Vaccination remains one of the greatest medical breakthroughs in human history and has resulted in the near eradication of many formerly lethal diseases in many countries, including the complete eradication of smallpox. However, there remain a number of diseases for which there are no or only partially effective vaccines. There are numerous hurdles in vaccine development, of which knowing the appropriate immune response to target is one of them.
KEYWORDS: DNA vaccines, intradermal, Leishmania, PEPCK, SEP
ABSTRACT
Vaccination remains one of the greatest medical breakthroughs in human history and has resulted in the near eradication of many formerly lethal diseases in many countries, including the complete eradication of smallpox. However, there remain a number of diseases for which there are no or only partially effective vaccines. There are numerous hurdles in vaccine development, of which knowing the appropriate immune response to target is one of them. Recently, tissue-resident T cells have been shown to mediate high levels of protection for several infections, although the best way to induce these cells is still unclear. Here we compare the ability to generate skin-resident T cells in sites distant from the immunization site following intramuscular and intradermal injection using optimized synthetic DNA vaccines. We found that mice immunized intradermally with a synthetic consensus DNA HIV envelope vaccine by electroporation (EP) are better able to maintain durable antigen-specific cellular responses in the skin than mice immunized by the intramuscular route. We extended these studies by delivering a synthetic DNA vaccine encoding Leishmania glycosomal phosphoenolpyruvate carboxykinase (PEPCK) by EP and again found that the intradermal route was superior to the intramuscular route for generating skin-resident PEPCK-specific T cells. We observed that when challenged with Leishmania major parasites, mice immunized intradermally exhibited significant protection, while mice immunized intramuscularly did not. The protection seen in intradermally vaccinated mice supports the viability of this platform not only to generate skin-resident T cells but also to promote durable protective immune responses at relevant tissue sites.
INTRODUCTION
The most successful approach to controlling infectious diseases is the development of protective vaccines, but unfortunately, there remain several diseases for which no vaccines are available. Resolving this deficit will require identifying the immune responses that provide protection and then understanding how best to generate them. Recent studies with several diseases have reported that T cells residing in the tissues (resident memory T cells or Trm cells) can often provide greater protection than those that are circulating (1–3), although how to best generate Trm cells through a vaccine remains poorly understood. For example, some studies suggest that intradermal (i.d.) immunizations or skin scarification may be particularly effective at generating skin Trm cells (4–10, 38), while other studies are less clear on this practical issue. We wanted to address this issue in part by comparing the generation of skin-resident T cells and protection against cutaneous leishmaniasis following DNA immunizations via the intramuscular (i.m.) route, most often used for currently approved vaccines, and the intradermal route.
Leishmania infection occurs in over 88 countries, with an estimated 12 million people currently infected and over 350 million people at risk (11–13). The parasitic infection is spread by the sand fly, primarily impacting people in resource-strained settings. There are a number of barriers that bar access to the few treatments available for leishmaniasis, including high costs, quality control issues, low production capacities, and physical geography (14, 15). However, evidence that people who recover from clinical disease are generally protected from future infection suggests that a vaccine approach is feasible, and there are currently a large number of potential vaccines being tested in both experimental animal models and clinical trials (16–18). However, none of these options are currently available for human leishmaniasis. Clinical trials that have used heat-killed whole Leishmania parasite antigens have resulted in disappointing outcomes where any observed efficacy is short-lived, and as such, they are unlikely to mount protective immune responses (19–25). Live parasites have also been used in the past to induce immunity with some success; however, concerns with protracted and nonhealing lesions as well as parasite passage issues have caused this option to fall out of favor (26–28). A major problem in the field of vaccine development for leishmaniasis has been the lack of an identified immunodominant leishmanial antigen. Recently, however, this deficit has been rectified with the discovery of a leishmania protein, Leishmania phosphoenolpyruvate carboxykinase (PEPCK), which is an enzyme that is critical for gluconeogenesis. At the peak of Leishmania infection, nearly 20% of all Leishmania-reactive CD4+ T cells are PEPCK specific (29). Furthermore, the authors of that study found that peripheral blood mononuclear cells (PBMCs) from people who have recovered from zoonotic Leishmania major infection recognize PEPCK, express high levels of interferon gamma (IFN-γ) and granzyme B, and have increased cell proliferation compared to PBMCs from healthy noninfected people when stimulated with recombinant PEPCK protein, suggesting the potential of clinical benefit.
