 |
| DBP (mm Hg) | −0.213 | 0.030 |
| LV ejection fraction (%) | 0.142 | 0.158 |
| LV mass/height2.7 | −0.280 | 0.007 |
| E/e′ ratio | −0.213 | 0.031 |
| LA Vi (mL/m2) | −0.230 | 0.022 |
| RV strain (%) | 0.245 | 0.015 |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; E/e′, ratio of the E wave mitral inflow by the mean e′; HR, heart rate; LA, left atrial; LV, left ventricular; RV strain, right ventricular Doppler‐based strain; SBP, systolic blood pressure.
To estimate the relative contributions of the clinical, demographic, and echocardiographic variables to LV strain, the multivariate model was constructed selecting both variables that were significant in univariate analyses and clinically relevant variables that could have influenced LV function. Although BMI did not correlate with LV strain at univariate analyses (r = 0.08; P = 0.236), it was selected to enter in the multivariate model because of its potential contribution to LV strain. Similarly, diabetes and hypertension were selected to enter in the multivariate model as dichotomized variables. In the multiple linear regression analysis adjusted by these variables, the presence of obesity remained independently associated with LV strain.
Reproducibility
Intraobserver variability of global LV strain and strain rate were 5.1% and 5.3%, and for RV strain and strain rate 13.3% and 19.4%, respectively. Interobserver variability for global LV strain was 6.7%, for strain rate 5.5%, for RV strain 13.4%, and RV strain rate 16.7%.
DISCUSSION
Obesity is an important risk factor for the development of heart failure, with several studies among obese and overweight patients showing biventricular involvement.5, 16, 17, 18 However, most studies have used a BMI cutoff ≥30 kg/m2, and data on morbidly obese patients are scarce.19 The present study has shown that morbidly obese patients have lower indices of LV and RV systolic and diastolic function when compared with healthy controls.
LV Function in Obesity
Obesity may contribute to the development and progression of cardiac dysfunction through several mechanisms. Ventricular remodeling is common, with LV eccentric hypertrophy developing in response to the expanded intravascular volume present in obesity. Afterload is elevated, not only because of increased preload, but also because of elevated vascular resistance caused by excess adipose tissue and higher conduit artery stiffness.20 Similar to other studies, we found that morbidly obese patients had thicker walls with larger LV mass than healthy controls. Increased mass has been shown to be related to worse prognosis in hypertension21 and coronary artery diseases,22 among others. In the present study, LV mass was found to correlate with a decrease in global LV strain, suggesting that the increased mass can lead to incipient ventricular dysfunction in obesity.
LV systolic indices were lower in morbidly obese patients. Although LVEF was not different from controls, LV global strain was lower in obese patients, suggesting incipient systolic involvement. These findings challenge previous studies that have shown normal systolic function in obesity.5 The reason for this discrepancy may be the fact that these studies used only traditional parameters to analyze systolic function, such as LVEF, which are relatively insensitive to detect incipient preclinical changes in obese patients. Studies using newer and more sensitive parameters, such as strain and strain rate, have shown incipient systolic dysfunction, even in the presence of a normal LVEF.6, 16, 23
LV diastolic dysfunction has also been described in obese children24, 25, 26 and adults.5, 16, 27 However, there are few reports showing normal diastolic function in obese patients.28, 29 The present study showed that morbidly obese patients not only had a more prolonged relaxation of the LV, but also a higher E/e′ ratio than controls.
RV Function in Obesity
RV function abnormalities have been shown in moderate obesity in the absence of associated comorbidities.9, 16, 28 These abnormalities are multifactorial.3, 30 In our study, RV strain in obese patients was lower than in healthy controls. Severe pulmonary hypertension was not present in our obese patients, and RV strain did not correlate with systolic pulmonary pressure. This suggests that RV strain may be a sensitive index to detect incipient RV dysfunction, unrelated to pulmonary hypertension in obese patients.
Subclinical RV dysfunction has been described in the absence of obstructive sleep apnea or other associated comorbidities by Wong et al.9 In their study, normal‐weight patients with obstructive sleep apnea did not have TDI evidence of RV dysfunction, suggesting that obesity, and not the sleep abnormality, is responsible for RV dysfunction.9
Morbidly obese patients do not have severe pulmonary hypertension, even in the presence of obstructive sleep apnea,9 indicating an unexpected degree of adaptation to an extreme physiological condition. In addition, LV filling pressures are not elevated enough to cause passive pulmonary hypertension.31 In fact, we found normal E/e′ ratio despite severe obesity.
