The major problem with Chagas disease is evolution of the chronic indeterminate form to a progressive cardiac disease. Treatment diminishes parasitemia but not clinical progression, and the immunological features involved are unclear. Here, we studied the clinical course and the immune response in patients with chronic-phase Chagas disease at 48 months after benznidazole treatment.
KEYWORDS: Chagas disease, T cells, benznidazole
ABSTRACT
The major problem with Chagas disease is evolution of the chronic indeterminate form to a progressive cardiac disease. Treatment diminishes parasitemia but not clinical progression, and the immunological features involved are unclear. Here, we studied the clinical course and the immune response in patients with chronic-phase Chagas disease at 48 months after benznidazole treatment. Progression to the cardiac form of Chagas disease or its aggravation was associated with higher in vitro antigen-specific production of interferon gamma (IFN-γ) in patients with cardiac Chagas disease than in patients with the indeterminate form. Predominance of IFN-γ production over interleukin-10 (IL-10) production in antigen-specific cultures was associated with cardiac involvement. Significantly higher numbers of antigen-specific T helper 1 cells (T-Bet+ IFN-γ+) and a significantly higher IFN-γ+/IL-10+ ratio were observed in patients with cardiac Chagas disease than in patients with the indeterminate form. Cardiac damage was associated with higher numbers of T helper cells than cytotoxic T lymphocytes producing IFN-γ. Patients with cardiac Chagas disease had predominant CD25− and CD25low T regulatory (Treg) subpopulations, whereas patients with the indeterminate form manifested a higher relative mean percentage of CD25high Treg subpopulations. These findings suggest that at 48 months after benznidazole treatment, the disease can worsen or progress to the cardiac form. The progression may be related to increased IFN-γ production (mostly from CD4+ T cells) relative to IL-10 production and increased Treg percentages. Patients with the indeterminate form of Chagas disease show a more balanced ratio of proinflammatory and anti-inflammatory cytokines.
INTRODUCTION
Chagas disease is a parasitic disease caused by infection with the hemoflagellate Trypanosoma cruzi. It remains a leading neglected tropical disease in America and is associated with 546,000 disability-adjusted life years and 10,300 annual deaths. Approximately 7.5 million people are living with Chagas disease, mostly in impoverished regions of Central and South America, although thousands of T. cruzi-infected individuals have migrated to the United States and various European countries (1–4). Despite important achievements with respect to the transmission control measures aimed at reducing the disease incidence (Southern Cone Initiative to Control/Eliminate Chagas Disease), Brazil still shows 1.9 million cases of chronic Chagas disease (3, 5).
Regardless of the existence and importance of the acute phase, which is predominantly short and has nonspecific symptoms, the major problem with Chagas disease is the development of chronic manifestations after various durations of the indeterminate form of this disease, during which the patients can progressively develop cardiac and/or digestive dysfunctions (determinate forms). Cardiac manifestations lead to a progressive, irreversible, and disabling form of cardiomyopathy, culminating in congestive heart failure, which is the major cause of death among such individuals in areas of endemicity (6, 7).
Mechanisms underlying this progression include host immune response quality and intensity, parasite persistence, and T. cruzi genotypes (8–11). A sustained inflammatory response featuring progressive tissue damage and fibrosis-related repair leads to chronic cardiac disability due to destruction and rearrangement of cardiomyocytes, microvasculature damage, and contractile alterations (12). Murine reinfections by T. cruzi are associated with cardiac damage and higher levels of inflammatory cytokines (13). In humans, the cellular immune response pattern seems to be related, at least in part, to the clinical forms. Although patients with the indeterminate form usually present a more regulated immune response, patients with cardiac Chagas disease show a more intense proinflammatory pattern (14–16). Patients with more severe cardiac damage manifest greater production of antigen-specific interferon gamma (IFN-γ) (17), and the cardiac milieu features the predominance of IFN-γ-producing T-cell clones (16).
Specific antiparasite chemotherapy is recommended in acute Chagas disease because this modality can reduce parasitemia and prevent disease progression (18–21). However, in patients with established Chagas cardiomyopathy, benznidazole treatment can significantly reduce the counts of detectable circulating parasites but does not reduce cardiac clinical progression (22). Benznidazole treatment during the chronic phase can simultaneously induce proinflammatory cytokines and interleukin-10 (IL-10), indicating a double effect in the control of parasite replication and tissue damage (23).
The mechanisms underlying the control and establishment of immunoregulatory networks in patients with chronic Chagas disease remain to be investigated in the context of Chagas disease (8, 24). Patients with chronic Chagas disease living in areas of endemicity still lack an effective antiparasite treatment in the current health care settings. We tested the hypothesis that benznidazole therapy affects the long-term clinical progression and immunological pattern associated with staying with the stable indeterminate form or progression to cardiac damage.
RESULTS
Progression to the cardiac form of Chagas disease after benznidazole treatment in the chronic phase is associated with predominance of antigen-specific IFN-γ over IL-10.
Among several factors that influence the progression of Chagas disease (such as parasite load, exposure time, and reexposure) and factors inherent to the host and parasite genetics, the host immune response is important, in particular, the pattern of the cellular immune response. Therefore, evaluating the production of cytokines by T lymphocytes may reveal characteristics that indicate immunological mechanisms associated with the different clinical forms that developed after benznidazole treatment. Accordingly, we evaluated the production of IFN-γ, IL-4, IL-17, and IL-10 in cultures of peripheral blood mononuclear cells (PBMCs) maintained without stimulation or in the presence of T. cruzi antigens.
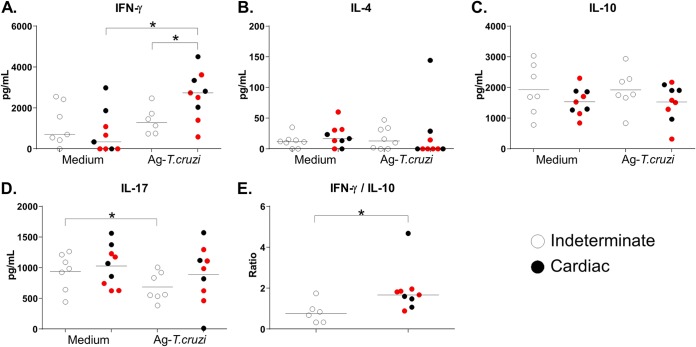
Progression to the cardiac form of Chagas disease or aggravation of the cardiac form was associated with higher levels of in vitro antigen-specific production of IFN-γ than were seen with patients with the indeterminate form (P = 0.03, unpaired t test). Cultures of specimens from patients with cardiac Chagas disease displayed an incremental change in IFN-γ antigen-specific production levels compared to unstimulated cultures, whereas those from patients with the indeterminate form did not (P = 0.0039; Wilcoxon’s test) (Fig. 1A). We did not observe differences in IL-4 production levels among culture conditions or among the clinical forms of the disease (Fig. 1B). Patients with the indeterminate form showed inhibited production of IL-17 in antigen-stimulated cultures (P = 0.041 [Wilcoxon’s test; T. cruzi antigen versus medium]) (Fig. 1C). Analysis of the levels of IL-10, a classic immunoregulatory cytokine, did not reveal statistically significant differences between clinical conditions after benznidazole treatment, although a trend of lower levels of antigen-specific production was observed in patients with cardiac Chagas disease (P = 0.22; Mann-Whitney test) (Fig. 1D). The final result of a multiple-cytokine production scenario can be defined by the individual concentrations of the cytokines and also by the relations among their production mechanisms. Therefore, to evaluate the balance between the levels of proinflammatory and anti-inflammatory cytokines in different clinical forms of Chagas disease after antiparasitic therapy, we determined the IFN-γ/IL-10 ratio in each patient. IFN-γ production predominated over IL-10 production in the antigen-specific cultures associated with cardiac involvement (P = 0.012; Mann-Whitney test) (Fig. 1E).
