Abstract
Cardiac sarcoid is an infiltrative, granulomatous disease of the myocardium. It is more prevalent entity than once believed, especially subclinical disease. It affects heart mechanics causing ventricular failure, and disrupts the cardiac electrical system leading to third degree heart block, malignant ventricular arrhythmias, and sudden cardiac death. This makes early diagnosis and treatment of this devastating disease essential. Based on reviewed literature this paper proposes step‐wise diagnostic and therapeutic algorithms for patients with suspected cardiac sarcoidoisis who do or do not have prior history of systemic sarcoidosis. Clin. Cardiol. 2012 DOI: 10.1002/clc.21982
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Special thanks to Dr. Maziar Zafari for his help with the editing process.
Introduction
Sarcoidosis is a multisystem granulomatous disease of unknown etiology. Postmortem studies indicate that cardiac involvement ranges from 20% to 25%, with a higher prevalence in Japan and Scandinavia.1, 2, 3 At early stages of the disease, the majority of cardiac sarcoidosis (CS) cases are clinically “silent.” The incidence of progressive heart failure, malignant arrhythmias, and sudden cardiac death (SCD) increases dramatically as CS becomes clinically recognizable.4, 5, 6, 7, 8
The diagnosis of CS is difficult to make. A recent Delphi study of leading national CS experts indicates that the medical community lacks a consensus on the approach to the diagnosis and treatment of CS.9 The revised 2006 Japanese Guidelines is the official diagnostic guide that helps to identify patients with CS.8 However, these guidelines do not provide an early rational, cost‐effective diagnostic approach for clinicians. A stepwise diagnostic algorithm that will assist cardiologists in early diagnosis of CS is needed.7
Clinical Manifestations and Electrocardiogram Findings
Cardiac sarcoid rarely precedes involvement of other organs, but in the worst‐case scenario it may be asymptomatic prior to presentation as SCD.10 There is a clear imperative for a clinician to screen for silent CS in patients with extracardiac disease (Figure 1) as well as patients suspected of isolated CS (Figure 2).8 The screening process starts with a detailed history and physical examination, electrocardiogram (ECG), and chest x‐ray.11 Symptoms of heart failure may be early indicators, sensitive but not specific for CS.12 Mehta et al found a significant prevalence of cardiac symptoms in systemic sarcoid patients compared with healthy controls (46% vs 5%, respectively; P < 0.001).12 Although abnormal ECG findings, such as complete bundle branch block, new atrioventricular (AV) block, frequent premature ventricular complexes (PVC), ventricular tachycardia (VT), pathologic Q waves, or ST‐T changes have a low sensitivity in asymptomatic patients with subclinical CS,6, 12 their presence should prompt further investigation. Patients with symptoms, an abnormal ECG, or cardiomegaly on chest x‐ray should be referred for transthoracic echocardiogram and Holter monitoring and subsequent advanced imaging.
Figure 1.
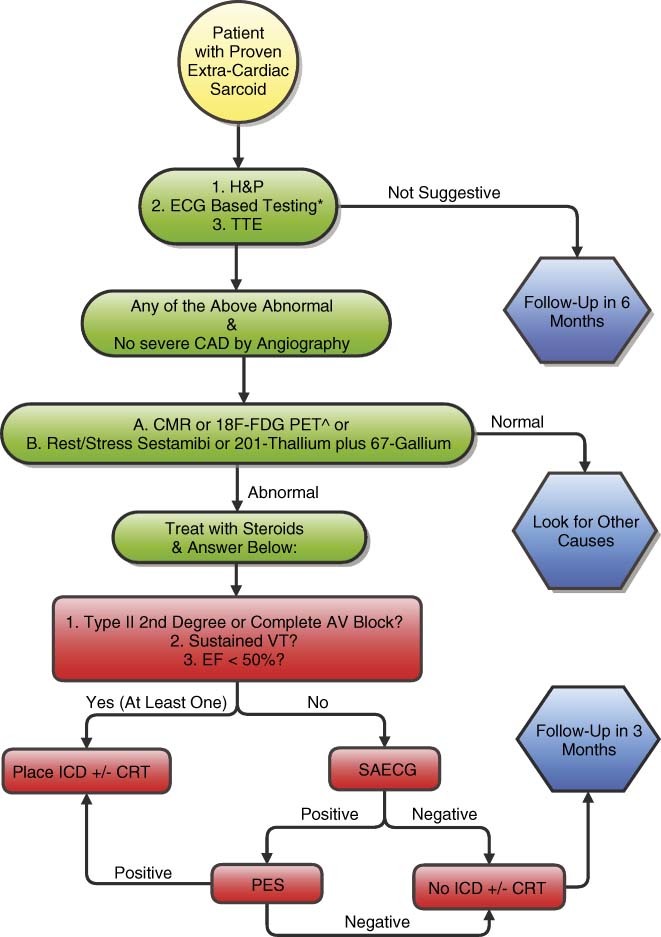
Diagnostic and treatment algorithm for a patient with extracardiac sarcoid. *Abnormal ECG includes: VT (monomorphic or polymorphic) or Mobitz type II or complete heart block on 12‐lead, >100 PVCs on 24‐hour Holter, T wave alternans. Group A: CMR or 18F‐FDG PET are the preferred imaging modalities. They are the most sensitive and specific tests available for cardiac sarcoid. Abbreviations: AV, atrioventricular; CAD, coronary artery disease; CMR, cardiac magnetic resonance; CRT, cardiac resynchronization therapy; ECG, electrocardiogram; EF, ejection fraction; FDG PET, fludeoxyglucose positron‐emission tomography; H&P, history and physical; ICD, implantable cardioverter‐defibrillator; PES; programmed electrical stimulation; PVCs, premature ventricular complexes; SAECG, signal‐averaged electrocardiogram; TTE, transthoracic echocardiogram; VT, ventricular tachycardia.