Clinical Implications
TDI and strain measurements are more sensitive in detecting incipient ventricular dysfunction. Prevalence of subclinical LV and RV dysfunction in obesity and its possible progression to symptomatic heart failure remains to be determined. However, our findings suggest that identification and prophylactic management of at‐risk patients are important issues.
Limitations of the Study
The exclusion of 9 patients (9%) due to technically inadequate echocardiograms is somewhat high. However, it can be explained by the fact that, different from studies in overweight or moderate obesity, all our patients were morbidly obese, with a very high BMI (mean, 53 kg/m2). For patients to be included, walls had to be adequately visualized to allow for strain measurements.
Hypertension and diabetes by themselves may explain some of the abnormalities detected in the present study. Because hypertension, diabetes, dyslipidemia, and sleep apnea are very common in morbid obesity, obtaining a large number of morbidly obese patients without these associated comorbidities would be very difficult. However, similar to our study, in some reports of the literature5, 19 comorbidities were not excluded. In addition, at multivariate analysis, obesity remained a significant determinant of global LV strain, independent of associated comorbidities.
Conclusion
In a group of morbidly obese patients with normal LV systolic function, myocardial ventricular strain was lower when compared to healthy controls, suggesting subclinical ventricular dysfunction. Strain imaging is helpful in the early detection of myocardial dysfunction and may provide additional data for patient management.
References
- 1. Flegal KM, Carrol MD, Ogden CI. Prevalence and trends in obesity among US adults, 1999‐2000. JAMA. 2002;288:1772–1773. [DOI] [PubMed] [Google Scholar]
- 2. Murphy NF, MacIntyre K, Stewart S, et al. Long‐term cardiovascular consequences of obesity: 20‐year follow‐up of more than 15000 middle‐aged men and women (the Renfrew‐Paisley study). Eur Heart J. 2006;27:96–106. [DOI] [PubMed] [Google Scholar]
- 3. Alpert MA. Obesity cardiomyopathy: pathophysiology and the evolution of the clinical syndrome. Am J Med Sci. 2001;321:225–236. [DOI] [PubMed] [Google Scholar]
- 4. Vasan RS. Cardiac function and obesity. Heart. 2003;89:1127–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Powell BD, Redfield MM, Bybee KA, et al. Association of obesity with left ventricular remodeling and diastolic dysfunction in patients without coronary artery disease. Am J Cardiol 2006;98:116–120. [DOI] [PubMed] [Google Scholar]
- 6. Wong CY, O' Moore‐Sullivan T, Leano R, et al. Alterations of left ventricular characteristics associated with obesity. Circulation. 2004;110:3081–3087. [DOI] [PubMed] [Google Scholar]
- 7. Greenberg NL, Firstenberg MS, Castro PL, et al. Doppler derived myocardial systolic strain rate is a strong index of left ventricular contractility. Circulation. 2002;105:99–105. [DOI] [PubMed] [Google Scholar]
- 8. Urheim S, Edvardsen T, Torp H, et al. Myocardial strain by Doppler echocardiography. Validation of a new method to quantify regional myocardial function. Circulation. 2000;102:1158–1164. [DOI] [PubMed] [Google Scholar]
- 9. Wong CY, O' Moore‐Sullivan T, Leano R, et al. Association of subclinical right ventricular dysfunction with obesity. J Am Coll Cardiol. 2006;47:611–616. [DOI] [PubMed] [Google Scholar]
- 10. Lang RM, Bierig M, Devereux RB, et al. Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography . Recommendations for chamber quantification: a report from the American Society of Echocardiography' s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 11. Lester SJ, Ryan EW, Schiller NB, et al. Best method in clinical practice and in research studies to determine left atrial size. Am J Cardiol. 1999;84:829–832. [DOI] [PubMed] [Google Scholar]