FIG 1.
(A to D) Production of IFN-γ (A), IL-4 (B), IL-10 (C), and IL-17 (D) by PBMCs cultured without a stimulus (Medium) or with T. cruzi antigens (4 μg/ml; Ag-T.cruzi) for 72 h at 37°C in a humidified atmosphere with 5% CO2. In panel E, we present the ratio of IFN-γ production to IL-10 production under the Ag-Tc culture conditions. For the patients with cardiac Chagas disease (n = 9), red dots represent patients who progressed from the indeterminate form to the cardiac form or worsened at 48 months after benznidazole treatment (n = 5). Horizontal lines represent the median. *, P < 0.05 (Mann-Whitney or Wilcoxon’s test).
Patients with the cardiac form of Chagas disease treated with benznidazole manifest predominance of IFN-γ over IL-10 and regulatory T cells (Tregs).
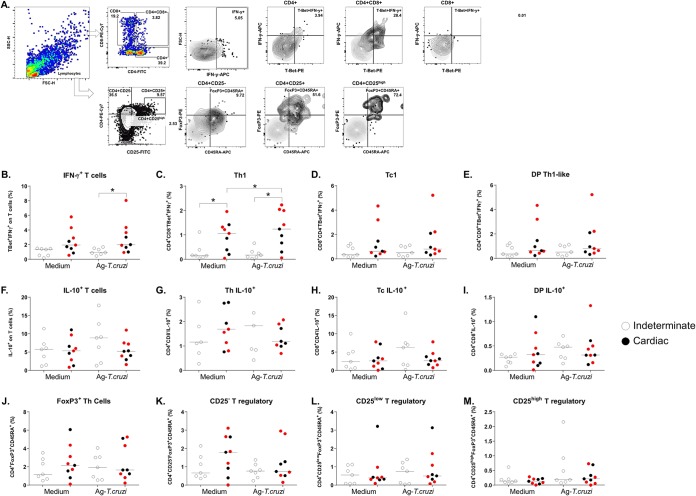
Differing levels of production of cytokines derived from T lymphocytes between patients with the indeterminate and cardiac clinical forms of Chagas disease as well as worsening clinical status after benznidazole therapy were noted. To determine the major T-cell sources of the cytokines, we simultaneously investigated the percentages of cytokine-producing T cells among PBMCs. For this purpose, we characterized the immunophenotypic profile of PBMCs maintained without stimulation or in the presence of T. cruzi antigens for CD4+ (T helper cells), CD8+ (cytotoxic T lymphocytes), and CD4+ CD8+ (doubly positive [DP] T cells) populations and according to the transcription factors and cytokines that characterize populations with different functionalities (Th1 and Tc1, Th2 and Tc2, Th17, and Tc17 as well as IL-10 producers and Tregs) by flow cytometry. The data acquisition strategy is depicted in Fig. 2A.
FIG 2.
The percentage of T lymphocytes after PBMC culture with T. cruzi antigens. (A) Gating strategy to determine the levels of IFN-γ and IL-10 expression in CD4+, CD8+, and CD4+ CD8+ DP T cells and in Treg subpopulations. FSC, forward scatter; SSC, side scatter. (B) IFN-γ+ T cells. (C) Th1, T-Bet+ IFN-γ+ in the CD4+ population. (D) Tc1, T-Bet+ IFN-γ+ in the CD8+ population. (E) DP-Th1-like, T-Bet+ IFN-γ+ among DP cells. (F to I) IL-10 expression in the same subpopulations. (J) FoxP3+ T cells. (K) CD4+ CD25− FoxP3+ T cells. (L) CD4+ CD25Low FoxP3+ T cells. (M) CD4+ CD25High FoxP3+ T cells. PBMCs were cultured without a stimulus (Medium) or with T. cruzi antigens (4 μg/ml; Ag-T.cruzi) for 72 h at 37°C in a humidified atmosphere with 5% CO2. Brefeldin A was added for incubation during the last 6 h, and the cells were stained with specific fluorochrome-conjugated antibodies against surface and intracellular molecules. Among the patients with cardiac Chagas disease (n = 9), red dots represent patients who progressed from the indeterminate form to the cardiac form or worsened at 48 months after benznidazole treatment (n = 5). Horizontal lines represent the median. *, P < 0.05 (Mann-Whitney or Wilcoxon’s test).
The percentages of T cells expressing T-Bet and IFN-γ differed significantly between patients with cardiac and indeterminate Chagas disease in antigen-specific cultures (P = 0.0079; Mann-Whitney test) (Fig. 2B). In particular, patients with cardiac Chagas disease displayed significantly more cells with a Th1 profile (T-Bet+ IFN-γ+) in antigen-stimulated cultures than in unstimulated cultures (P = 0.039; Wilcoxon’s test) (Fig. 2C). Patients with cardiac Chagas disease also showed significant differences in the percentages of Th1 cells under both culture conditions (P = 0.038 [Mann-Whitney test] and P = 0.0062 [unpaired t test]) (Fig. 2C). There were no differences seen with Tc1 cells (percentage of T-Bet+ IFN-γ+ among CD8+ cells) (Fig. 2D) or with respect to the Th1/Tc1-like phenotype among DP T cells (CD4+ CD8+) (Fig. 1E). Furthermore, no differences were evident in Th2/Tc2 or Th17/Tc17 comparisons among T cells or in IL-10 expression levels in T cells and the cell subpopulations. However, under all conditions, patients with cardiac Chagas disease showed a trend toward a lower percentage of IL-10+ cells in antigen-stimulated cultures than did patients with the indeterminate form, in a pattern similar to that seen with secreted IL-10 (Fig. 2F to I). Because Tregs comprise several subpopulations, analyses were performed on the major cell population expressing transcription factor FoxP3 and surface marker CD45RA (effector Tregs) and on three subpopulations (CD4+ CD25−, CD4+ CD25+, and CD4+ CD25high) on the basis of CD25 expression. We did not find any significant differences among the evaluated T cells expressing FoxP3 and their subpopulations (Fig. 2J to M).
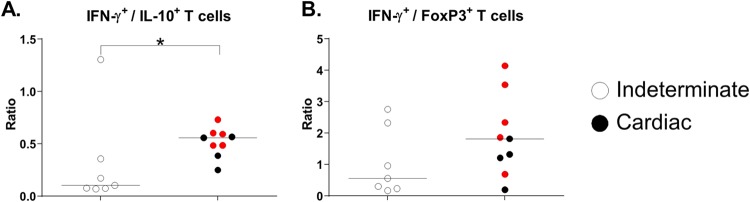
On the basis of these results, we decided to investigate the relation between the global T-cell expression levels of IFN-γ and IL-10 or Treg patterns (FoxP3+) in antigen-stimulated cultures. The IFN-γ+/IL-10+ ratio was significantly higher in patients with cardiac Chagas disease than in patients with the indeterminate form (P = 0.022; Mann-Whitney test) (Fig. 3A). In addition, patients with cardiac Chagas disease manifested no difference in levels of IFN-γ+/FoxP3+ cells (Fig. 3B) but showed a trend toward a higher ratio than was seen with patients with the indeterminate form.
