Galleria mellonella larvae have been used as a host model to study interactions between pathogens and hosts for several years. However, whether the model is useful to interrogate Riemerella anatipestifer infection biology remained unknown. This study aimed to exploit the potential of G. mellonella larvae and reveal their limitations as a host model for R. anatipestifer infection. G. mellonella larvae were shown to be effective for virulence evaluations of different R. anatipestifer strains.
KEYWORDS: Galleria mellonella, R. anatipestifer, virulence
ABSTRACT
Galleria mellonella larvae have been used as a host model to study interactions between pathogens and hosts for several years. However, whether the model is useful to interrogate Riemerella anatipestifer infection biology remained unknown. This study aimed to exploit the potential of G. mellonella larvae and reveal their limitations as a host model for R. anatipestifer infection. G. mellonella larvae were shown to be effective for virulence evaluations of different R. anatipestifer strains. Furthermore, the virulent strain R. anatipestifer CH-1 had a stronger ability to proliferate than the attenuated strain R. anatipestifer ATCC 11845 in both G. mellonella larvae and ducklings. Unconventionally it was shown that G. mellonella larvae cannot be used to evaluate the efficacy of antimicrobials and their combinations. Additionally, it was shown that certain virulence factors, such as OmpA (B739_0861), B739_1208, B739_1343, and Wza (B739_1124), were specific only for ducklings, suggesting that G. mellonella larvae must be cautiously used to identify virulence factors of R. anatipestifer. Evaluation of heme uptake-related virulence genes, such as tonB1 and tonB2, required preincubating the strains with hemoglobin before infection of G. mellonella larvae since R. anatipestifer cannot obtain a heme source from G. mellonella larvae. In conclusion, this study revealed the applicability and limitations of G. mellonella as a model with which to study the pathogen-host interaction, particularly in the context of R. anatipestifer infection.
INTRODUCTION
After Robert Koch’s work, Stanley Falkow established the molecular version of Koch’s postulates, which have guided the study of the microbial virulence determinants in infectious diseases since the late 1980s (1). One of the key purposes of the molecular version of Koch’s postulates is to test whether a microorganism is attenuated when a candidate virulence gene is inactivated. Thus, the use of animal models to identify the virulence factors of pathogens is indispensable. However, animal models are accompanied by ethical problems and cannot be used in large numbers for statistical analysis. Recently, nonmammalian models, including Caenorhabditis elegans, Drosophila melanogaster, Danio rerio, and larvae of the greater wax moth, Galleria mellonella, have been used for biological research (2–4). These organisms share many advantages over mammalian models, including being easy to obtain, cost-effective, and more ethically acceptable. However, the optimal temperature for C. elegans, D. melanogaster, and Danio rerio is below 28°C (5, 6). These models are defective since the optimum temperature for most mammal pathogens is 37°C. In contrast, the G. mellonella larva model is able to survive at 37°C. Moreover, the immune system of G. mellonella larvae shares functional homology with the innate immune systems of mammals (7, 8). For instance, the killing of pathogens by G. mellonella larvae is achieved similarly to that in mammals, i.e., by lysozymes, reactive oxygen species, and antimicrobial peptides (9). Also, G. mellonella larvae employ recognition of non-self microbe-associated molecular patterns by germ line-encoded receptors (e.g., Toll and peptidoglycan recognition proteins) (10).
To date, this infection model has been used to study the virulence factors, host response, and antimicrobial delivery of many pathogens (11–13). However, whether the G. mellonella larva model also can be used for the study of Riemerella anatipestifer infection remains unknown.
R. anatipestifer is a Gram-negative bacterium belonging to the family Flavobacteriaceae (14). It has been reported as the most important pathogen in ducks and other birds around the world and is difficult to eradicate (15). At present, more than 21 serotypes have been identified worldwide, and no cross protection exists between serotypes (16, 17). R. anatipestifer is naturally resistant to various antibiotics (18–21). Using the duckling infection model, some virulence factors, such as OmpA (22), TonB-dependent receptors B739_1208 (23) and B739_1343 (24), lipopolysaccharide biosynthesis gene (25, 26), and capsule biosynthesis gene (27), have been identified. In this study, the availability of the G. mellonella larva model and its limitations were studied using R. anatipestifer as a proof of concept.
RESULTS
Virulence evaluation of different R. anatipestifer isolates in ducklings and G. mellonella larvae.
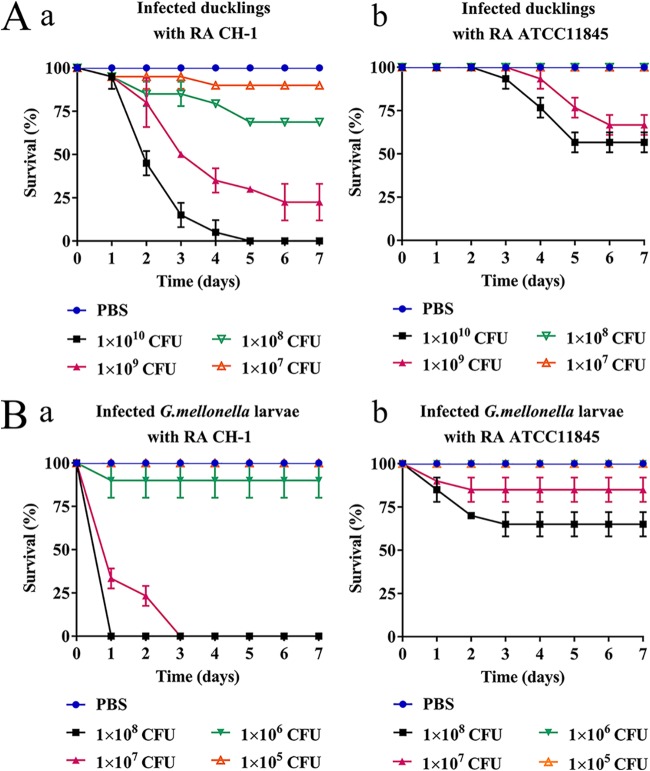
The degrees of virulence of two different R. anatipestifer isolates, R. anatipestifer ATCC 11845 and R. anatipestifer CH-1 (28), were first assessed in the duckling infection model. As Fig. 1A shows, at an inoculum of 1 × 1010 CFU/duckling for R. anatipestifer CH-1, 100% mortality was observed on day 7. However, at an inoculum of 1 × 1010 CFU/duckling for R. anatipestifer ATCC 11845, the mortality was 50%. At an inoculum rate of 1 × 108 CFU/duckling for strain CH-1, the mortality rate was 30% (Fig. 1Aa); in contrast, the same dose of strain ATCC 11845 was avirulent for ducklings (Fig. 1Ab). These results indicated that compared to R. anatipestifer CH-1, R. anatipestifer ATCC 11845 was weakly virulent in ducklings.
FIG 1.
Virulence evaluation of R. anatipestifer CH-1 and R. anatipestifer ATCC 11845 in ducklings and G. mellonella larvae. (A) Survival rate of ducklings infected with different doses of R. anatipestifer CH-1 (a) and R. anatipestifer ATCC 11845 (b) for 7 days. (B) Survival rate of G. mellonella larvae infected with different doses of R. anatipestifer CH-1 (a) and R. anatipestifer ATCC 11845 (b) for 7 days. PBS injection was used as a negative control. Ten ducklings or larvae were infected in each experimental group. All assays were performed at least three times independently. Survival data were plotted using GraphPad Prism v 7.00.
To evaluate whether G. mellonella larvae can be used as an infection host model for R. anatipestifer, larvae were first infected with strain CH-1 at different inoculum levels. We tried to keep the larvae at 42°C to mimic the duck temperature; however, the larvae were not able to survive at 42°C (data not shown). Thus, the following experiments refer to larvae studied at 37°C. As shown in Fig. 1Ba, at an inoculum of 1 × 108 CFU/larva for strain CH-1, we observed 100% mortality of the larvae within 24 h and approximately 70% mortality of the larvae at the same time after infection with 1 × 107 CFU/larva. Differences in mortality were less apparent at lower doses, and no deaths were recorded when larvae were injected with phosphate-buffered saline (PBS) alone (Fig. 1Ba). To confirm that the mortality was not due to other, nonspecific factors, larvae were also injected with multigelation-treated bacteria. No larval death was observed when infected with multigelation-killed bacteria (1 × 108 CFU/larva). These results clearly demonstrated that R. anatipestifer CH-1 could kill the larvae in a dose-dependent fashion.
Strain ATCC 11845 led to a lower larval mortality than strain CH-1 at the same bacterial inoculum doses (1 × 108 CFU, 1 × 107 CFU, and 1 × 106 CFU) (Fig. 1Bb). This finding indicated that the CH-1 strain had higher virulence in both the G. mellonella larva model and the duckling model than that of the ATCC 11845 strain.