Figure 2.
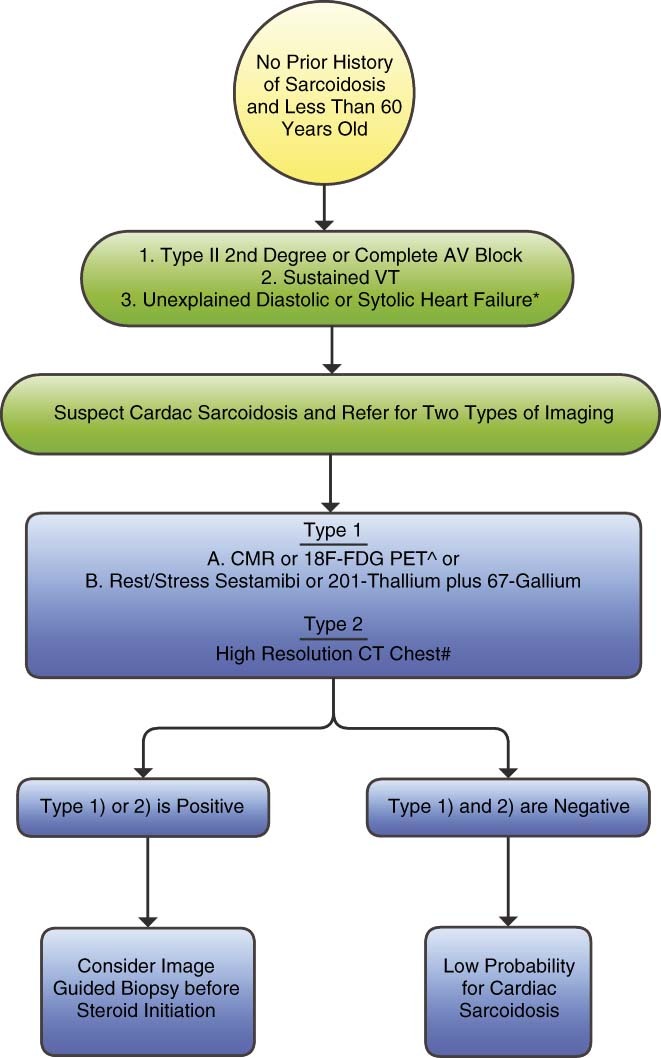
Diagnostic algorithm for a patient with suspicion for isolated cardiac sarcoidosis. *Absence of coronary artery disease by selective coronary angiography and no comorbidity that could alternatively explain heart failure. Group A: CMR or 18F‐FDG PET are the preferred imaging modalities. They are the most sensitive and specific tests available for cardiac sarcoid. #With particular attention to upper chest mediastinal lymph nodes. Abbreviations: AV, atrioventricular; CMR, cardiac magnetic resonance; CT, computed tomography; FDG PET, fludeoxyglucose positron‐emission tomography; VT, ventricular tachycardia.
Echocardiography, Holter Monitoring, Signal‐Averaged Electrocardiography, and Microvolt T‐Wave Alternans
Echocardiography is a valuable modality in the diagnostic work‐up of patients with suspected CS.12, 13, 14 Ventricular systolic and diastolic dysfunction, wall‐motion abnormalities, abnormal septal thickness, and Doppler filling pattern (Figure 3) are the most frequent findings suggestive of CS.15 Given its availability and low complication risk, echocardiography is an important screening tool in such patients.
Figure 3.