In order to determine if the route of vaccination (i.m. versus i.d.) would influence the generation of skin-resident T cells, we first tested the ability of a well-defined DNA vaccine developed for HIV to generate these cells by these two routes. We then created two synthetic consensus DNA plasmids that encode PEPCK, which represent a consensus of PEPCK sequences from six Leishmania parasites, including Leishmania infantum, L. donovani, L. major, L. mexicana, L. braziliensis, and L. panamensis. These species represent both Old and New World Leishmania parasites that can cause cutaneous, mucosal, and visceral leishmaniasis. We immunized mice with these plasmids either intramuscularly or intradermally by Cellectra electroporation (EP) to evaluate vaccine-induced immunity and assess protection following challenge. We found that mice immunized intradermally were better protected against L. major challenge than mice immunized intramuscularly. The levels of protection seen in intradermally immunized mice were similar to those seen in immune mice that were previously infected with parasites, as measured by lesion size and parasite burden, and suggest that the intradermal route may be most efficient at generating Trm cells and protection against leishmania parasites.
RESULTS
Intradermal Env vaccination leads to durable immune responses in the skin compared to intramuscular delivery.
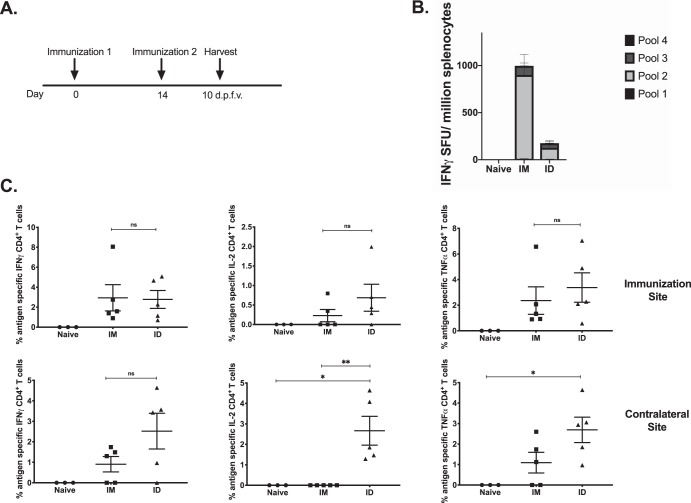
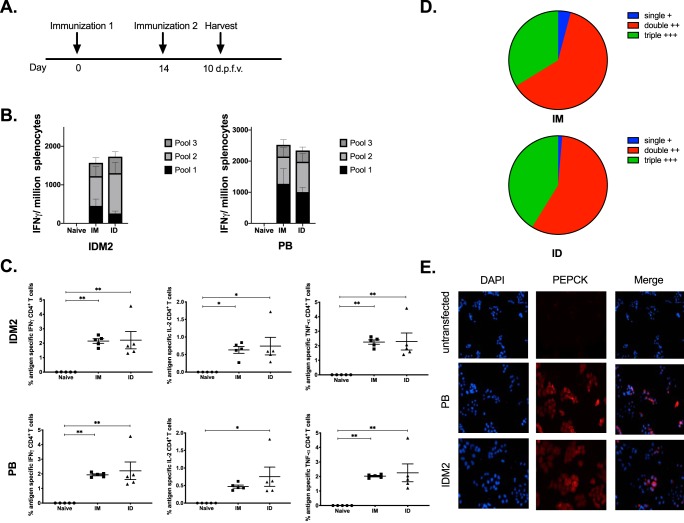
An important outcome for vaccination is to generate long-lived immunity to protect against future pathogen exposure. Therefore, we sought to examine in pilot experiments the immune responses elicited postvaccination as well as the response at a memory time point using defined constructs. C57BL/6 mice (n = 5) were immunized with 25 μg of an HIV Env DNA vaccine either intramuscularly with Cellectra EP or i.d. in the abdominal flank with surface electroporation (SEP), twice, 2 weeks apart (Fig. 1A). Spleens and skin at the injection site were collected 10 days after the final vaccination to analyze antigen-specific responses. A quantitative enzyme-linked immunosorbent spot (ELISpot) assay was performed to analyze the IFN-γ response in the spleen, and we found that mice immunized intramuscularly had an average of 1,000 spot-forming units (SFU) per million splenocytes, while intradermally vaccinated mice had an average of 250 SFU (Fig. 1B). In the skin, there was no significant difference between i.m. and i.d. immunized mice in the frequencies of IFN-γ-, interleukin-2 (IL-2)-, and tumor necrosis factor alpha (TNF-α)-secreting CD4+ T cells at the site of immunization; however, there was a significant difference in the frequencies of IL-2- and TNF-α-producing CD4+ T cells at the contralateral site, suggesting some enhanced homing and mobility of these antigen-specific T cells induced in the skin by i.d. immunization (Fig. 1C).