To further demonstrate the consistency of the virulence of R. anatipestifer in both models, we evaluated the virulence of three other strains: RCAD_0404, RCAD_0405, and RCAD_0414. For the duckling model, we chose 1 × 109 CFU as the dose of inoculation since the virulence of tested strains revealed significant differences under this condition. For the G. mellonella larva model, the dose of inoculation was 1 × 107 CFU, as the degrees of virulence of tested strains were significantly different under this condition. The mortality rates of ducklings were 77.5% for R. anatipestifer CH-1, 60% for RCAD_0404, 52.8% for RCAD_0405, 40% for RCAD_0414, and 33% for R. anatipestifer ATCC 11845 (see Fig. S1A in the supplemental material). Correspondingly, the mortality rates of G. mellonella larvae were 100% for strain CH-1, 45% for RCAD_0404, 30% for RCAD_0405, 20% for RCAD_0414, and 10% for ATCC 11845 (Fig. S1B). Consistently, the 50% lethal doses (LD50) of the tested strains in G. mellonella larvae were 1.7 × 106 CFU for CH-1, 1.6 × 107 CFU for RCAD_0404, 2.3 × 107 CFU for RCAD_0405, 4.2 × 107 CFU for RCAD_0414, and 3.2 × 108 CFU for ATCC 11845. Taken together, isolates reported as virulent in ducklings showed dose-dependent virulence in G. mellonella larvae. Isolates reported as weakly virulent in ducklings showed attenuated virulence in G. mellonella larvae.
Proliferation of R. anatipestifer CH-1 and R. anatipestifer ATCC 11845 in G. mellonella larvae and ducklings.
To further determine whether the mortality of G. mellonella was associated with the bacterial growth of R. anatipestifer in the infected larvae, the change in intracellular bacterial numbers of R. anatipestifer was evaluated in G. mellonella larvae at 2, 12, 24, 36, and 48 h after inoculation with 1 × 106 CFU/larva. At this dose of inoculation, most of the G. mellonella larvae survived during the experiment. Thus, it was convenient for detecting the bacterial loading in G. mellonella larvae. For R. anatipestifer CH-1, initially, the detectable clones of the infection were (1.12 ± 0.12) × 106 CFU/larva and remained fairly constant, only reaching (1.07 ± 0.16) × 106 CFU/larva at 12 h postinfection. In contrast, at 24 h, the bacterial loads of CH-1 in the hemocoel of the larvae increased ∼55-fold to (5.9 ± 0.2) × 107 CFU/larva and began to decrease at 36 h postinfection (Fig. 2A). These observations supported the consistency between the ability to grow inside the larvae and the killing abilities.
FIG 2.
Kinetics of proliferation of R. anatipestifer CH-1 and R. anatipestifer ATCC 11845 inside G. mellonella larvae and ducklings. (A) G. mellonella larvae were infected with 106 CFU of CH-1 and ATCC 11845. After 2, 12, 24, 36, and 48 h, the bacterial load in larvae was recorded. Six live larvae per time point were killed for these assays. (B) Three-day-old ducklings were infected with 108 CFU of CH-1 and ATCC 11845. After 24 and 48 h, the bacterial loads in the blood, liver, and spleen were recorded. Six ducklings at the time point were killed for these assays. These results were repeated at least three times. Each error bar represents 1 SD from the mean. Differences were assessed for statistical significance with one-way analysis of variance (ANOVA).
However, infection of G. mellonella larvae with R. anatipestifer ATCC 11845 did not result in a significant increase in the CFU at 24 h postinfection (Fig. 2A). Nearly no bacteria were detected in the larvae at 48 h postinfection. These results indicated that compared to CH-1, ATCC 11845 had a decreased ability to replicate inside G. mellonella larvae.
To further investigate whether CH-1 and ATCC 11845 also have different levels of proliferation inside ducklings, 108 CFU bacteria were injected into the legs of 3-day-old ducklings. At this dose of inoculation, most ducklings survived within 48 h. As Fig. 2B shows, at 24 h postinoculation, the bacterial load of ATCC 11845 in the heart blood, liver, and spleen of the ducks was significantly reduced compared with that of CH-1. At 48 h postinoculation, ATCC 11845 was undetectable by plating, whereas CH-1 remained detectable (Fig. 2B). Thus, consistent with the G. mellonella larva model, ATCC 11845 had a lower proliferative ability in ducklings.
R. anatipestifer CH-1 stimulated a stronger immune response than R. anatipestifer ATCC 11845 in G. mellonella larvae.
Furthermore, we investigated whether R. anatipestifer ATCC 11845 was more sensitive to host immune response than R. anatipestifer CH-1 and was thus more easily cleared away. The immune response of the larvae stimulated by ATCC 11845 and CH-1 was evaluated. Larvae were infected with PBS, ATCC 11845 (104 CFU), and CH-1 (104 CFU) separately. The dose of inoculation was stimulatory but not lethal to G. mellonella larvae. The results showed that the amounts of hemolin, gallerimycin, and lysozyme mRNAs were induced at 2 h after infection with CH-1 or ATCC 11845, and the induction was more pronounced at 6 h after injection (Fig. 3A). However, anionic peptide mRNA levels increased up to 48 h (Fig. 3A). Interestingly, CH-1 stimulated a stronger immune response than ATCC 11845 at the same time point postinfection (Fig. 3A).
FIG 3.
Comparison of immune-responsive G. mellonella larvae stimulated by R. anatipestifer CH-1 and ATCC 11845. (A) Transcription of immune-responsive genes following infection by CH-1 and ATCC 11845 at 2 h, 6 h, 12 h, 24 h, 36 h, and 48 h postinfection. The transcription levels of hemolin, gallerimycin, lysozyme, and anionic peptide were determined by RT-qPCR and are shown relative to the expression levels in PBS-injected larvae. All the results represent means and SDs for at least three independent determinations. (B) Larvae were infected with 104 CFU of CH-1 and 104 CFU of ATCC 11845 and, after 6 h, infected with 107 CFU of CH-1. The survival rate of the G. mellonella larvae preimmunized with CH-1 or ATCC 11845 was monitored for 7 days. Ten larvae were infected in each experimental group. All the results were repeated at least three times. (C) Larvae were inoculated with 106 CFU of heat-killed E. coli and, after 24 h, challenged with CH-1 (107 CFU, the lethal dose) and ATCC 11845 (1.2 × 108 CFU, the lethal dose). The survival rate of the G. mellonella larvae was monitored for 7 days. Ten larvae were infected in each experimental group. These results were repeated at least three times.
To further verify this conclusion, we examined whether the prior induction of immune responses in G. mellonella would protect against subsequent infection by CH-1. Larvae were injected with 104 CFU of CH-1 and ATCC 11845 separately and then challenged by injecting a dose of 107 CFU of CH-1 (the lethal dose) at 6 h postinfection. As shown in Fig. 3B, G. mellonella preimmunized by CH-1 had a higher protection rate than larvae preimmunized by ATCC 11845.
To further investigate whether the immune response of G. mellonella larvae confers cross protection against different pathogens, larvae were inoculated with 106 CFU of heat-killed Escherichia coli and after 24 h challenged with CH-1 (107 CFU, the lethal dose) and ATCC 11845 (1.2 × 108 CFU, the lethal dose). As Fig. 3C shows, larvae preimmunized with heat-killed E. coli showed increased protection against subsequent infection with a lethal dose of CH-1 and ATCC 11845, and without a significant difference in the protective efficacy for the two strains. These results suggest that activation of immunity in G. mellonella increases the host defense against R. anatipestifer infection. It seems that there is no significant difference in the immune responses of G. mellonella larvae to the two strains.
To further demonstrate this hypothesis, we tested the susceptibility of CH-1 and ATCC 11845 to E. coli-elicited G. mellonella larval antimicrobial peptides which were isolated from the hemolymph in vitro. Indeed, the survival rates of CH-1 and ATCC 11845 in the hemolymph were 40% ± 5% and 37% ± 6% at 1 h, and all of the bacteria were killed within 3 h, suggesting that there is no significant difference of the two strains in susceptibility to the antimicrobial factors present in the hemolymph of G. mellonella larvae.
G. mellonella larvae cannot to be used for the assessment of antimicrobials and their combinations for R. anatipestifer infection.
Treatment of R. anatipestifer infection often requires the use of several antibiotics in combination. However, which combinations are ideal is unknown, since some combinations may display synergy or antagonism (29). In this study, we tested whether the G. mellonella larva model can be used for the assessment of antimicrobials and their combinations for CH-1 infection.
Larvae were infected with 107 CFU of CH-1 (the lethal dose) and divided into 3 treatment groups: single antibiotic, antibiotic combination, and PBS treatment control. Antibiotics were injected 2 h postinoculation. As expected, treatment with cefoxitin alone (Fig. S2Ab) and spectinomycin alone (Fig. S2Bb) rescued the larvae at suitable concentrations. However, the group treated with gentamicin had a less pronounced effect than the control (Fig. S2Cb). Cefoxitin-spectinomycin combinations and spectinomycin-gentamicin combinations acted additively by increasing the rates of larval survival during infection compared to those with the single antibiotics alone (Fig. 4Ab and Cb). However, compared to cefoxitin treatment alone, the cefoxitin-gentamicin combination did not lead to an increase in the survival rate (Fig. 4Bb), indicating that this combination exhibited antagonistic effects.
FIG 4.