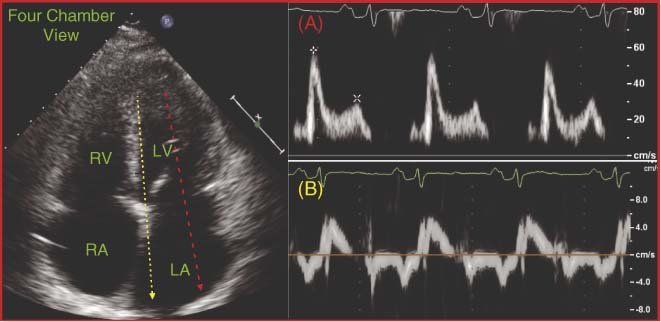
Transmitral and tissue Doppler images. (A) Transmitral Doppler: E & A velocity approach a 2:1 proportion, respectively. (B) Tissue Doppler demonstrates decreased E′ and increased E/E′ ratio, indicative of restrictive physiology. Abbreviations: LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
When the available evidence is considered, Holter monitoring also plays an important part in the diagnostic work‐up. Greater than 100 ventricular ectopic beats in 24 hours has been proposed as a screening criteria.16 A prospective trial found that 8 of 12 (67%) patients with CS, 2 of 26 (8%) patients with systemic sarcoidosis, and 3 of 58 (5%) healthy controls had ≥100 PVCs per 24 hours.16 This translated to a sensitivity and specificity of 67% and 80%, respectively, for diagnosis of cardiac involvement in patients with systemic sarcoidosis. Mehta et al confirmed that patients with CS had more PVCs than those without CS (50% vs 3%, respectively; P < 0.001).12
The signal‐averaged electrocardiogram (SAECG) measures ventricular late potentials, low‐amplitude, high‐ frequency waveforms, in the terminal QRS complex of the ECG.15 Those could potentially originate from areas of fibrogranulomatous infiltration of the ventricular myocardium and lead to delayed and asynchronous ventricular activation.17 In a prospective study, Yogodawa et al found that 8 of 10 (80%) patients with CS, 25 of 52 (46.2%) with pulmonary sarcoid, and only 3 of 52 (5.8%) healthy controls had SAECG abnormalities.15 From a cohort of 88 patients (27 of them with cardiac sarcoidosis), Schuller et al found abnormalities on SAECG in 14 of 27 patients with CS and 11 of 61 patients with extracardiac sarcoidosis, which translates to a sensitivity and specificity of SAECG of 52% and 82%, respectively.18 Microvolt T‐wave alternans (MVTWA) evaluates T‐wave changes at the microvolt level due to variations in the action potential duration of the transmural gradient. This test in a recent small 35‐patient study revealed ability to detect cardiac sarcoidosis with 85.7% sensitivity and 92.8% specificity.19
Echocardiography, Holter monitoring, SAECG, and MVTWA are all relatively inexpensive and readily available tools that can help to risk‐stratify patients early for the need for further diagnostic evaluation.
Cardiac Magnetic Resonance, Fludeoxyglucose–Positron‐Emission Tomography, Single Photon‐Emission Computed Tomography, Gallium Scanning
Cardiac magnetic resonance (CMR) with late gadolinium enhancement (LGE) imaging (Figure 4) emerged as the gold standard for diagnosis of cardiac involvement in sarcoidosis.20, 21, 22 Unfortunately, the test is not always available, it is expensive, and it cannot be effectively used in patients with renal dysfunction (glomerular filtration rate <30 mL/min/1.73 m2) and in patients with pacemakers and implantable cardioverter‐defibrillator (ICD) devices. Early enhancement of sarcoid granulomas in T2‐weighted gadolinium images suggests presence of inflammation and edema, whereas late enhancement indicates fibrotic changes and scarring.4, 7 The most common areas of distribution are usually midmyocardial, with preferential involvement of the basal segments of the septum and lateral walls.23 In a cohort of 16 patients with proven CS by revised Japanese Criteria, Smedema et al found 12 (75%) with LGE.24 Cardiac magnetic resonance and 201‐thallium single photon‐emission computed tomography (SPECT) performed on 10 CS patients revealed abnormalities in 8 and 4 patients, respectively.24 Smedema et al also imaged a small cohort of patients strongly suspected of CS and reported 100% sensitivity and 78% specificity of CMR in detection of the disease.24, 25 Ohira et al found a CMR specificity of 76.9% compared with 38.5% for fludeoxyglucose (FDG; 18F) positron‐emission tomography (PET), but both techniques had comparable sensitivities.21 Cheong et al showed in a cohort of 31 patients with systemic sarcoidosis 8 patients with LGE on CMR, but none were formally diagnosed with CS by the revised Japanese guidelines.20 In 4 biopsy‐proven CS patients, Patel et al found that 4 of 4 had defects on CMR and only 2 of 4 were diagnosed with CS by the guidelines.26 Based on current evidence, CMR, if available, should be the study of choice in patients suspected of CS (Table 1).
Figure 4.