FIG 3.
Ratios of (A) IFN-γ+ to IL-10+ T cells and (B) IFN-γ+ to Tregs (FoxP3+). PBMCs were cultured with T. cruzi antigens (4 μg/ml; Ag-Tc) for 72 h at 37°C in a humidified atmosphere with 5% CO2. Brefeldin A was added for incubation during the last 6 h, and the cells were stained with appropriate fluorochrome-conjugated antibodies against surface and intracellular molecules. Among the patients with cardiac Chagas disease (n = 9), red dots represent patients who progressed from the indeterminate form to the cardiac form or worsened at 48 months after benznidazole treatment (n = 5). Horizontal lines represent the median. *, P < 0.05 (Mann-Whitney test).
These results collectively indicated that patients with indeterminate and cardiac forms of Chagas disease have significant differences in antigen-specific T cells and their subpopulations expressing IFN-γ. Furthermore, despite the lack of differences in the percentages of global IL-10+ cells and Tregs, individual evaluation of the ratio of these populations revealed a strong bias toward IFN-γ-producing cells.
Patients with the cardiac and indeterminate forms of Chagas disease treated with benznidazole display different T-cell sources of antigen-specific IFN-γ and Treg subpopulations.
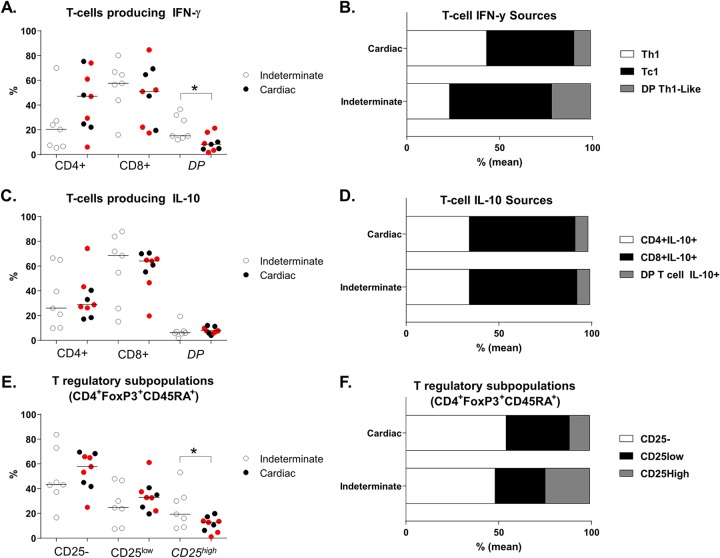
According to the percentages obtained in the analysis of the three major T cell populations (T helper cells, cytotoxic T lymphocytes, and DP T cells), we determined the contribution of each population to the global expression levels of IFN-γ and IL-10 and the distribution of Treg subpopulations in antigen-stimulated cultures. A significant difference was evident in the distribution of T-cell sources in terms of the mean percentages of IFN-γ-producing T cells. The patients with cardiac Chagas disease manifested a greater prevalence of T helper cells producing IFN-γ, with percentages similar to those determined for the cytotoxic T lymphocytes, whereas the patients with the indeterminate form displayed a clear predominance of cytotoxic T lymphocytes and more DP Th-like cells (P = 0.0032; χ2 test) (Fig. 4A and B). Despite the trend toward the lower prevalence of IL-10-producing T cells in patients with cardiac Chagas disease, the relative mean percentages seen under the two clinical conditions were almost identical (P = 0.99; χ2 test) (Fig. 4C and D). Furthermore, there were differences in Treg population composition: the patients with cardiac Chagas disease showed a predominance of CD25− and CD25low Treg subpopulations, whereas the patients with the indeterminate form displayed a higher relative mean percentage of CD25high Tregs (P = 0.05; χ2 test) (Fig. 4E and F).
FIG 4.
(A and B) T-cell sources of IFN-γ. (C and D) T-cell sources of IL-10 in CD4+, CD8+, and DP T populations. (E and F) T-cell sources of FoxP3+ Treg subpopulations after stimulation with T. cruzi antigens. PBMCs were cultured with T. cruzi antigens (4 μg/ml; Ag-Tc) for 72 h at 37°C in a humidified atmosphere with 5% CO2. Brefeldin A was added for incubation during the last 6 h, and the cells were stained with appropriate fluorochrome-conjugated antibodies against surface and intracellular molecules. Among the patients with cardiac Chagas disease (n = 9), red dots represent patients who progressed from the indeterminate form to the cardiac form or worsened at 48 months after benznidazole treatment (n = 5). Horizontal lines represent the median. *, P < 0.05 (Mann-Whitney test or χ2 test).
DISCUSSION
Knowledge of the immune response and cytokine production patterns in patients with Chagas disease is a cornerstone for identifying the clinical course, which can be unpredictable, and enables identification of cells and molecules that are associated with a better prognosis. The resistance or susceptibility to T. cruzi infection may depend on the cytokine profile, with Th1 cytokines (IFN-γ, tumor necrosis factor alpha [TNF-α], and IL-12) promoting resistance (25–28) and regulatory or Th2 cytokines (IL-10, IL-4, and transforming growth factor β [TGF-β]) promoting susceptibility (29).
In this study, some patients with chronic Chagas disease treated with benznidazole progressed from the indeterminate form to the cardiac form or manifested worsened clinical status. In the BENEFIT study, antiparasite treatment significantly reduced parasitemia but did not change with respect to clinical deterioration after 5 years of follow-up (24). Thus, the findings of our study reinforce those of the BENEFIT study and provide stronger evidence of the importance of cellular immunity. In our study, an increase in T. cruzi–specific IFN-γ production was evident, as was the greater percentage of antigen-specific T cells producing IFN-γ, specifically, Th1 cells associated with the cardiac form. In addition, these patients displayed a trend toward lower production of IL-10 and lower percentages of T cells expressing this cytokine after antigen stimulation. This relation between IFN-γ and IL-10 became even more clearly evident when we evaluated the cytokine ratio or positive-cell percentage ratio, where the indeterminate form was associated with a more highly regulated milieu, whereas patients with cardiac Chagas disease clearly showed a predominance of IFN-γ over IL-10. Progression to the cardiac form likely involved tissue damage that was mediated by IFN-γ. The role of IFN-γ and its relationship with regulatory cytokines in infection control and cardiomyopathy progression have been widely discussed; in several studies, higher levels of IFN-γ were found to lead to a more rapid progression to determinate forms, and higher IL-10 levels favored stabilization of the indeterminate form, probably owing to the immunoregulatory status (15, 17, 30). Besides, the maintenance of functional T cells has been discussed as a mechanism of control over parasite replication without tissue damage; in this sense, IL-10 has emerged as a control factor that balances Th1 action and preserves cardiac function (31), even in patients with the cardiac form (32).