Effects of different antibiotic treatments on the survival of G. mellonella larvae and ducklings infected with R. anatipestifer CH-1. Ducklings (Aa, Ba, and Ca) or G. mellonella larvae (Ab, Bb, and Cb) were each infected with 109 CFU of CH-1 or 107 CFU of CH-1, respectively, and then subsequently treated with PBS, with single drugs, or with a combination of drugs 2 h after injection. Each group contained 10 larvae or ducklings. The percentage of surviving larvae was monitored at the indicated intervals after infection. The averages of three biological replicates are shown. The MICs of the combinations Cfx plus Spc, Cfx plus Gen, and Spc plus Gen against R. anatipestifer CH-1 are 1 μg/ml, 0.5 μg/ml, and 20 μg/ml, respectively. Cfx, cefoxitin; Spc, spectinomycin; Gen, gentamicin.
In parallel, the 3-day-old ducklings that had been injected with 109 CFU of CH-1 (the lethal dose), antibiotics (single antibiotic or antibiotic combination), or PBS were injected 2 h postinoculation. As Fig. S2 shows, treatment with cefoxitin alone, spectinomycin alone, or gentamicin alone has a dose-dependent protection effect in the duckling infection model. Cefoxitin-spectinomycin combinations, cefoxitin-gentamicin combinations, and spectinomycin-gentamicin combinations all acted additively by increasing the rates of duckling survival during infection compared to those with the single antibiotics alone (Fig. 4Aa, Ba, and Ca).
Taken together, the results show that the efficacies of the antibiotics and their combinations were not always consistent in the duckling and G. mellonella larva models. It was suggested that in the case of infection with R. anatipestifer, G. mellonella larvae cannot be used to evaluate the efficacy of antimicrobials and their combinations in vivo.
Assessment of the virulence genes for R. anatipestifer infection in the G. mellonella larva model.
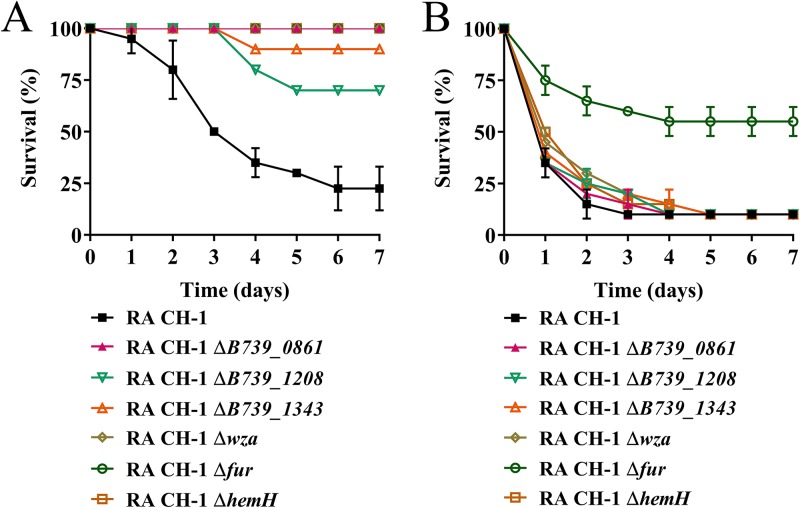
Previous work identified several virulence factors that affect virulence in ducklings, including ompA (B739_0861) (22), B739_1208 (23), B739_1343 (24), wza (B739_1124) (27), and fur (30). As proof of principle, we tested whether the G. mellonella larva model could be used to assess these known virulence factors for R. anatipestifer infection. As Fig. 5 shows, compared to the wild type, the ompA mutant, B739_1208 mutant, B739_1343 mutant, and wza mutant strains were attenuated only in the duckling model and not in the G. mellonella larva model, suggesting a specific role for these genes for virulence in duckling hosts. The fur mutant was attenuated in both models (Fig. 5). Additionally, we evaluated the importance of the heme synthesis gene, hemH, in virulence in both models. The result showed that the R. anatipestifer CH-1 hemH mutant was attenuated in the duckling model but not in the G. mellonella larva model (Fig. 5). Taken together, these results suggest that R. anatipestifer does not always use the same virulence factor to cause disease in different hosts.
FIG 5.
Assessment of the virulence genes of R. anatipestifer CH-1 in the duckling model and G. mellonella larva model. (A) Survival rates of ducklings infected with 109 CFU of wild-type and mutant strains for 7 days. (B) Survival rates of G. mellonella larvae infected with 5 × 106 CFU of wild-type and mutant strains for 7 days. Ten ducklings or larvae were infected in each experimental group. All assays were performed at least three times independently. Survival data were plotted using GraphPad Prism v 7.00.
R. anatipestifer can utilize the hemocyte lysates of G. mellonella larvae as an iron source but not as a heme source.
Different host microenvironments between G. mellonella larvae and ducklings could be a reason why R. anatipestifer uses different virulence factors. Since heme and iron are essential for most bacteria (31, 32), we checked the iron and heme sources of R. anatipestifer in ducklings and G. mellonella larvae. In vitro, R. anatipestifer CH-1 was able to grow on the iron-limited medium supplemented with duck hemoglobin (Hb) (Fig. S3A), suggesting that R. anatipestifer was able to obtain an iron source from ducklings. When hemH, which is involved in heme synthesis (33), was deleted in CH-1, the bacteria failed to grow in GC broth (GCB) (Fig. S3B). The growth of CH-1 ΔhemH was restored by the addition of 2 μM duck hemoglobin in GCB (Fig. S3B). This result suggested that R. anatipestifer was able to obtain a heme source from ducklings.
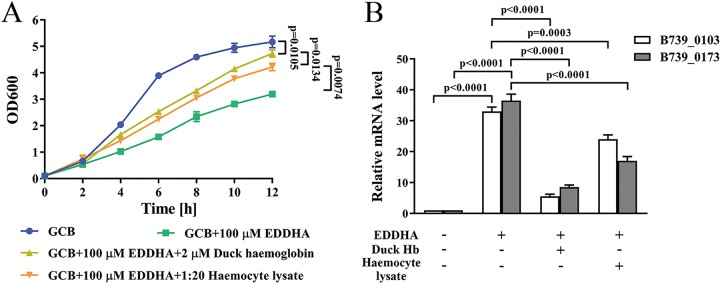
Next, we investigated whether CH-1 was able to obtain iron from G. mellonella larvae. To address this question, we first grew CH-1 in GCB, GCB with 100 μM EDDHA (ethylenediamine-di-o-hydroxyphenylacetic acid), GCB with 100 μM EDDHA supplemented with 2 μM duck Hb and GCB with 100 μM EDDHA supplemented with a 1:20 dilution of hemocyte lysate. As shown in Fig. 6A, similar to the growth in GCB with 100 μM EDDHA supplemented with 2 μM duck Hb, the growth of CH-1 was rescued in GCB with 100 μM EDDHA supplemented with the 1:20 dilution of hemocyte lysate. Consistently, it was found that the iron-regulated genes B739_0103 and B739_0173 (34, 35) were upregulated ∼30-fold in GCB with 100 μM EDDHA compared to that in GCB. The expression levels of these two genes were strongly decreased after the addition of duck Hb or hemocyte lysate in the iron-depleted medium (Fig. 6B). Taken together, these results indicated that CH-1 could potentially obtain iron from G. mellonella larvae.
FIG 6.
Hemocyte lysate of G. mellonella larvae was used as an iron source for R. anatipestifer CH-1. (A) The growth curve of R. anatipestifer CH-1 in GCB, GCB together with 100 μM EDDHA, GCB together with 100 μM EDDHA supplemented with 2 μM duck hemoglobin, and GCB together with 100 μM EDDHA supplemented with hemocyte lysate. (B) Iron-responsive transcription of B739_0103 and B739_0173 in R. anatipestifer CH-1, which was grown in GCB, GCB containing 100 μM EDDHA, 100 μM EDDHA together with 2 μM duck hemoglobin, or 100 μM EDDHA together with hemocyte lysate. Transcription was measured by qRT-PCR. Representative fold changes in comparison with growth in GCB medium. Differences were assessed for statistical significance with a one-way ANOVA.
To determine whether CH-1 was able to obtain a heme source in G. mellonella larvae, strain CH-1 ΔhemH was grown on a GCB plate supplemented with duck Hb or a hemocyte lysate of G. mellonella larvae. As shown in Fig. 7A, growth around discs containing duck Hb was observed by 24 h and continued to expand over the course of the experiment. In contrast, there were no live bacteria detected around the hemocyte lysate-containing disc until approximately 72 h after inoculation; the visible circle around the disc was because the hemocyte lysate of G. mellonella larvae became pigmented easily. In vivo, 106 CFU of CH-1/pLMF03, CH-1 ΔhemH/pLMF03, and CH-1ΔhemH/pLMF03::hemH was injected into the G. mellonella larvae. As shown in Fig. 7, CH-1ΔhemH/pLMF03 (Fig. 7C) bacteria were not detected at 24 h postinfection. However, the number of bacteria was increased 24 h postinfection for the wild type (Fig. 7B) and the complemented strain (Fig. 7D). These findings demonstrated that CH-1 has evolved to acquire heme from duck Hb but not from hemocyte lysates of G. mellonella larvae.
FIG 7.