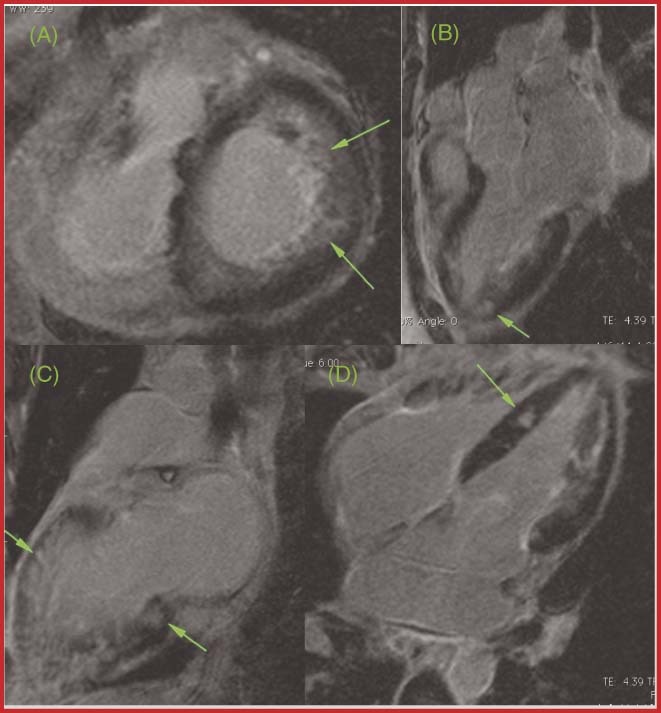
A CS patient's CMR. Green arrows designate delayed gadolinium enhancement in (A) axial view, (B,C) 3‐chamber view, and (D) 4‐chamber view. Abbreviations: CMR, cardiac magnetic resonance; CS, cardiac sarcoid.
Table 1.
Imaging Studies and Their Corresponding Sensitivities and Specificities in Patients Suspected of Cardiac Sarcoid
| Authors | Patient Population | Imaging Modality | Sensitivity, % | Specificity, % |
|---|---|---|---|---|
| Cheong et al, 200920 | 31 patients with biopsy‐confirmed systemic sarcoidosis | cMRI with gadolinium | NA | NA |
| Ohira et al, 200821 | 21 patients with suspected CS by ECG, Holter | cMRI with gadolinium | 75 | 77 |
| Tadamura et al, 200522 | 10 patients with histologically and clinically diagnosed CS | cMRI with gadolinium | 100 | NA |
| Smedema et al, 200524 | 58 patients with biopsy‐proven pulmonary sarcoid | cMRI with gadolinium | 100 | 58 |
| Smedema et al, 200525 | 88 patients with biopsy‐proven pulmonary sarcoid | cMRI with gadolinium | 100 | 83 |
| Ohira et al, 200821 | 21 patients suspected for CS by ECG, Holter | 18F‐FDG PET | 88 | 39 |
| Yamagishi et al, 200327 | Retrospective study of 17 patients with histologically proven CS | 18F‐FDG PET | 100 | NA |
| Okumura et al, 200428 | 22 patients with histologically diagnosed CS | 18F‐FDG PET | 100 | 91 |
| Ishimaru et al, 200529 | 32 patients clinically diagnosed with systemic sarcoid | 18F‐FDG PET | 100 | 82 |
| Langah et al, 200930 | 65 patients with suspected CS | 18F‐FDG PET | 85 | 90 |
| 99mTc‐sestamibi6, 30, 31, 39 | 64–80 | 93–100 | ||
| Gallium‐678, 24, 30, 31, 32 | 0–36 | 80–100 | ||
| Thallium‐20139 | 24–58 | Insufficient data |
Abbreviations: cMRI, cardiac magnetic resonance imaging; CS, cardiac sarcoid; ECG, electrocardiogram; FDG PET, fludeoxyglucose positron‐emission tomography; NA, not applicable.
It has been documented that FDG uptake is increased in the myocardium of patients with suspected CS.27, 28 Yamagishi et al studied a cohort of 17 CS patients with 13N‐NH3 and 18F‐FDG PET and found abnormalities in 15 of 17 patients.27 Okumura et al confirmed the higher sensitivity of the 18F‐FDG PET in the diagnosis of CS, superior to the sensitivity of 99mTc‐sestamibi SPECT and gallium scintigraphy (100% vs 63.6% vs 36.3%).28 However, 18F‐FDG PET did not confer a similar improvement in specificity.28 Ishimaru et al echoed the findings of previous studies.29 They used 18F‐FDG PET, 99mTc‐sestamibi, and gallium scintigraphy to identify abnormalities suggestive of CS in a cohort of 32 patients with systemic sarcoidosis, and found a respective prevalence of 31%, 12.5%, and 0% for each.29 Furthermore, in a retrospective examination of 76 patients with suspected CS who underwent 18F‐FDG PET and/or gallium scintigraphy for evaluation of CS, Langah et al found that 18F‐FDG PET had a sensitivity and specificity of 85% and 90%, respectively, vs 15% and 80%, respectively, for gallium scintigraphy.30 Overall it can be concluded that 18F‐FDG PET has the potential to detect subtle sarcoid‐induced changes in myocardium missed by other radionuclide studies, but the test is commonly limited to large tertiary referral centers (Table 1).