Higher IFN-γ levels have been associated with therapeutic efficacy (33, 34), and incremental changes in IFN-γ and TNF-α levels have been associated with lower IL-10 and IL-4 levels in the cardiac form (14, 16, 17, 35, 36). Other studies have suggested the induction of natural killer cells with a mixed Th1/Th2 pattern (IFN-γ, TNF-α, and IL-4) (23, 37) or inhibited synthesis of IL-12 and TNF-α (38). Other studies have revealed no differences in cytokine production among the chronic forms of Chagas disease (39, 40). The data collectively highlight the complexity of the relationship between the immune system and clinical status in patients with Chagas disease. According to the classical concept in this field, in chagasic infection, T helper and cytotoxic T lymphocytes are the major cellular sources of IFN-γ, which activates macrophages, which in turn destroy internalized parasites, inducing T. cruzi–specific antibodies (41, 42). Furthermore, beyond IFN-γ production, cytotoxic T lymphocytes have an important role in the destruction of infected cells (43). Here, we demonstrated that patients with the indeterminate form of Chagas disease showed a predominance of cytotoxic T lymphocytes over T helper cells as a source of IFN-γ (a ratio of approximately 3:1) and a relevant percentage of DP IFN-γ+ T cells (approximately 10% of all IFN-γ+ T cells). Previous reports uncovered an association of greater numbers of antigen-specific cytotoxic T lymphocytes and the indeterminate form, especially with a memory phenotype (44, 45). However, other reports associate IFN-γ+ cytotoxic T lymphocytes with cardiac Chagas disease (46). Benznidazole has been found to be associated with increased IFN-γ expression in infants after 1 year of treatment (23) and with a higher percentage of TNF-α+ cytotoxic T lymphocytes and IFN-γ+ cytotoxic T lymphocytes in adults with no evidence of cardiac Chagas disease (47). According to the finding that the indeterminate form is associated with lower production of IFN-γ, we believe that cytotoxic T lymphocytes are more extensively involved in parasite control and in elimination of parasite cells than in continuous production of IFN-γ.
Much attention has been given to the role of Treg subsets in chronic manifestations of Chagas disease. Here, we did not observe significant differences in the percentage of Tregs, either in the global evaluation of their signature transcription factor (FoxP3) or in Treg subsets (based on CD25 expression in CD25−, CD25low, and CD25high T helper cells), between patients with cardiac Chagas disease and patients with the indeterminate form. However, as in the case of the IFN-γ+/IL-10+ ratio, we observed a trend toward an imbalance between IFN-γ–expressing and Foxp3-expressing T cells in patients with cardiac Chagas disease. Several studies have pointed out that in the indeterminate form, a Th1 pattern is accompanied by regulatory mechanisms (37, 48–53). In fact, in a murine model of T. cruzi infection, inhibition of Treg function leads to more severe myocarditis and greater mortality that was probably secondary to reductions in the levels of IL-10 and TGF-β (54). In humans, the indeterminate form has been associated with higher numbers of peripheral-blood and cardiac CD4+ CD25high FoxP3+ cells. Other studies also indicated Treg participation in the prevention of excessive immune stimulation and consequent cardiac dysfunction and/or mortality (55, 56).
In addition to the lack of a statistically significant difference in the Treg percentages, we observed different distributions of Treg subpopulations between patients with cardiac Chagas disease and patients with the indeterminate form. The indeterminate form was associated with a higher proportion of CD25high Tregs but lower proportions of CD25− or CD25low Tregs than the cardiac form was. Some reports indicate that different Treg subpopulations use different major mechanisms to control the immune response, such as a contact-dependent mechanism in CD25− cells and cytokine production in CD25+ cells. Furthermore, among CD25+ Tregs, CD25high FoxP3+ cells have a more highly suppressive function, which might be involved in differential antigen-specific cytokine production in patients with cardiac Chagas disease versus patients with the indeterminate form.
T cells that simultaneously express CD4 and CD8 (DP T cells) have emerged as important cytokine sources. However, their importance in infectious diseases is unclear. The present findings indicate that besides the absence of the difference in the percentages of DP cells that express IFN-γ or IL-10, in the patients with the indeterminate form of Chagas disease, higher proportions of these cells are committed to antigen-specific IFN-γ expression (mean of 21% versus 9%).
This report provides a more complete picture of the production of T-cell-derived cytokines and the percentages of major T-cell subpopulations after in vitro antigenic stimulation in patients with chronic-phase Chagas disease at 4 years after benznidazole treatment. Taken together, our results show that this treatment does not stop disease evolution, and patients who progress to the cardiac form, or who experience aggravation of the cardiac form, show bias in their inflammatory immune response, with more IFN-γ and Th1 cells and an increased IFN-γ/IL-10 ratio. The maintenance of the indeterminate form was associated with lower IFN-γ production and fewer IFN-γ+ cells, a higher proportion of cytotoxic T lymphocytes as an IFN-γ source, inflammatory/anti-inflammatory ratios favoring IL-10 and Tregs, and a higher proportion of CD25high Tregs. Thus, benznidazole treatment seems to increase IFN-γ production but does not modify the progression of clinical forms of Chagas disease.
MATERIALS AND METHODS
Patient population.
Following approval by the Ethics in Research Committee of the Universidade Federal do Triângulo Mineiro (UFTM) Uberaba, Minas Gerais, Brazil (protocol no. 1030), a prospective study was conducted between September 2012 and December 2017. Individuals with positive serology for chronic-stage Chagas disease who visited the Chagas disease Outpatient Service of UFTM and who resided in the city of Uberaba and the region were analyzed. The study population consisted of patients who provided blood samples that showed positive serology for T. cruzi, who were referred by the Hemocenter, and who met the inclusion criteria. These criteria were an age of 18 to 60 years, informed consent to participate in the study, and blood culture and PCR results positive for T. cruzi at the time of the intervention. The exclusion criteria were age under 18 and over 60 years, treatment with benznidazole within the previous 5 years, a lack of consent to participate, blood culture or PCR results negative for T. cruzi, the cardiac form of Chagas disease (New York Heart Association functional class III and IV, corresponding to C and D classification in the 2nd Brazilian Consensus on Chagas Disease [2]) (because these patients had severe cardiomyopathy and long-lasting follow-up was not possible), and treatment with specific medications (including antiarrhythmic drugs, oral contraceptives, hormone replacement, and centrally acting antihypertensive drugs).
Experimental protocol.
Taking of clinical histories and physical examinations were performed and followed the usual preliminary norms. During the selection and formation of the groups, the following tests were carried out: a test of cardiac autonomic function and tests to classify the clinical forms (12-lead electrocardiogram [ECG], echocardiogram, chest X-ray imaging, esophagogram, and a barium enema), serological testing to diagnose infection with T. cruzi (indirect immunofluorescence, indirect hemagglutination, enzyme-linked immunosorbent assay [ELISA]), hemoculture for T. cruzi, and PCR analysis for T. cruzi. T. cruzi infection was confirmed by seropositivity in at least two of the three techniques used. Biochemical tests measuring blood glucose, total cholesterol, high-density lipoprotein cholesterol, triglycerides, urea, creatinine, sodium, potassium, alanine aminotransferase, aspartate aminotransferase, γ glutamyl transferase, total and indirect bilirubin, and hemogram were also conducted.
In accordance with the biochemical results, the patients were analyzed for the presence of other diseases (diabetes, lipid disorders, thyroid disorders, electrolyte imbalances, and kidney and liver function aberrations). Chagas disease was classified using the criteria of the 2nd Brazilian Consensus on Chagas Disease (2).
Between September 2007 and December 2008, 1,254 cases were evaluated. Among these, 123 patients agreed to participate. Of these 123 patients, 39 met the inclusion criteria and did not meet the exclusion criteria. Ultimately, 32 agreed to participate. Benznidazole treatment was initiated (Table 1) and lasted for 60 days; the patients were followed during and after the treatment. Three patients did not complete the treatment: one withdrew consent, and two developed adverse reactions to benznidazole and were removed from the study.
TABLE 1.