Measurement of hemocyte lysate of G. mellonella larvae used as a heme source for R. anatipestifer CH-1 in vitro and in vivo. In vitro, strain CH-1 ΔhemH was tested for heme utilization efficiency on a GCB plate as described in Materials and Methods. After 24 h, growth around discs containing duck Hb or hemocyte lysate from G. mellonella larvae was observed (A). In vivo, strains CH-1/pLMF03 (B), CH-1 ΔhemH/pLMF03 (C), and CH-1 ΔhemH/pLMF03::hemH (D) were infected with G. mellonella larvae. After 2, 12, 24, 36, and 48 h, the bacterial load in larvae was recorded. Six live larvae at each time point were killed for these assays. These results were repeated 4 times. Each error bar represents 1 SD from the mean.
Evaluation of the heme uptake-related virulence gene in the G. mellonella larva model.
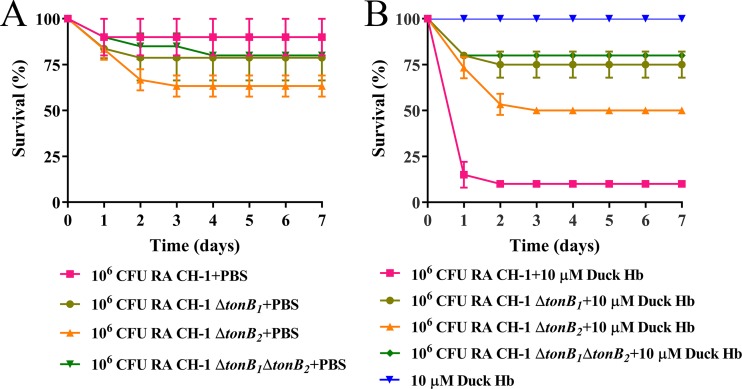
Since it had been demonstrated that R. anatipestifer was able to obtain iron but not a heme source from G. mellonella larva infection, we wondered whether the G. mellonella larva model could be used to evaluate virulence genes related to heme uptake. As a proof of concept, we evaluated the virulence of the tonB1 mutant, tonB2 mutant, and tonB1 tonB2 mutant in the G. mellonella larva model since it has been shown that both tonB1 and tonB2 are involved in heme transport (36) and virulence (37) in ducklings. In the duckling model, consistent with a previous report (37), injection with 109 CFU of the tonB1 mutant, tonB2 mutant, and tonB1 tonB2 mutant resulted in no death within 7 days after injection (Fig. S4A); parallel injection with the wild-type strain resulted in a mortality rate of 78% for ducklings 7 days after injection (P < 0.05) (Fig. S4A). In the G. mellonella larva model, injection with 107 CFU of the tonB1 mutant, tonB2 mutant, and tonB1 tonB2 mutant resulted in mortality rates of 89%, 100%, and 97%; these levels of virulence were not significantly different from that of the parental strain (Fig. S4B). This result suggested that compared to the wild type, the tonB1 mutant, tonB2 mutant, and tonB1 tonB2 mutant strains were attenuated only in the duckling model and not in the G. mellonella larva model. Next, G. mellonella larvae were infected with 106 CFU of all these strains or strains preincubated with 10 μM duck Hb. The addition of 10 μM duck Hb to the inoculum resulted in increased mortality for all strains tested compared to those for all strains alone (Fig. 8). Injection with the wild type and duck Hb led to a 90% mortality rate for the G. mellonella larvae 7 days after injection. However, injection with the tonB1 mutant with duck Hb, the tonB2 mutant with duck Hb, and the tonB1 tonB2 mutant with duck Hb led to 25%, 50%, and 20% mortality rates, respectively, for the G. mellonella larvae 7 days after injection (Fig. 8B). The difference in mortality rates between the tonB mutant and the parental strain was significant (P < 0.05). Using bovine hemoglobin to substitute for duck hemoglobin, we obtained a similar result (data not shown). All these findings suggest that preincubation with a heme source is required for evaluating heme uptake-related virulence genes.
FIG 8.
Virulence evaluation of the heme uptake-related gene in the G. mellonella larva model. (A) Survival rate of G. mellonella larvae infected with 106 CFU of CH-1, CH-1 ΔtonB1, CH-1 ΔtonB2, or CH-1 ΔtonB1 ΔtonB2 for 7 days. (B) Survival rate of G. mellonella larvae infected with 106 CFU of CH-1, CH-1 ΔtonB1, CH-1 ΔtonB2, or CH-1 ΔtonB1 ΔtonB2 (which were preincubated with 10 μM duck Hb), and duck Hb alone. Ten larvae were infected in each experimental group. All assays were performed at least three times independently. Survival data were recorded daily for a period of 7 days and plotted using GraphPad Prism v. 7.00.
DISCUSSION
Recently, the larva of G. mellonella has been reported as an easy-to-use model organism for several pathogenic bacteria (11, 12, 38, 39). This model host represents a valuable tool for the study of host-pathogen interactions because it is convenient for distinguishing virulent and avirulent strains and identifying bacterial virulence factors and has innate immune responses similar to those of animal hosts (40). However, it was reported that this model cannot be used for the identification of most virulence factors for Burkholderia cepacia (41). Nonetheless, we wondered whether this model could also be used as a host model for the duck pathogen R. anatipestifer and whether this model could completely substitute for animal models since G. mellonella larvae have different microenvironments from the animal hosts. In this study, we characterized G. mellonella larvae as a new infection model for R. anatipestifer and revealed new perspectives of G. mellonella larvae as a host model.
Although the G. mellonella larva model has been used to identify the virulence of different bacterial isolates, such as Vibrio cholerae (38) and enteroaggregative Escherichia coli (11), the correlation of the bacterial virulence in the G. mellonella larva model and the animal model has not been evaluated broadly. In this study, first, it was shown that different isolates of R. anatipestifer strains, namely, CH-1 and ATCC 11845, had different kinetics of survival and larval mortality. Furthermore, it was demonstrated that CH-1 killed the larvae in a dose- and time-dependent manner. To confirm that other R. anatipestifer isolates were also pathogenic in the G. mellonella larva model, we tested an additional 3 R. anatipestifer strains and observed a wide variation in responses. Moreover, there was a correlation between virulence in the G. mellonella larva model and in ducklings for these R. anatipestifer strains. A correlation was observed between the G. mellonella larva model and well-established duckling models, suggesting that this model is a quick and inexpensive tool with which to compare the degrees of virulence of different R. anatipestifer isolates. Combining our results with the results obtained for other bacteria, it can be inferred that the G. mellonella larva model can be used extensively to identify the virulence of different bacterial isolates (11, 38).
Analysis of R. anatipestifer CH-1 replication in G. mellonella larvae demonstrated that bacterial CFU counts increased 55-fold from the inoculum by 24 h postinfection and began to decrease at 36 h postinfection. R. anatipestifer ATCC 11845 was cleared more quickly than CH-1 by the larvae of G. mellonella. Consistently, it was shown that ATCC 11845 was cleared more quickly than CH-1 in the ducklings. To further investigate why CH-1 had a higher proliferative capacity than ATCC 11845 inside G. mellonella larvae, we compared the immune responses of G. mellonella larvae to the two strains. It was shown that CH-1 stimulates a stronger immune response than ATCC 11845. However, the resistances of the two strains to Galleria antimicrobial peptides did not show significant difference. It was suggested that the proliferative capacity of CH-1 was not related to the immune response of G. mellonella larvae. Similarly, under the laboratory conditions, the growth of CH-1 is more rapid than that of ATCC 11845 (data not shown). The result of proliferation inside G. mellonella larvae was not consistent with the results for enteroaggregative Escherichia coli, Pseudomonas aeruginosa, or Streptococcus pyogenes, which showed that the bacterial burden increased within the larvae over time (11, 42, 43). It is possible that these widely distributed bacteria had higher adaptability in G. mellonella larvae than R. anatipestifer.
The spread of antimicrobial resistance has become a serious public health concern worldwide (29, 44). R. anatipestifer is naturally resistant to multiple antibiotics (18, 19, 21, 45); thus, evaluation of single drugs and drug combinations can help fight R. anatipestifer and avoid misuse. In previous studies, it was reported that G. mellonella larvae were used for the assessment of the efficacies of single antimicrobials and antimicrobial combinations for Mycobacterium abscessus infection (46) and for Pseudomonas aeruginosa infection (39); however, it remains unknown whether the efficacies of single antimicrobials and antimicrobial combinations are always consistent in G. mellonella larvae and the host, since a variety of factors can impact the efficacy of antimicrobials administered to a given host, such as route of delivery, toxicity, host metabolism, and elimination. Here we have clearly shown that the efficacies of single antimicrobials and combinations were not always consistent in G. mellonella larvae and the host, at least for R. anatipestifer infection. Thus, we propose that G. mellonella larvae no longer be used as a model for evaluating the efficacy of antimicrobials.