Among the other available imaging modalities, current evidence supports the superiority of 99mTc‐sestamibi SPECT above 201‐thallium SPECT, and both those nuclear tests outperform 67‐gallium scanning in detecting CS. When comparing sestamibi with thallium, Le Guludec et al: showed that sestamibi SPECT detects significantly larger defects (28.1% ± 3.2 vs 17.2% ± 12.8% of bull's‐eye area, P < 0.001) as well as more abnormalities (24 vs 17) than 201‐thallium.31 In comparing sestamibi SPECT to gallium scintigraphy, Eguchi et al found a greater prevalence of perfusion defects with 99mTc‐sestamibi in patients with CS (21 of 36; 60%) compared with systemic sarcoidosis (19 of 60; 32%) and healthy controls (11 of 150; 7%).32 Also, 99mTc‐sestamibi detected defects in 4 of 6 patients with known CS, whereas gallium only detected defects in 1 of 6 patients.32
To assess the activity of the disease and its potential response to systemic steroid therapy, a dual (99mTc‐sestamibi + 67‐gallium) approach might be beneficial. In a study by Nakazawa et al,33 14 patients with systemic sarcoidosis received a dual scan. Nine out of 14 patients had an abnormal cardiac infiltrative pattern detected by 67‐gallium uptake. This pattern disappeared on repeat scanning after 60 days of steroid therapy. In the 5 patients without abnormalities on 67‐gallium, 2 had reduced uptake on 99mTc‐sestamibi. Interestingly, both patients (67‐gallium negative, yet 99mTc‐sestamibi positive) were previously treated with steroids. Rest sestamibi followed by dipyridamole infusion and repeat SPECT follows the same principles as the dual scan discussed above. In the Le Guludec et al trial, rest sestamibi detected cardiac defects in 24 of 37 patients with clinical suspicion for CS.31 Dipyridamole was then infused and patients underwent repeat sestamibi scanning. Interestingly, the number of defects decreased significantly (28.1 ± 13.2% vs 15.2 ± 12.3%, P < 0.001). The inability to detect tracer activity by sestamibi after dipyridamole infusion suggests that the vasodilator allows the tracer to disperse more quickly from actively inflamed tissue, which is called reverse redistribution pattern. Conversely, areas of fibrosis do not have blood supply and thus are unaffected by vasodilators. There was a high linear correlation between the improvement of the defect after dipyridamole infusion and improvement following corticosteroid therapy (r = 0.85, P < 0.001), suggesting that the acute response under vasodilators predicts steroid efficacy.31
As a result, in a community setting in the absence of more sophisticated imaging techniques, dual imaging (99mTc‐sestamibi + 67‐gallium) or rest 99mTc‐sestamibi scanning followed by dipyridamole infusion and repeat imaging may be used to both identify CS with adequate specificity and sensitivity and to guide steroid therapy in patients with signs of active inflammation on imaging studies.
A clinical diagnostic algorithm (Figure 1), based on available data, could be utilized by the general internist or cardiologist to screen for subclinical CS in patients with known extracardiac sarcoidosis.