Demographic and clinical dataa
| Patient group (mean age [yrs] ± SD) |
Patient ID |
Patient sex |
Clinical form at treatment initiationb |
Clinical formb at 48 mo after treatment |
Progression to cardiac form or cardiac form worsening?b |
Hemoculture for T. cruzi 60 days after treatment |
Hemoculture for T. cruzi 48 mo after treatment |
Age (yrs) at 48 mo after treatment |
|---|---|---|---|---|---|---|---|---|
| 4 males/4 females (53.1 ± 6.6) | P1 | Male | Indeterminate | Indeterminate | No | Negative | Negative | 42 |
| P2 | Female | Indeterminate | Indeterminate | No | Negative | Negative | 53 | |
| P3 | Female | Indeterminate | Indeterminate | No | Negative | Negative | 61 | |
| P4 | Male | Indeterminate | Indeterminate | No | Negative | Negative | 62 | |
| P5 | Male | Indeterminate | Indeterminate | No | Negative | Negative | 56 | |
| P6 | Female | Indeterminate | Indeterminate | No | Negative | Negative | 49 | |
| P7 | Male | Indeterminate | Indeterminate | No | Negative | Negative | 53 | |
| P8 | Female | Indeterminate | Indeterminate | No | Negative | Negative | 49 | |
| 5 males/4 females (55.3 ± 10.6) | P9 | Male | Cardiac | Cardiac | No | Negative | Negative | 66 |
| P10 | Male | Indeterminate | Cardiac | Yes | Negative | Negative | 37 | |
| P11 | Male | Cardiac | Cardiac | No | Negative | Negative | 65 | |
| P12 | Female | Indeterminate | Cardiac | Yes | Negative | Negative | 44 | |
| P13 | Male | Cardiac | Cardiac | Yes | Negative | Negative | 58 | |
| P14 | Female | Cardiac | Cardiac | No | Negative | Negative | 65 | |
| P15 | Female | Indeterminate | Cardiac | Yes | Negative | Negative | 46 | |
| P16 | Male | Cardiac | Cardiac | Yes | Negative | Negative | 62 | |
| P17 | Female | Cardiac | Cardiac | No | Negative | Negative | 55 |
All patients were subjected to the following treatment regimen (according to guidelines from the 2nd Brazilian Consensus on Chagas Disease, 2016): benznidazole administered at 5 mg/kg of body weight for 60 days, three doses/day; maximum daily dose, 300 mg. P value, >0.99 (by Fisher's exact test) for patient sex; P value, 0.62 (by Student’s t test) for age. ID, identifier.
Data were determined according to guidelines from the 2nd Brazilian Consensus on Chagas Disease, 2016.
All the treated patients yielded hemoculture results negative for T. cruzi after 60 days of benznidazole treatment, and all of them remained parasite negative in terms of hemoculture during 48 months of follow-up, thus showing the antiparasite effectiveness of benznidazole. At that time, the studied patients were reclassified, and those with mixed forms of the disease were excluded for rigor of immunological evaluation. Three patients were lost to follow-up, and six patients did not agree to participate again at the final evaluation. The final classification was modified because three patients with the indeterminate form of Chagas disease progressed to the cardiac form, and the cardiac form of the disease worsened in two patients. Thus, our study evaluated eight patients with indeterminate Chagas disease who completed treatment and nine patients with cardiac Chagas disease who completed treatment. Disease progression was determined according to the 2nd Brazilian Consensus on Chagas Disease (2). Demographic and clinical data are presented in Table 1.
Preparation of soluble antigens of T. cruzi.
Epimastigote forms of T. cruzi, Y strain, were cultivated in Schneider medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 2 mM l-glutamine (Gibco, Grand Island, NY, USA), calcium carbonate (Sigma-Aldrich), and 20% inactivated fetal calf serum (Gibco) at 29°C in a humidified atmosphere with 5% CO2. At the end of the logarithmic phase, the parasites were collected, washed three times with sterile phosphate-buffered saline (PBS) (with centrifugation at 800 × g and 4°C), and resuspended in sterile water at a concentration of 108 parasites/ml. The suspension was frozen and thawed five times to lyse the parasites. The final preparation was centrifuged at 10,000 × g at 4°C for 30 min, and the supernatant (protein fraction) was collected and passed through a 0.22-μm-pore-size filter (Millipore, Molsheim, France). Protein concentrations were quantified by the Bradford method (Pierce, Rockford, IL, USA), and the protein fraction was adjusted to a concentration of 5 mg/ml in sterile PBS and stored at −70°C.
Blood collection and isolation and culture of PBMCs.
After the 48-month treatment with benznidazole, venous blood (30 ml) was collected by venipuncture into heparinized tubes. PBMCs were isolated by Ficoll-Hypaque density gradient centrifugation (GE Healthcare, Uppsala, Sweden) at 400 × g and 21°C for 30 min. The cells were resuspended in RPMI 1640 medium (GE Healthcare) containing 50 mM HEPES buffer (Gibco), 10% inactivated fetal calf serum (Gibco), 2 mM l-glutamine (Gibco), 50 mM β-mercaptoethanol (Gibco), and 40 μg/ml gentamicin (Neoquímica, Anápolis, GO, Brazil) to reach a final concentration of 2 × 106 cells/ml. PBMCs were cultured in 24-well microplates (Falcon, San Jose, CA, USA) in the presence of 5 μg/ml T. cruzi antigens or maintained in a culture medium at 37°C in a humidified atmosphere with 5% CO2. The cells were collected after 72 h for immunophenotyping and cytokine titration.
Analysis of transcription factors and intracellular cytokines associated with T-lymphocyte subsets.
For analysis of T-cell profiles, PBMCs cultured for 72 h were resuspended (5 × 105 cells/ml) in Hanks’ medium (Sigma-Aldrich), washed three times (with centrifugation at 400 × g, 4°C, 10 min), and incubated in Hanks’ medium supplemented with 10% of inactivated human AB+ serum. After that, the samples were surface labeled with corresponding antibodies for each T-cell profile. The cells were washed to remove unbound antibodies and then fixed and permeabilized with Cytofix/Cytoperm (BD Biosciences, San Jose, CA, USA) (4°C, 30 min). The samples were labeled intracellularly with the corresponding antibodies for each T-cell profile and washed again to remove unbound antibodies. Finally, the cells were resuspended in PBS containing 2% of paraformaldehyde and stored at 4°C in the dark until acquisition of flow-cytometric data. The samples were labeled with antibodies for T-cell profiles, including Th1/Tc1 (CD4-fluorescein isothiocyanate [FITC], T-Bet–phycoerythrin [T-Bet-PE], CD8-peridinin chlorophyll protein [PerCP], and IFN-γ–allophycocyanin [APC]), Th2/Tc2 (CD4-FITC, GATA-3-PE, CD8-PerCP, and IL-4–APC), Th17 (CD4-FITC, RORγT-PE, CD8-PerCP, and IL-17–APC), and Treg (CD25-FITC, FoxP3-PE, CD4-PerCP, and CD45RA-APC) (BD Biosciences, San Jose, CA, USA). A FACSCalibur cytometer (Becton, Dickinson, Mountain View, CA, USA) was used for the acquisition of events (100,000 events/sample), and the data were analyzed in FlowJo software (Tree Star Inc., Ashland, OR, USA).
Cytokine quantification.