Evidence accumulated in recent years has demonstrated that G. mellonella larvae can be used as an attractive alternative model to identify the virulence of various pathogens (42, 47–50). In this study, we investigated the extent to which the virulence factors required for pathogenesis in both ducklings and G. mellonella larvae overlap. The identified virulence factors include ompA (22), B739_1208 and B739_1343, which encode TonB-dependent receptors (23, 24), wza, which is involved in capsule biosynthesis (27), and a ferric uptake regulator, fur (30). Using these genes as a proof of concept, we evaluated the effects of mutants on the pathogenicity in both ducklings and G. mellonella larvae. It was shown that the fur knockout mutant exhibited reduced virulence compared with that of the wild-type strain in both models. However, the ompA mutant, the B739_1208 mutant, the B739_1343 mutant, and the wza mutant were attenuated in ducklings but not in G. mellonella larvae. These studies demonstrated that several virulence factors were host specific, with little correlation seemingly related to the duckling model. R. anatipestifer strains appear to utilize different virulence mechanisms to compete for survival in G. mellonella larvae and ducklings. We identified only a few virulence factors in R. anatipestifer, and we therefore believe that extrapolations from G. mellonella larva infection models to mammalian, anatine, and other infections must be made with caution. Similarly, it has been shown that most of the virulence factors in Burkholderia cepacia are specific for one infection model but not for other infection models (41). It was speculated that the G. mellonella larva infection model was not able to be used to identify all the virulence of other bacteria.
Different host environments present different nutritional possibilities. The bacterial pathogen must overcome these challenges. One of these challenges is the acquisition of iron, a micronutrient that is essential for almost all living cells (51). It has also been shown that iron utilization-related systems are involved in the virulence of most bacteria (31, 51), including R. anatipestifer (23). Although many studies have shown that the bacteria are able to proliferate in G. mellonella larvae (11, 47, 52), the iron source in G. mellonella larvae has not been evaluated. Thus, it was first evaluated whether R. anatipestifer is able to obtain an iron source from G. mellonella larvae in vitro. The addition of hemocyte lysate was able to restore the growth of strain CH-1 under iron-limited conditions. Moreover, the iron-regulated genes B739_0103 and B739_0173 were downregulated with the addition of a hemocyte lysate in iron-limited medium. These data strongly suggested that CH-1 was able to obtain an iron source from G. mellonella larvae, supporting the growth of this strain inside the larvae. Accordingly, it has been shown that the iron-binding proteins are present within the hemolymph of the larvae (53). The components that could be used by R. anatipestifer remain unknown at this time. Regardless, these results indicate that R. anatipestifer can use iron from larvae during colonization and infection under iron-limiting conditions, which is similar to the case in the vertebrate host. Since other bacteria, such as Listeria monocytogenes (48), Streptococcus (49), and Escherichia coli (11), were also able to proliferate inside G. mellonella larvae, it was suggested that other infective bacteria were able to get the iron source from them.
Bacteria obtain heme through either synthesis or uptake from the host (32). Whether bacteria are also able to obtain heme from G. mellonella larvae was an important unanswered question. In this study, it was shown that R. anatipestifer CH-1 was unable to obtain a heme source from G. mellonella larvae both in vivo and in vitro. Moreover, we did not find the E. coli strain C600 ΔhemA heme source from G. mellonella larvae (data not shown). Thus, we predicted that G. mellonella larvae were not heme available. Since CH-1 was not able to obtain heme from G. mellonella larvae, we investigated whether the G. mellonella larva model could be used to evaluate the virulence of heme uptake-related genes. To this end, we evaluated the effect of the tonB1 mutant, tonB2 mutant, and tonB1 tonB2 mutant on pathogenicity in both ducklings and G. mellonella larvae. It was shown that the tonB1 mutant, tonB2 mutant, and tonB1 tonB2 mutant were attenuated in ducklings but not in G. mellonella larvae. However, it is interesting that the killing of G. mellonella larvae by the CH-1 strain when injected with supplemental duck hemoglobin was faster than the response obtained with infections in which the inocula were not supplemented with hemoglobin. Moreover, compared to the wild type, the tonB1 mutant, tonB2 mutant, and tonB1 tonB2 mutant were attenuated significantly in G. mellonella larvae when all the strains were injected with supplemental duck hemoglobin. Whether other bacteria are able to get heme from G. mellonella larvae and whether this theory is able to be used to identify other bacterial heme uptake-related genes require experimental demonstration in the future. Nevertheless, these results suggested that supplementation of certain nutrients was required for the virulence evaluation when the bacteria could not be obtained from G. mellonella larvae.
In conclusion, we demonstrated that G. mellonella larvae are susceptible to R. anatipestifer infection and that this model reproduces virulence phenotypes in duckling infection models. Despite the clear utility of G. mellonella larvae as a surrogate model with which to assess the virulence of R. anatipestifer, this model is unable to fully recapitulate R. anatipestifer pathogenesis in ducklings. Moreover, the enormous evolutionary distance between G. mellonella larvae and ducks does not allow the observation of host-specific phenomena. G. mellonella larval iron sources for the pathogens were different from those of the animal host. Nevertheless, this study demonstrated that inoculating R. anatipestifer together with hemoglobin as an iron or heme source provides valuable tools for modeling infection. Importantly, many bacterial pathogens utilize hemoglobin as an iron source; therefore, this model may be valuable for studies of a variety of infectious diseases.
MATERIALS AND METHODS
Bacterial strains, plasmids, and primers.
The strains and plasmids used in this study are listed in Table S1. The primers used in this study are listed in Table S2.
Growth conditions.
E. coli strains were grown routinely on LB medium (Sigma-Aldrich; product number L3522) aerobically at 37°C with shaking. The solid media contained 1.5% Difco agar. R. anatipestifer strains were cultured routinely on LB agar supplemented with 5% sheep blood or in GCB (54) liquid medium at 37°C with shaking. Bovine hemoglobin (Sigma, USA) was dissolved in 100 mM NaCl and filter sterilized with 0.45-μm-pore-size Millipore filters for heme utilization experiments. EDDHA (ethylenediamine-di-o-hydroxyphenylacetic acid) (Alfa Chemistry, Protheragen, Inc., USA) was dissolved in H2O to a final concentration of 20 mM. The number of bacteria was measured by plating aliquots on blood agar to calculate the bacterial CFU per milliliter. Antibiotics were used at the following concentrations when needed: ampicillin (Amp), 100 μg/ml, and kanamycin (Kan), 50 μg/ml, for E. coli; cefoxitin (Cfx), 1 μg/ml, spectinomycin (Spec), 60 μg/ml, and gentamicin (Gen), 20 μg/ml, for R. anatipestifer.
Duck hemoglobin preparation.
Duck hemoglobin was prepared with a polyethylene glycol (PEG)/salt aqueous two-phase system with a slight modification (55). Briefly, fresh duck blood was obtained from a 1-month-old duck. Then 10 ml of whole blood was centrifuged at 5,000 rpm for 15 min. The supernatant was discarded, and the pellet was washed three times with 2 volumes of 0.9% NaCl. The blood cells were hemolysed by the addition of 3 volumes of distilled water and incubation for 30 min at room temperature, and the cell debris was removed by centrifugation at 12,000 × g for 30 min. (NH4)2SO4 was added to the supernatant to obtain a 60% final concentration, and the mixture was incubated for 30 min at 4°C. After centrifugation at 12,000 × g for 30 min, the red pellet was dissolved in 0.9% NaCl. The samples were centrifuged again at 12,000 × g for 30 min. The supernatant was sterilized using a 0.45-μm filter. Final hemoglobin concentrations were measured using a hemoglobin colorimetric assay kit (item number 700540; Cayman Chemical, MI).
Virulence evaluation of R. anatipestifer in ducklings.
Three-day-old Pekin ducklings were randomly divided into four groups (10 ducks/group). The ducklings were injected by leg with 107, 108, 109, or 1010 CFU of each bacterial strain. The initial bacterial number was estimated by optical density at 600 nm (OD600) and counted by plating the blood plates. The ducklings were observed every 4 to 6 h for 7 days. Once the ducklings exhibited moribund signs, they had to be euthanized with forced inhalation of CO2. Dead ducklings were subjected to R. anatipestifer identification by PCR and Gram staining. The mortality of the ducklings was recorded daily for a period of 7 days postchallenge.
Changes in populations of R. anatipestifer in inoculated ducklings.
To assess bacterial population changes in ducklings, 3-day-old ducklings were infected with R. anatipestifer (108 CFU). The initial bacterial number was estimated by OD600 and counted by plating the blood plates. At the time points of 24 and 48 h postinfection, six surviving ducklings in each test group were randomly selected and euthanized by forced inhalation of CO2. Blood, liver, and spleen tissue were collected and weighed. The samples were homogenized in PBS (0.1 g of sample/0.9 ml of PBS) using a Nasco WHIRL-PAK (B01245WA, USA) as described previously (23). The homogenized contents were serially diluted in PBS buffer and plated on blood agar plates supplemented with 50 μg/ml of kanamycin, since R. anatipestifer was naturally resistant to kanamycin (36), and incubated at 37°C overnight to determine the CFU of bacteria.
Galleria mellonella larvae.
G. mellonella larvae were purchased from Hui Yude Biological Technology Co., Ltd. (Tianjin, China), stored in the dark at room temperature, and used within 7 days. G. mellonella larvae were evaluated for health and used in experiments based on lack of melanization and movement in response to touch. Larvae weighing between 0.3 and 0.4 g were chosen for experiments. For each experiment, 10 larvae were used per strain, and experiments were repeated three times using larvae from different batches.