Endomyocardial Biopsy
Endomyocardial biopsy is the most specific detection method for CS, but a small tissue sample taken blindly from a myocardium with patchy granulomatous infiltration leads to diagnostic yield of only 20%,34, 35 precluding its routine usage. However, image‐guided biopsy should be considered in patients without a prior history of systemic sarcoid who have unexplained arrhythmias or heart failure (Figure 2).8 Kandolin et al retrospectively reviewed 52 patients with histologically proven cardiac sarcoid, 33 of whom had disease isolated to the heart, and found that imaging increased the diagnostic yield by 31%.36 Imaging also helped identify and guide the biopsy of granulomatous infiltration in mediastinal lymph nodes, which could aid diagnosis in a patient with no known extracardiac sarcoid.36
Treatment
Steroids
If a diagnostic work‐up reveals active CS, corticosteroids should be initiated. There are no randomized controlled trials that have confirmed their efficacy in CS. Current evidence based on several small cohort studies suggests the use of 30 mg/day or 60 mg/every other day of oral prednisone for 8–12 weeks, with gradual tapering of the dose to 10–20 mg every other day over a period of 6–12 months to establish the minimal effective dose.4, 5, 6, 34, 35, 37 Chiu et al stratified patients into 3 groups based on left ventricular (LV) ejection fraction (LVEF) and found a statistically significant improvement in the group with an initial LVEF of 30%–54%.38 However, no such improvement was found in the group with an LVEF <30%.38 Similarly, Yazaki et al found that 75 patients treated with steroids had a significantly better 5‐year survival (75% vs 10%) compared with untreated patients.39 Most compelling was 89% 5‐year survival when steroids were started with the LVEF >50%.39 Yogodawa also found that CS patients with a preserved LVEF showed a significant reduction in the number of PVCs and prevalence of nonsustained ventricular tachycardia compared with those with advanced LV dysfunction.40 Yogodawa et al found from a cohort of 31 CS patients that the group with less‐advanced LV dysfunction showed a significantly higher prevalence of gallium‐67 uptake compared with the advanced–LV dysfunction group.40
Based on these observations, steroid therapy in patients with established CS and active inflammation should be initiated before LV systolic function declines. Traditionally, steroids have been reserved for patients with LVEF <50%, advanced AV block, VT, or positive cardiac biopsy,6 but the approach was bound with high mortality risk.38, 39 These findings suggest that there is a tipping point of steroid efficacy where responsive active granulomatous inflammation/infiltration transforms toward nonresponsive fibrosis. Prolonged steroid therapy is not without risk, but an ominous mortality curve and resistance to treatment of advanced disease compel early action.
Programmed Electrical Stimulation
Granuloma formation and subsequent fibrosis may be the substrate for abnormal automaticity and electrical depolarization/repolarization process, a nidus for reentrant ventricular arrhythmias.41, 42 Programmed electrical stimulation (PES) has the potential to identify CS patients with electrical instability and may help to determine if patients should get an ICD.6 Mehta et al studied 76 patients with extracardiac sarcoidosis and found 8 with a positive study, and all had ICDs placed.42 Of these 8, 4 received appropriate shocks and 2 died of SCD. Only 1 patient died from the 68 whose PES was negative and none had symptomatic VT or required an ICD after 5 years of follow‐up.42 Similarly, in 27 systemic sarcoidosis patients with suspected cardiac involvement, Aizer et al found a hazard ratio, for SCD or ICD shock, of 6.3 (95% confidence interval: 1.81‐21.95) in subjects with spontaneous VT.41 They also found a hazard ratio of 6.97 (95% confidence interval: 1.27‐38.27) in patients with inducible VT by PES but no history of spontaneous VT. These studies demonstrate that PES can detect electrically labile myocardium in subclinical CS. Although limited, current evidence suggests that an ICD could prevent dangerous arrhythmias or SCD even in patients with a relatively preserved LVEF. For patients with diagnosed CS, we suggest the following treatment algorithm (Figure 1) based on currently available management data.
Cardiac Transplantation
Young patients with progressive cardiomyopathy not responsive to immunosuppressive therapy should undergo evaluation for cardiac transplantation. Zaidi et al reviewed 65 patients with documented systemic sarcoid who underwent orthotropic heart transplant and found better 1‐year survivability than nonsarcoid patients, 88% vs 85%.43
Conclusion
Cardiac sarcoid is a relatively uncommon disease, but the risk of SCD, malignant arrhythmias, and progressive heart failure are often the first signs of cardiac involvement in the heart, at which point mortality increases dramatically while intervention efficacy diminishes. As a result, clinicians should use low‐cost, easily accessible technology to screen for CS in all patients with extracardiac sarcoid. In addition, patients with unexplained VT, type II second‐degree or complete AV block, or cardiomyopathy should undergo an algorithm‐based evaluation for this rare but insidious condition. This is especially true for young patients from regions where the occurrence of the disease is high, such as Japan, Scandinavia, or Africa. Early diagnosis and treatment of CS is essential and a cost‐effective algorithmic approach can be successfully used in diagnosis and treatment of CS patients.