- 12. de Simone G, Daniels SR, Devereux RB, et al. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–1260. [DOI] [PubMed] [Google Scholar]
- 13. McQuillan BM, Picard MH, Leavitt M, et al. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation. 2001;104:2797–2802. [DOI] [PubMed] [Google Scholar]
- 14. Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. [DOI] [PubMed] [Google Scholar]
- 15. Marwick TH. Measurement of strain and strain rate by echocardiography. Ready for prime time? J Am Coll Cardiol. 2006;47: 1313–1327. [DOI] [PubMed] [Google Scholar]
- 16. Orhan AL, Uslu N, Dayi SU, et al. Effects of isolated obesity on left and right ventricular function: a tissue Doppler and strain rate imaging study. Echocardiography. 2010;27:236–243. [DOI] [PubMed] [Google Scholar]
- 17. Di Stante B, Galandauer I, Aronow WS, et al. Prevalence of left ventricular diastolic dysfunction in obese persons with and without diabetes. Am J Cardiol. 2005;95:1527–1528. [DOI] [PubMed] [Google Scholar]
- 18. Di Bello V, Santini F, Di Cori A, et al. Relationship between preclinical abnormalities of global and regional left ventricular function and insulin resistance in severe obesity: a color Doppler imaging study. Int J Obes (Lond). 2006;30:948–956. [DOI] [PubMed] [Google Scholar]
- 19. Willens HJ, Chakko SC, Lowery MH, et al. Tissue Doppler imaging of the right and left ventricle in severe obesity (body mass index ≥35 kg/m2). Am J Cardiol. 2004;94: 1087–1090. [DOI] [PubMed] [Google Scholar]
- 20. Di Bello V, Santini F, Di Cori A, et al. Obesity cardiomyopathy: is it a reality? An ultrasonic tissue characterization study. J Am Soc Echocardiogr. 2006;19:1063–1071. [DOI] [PubMed] [Google Scholar]
- 21. Krauser DG, Devereux RD. Ventricular hypertrophy and hypertension: prognosis elements and implications for management. Herz. 2006;31:305–316. [DOI] [PubMed] [Google Scholar]
- 22. Turakhia MP, Schiller NB, Whooley MA. Prognostic significance of increased left ventricular mass index to mortality and sudden death in patients with stable coronary heart disease (from the Heart and Soul Study). Am J Cardiol. 2008;102:1131–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Litwin SE. The growing problem of obesity and the heart. J Am Coll Cardiol. 2006;47:617–619. [DOI] [PubMed] [Google Scholar]
- 24. Sharpe JA, Naylor LH, Jones TW, et al. Impact of obesity on diastolic function in subjects ≤16 years of age. Am J Cardiol. 2006;98:691–693. [DOI] [PubMed] [Google Scholar]
- 25. Mehta SK, Holliday C, Hayduk L, et al. Comparison of myocardial function in children with body mass indexes ≥25 versus those ≤25 kg/m2 . Am J Cardiol. 2004;93:1567–1569. [DOI] [PubMed] [Google Scholar]
- 26. Di Salvo G, Pacileo G, Del Giudice EM, et al. Atrial myocardial deformation properties in obese nonhypertensive children. J Am Soc Echocardiogr. 2008;21:151–156. [DOI] [PubMed] [Google Scholar]
- 27. Kosmala W, Wong C, Kulieczkowska J, et al. Use of body weight and insulin resistance to select obese patients for echocardiographic assessment of subclinical left ventricular dysfunction. Am J Cardiol. 2008;101:1334–1340. [DOI] [PubMed] [Google Scholar]
- 28. Otto ME, Belohlavek M, Khandheria B, et al. Comparison of right and left ventricular function in obese and nonobese men. Am J Cardiol. 2004;93:1569–1572. [DOI] [PubMed] [Google Scholar]
- 29. Kinoshita N, Onishi S, Yamamoto S, et al. Unusual left ventricular dilation without functional or biochemical impairment in normotensive extremely overweight Japanese professional wrestlers. Am J Cardiol. 2003;91:699–703. [DOI] [PubMed] [Google Scholar]
- 30. Vgontzas AN, Tan TL, Bixker EO, et al. Sleep apnea and sleep disruption in obese patients. Arch Intern Med. 1994;154: 1705–1711. [PubMed] [Google Scholar]