Virulence evaluation of R. anatipestifer in G. mellonella larvae.
R. anatipestifer strains were grown in GCB at 37°C with shaking and washed twice in PBS. Quantification of the bacterial cell number was carried out by plating serial dilutions of the inoculum onto blood agar plates. Infection of G. mellonella larvae with R. anatipestifer strains was performed as described previously for other bacterial pathogens (43, 52). Briefly, the larvae were infected by microinjection into the right foremost proleg with 50 μl of inocula of different R. anatipestifer strains containing between 1 × 105 CFU and 1 × 108 CFU. The initial bacterial number was estimated by OD600 and counted by plating the blood plates. For the negative control, 10 larvae were inoculated with PBS. Ten larvae were not injected to control for background larval mortality. The larvae were incubated at 37°C, and survival was recorded for all strains at 24-h intervals for 7 days. Larvae were scored as dead when they stopped moving and failed to respond when gently manipulated with a pipette tip. All assays were performed at least three times independently.
Antibiotic treatment.
Antibiotic stock solutions were freshly prepared and diluted in 1× PBS to the required concentration. Antibiotic toxicity was preevaluated by injection of serial dilutions of either single antibiotics or antibiotic combinations. For studies of antibiotic efficacy, an inoculum of 1 × 107 CFU of CH-1 or 1 × 109 CFU of CH-1 was used for G. mellonella larvae or ducklings, respectively. The initial bacterial number was estimated by OD600 and counted by plating the blood plates. For final experiments, groups of 10 larvae or ducklings were injected with the strains and different concentrations of antibiotic or antibiotic combinations were administered 2 h postinfection. In a control experiment, PBS was administered. Additional control groups were assessed for the toxicity of the antibiotic treatment. Time-survival curves were generated. Each strain-antibiotic combination was evaluated in three independent experiments.
Changes in populations of R. anatipestifer in inoculated G. mellonella larvae.
To assess bacterial population changes, G. mellonella larvae were infected with R. anatipestifer (106 CFU/larva). The initial bacterial number was estimated by OD600 and counted by plating the blood plates. At the time points of 2, 12, 24, 36 and 48 h postinfection, six surviving larvae in each test group were randomly selected and destroyed. Appropriate dilutions were plated on blood agar plates supplemented with 50 μg/ml of kanamycin, since R. anatipestifer was naturally resistant to kanamycin (36), and incubated at 37°C overnight. The growing bacteria on plates were identified using PCR to amplify the specific 16S rRNA gene of R. anatipestifer. At least three independent experiments were performed.
G. mellonella larva hemocyte lysis preparation.
G. mellonella larvae were bled by inserting a lancet into the hemocoel. Hemolymph was collected with ice-cold anticoagulant saline. Hemolymph was destroyed by MP FastPrep-24 and then centrifuged to remove the cell debris, and the supernatant was used for cell lysis. The cell lysis was diluted at 1:20 by GCB and passed through a 0.45-μm filter to use.
In vitro growth rate determination.
The in vitro growth rates of the test strains were determined by measuring the OD600 with a spectrophotometer (Eppendorf Biophotometer, Germany). Briefly, early-exponential-phase cultures of R. anatipestifer CH-1 were inoculated into 20 ml of GCB or GCB supplemented with 100 μM EDDHA, GCB together with 100 μM EDDHA supplemented with 1 μM hemoglobin, and GCB together with 100 μM EDDHA supplemented with hemocyte lysate, followed by incubation at 37°C with shaking at 180 rpm. The OD was determined at 600 nm every 2 h for 14 h. The experiment was performed using three independent experiments, with two replicate samples for each experiment.
Growth of R. anatipestifer on solid medium supplemented by different heme sources.
The strain R. anatipestifer CH-1 ΔhemH (OD600 = 1.0) was mixed with soft agar and poured onto the GCB plate. Sterile Whatman discs were impregnated with 10 μl of PBS, duck hemoglobin, or hemocyte lysate of G. mellonella larvae, plated onto agar, and incubated at 37°C. Pictures were taken at 24 h and 72 h. Growth was measured by quantifying the distance between the edge of the disc and the edge of the zone of growth.
Mutagenesis by allelic exchange and trans-complementation.
The deletion of the genes was performed according to the natural transformation-based knockout method as described in a previous study (54). Briefly, the ∼800-bp left flanking sequence and ∼800-bp right flanking sequence of the target gene were amplified by PCR using primers listed in Table S2. The 1,140-bp sequence containing the SpcR cassette was amplified from plasmid pAM238 using primers SpcRP1 and SpcRP2. The PCR fragments (upstream, SpcR cassette, and downstream) were ligated using the overlap PCR method (56). The fused PCR fragments were purified and incubated with 300 μl of R. anatipestifer CH-1 for 1 h at 37°C. Then 100-μl samples of the mixture were plated onto plates supplemented with spectinomycin and incubated overnight at 37°C. Six clones were isolated on selective media and identified using PCR.
To construct the trans-complementation strain, the genes were amplified using the primers listed in Table S2. The fragments were cloned into the shuttle vector pLMF03 using a routine method. The resulting plasmid was introduced into E. coli S17-1. Then it was introduced into R. anatipestifer by conjugation as described in a previous study (57). The strain was selected on blood-containing agar plates supplemented with Kan (20 μg/ml) and Cfx (1 μg/ml). The correct strain was identified using PCR.
Real-time PCR detection of gene expression in R. anatipestifer.
RNA preparations of R. anatipestifer from different growth conditions and real-time PCR were performed as described in a previous study (57). Briefly, RNA was extracted by the RNeasy minikit procedure (Qiagen). First-strand cDNA synthesis was performed with reverse transcriptase (HiScript Q RT SuperMix for qPCR +gDNA wiper, R223-01; Vazyme, Nanjing, China). Real-time PCR was performed with an AceQ quantitative PCR (qPCR) SYBR green master mix (Q111-03; Vazyme) using a CFX connect real-time PCR detection system (Bio-Rad Laboratories, Hercules, CA). Fold change was calculated as described previously (58) with the threshold cycle (ΔΔCT) method considering the efficiency of the PCR for each target. Quantitative measurements were performed on biological samples in triplicate, and the results were normalized to findings with the R. anatipestifer housekeeping gene recA (57).
Real-time PCR detection of gene expression in G. mellonella larvae.
Larvae were infected with approximately 104 CFU of R. anatipestifer CH-1 and R. anatipestifer ATCC 11845; after different time intervals, three larvae were homogenized using MP FastPrep-24 (MP, USA). Total RNA was extracted using RNAiso Plus reagent (9108; TaKaRa, China) according to the manufacturer’s instructions. cDNA was obtained as described above. The transcriptional levels of genes encoding the G. mellonella antimicrobial peptides hemolin, gallerimycin, lysozyme, and anionic peptide were determined with AceQ qPCR SYBR green master mix using a CFX Connect real-time PCR detection system as described above. All samples were analyzed in triplicate, and 18S rRNA genes were amplified as housekeeping genes.
Preimmune activation of G. mellonella larvae and antimicrobial peptide resistance assay.
Larvae were infected with 104 CFU of R. anatipestifer CH-1 or 104 CFU of R. anatipestifer ATCC 11845 to increase the levels of antimicrobial factors in the hemolymph. Then the larvae were challenged by injection with a dose of 107 CFU of CH-1 6 h later. To investigate the cross protection of the strains, 106 CFU of heat-killed (65°C for 15 min) E. coli DH5α cells was injected into larvae and after 24 h, 107 CFU of CH-1 or 1.2 × 108 CFU of ATCC 11845 was injected into each larva for survival counts. To investigate the presence of antimicrobial activities in G. mellonella killing viable CH-1 and ATCC 11845, the larvae were infected with heat-killed E. coli and after 24 h were injured with a sterile needle. Hemolymph pooled from 6 larvae in each group was centrifuged at 6,000 × g for 10 min at 4°C. Then 200 μl of the bacteria-free hemolymph was mixed with 20 μl of CH-1 or ATCC 11845 suspension containing 8 × 105 CFU per ml and the mixture was incubated at 37°C for 0 h, 1 h, and 3 h. Serial dilutions of the mixture were plated on blood plate, and colonies were counted after incubation at 37°C for 24 h. Results are expressed as percentages of the number of bacteria not exposed to antibacterial agents. All measurements were done in triplicate on three separate occasions.
Ethics statement.
One-day-old Pekin ducks were purchased from Grimaud farms in Chengdu (Sichuan, China) and housed at our animal facilities with free access to food and water. This study was carried out in accordance with the recommendations of the local animal welfare bodies and the Sichuan Agricultural University ethics committee. The protocol was approved by the Sichuan Agricultural University ethics committee.
Statistical analysis.
All experimental data are expressed as the mean ± 1 standard deviation (SD). The independent Student t test was utilized to compare two groups, and the one-way analysis of variance (ANOVA) was used to compare multiple groups. Survival data were plotted using the Kaplan-Meier method, and comparisons were made between groups using the log rank test with GraphPad Prism 7.00 (GraphPad Software, CA). A P value of <0.05 was considered significant.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (grant no. 31772772), the China Agricultural Research System (CARS-42-17), the Special Fund for Key Laboratory of Animal Disease and Human Health of Sichuan Province (2016JPT0004), and the Sichuan Veterinary Medicine and Drug Innovation Group of the China Agricultural Research System under grant no. CARS-SVDIP.