References
- 1. Roberts WC, McAllister HA Jr, Ferrans VJ. Sarcoidosis of the heart: a clinicopathologic study of 35 necropsy patients (group I) and review of 78 previously described necropsy patients (group II). Am J Med. 1977;63:86–108. [DOI] [PubMed] [Google Scholar]
- 2. Silverman K, Hutchins G, Bulkley B. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978;58:1204–1211. [DOI] [PubMed] [Google Scholar]
- 3. Tavora F, Cresswell N, Li L, et al. Comparison of necropsy findings in patients with sarcoidosis dying suddenly from cardiac sarcoidosis versus dying suddenly from other causes. Am J Cardiol. 2009;104:571–577. [DOI] [PubMed] [Google Scholar]
- 4. Kim JS, Judson MA, Donnino R, et al. Cardiac sarcoidosis. Am Heart J. 2009;157:9–21. [DOI] [PubMed] [Google Scholar]
- 5. Pierre‐Louis B, Prasad A, Frishman WH. Cardiac manifestations of sarcoidosis and therapeutic options. Cardiol Rev. 2009;17:153–158. [DOI] [PubMed] [Google Scholar]
- 6. Soejima K, Yada H. The work‐up and management of patients with apparent or subclinical cardiac sarcoidosis: with emphasis on the associated heart rhythm abnormalities. J Cardiovasc Electrophysiol. 2009;20:578–583. [DOI] [PubMed] [Google Scholar]
- 7. Dubrey SW, Falk RH. Diagnosis and management of cardiac sarcoidosis. Prog Cardiovasc Dis. 2010;52:336–346. [DOI] [PubMed] [Google Scholar]
- 8. Youssef G, Beanlands RS, Birnie DH, et al. Cardiac sarcoidosis: applications of imaging in diagnosis and directing treatment. Heart. 2011;97:2078–2087. [DOI] [PubMed] [Google Scholar]
- 9. Hamzeh NY, Wamboldt FS, Weinberger HD. Management of cardiac sarcoidosis in the United States: a Delphi study. Chest. 2012;141:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yoshida Y, Morimoto S, Hiramitsu S, et al. Incidence of cardiac sarcoidosis in Japanese patients with high‐degree atrioventricular block. Am Heart J. 1997;134:382–386. [DOI] [PubMed] [Google Scholar]
- 11. Statement on sarcoidosis. Am J Respir Crit Care Med 1999;160:736–755. [DOI] [PubMed] [Google Scholar]
- 12. Mehta D, Lubitz SA, Frankel Z, et al. Cardiac involvement in patients with sarcoidosis. Chest. 2008;133:1426–1435. [DOI] [PubMed] [Google Scholar]
- 13. Burstow DJ, Tajik AJ, Bailey KR, et al. Two‐dimensional echocardiographic findings in systemic sarcoidosis. Am J Cardiol. 1989;63: 478–482. [DOI] [PubMed] [Google Scholar]
- 14. Aydin Kaderli A, Gullulu S, Coskun F, et al. Impaired left ventricular systolic and diastolic functions in patients with early grade pulmonary sarcoidosis. Eur J Echocardiogr. 2010;11:809–813. [DOI] [PubMed] [Google Scholar]
- 15. Yodogawa K, Seino Y, Ohara T, et al. Non‐invasive detection of latent cardiac conduction abnormalities in patients with pulmonary sarcoidosis: application of signal averaged electrocardiogram. Circ J. 2007;71:540–545. [DOI] [PubMed] [Google Scholar]
- 16. Suzuki T, Kanda T, Kubota S, et al. Holter monitoring as a noninvasive indicator of cardiac involvement in sarcoidosis. Chest. 1994;106:1021–1024. [DOI] [PubMed] [Google Scholar]
- 17. Goldner B, Brandspiegel HZ, Horwitz L, et al. Utility of QT dispersion combined with the signal‐averaged electrocardiogram in detecting patients susceptible to ventricular tachyarrhythmia. Am J Cardiol. 1995;76:1192–1194. [DOI] [PubMed] [Google Scholar]
- 18. Schuller JL, Lowery CM, Zipse M, et al. Diagnostic utility of signal‐averaged electrocardiography for detection of cardiac sarcoidosis. Ann Noninvasive Electrocardiol. 2011;16:70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matsumoto S, Hirayama Y, Saitoh H, et al. Noninvasive diagnosis of cardiac sarcoidosis using microvolt T‐wave alternans. Int Heart J. 2009;50:731–739. [DOI] [PubMed] [Google Scholar]
- 20. Cheong BY, Muthupillai R, Nemeth M, et al. The utility of delayed‐enhancement magnetic resonance imaging for identifing nonischemic myocardial fibrosis in asymptomatic patients with biopsy‐proven systemic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2009;26:39–46. [PubMed] [Google Scholar]
- 21. Ohira H, Tsujino I, Ishimaru S, et al. Myocardial imaging with 18‐F‐fluoro‐2‐deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging. 2008;35:933–941. [DOI] [PubMed] [Google Scholar]