Concentrations of IL-4, IL-10, IFN-γ (BD Biosciences), and IL-17 (R&D Systems, Minneapolis, MN, USA) in culture supernatants were measured by ELISAs using pairs of monoclonal antibodies, in accordance with the manufacturer’s specifications. Briefly, wells of high-affinity 96-well plates (Nunc, Roskilde, Denmark) were coated with cytokine-specific monoclonal antibodies overnight at 4°C, followed by blocking with PBS containing 10% fetal calf serum (Sigma-Aldrich) for 4 h at room temperature. The supernatants and recombinant cytokines were added, and the plates were incubated overnight at 4°C, washed, and incubated with a biotinylated anticytokine monoclonal antibody at room temperature for 4 h, followed by washing and incubation with horseradish peroxidase-conjugated streptavidin at room temperature for 4 h. The reaction was developed with tetramethylbenzidine (BD Biosciences) according to the manufacturer’s specifications. Absorbance was determined by subtracting the absorbance at 570 nm from the absorbance at 450 nm using an Inspire microplate reader (Perkin-Elmer, Waltham, MA, USA). Cytokine concentrations were calculated by 5PL regression analysis of absorbance values obtained for the recombinant cytokines, and the results were expressed in picograms per milliliter. The sensitivity of the tests ranged from 2 to 20 pg/ml.
Statistical analyses.
Data were analyzed in GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA, USA). The Mann-Whitney (U) test was conducted for comparisons between two groups. Qualitative variables were expressed as percentages, and the associations between them were analyzed by the χ2 test. Results were considered statistically significant at P values of <0.05.
Ethics statement.
The study protocol was approved by the Ethics Committee of Federal University of Triângulo Mineiro, Uberaba, Minas Gerais state, Brazil (protocol no. 1030), and all participants signed the Free and Informed Consent Form.
ACKNOWLEDGMENTS
This work was funded by CNPq, FAPEMIG, CAPES, FUNEPU, NIDR, and CEFORES. The funders had no role in the study design, data collection, and analysis, the decision to publish, or preparation of the paper.
REFERENCES
- 1.WHO. 2015. Investing to overcome the global impact of neglected tropical diseases: third WHO report on neglected diseases 2015. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Dias JC, Ramos AN Jr, Gontijo ED, Luquetti A, Shikanai-Yasuda MA, Coura JR, Torres RM, Melo JR, Almeida EA, Oliveira W Jr, Silveira AC, Rezende JM, Pinto FS, Ferreira AW, Rassi A, Fragata AAF, Sousa AS, Correia D, Jansen AM, Andrade GM, Britto CF, Pinto AY, Rassi A Jr, Campos DE, Abad-Franch F, Santos SE, Chiari E, Hasslocher-Moreno AM, Moreira EF, Marques DS, Silva EL, Marin-Neto JA, Galvao LM, Xavier SS, Valente SA, Carvalho NB, Cardoso AV, Silva RA, Costa VM, Vivaldini SM, Oliveira SM, Valente VD, Lima MM, Alves RV. 2016. 2nd Brazilian consensus on Chagas disease, 2015. Rev Soc Bras Med Trop 49(Suppl 1):3–60. doi: 10.1590/0037-8682-0505-2016. [DOI] [PubMed] [Google Scholar]
- 3.Bern C. 2015. Chagas' disease. N Engl J Med 373:456–466. doi: 10.1056/NEJMra1410150. [DOI] [PubMed] [Google Scholar]
- 4.Rassi A Jr, Marin JAN, Rassi A. 2017. Chronic Chagas cardiomyopathy: a review of the main pathogenic mechanisms and the efficacy of aetiological treatment following the BENznidazole Evaluation for Interrupting Trypanosomiasis (BENEFIT) trial. Mem Inst Oswaldo Cruz 112:224–235. doi: 10.1590/0074-02760160334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rassi A Jr, Rassi A, Marin-Neto JA. 2010. Chagas disease. Lancet 375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 6.Rassi A Jr, Rassi A, Rassi SG. 2007. Predictors of mortality in chronic Chagas disease: a systematic review of observational studies. Circulation 115:1101–1108. doi: 10.1161/CIRCULATIONAHA.106.627265. [DOI] [PubMed] [Google Scholar]
- 7.Elizari MV. 1999. Chagasic myocardiopathy: historical perspective. Medicina (B Aires) 59(Suppl 2):25–40. (In Spanish.) [PubMed] [Google Scholar]
- 8.Dutra WO, Menezes CA, Magalhaes LM, Gollob KJ. 2014. Immunoregulatory networks in human Chagas disease. Parasite Immunol 36:377–387. doi: 10.1111/pim.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morel CM, Lazdins J. 2003. Chagas disease. Nat Rev Microbiol 1:14–15. doi: 10.1038/nrmicro735. [DOI] [PubMed] [Google Scholar]
- 10.Higuchi MDL, Benvenuti LA, Martins Reis M, Metzger M. 2003. Pathophysiology of the heart in Chagas' disease: current status and new developments. Cardiovasc Res 60:96–107. doi: 10.1016/S0008-6363(03)00361-4. [DOI] [PubMed] [Google Scholar]
- 11.Marin-Neto JA, Cunha-Neto E, Maciel BC, Simões MV. 2007. Pathogenesis of chronic Chagas heart disease. Circulation 115:1109–1123. doi: 10.1161/CIRCULATIONAHA.106.624296. [DOI] [PubMed] [Google Scholar]
- 12.Kumar S, Tarleton RL. 2001. Antigen-specific Th1 but not Th2 cells provide protection from lethal Trypanosoma cruzi infection in mice. J Immunol 166:4596–4603. doi: 10.4049/jimmunol.166.7.4596. [DOI] [PubMed] [Google Scholar]
- 13.Reis Machado J, Silva MV, Borges DC, da Silva CA, Ramirez LE, dos Reis MA, Castellano LR, Rodrigues V, Rodrigues DB. 2014. Immunopathological aspects of experimental Trypanosoma cruzi reinfections. Biomed Res Int 2014:648715. doi: 10.1155/2014/648715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Avila DA, Guedes PM, Castro AM, Gontijo ED, Chiari E, Galvao LM. 2009. Immunological imbalance between IFN-gamma and IL-10 levels in the sera of patients with the cardiac form of Chagas disease. Mem Inst Oswaldo Cruz 104:100–105. doi: 10.1590/S0074-02762009000100015. [DOI] [PubMed] [Google Scholar]
- 15.Brener Z, Gazzinelli RT. 1997. Immunological control of Trypanosoma cruzi infection and pathogenesis of Chagas' disease. Int Arch Allergy Immunol 114:103–110. doi: 10.1159/000237653. [DOI] [PubMed] [Google Scholar]
- 16.Abel LC, Rizzo LV, Ianni B, Albuquerque F, Bacal F, Carrara D, Bocchi EA, Teixeira HC, Mady C, Kalil J, Cunha-Neto E. 2001. Chronic Chagas' disease cardiomyopathy patients display an increased IFN-gamma response to Trypanosoma cruzi infection. J Autoimmun 17:99–107. doi: 10.1006/jaut.2001.0523. [DOI] [PubMed] [Google Scholar]