We declare that we have no competing interests.
M.L., A.C., and F.B. conceived and designed the experiments; M.H., H.L., D.Z., M.W., Y.L., L.Z., X.C., and Y.Y. performed the experiments; M.W., R.J., S.C., and X.Z. analyzed the data; Y.W., Q.Y., and S.Z. contributed reagents/materials/analysis tools; and M.L., F.B., and A.C. wrote the paper. All authors have reviewed the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00072-19.
REFERENCES
- 1.Falkow S. 1988. Molecular Koch’s postulates applied to microbial pathogenicity. Rev Infect Dis 10(Suppl 2):S274–S276. doi: 10.1093/cid/10.Supplement_2.S274. [DOI] [PubMed] [Google Scholar]
- 2.Glavis-Bloom J, Muhammed M, Mylonakis E. 2012. Of model hosts and man: using Caenorhabditis elegans, Drosophila melanogaster and Galleria mellonella as model hosts for infectious disease research. Adv Exp Med Biol 710:11–17. doi: 10.1007/978-1-4419-5638-5_2. [DOI] [PubMed] [Google Scholar]
- 3.Mahajan-Miklos S, Rahme LG, Ausubel FM. 2000. Elucidating the molecular mechanisms of bacterial virulence using non-mammalian hosts. Mol Microbiol 37:981–988. doi: 10.1046/j.1365-2958.2000.02056.x. [DOI] [PubMed] [Google Scholar]
- 4.Meijer AH, Spaink HP. 2011. Host-pathogen interactions made transparent with the zebrafish model. Curr Drug Targets 12:1000–1017. doi: 10.2174/138945011795677809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trinder M, Daisley BA, Dube JS, Reid G. 2017. Drosophila melanogaster as a high-throughput model for host-microbiota interactions. Front Microbiol 8:751. doi: 10.3389/fmicb.2017.00751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bermudez LE, Rose SJ, Everman JL, Ziaie NR. 2018. Establishment of a host-to-host transmission model for Mycobacterium avium subsp. hominissuis using Caenorhabditis elegans and identification of colonization-associated genes. Front Cell Infect Microbiol 8:123. doi: 10.3389/fcimb.2018.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson R, Chen C, Ratcliffe NA. 1999. Innate immunity in insects: the role of multiple, endogenous serum lectins in the recognition of foreign invaders in the cockroach, Blaberus discoidalis. J Immunol 162:1590–1596. [PubMed] [Google Scholar]
- 8.Hoffmann JA. 1995. Innate immunity of insects. Curr Opin Immunol 7:4–10. doi: 10.1016/0952-7915(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 9.Bergin D, Murphy L, Keenan J, Clynes M, Kavanagh K. 2006. Pre-exposure to yeast protects larvae of Galleria mellonella from a subsequent lethal infection by Candida albicans and is mediated by the increased expression of antimicrobial peptides. Microbes Infect 8:2105–2112. doi: 10.1016/j.micinf.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Seitz V, Clermont A, Wedde M, Hummel M, Vilcinskas A, Schlatterer K, Podsiadlowski L. 2003. Identification of immunorelevant genes from greater wax moth (Galleria mellonella) by a subtractive hybridization approach. Dev Comp Immunol 27:207–215. doi: 10.1016/S0145-305X(02)00097-6. [DOI] [PubMed] [Google Scholar]
- 11.Jonsson R, Struve C, Jenssen H, Krogfelt KA. 2017. The wax moth Galleria mellonella as a novel model system to study enteroaggregative Escherichia coli pathogenesis. Virulence 8:1894–1899. doi: 10.1080/21505594.2016.1256537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heitmueller M, Billion A, Dobrindt U, Vilcinskas A, Mukherjee K. 2017. Epigenetic mechanisms regulate innate immunity against uropathogenic and commensal-like Escherichia coli in the surrogate insect model Galleria mellonella. Infect Immun 85:e00336-17. doi: 10.1128/IAI.00336-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Astvad KMT, Meletiadis J, Whalley S, Arendrup MC. 2017. Fluconazole pharmacokinetics in Galleria mellonella larvae and performance evaluation of a bioassay compared to liquid chromatography-tandem mass spectrometry for hemolymph specimens. Antimicrob Agents Chemother 61:e00895-17. doi: 10.1128/AAC.00895-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Segers P, Mannheim W, Vancanneyt M, De Brandt K, Hinz KH, Kersters K, Vandamme P. 1993. Riemerella anatipestifer gen. nov., comb. nov., the causative agent of septicemia anserum exsudativa, and its phylogenetic affiliation within the Flavobacterium-Cytophaga rRNA homology group. Int J Syst Bacteriol 43:768–776. doi: 10.1099/00207713-43-4-768. [DOI] [PubMed] [Google Scholar]
- 15.Hess C, Enichlmayr H, Jandreski-Cvetkovic D, Liebhart D, Bilic I, Hess M. 2013. Riemerella anatipestifer outbreaks in commercial goose flocks and identification of isolates by MALDI-TOF mass spectrometry. Avian Pathol 42:151–156. doi: 10.1080/03079457.2013.775401. [DOI] [PubMed] [Google Scholar]
- 16.Pathanasophon P, Sawada T, Tanticharoenyos T. 1995. New serotypes of Riemerella anatipestifer isolated from ducks in Thailand. Avian Pathol 24:195–199. doi: 10.1080/03079459508419059. [DOI] [PubMed] [Google Scholar]
- 17.Pathanasophon P, Phuektes P, Tanticharoenyos T, Narongsak W, Sawada T. 2002. A potential new serotype of Riemerella anatipestifer isolated from ducks in Thailand. Avian Pathol 31:267–270. doi: 10.1080/03079450220136576. [DOI] [PubMed] [Google Scholar]
- 18.Zhong CY, Cheng AC, Wang MS, Zhu DK, Luo QH, Zhong CD, Li L, Duan Z. 2009. Antibiotic susceptibility of Riemerella anatipestifer field isolates. Avian Dis 53:601–607. doi: 10.1637/8552-120408-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Wang MS, Liu MF, Zhu DK, Biville F, Jia RY, Chen S, Sun KF, Yang Q, Wu Y, Zhao XX, Chen XY, Cheng AC. 2017. Contribution of RaeB, a putative RND-type transporter to aminoglycoside and detergent resistance in Riemerella anatipestifer. Front Microbiol 8:2435. doi: 10.3389/fmicb.2017.02435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu DK, Luo HY, Liu MF, Zhao XX, Jia RY, Chen S, Sun KF, Yang Q, Wu Y, Chen XY, Cheng AC, Wang MS. 2018. Various profiles of tet genes addition to tet(X) in Riemerella anatipestifer isolates from ducks in China. Front Microbiol 9:585. doi: 10.3389/fmicb.2018.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo HY, Liu MF, Wang MS, Zhao XX, Jia RY, Chen S, Sun KF, Yang Q, Wu Y, Chen XY, Biville F, Zou YF, Jing B, Cheng AC, Zhu DK. 2018. A novel resistance gene, lnu(H), conferring resistance to lincosamides in Riemerella anatipestifer CH-2. Int J Antimicrob Agents 51:136–139. doi: 10.1016/j.ijantimicag.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 22.Hu Q, Han X, Zhou X, Ding C, Zhu Y, Yu S. 2011. OmpA is a virulence factor of Riemerella anatipestifer. Vet Microbiol 150:278–283. doi: 10.1016/j.vetmic.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 23.Wang M, Zhang P, Zhu D, Wang M, Jia R, Chen S, Sun K, Yang Q, Wu Y, Chen X, Biville F, Cheng A, Liu M. 2017. Identification of the ferric iron utilization gene B739_1208 and its role in the virulence of R. anatipestifer CH-1. Vet Microbiol 201:162–169. doi: 10.1016/j.vetmic.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 24.Liu M, Huang M, Shui Y, Biville F, Zhu D, Wang M, Jia R, Chen S, Sun K, Zhao X, Yang Q, Wu Y, Chen X, Cheng A. 2018. Roles of B739_1343 in iron acquisition and pathogenesis in Riemerella anatipestifer CH-1 and evaluation of the RA-CH-1DeltaB739_1343 mutant as an attenuated vaccine. PLoS One 13:e0197310. doi: 10.1371/journal.pone.0197310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dou Y, Wang X, Yu G, Wang S, Tian M, Qi J, Li T, Ding C, Yu S. 2017. Disruption of the M949_RS01915 gene changed the bacterial lipopolysaccharide pattern, pathogenicity and gene expression of Riemerella anatipestifer. Vet Res 48:6. doi: 10.1186/s13567-017-0409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Ding C, Wang S, Han X, Hou W, Yue J, Zou J, Yu S. 2014. The AS87_04050 gene is involved in bacterial lipopolysaccharide biosynthesis and pathogenicity of Riemerella anatipestifer. PLoS One 9:e109962. doi: 10.1371/journal.pone.0109962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yi H, Yuan B, Liu J, Zhu D, Wu Y, Wang M, Jia R, Sun K, Yang Q, Chen S, Liu M, Chen X, Cheng A. 2017. Identification of a wza-like gene involved in capsule biosynthesis, pathogenicity and biofilm formation in Riemerella anatipestifer. Microb Pathog 107:442–450. doi: 10.1016/j.micpath.