- 22. Tadamura E, Yamamuro M, Kubo S, et al. Effectiveness of delayed enhanced MRI for identification of cardiac sarcoidosis: comparison with radionuclide imaging. Am J Roentgenol. 2005;185:110–115. [DOI] [PubMed] [Google Scholar]
- 23. Vignaux O, Dhote R, Duboc D, et al. Detection of myocardial involvement in patients with sarcoidosis applying T2‐weighted, contrast‐enhanced, and cine magnetic resonance imaging: initial results of a prospective study. J Comput Assist Tomogr. 2002;26: 762–767. [DOI] [PubMed] [Google Scholar]
- 24. Smedema JP, Snoep G, van Kroonenburgh MP, et al. Cardiac involvement in patients with pulmonary sarcoidosis assessed at two university medical centers in the Netherlands. Chest. 2005;128:30–35. [DOI] [PubMed] [Google Scholar]
- 25. Smedema JP, Snoep G, van Kroonenburgh MP, et al. Evaluation of the accuracy of gadolinium‐enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol. 2005;45:1683–1690. [DOI] [PubMed] [Google Scholar]
- 26. Patel MR, Cawley PJ, Heitner JF, et al. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120: 1969–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamagishi H, Shirai N, Takagi M, et al. Identification of cardiac sarcoidosis with 13(N)‐NH(3)/ (18)F‐FDG PET. J Nucl Med. 2003; 44:1030–1036. [PubMed] [Google Scholar]
- 28. Okumura W, Iwasaki T, Toyama T, et al. Usefulness of fasting 18F‐FDG PET in identification of cardiac sarcoidosis. J Nucl Med. 2004; 45:1989–1998. [PubMed] [Google Scholar]
- 29. Ishimaru S, Tsujino I, Takei T, et al. Focal uptake on 18F‐fluoro‐2‐deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur Heart J. 2005;26: 1538–1543. [DOI] [PubMed] [Google Scholar]
- 30. Langah R, Spicer K, Gebregziabher M , et al. Effectiveness of prolonged fasting 18F‐FDG PET‐CT in the detection of cardiac sarcoidosis. J Nucl Cardiol. 2009;16:801–810. [DOI] [PubMed] [Google Scholar]
- 31. Le Guludec DL, Menad F, Faraggi M, et al. Myocardial sarcoidosis. Chest. 1994;106:1675–1682. [DOI] [PubMed] [Google Scholar]
- 32. Eguchi M, Tsuchihashi K, Hotta D, et al. Technetium‐99m sestamibi/tetrofosmin myocardial perfusion scanning in cardiac and noncardiac sarcoidosis. Cardiology. 2000;94:193–199. [DOI] [PubMed] [Google Scholar]
- 33. Nakazawa A, Ikeda K, Ito Y, et al. Usefulness of dual 67Ga and 99mTc‐sestamibi single‐photon‐emission CT scanning in the diagnosis of cardiac sarcoidosis. Chest. 2004;126:1372–1376. [DOI] [PubMed] [Google Scholar]
- 34. Ratner SJ, Fenoglio JJ, Ursell PC. Utility of endomyocardial biopsy in the diagnosis of cardiac sarcoidosis. Chest. 1986;90:528–533. [DOI] [PubMed] [Google Scholar]
- 35. Uemura A, Morimoto S, Hiramitsu S, et al. Histologic diagnostic rate of cardiac sarcoidosis: evaluation of endomyocardial biopsies. Am Heart J. 1999;138:299–302. [DOI] [PubMed] [Google Scholar]
- 36. Kandolin R, Lehtonen J, Graner M, et al. Diagnosing isolated cardiac sarcoidosis. J Intern Med. 2011;270:461–468. [DOI] [PubMed] [Google Scholar]
- 37. Doughan AR, Williams BR. Cardiac sarcoidosis. Heart. 2006;92: 282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chiu CZ, Nakatani S, Zhang G, et al. Prevention of left ventricular remodeling by long‐term corticosteroid therapy in patients with cardiac sarcoidosis. Am J Cardiol. 2005;95:143–146. [DOI] [PubMed] [Google Scholar]
- 39. Yazaki Y, Isobe M, Hiroe M, et al. Prognostic determinants of long‐term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol. 2001;88:1006–1010. [DOI] [PubMed] [Google Scholar]
- 40. Yodogawa K, Seino Y, Ohara T, et al. Effect of corticosteroid therapy on ventricular arrhythmias in patients with cardiac sarcoidosis. Ann Noninvasive Electrocardiol. 2011;16:140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aizer A, Stern EH, Gomes JA, et al. Usefulness of programmed ventricular stimulation in predicting future arrhythmic events in patients with cardiac sarcoidosis. Am J Cardiol. 2005;96: 276–282. [DOI] [PubMed] [Google Scholar]
- 42. Mehta D, Mori N, Goldbarg SH, et al. Primary prevention of sudden cardiac death in silent cardiac sarcoidosis: clinical perspective. Circ Arrhythm Electrophysiol. 2011;4:43–48. [DOI] [PubMed] [Google Scholar]
- 43. Zaidi AR, Zaidi A, Vaitkus PT. Outcome of heart transplantation in patients with sarcoid cardiomyopathy. J Heart Lung Transplant. 2007;26:714–717. [DOI] [PubMed] [Google Scholar]