- 17.Gomes JA, Bahia-Oliveira LM, Rocha MO, Martins-Filho OA, Gazzinelli G, Correa-Oliveira R. 2003. Evidence that development of severe cardiomyopathy in human Chagas' disease is due to a Th1-specific immune response. Infect Immun 71:1185–1193. doi: 10.1128/IAI.71.3.1185-1193.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viotti R, Vigliano C, Armenti H, Segura E. 1994. Treatment of chronic Chagas' disease with benznidazole: clinical and serologic evolution of patients with long-term follow-up. Am Heart J 127:151–162. doi: 10.1016/0002-8703(94)90521-5. [DOI] [PubMed] [Google Scholar]
- 19.Garcia S, Ramos CO, Senra JF, Vilas-Boas F, Rodrigues MM, Campos-de-Carvalho AC, Ribeiro-Dos-Santos R, Soares MB. 2005. Treatment with benznidazole during the chronic phase of experimental Chagas' disease decreases cardiac alterations. Antimicrob Agents Chemother 49:1521–1528. doi: 10.1128/AAC.49.4.1521-1528.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rassi A, Rassi A Jr. 1998. Treatment of chronic Chagas' disease. Is the etiological treatment effective? Arq Bras Cardiol 71:643–646. (In Portuguese.) [PubMed] [Google Scholar]
- 21.Sosa Estani S, Segura EL, Ruiz AM, Velazquez E, Porcel BM, Yampotis C. 1998. Efficacy of chemotherapy with benznidazole in children in the indeterminate phase of Chagas' disease. Am J Trop Med Hyg 59:526–529. doi: 10.4269/ajtmh.1998.59.526. [DOI] [PubMed] [Google Scholar]
- 22.Marin-Neto JA, Rassi A Jr, Morillo CA, Avezum A, Connolly SJ, Sosa-Estani S, Rosas F, Yusuf S. 2008. Rationale and design of a randomized placebo-controlled trial assessing the effects of etiologic treatment in Chagas' cardiomyopathy: the BENznidazole Evaluation For Interrupting Trypanosomiasis (BENEFIT). Am Heart J 156:37–43. doi: 10.1016/j.ahj.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Sathler-Avelar R, Vitelli-Avelar DM, Massara RL, de Lana M, Pinto Dias JC, Teixeira-Carvalho A, Elói-Santos SM, Martins-Filho OA. 2008. Etiological treatment during early chronic indeterminate Chagas disease incites an activated status on innate and adaptive immunity associated with a type 1-modulated cytokine pattern. Microbes Infect 10:103–113. doi: 10.1016/j.micinf.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Morillo CA, Marin-Neto JA, Avezum A, Sosa-Estani S, Rassi A Jr, Rosas F, Villena E, Quiroz R, Bonilla R, Britto C, Guhl F, Velazquez E, Bonilla L, Meeks B, Rao-Melacini P, Pogue J, Mattos A, Lazdins J, Rassi A, Connolly SJ, Yusuf S, BENEFIT Investigators . 2015. Randomized trial of benznidazole for chronic Chagas' cardiomyopathy. N Engl J Med 373:1295–1306. doi: 10.1056/NEJMoa1507574. [DOI] [PubMed] [Google Scholar]
- 25.Silva JS, Morrissey PJ, Grabstein KH, Mohler KM, Anderson D, Reed SG. 1992. Interleukin 10 and interferon gamma regulation of experimental Trypanosoma cruzi infection. J Exp Med 175:169–174. doi: 10.1084/jem.175.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoft DF, Lynch RG, Kirchhoff LV. 1993. Kinetic analysis of antigen-specific immune responses in resistant and susceptible mice during infection with Trypanosoma cruzi. J Immunol 151:7038–7047. [PubMed] [Google Scholar]
- 27.Minoprio P, el Cheikh MC, Murphy E, Hontebeyrie-Joskowicz M, Coffman R, Coutinho A, O'Garra A. 1993. Xid-associated resistance to experimental Chagas' disease is IFN-gamma dependent. J Immunol 151:4200–4208. [PubMed] [Google Scholar]
- 28.Reed SG, Brownell CE, Russo DM, Silva JS, Grabstein KH, Morrissey PJ. 1994. IL-10 mediates susceptibility to Trypanosoma cruzi infection. J Immunol 153:3135–3140. [PubMed] [Google Scholar]
- 29.Savino W, Villa-Verde DMS, Mendes-da-Cruz DA, Silva-Monteiro E, Perez AR, Aoki MDP, Bottasso O, Guiñazú N, Silva-Barbosa SD, Gea S. 2007. Cytokines and cell adhesion receptors in the regulation of immunity to Trypanosoma cruzi. Cytokine Growth Factor Rev 18:107–124. doi: 10.1016/j.cytogfr.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Aliberti JC, Souto JT, Marino AP, Lannes-Vieira J, Teixeira MM, Farber J, Gazzinelli RT, Silva JS. 2001. Modulation of chemokine production and inflammatory responses in interferon-gamma- and tumor necrosis factor-R1-deficient mice during Trypanosoma cruzi infection. Am J Pathol 158:1433–1440. doi: 10.1016/S0002-9440(10)64094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dutra WO, Gollob KJ. 2008. Current concepts in immunoregulation and pathology of human Chagas disease. Curr Opin Infect Dis 21:287–292. doi: 10.1097/QCO.0b013e3282f88b80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Llaguno M, Pertili LA, da Silva MV, Bunazar P, Reges AM, Faleiros AC, Lages-Silva E, Rodrigues Junior V, da Silva VJ, Correia Filho D. 2011. The relationship between heart rate variability and serum cytokines in chronic chagasic patients with persistent parasitemia. Pacing Clin Electrophysiol 34:724–735. doi: 10.1111/j.1540-8159.2010.03025.x. [DOI] [PubMed] [Google Scholar]
- 33.Bahia-Oliveira LM, Gomes JA, Rocha MO, Moreira MC, Lemos EM, Luz ZM, Pereira ME, Coffman RL, Dias JC, Cançado JR, Gazzinelli G, Corrêa-Oliveira R. 1998. IFN-gamma in human Chagas' disease: protection or pathology? Braz J Med Biol Res 31:127–131. doi: 10.1590/S0100-879X1998000100017. [DOI] [PubMed] [Google Scholar]
- 34.Oliveira MA, Santiago HC, Lisboa CR, Ceravollo IP, Trinchieri G, Gazzinelli RT, Vieira LQ. 2000. Leishmania sp: comparative study with Toxoplasma gondii and Trypanosoma cruzi in their ability to initialize IL-12 and IFN-gamma synthesis. Exp Parasitol 95:96–105. doi: 10.1006/expr.2000.4523. [DOI] [PubMed] [Google Scholar]
- 35.Correa-Oliveira R, Gomes J, Lemos EM, Cardoso GM, Reis DD, Adad S, Crema E, Martins-Filho OA, Costa MO, Gazzinelli G, Bahia-Oliveira LM. 1999. The role of the immune response on the development of severe clinical forms of human Chagas disease. Mem Inst Oswaldo Cruz 94(Suppl 1):253–255. doi: 10.1590/S0074-02761999000700042. [DOI] [PubMed] [Google Scholar]
- 36.Ribeirao M, Pereira-Chioccola VL, Renia L, Augusto Fragata Filho A, Schenkman S, Rodrigues MM. 2000. Chagasic patients develop a type 1 immune response to Trypanosoma cruzi trans-sialidase. Parasite Immunol 22:49–53. doi: 10.1046/j.1365-3024.2000.00260.x. [DOI] [PubMed] [Google Scholar]