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Liu W, Zhu D, Yang L, Liu M, Yin S, Wang M, Jia R, Chen S, Sun K, Cheng A, Chen X. 2014. Comparative genomics of Riemerella anatipestifer reveals genetic diversity. BMC Genomics 15:479. doi: 10.1186/1471-2164-15-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brochado AR, Telzerow A, Bobonis J, Banzhaf M, Mateus A, Selkrig J, Huth E, Bassler S, Beas JZ, Zietek M, Ng N, Foerster S, Ezraty B, Py B, Barras F, Savitski MM, Bork P, Gottig S, Typas A. 2018. Species-specific activity of antibacterial drug combinations. Nature 559:259–263. doi: 10.1038/s41586-018-0278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo Y, Hu D, Guo J, Li X, Guo J, Wang X, Xiao Y, Jin H, Liu M, Li Z, Bi D, Zhou Z. 2017. The role of the regulator fur in gene regulation and virulence of Riemerella anatipestifer assessed using an unmarked gene deletion system. Front Cell Infect Microbiol 7:382. doi: 10.3389/fcimb.2017.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cassat JE, Skaar EP. 2013. Iron in infection and immunity. Cell Host Microbe 13:509–519. doi: 10.1016/j.chom.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choby JE, Skaar EP. 2016. Heme synthesis and acquisition in bacterial pathogens. J Mol Biol 428:3408–3428. doi: 10.1016/j.jmb.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dailey HA, Dailey TA, Gerdes S, Jahn D, Jahn M, O’Brian MR, Warren MJ. 2017. Prokaryotic heme biosynthesis: multiple pathways to a common essential product. Microbiol Mol Biol Rev 81:e00048-16. doi: 10.1128/MMBR.00048-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu M, Huang M, Zhu D, Wang M, Jia R, Chen S, Sun K, Yang Q, Wu Y, Biville F, Cheng A. 2017. Identifying the genes responsible for iron-limited condition in Riemerella anatipestifer CH-1 through RNA-Seq-based analysis. Biomed Res Int 2017:8682057. doi: 10.1155/2017/8682057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu M, Huang Y, Liu J, Biville F, Zhu D, Wang M, Jia R, Chen S, Zhao X, Yang Q, Wu Y, Zhang S, Chen X, Liu Y, Zhang L, You Y, Yu Y, Cheng A. 2018. Multiple genetic tools for editing the genome of Riemerella anatipestifer using a counterselectable marker. Appl Microbiol Biotechnol 102:7475–7488. doi: 10.1007/s00253-018-9181-4. [DOI] [PubMed] [Google Scholar]
- 36.Liao H, Cheng X, Zhu D, Wang M, Jia R, Chen S, Chen X, Biville F, Liu M, Cheng A. 2015. TonB energy transduction systems of Riemerella anatipestifer are required for iron and hemin utilization. PLoS One 10:e0127506. doi: 10.1371/journal.pone.0127506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miao S, Xing L, Qi J, Yu H, Jiang P, Sun B, Cui J, Ou C, Hu Q. 2015. Roles of the TonB1 and TonB2 proteins in haemin iron acquisition and virulence in Riemerella anatipestifer. Microbiology 161:1592–1599. doi: 10.1099/mic.0.000123. [DOI] [PubMed] [Google Scholar]
- 38.Bokhari H, Ali A, Noreen Z, Thomson N, Wren BW. 2017. Galleria mellonella is low cost and suitable surrogate host for studying virulence of human pathogenic Vibrio cholerae. Gene 628:1–7. doi: 10.1016/j.gene.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 39.Hill L, Veli N, Coote PJ. 2014. Evaluation of Galleria mellonella larvae for measuring the efficacy and pharmacokinetics of antibiotic therapies against Pseudomonas aeruginosa infection. Int J Antimicrob Agents 43:254–261. doi: 10.1016/j.ijantimicag.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Kavanagh K, Reeves EP. 2004. Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiol Rev 28:101–112. doi: 10.1016/j.femsre.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Uehlinger S, Schwager S, Bernier SP, Riedel K, Nguyen DT, Sokol PA, Eberl L. 2009. Identification of specific and universal virulence factors in Burkholderia cenocepacia strains by using multiple infection hosts. Infect Immun 77:4102–4110. doi: 10.1128/IAI.00398-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jander G, Rahme LG, Ausubel FM. 2000. Positive correlation between virulence of Pseudomonas aeruginosa mutants in mice and insects. J Bacteriol 182:3843–3845. doi: 10.1128/jb.182.13.3843-3845.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loh JM, Adenwalla N, Wiles S, Proft T. 2013. Galleria mellonella larvae as an infection model for group A streptococcus. Virulence 4:419–428. doi: 10.4161/viru.24930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown ED, Wright GD. 2016. Antibacterial drug discovery in the resistance era. Nature 529:336–343. doi: 10.1038/nature17042. [DOI] [PubMed] [Google Scholar]
- 45.Huang L, Yuan H, Liu MF, Zhao XX, Wang MS, Jia RY, Chen S, Sun KF, Yang Q, Wu Y, Chen XY, Cheng AC, Zhu DK. 2017. Type B chloramphenicol acetyltransferases are responsible for chloramphenicol resistance in Riemerella anatipestifer, China. Front Microbiol 8:297. doi: 10.3389/fmicb.2017.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meir M, Grosfeld T, Barkan D. 2018. Establishment and validation of Galleria mellonella as a novel model organism to study Mycobacterium abscessus infection, pathogenesis, and treatment. Antimicrob Agents Chemother 62:e02539-17. doi: 10.1128/AAC.02539-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martinez MR, Wiedmann M, Ferguson M, Datta AR. 2017. Assessment of Listeria monocytogenes virulence in the Galleria mellonella insect larvae model. PLoS One 12:e0184557. doi: 10.1371/journal.pone.0184557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Viegas SC, Mil-Homens D, Fialho AM, Arraiano CM. 2013. The virulence of Salmonella enterica serovar Typhimurium in the insect model Galleria mellonella is impaired by mutations in RNase E and RNase III. Appl Environ Microbiol 79:6124–6133. doi: 10.1128/AEM.02044-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Velikova N, Kavanagh K, Wells JM. 2016. Evaluation of Galleria mellonella larvae for studying the virulence of Streptococcus suis. BMC Microbiol 16:291. doi: 10.1186/s12866-016-0905-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Si M, Wang Y, Zhang B, Zhao C, Kang Y, Bai H, Wei D, Zhu L, Zhang L, Dong TG, Shen X. 2017. The type VI secretion system engages a redox-regulated dual-functional heme transporter for zinc acquisition. Cell Rep 20:949–959. doi: 10.1016/j.celrep.2017.06.081. [DOI] [PubMed] [Google Scholar]
- 51.Schaible UE, Kaufmann SH. 2004. Iron and microbial infection. Nat Rev Microbiol 2:946–953. doi: 10.1038/nrmicro1046. [DOI] [PubMed] [Google Scholar]
- 52.Giannouli M, Palatucci AT, Rubino V, Ruggiero G, Romano M, Triassi M, Ricci V, Zarrilli R. 2014. Use of larvae of the wax moth Galleria mellonella as an in vivo model to study the virulence of Helicobacter pylori. BMC Microbiol 14:228. doi: 10.1186/s12866-014-0228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nichol H, Law JH, Winzerling JJ. 2002. Iron metabolism in insects. Annu Rev Entomol 47:535–559. doi: 10.1146/annurev.ento.47.091201.145237. [DOI] [PubMed] [Google Scholar]
- 54.Liu M, Zhang L, Huang L, Biville F, Zhu D, Wang M, Jia R, Chen S, Sun K, Yang Q, Wu Y, Chen X, Cheng A. 2017. Use of natural transformation to establish an easy knockout method in Riemerella anatipestifer. Appl Environ Microbiol 83:e00127-17. doi: 10.1128/AEM.00127-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kan P, Lee CJ. 1994. Application of aqueous two-phase systems in separation/purification of stroma free hemoglobin from animal blood. Artif Cells Blood Substit Immobil Biotechnol 22:641–649. doi: 10.3109/10731199409117894. [DOI] [PubMed] [Google Scholar]
- 56.Xiong AS, Yao QH, Peng RH, Duan H, Li X, Fan HQ, Cheng ZM, Li Y. 2006. PCR-based accurate synthesis of long DNA sequences. Nat Protoc 1:791–797. doi: 10.1038/nprot.2006.103. [DOI] [PubMed] [Google Scholar]
- 57.Liu M, Wang M, Zhu D, Wang M, Jia R, Chen S, Sun K, Yang Q, Wu Y, Chen X, Biville F, Cheng A. 2016. Investigation of TbfA in Riemerella anatipestifer using plasmid-based methods for gene over-expression and knockdown. Sci Rep 6:37159. doi: 10.1038/srep37159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.