- 37.Sathler-Avelar R, Vitelli-Avelar DM, Massara RL, Borges JD, Lana M, Teixeira-Carvalho A, Dias JCP, Elói-Santos SM, Martins-Filho OA. 2006. Benznidazole treatment during early-indeterminate Chagas' disease shifted the cytokine expression by innate and adaptive immunity cells toward a type 1-modulated immune profile. Scand J Immunol 64:554–563. doi: 10.1111/j.1365-3083.2006.01843.x. [DOI] [PubMed] [Google Scholar]
- 38.Pascutti MF, Pitashny M, Nocito AL, Guermonprez P, Amigorena S, Wietzerbin J, Serra E, Bottasso O, Revelli S. 2004. Benznidazole, a drug used in Chagas' disease, ameliorates LPS-induced inflammatory response in mice. Life Sci 76:685–697. doi: 10.1016/j.lfs.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 39.Pérez-Fuentes R, López-Colombo A, Ordóñez-Toquero G, Gomez-Albino I, Ramos J, Torres-Rasgado E, Salgado-Rosas H, Romero-Díaz M, Pulido-Pérez P, Sánchez-Guillén MC. 2007. Correlation of the serum concentrations of tumour necrosis factor and nitric oxide with disease severity in chronic Chagas disease (American trypanosomiasis). Ann Trop Med Parasitol 101:123–132. doi: 10.1179/136485907X154593. [DOI] [PubMed] [Google Scholar]
- 40.Ward LS, Guariento ME, Fernandes GA, Maciel RM. 1999. Serum cytokines in chronic Chagas' disease. Rev Soc Bras Med Trop 32:285–289. doi: 10.1590/S0037-86821999000300010. [DOI] [PubMed] [Google Scholar]
- 41.Kumar S, Tarleton RL. 1998. The relative contribution of antibody production and CD8+ T cell function to immune control of Trypanosoma cruzi. Parasite Immunol 20:207–216. doi: 10.1046/j.1365-3024.1998.00154.x. [DOI] [PubMed] [Google Scholar]
- 42.Spinella S, Liegeard P, Hontebeyrie-Joskowicz M. 1992. Trypanosoma cruzi: predominance of IgG2a in nonspecific humoral response during experimental Chagas' disease. Exp Parasitol 74:46–56. doi: 10.1016/0014-4894(92)90138-Z. [DOI] [PubMed] [Google Scholar]
- 43.Abrahamsohn IA. 1998. Cytokines in innate and acquired immunity to Trypanosoma cruzi infection. Braz J Med Biol Res 31:117–121. doi: 10.1590/S0100-879X1998000100015. [DOI] [PubMed] [Google Scholar]
- 44.Albareda MC, Laucella SA, Alvarez MG, Armenti AH, Bertochi G, Tarleton RL, Postan M. 2006. Trypanosoma cruzi modulates the profile of memory CD8+ T cells in chronic Chagas' disease patients. Int Immunol 18:465–471. doi: 10.1093/intimm/dxh387. [DOI] [PubMed] [Google Scholar]
- 45.Lasso P, Mesa D, Cuéllar A, Guzmán F, Bolaños N, Rosas F, Velasco V, Thomas MDC, Lopez MC, Gonzalez JM, Puerta CJ. 2010. Frequency of specific CD8+ T cells for a promiscuous epitope derived from Trypanosoma cruzi KMP-11 protein in chagasic patients. Parasite Immunol 32:494–502. doi: 10.1111/j.1365-3024.2010.01206.x. [DOI] [PubMed] [Google Scholar]
- 46.Lorena VM, Lorena IM, Braz SC, Melo AS, Melo MF, Melo MG, Silva ED, Ferreira AG, Morais CN, Costa VM, Correa-Oliveira R, Gomes YM. 2010. Cytokine levels in serious cardiopathy of Chagas disease after in vitro stimulation with recombinant antigens from Trypanosoma cruzi. Scand J Immunol 72:529–539. doi: 10.1111/j.1365-3083.2010.02462.x. [DOI] [PubMed] [Google Scholar]
- 47.Sathler-Avelar R, Vitelli-Avelar DM, Elói-Santos SM, Gontijo ED, Teixeira-Carvalho A, Martins-Filho OA. 2012. Blood leukocytes from benznidazole-treated indeterminate Chagas disease patients display an overall type-1-modulated cytokine profile upon short-term in vitro stimulation with Trypanosoma cruzi antigens. BMC Infect Dis 12:123. doi: 10.1186/1471-2334-12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vitelli-Avelar DM, Sathler-Avelar R, Dias JCP, Pascoal VPM, Teixeira-Carvalho A, Lage PS, Elói-Santos SM, Corrêa-Oliveira R, Martins-Filho OA. 2005. Chagasic patients with indeterminate clinical form of the disease have high frequencies of circulating CD3+CD16-CD56+ natural killer T cells and CD4+CD25High regulatory T lymphocytes. Scand J Immunol 62:297–308. doi: 10.1111/j.1365-3083.2005.01668.x. [DOI] [PubMed] [Google Scholar]
- 49.Vitelli-Avelar DM, Sathler-Avelar R, Teixeira-Carvalho A, Pinto Dias JC, Gontijo ED, Faria AM, Elói-Santos SM, Martins-Filho OA. 2008. Strategy to assess the overall cytokine profile of circulating leukocytes and its association with distinct clinical forms of human Chagas disease. Scand J Immunol 68:516–525. doi: 10.1111/j.1365-3083.2008.02167.x. [DOI] [PubMed] [Google Scholar]
- 50.Dutra WO, Gollob KJ, Pinto-Dias JC, Gazzinelli G, Correa-Oliveira R, Coffman RL, Carvalho-Parra JF. 1997. Cytokine mRNA profile of peripheral blood mononuclear cells isolated from individuals with Trypanosoma cruzi chronic infection. Scand J Immunol 45:74–80. doi: 10.1046/j.1365-3083.1997.d01-362.x. [DOI] [PubMed] [Google Scholar]
- 51.Menezes CA, Rocha MO, Souza PE, Chaves AC, Gollob KJ, Dutra WO. 2004. Phenotypic and functional characteristics of CD28+ and CD28- cells from chagasic patients: distinct repertoire and cytokine expression. Clin Exp Immunol 137:129–138. doi: 10.1111/j.1365-2249.2004.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Souza PE, Rocha MO, Menezes CA, Coelho JS, Chaves AC, Gollob KJ, Dutra WO. 2007. Trypanosoma cruzi infection induces differential modulation of costimulatory molecules and cytokines by monocytes and T cells from patients with indeterminate and cardiac Chagas' disease. Infect Immun 75:1886–1894. doi: 10.1128/IAI.01931-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soares MB, Goncalves R, Pyrrho AS, Costa DA, Paiva CN, Gattass CR. 2003. Balanced cytokine-producing pattern in mice immunized with an avirulent Trypanosoma cruzi. An Acad Bras Cienc 75:167–172. doi: 10.1590/S0001-37652003000200005. [DOI] [PubMed] [Google Scholar]
- 54.Mariano FS, Gutierrez FR, Pavanelli WR, Milanezi CM, Cavassani KA, Moreira AP, Ferreira BR, Cunha FQ, Cardoso CR, Silva JS. 2008. The involvement of CD4+CD25+ T cells in the acute phase of Trypanosoma cruzi infection. Microbes Infect 10:825–833. doi: 10.1016/j.micinf.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 55.Hunter CA, Ellis-Neyes LA, Slifer T, Kanaly S, Grunig G, Fort M, Rennick D, Araujo FG. 1997. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. J Immunol 158:3311–3316. [PubMed] [Google Scholar]
- 56.Golgher D, Gazzinelli RT. 2004. Innate and acquired immunity in the pathogenesis of Chagas disease. Autoimmunity 37:399–409. doi: 10.1080/08916930410001713115. [DOI] [PubMed] [Google Scholar]