FIG 1.
HIV Env DNA vaccination at intramuscular and intradermal sites induces immune responses in the skin. (A) Immunization schedule for tissue-specific DNA vaccination. C57BL/6 mice were immunized twice, 2 weeks apart (n = 3 to 5 per group), either intramuscularly (IM) or intradermally (ID). Spleens and skin from the flank and TA were collected 10 days after the final vaccination to analyze antigen-specific immune responses. d.p.f.v., days post-final vaccination. (B) The frequency of Env-specific IFN-γ responses (spot-forming units per million splenocytes) induced after vaccination was determined by an IFN-γ ELISpot assay in response to pooled Env peptides. (C) Env-specific CD4+ T cell responses by intracellular cytokine staining at vaccination and contralateral sites. *, P < 0.05; **, P < 0.005; ***, P < 0.0005; ****, P < 0.0001; ns, not significant.
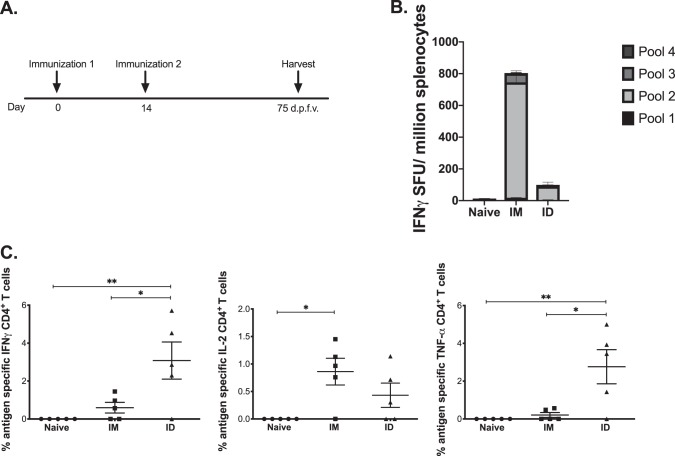
We next examined immune responses 75 days after the final vaccination to study the durability of the antigen-specific responses (Fig. 2A). There was some contraction of the IFN-γ response, with averages of 800 SFU/million splenocytes for the i.m. group and 150 SFU/million for the i.d. group (Fig. 2B), but strikingly, there was a significant number of IFN-γ- and TNF-α-secreting CD4+ T cells in the i.d. group compared to the i.m. group in the skin (Fig. 2C), suggesting that i.d. immunization has the potential to generate long-lasting immunity at the site of vaccination. We did not observe a significant number of antigen-specific CD4+ T cells at the contralateral site at the memory time point in this model. Encouraged by these data, we next studied intradermal vaccination in a Leishmania model for which a mouse challenge exists.
FIG 2.
HIV Env DNA vaccination to intramuscular and intradermal sites maintains durable immune responses at the memory time point. (A) Immunization schedule for tissue-specific DNA vaccination. C57BL/6 mice were immunized twice, 2 weeks apart (n = 4 to 5 per group), either intramuscularly or intradermally. Spleens and skin from the flank and TA were collected 75 days after the final vaccination to analyze antigen-specific immune responses. (B) The frequency of Env-specific IFN-γ (spot-forming units per million splenocytes) maintained at the memory time point was determined by an IFN-γ ELISpot assay in response to pooled Env peptides. (C) Env-specific CD4+ T cell responses by intracellular cytokine staining at the vaccination site.
Development of a consensus Leishmania PEPCK vaccine.
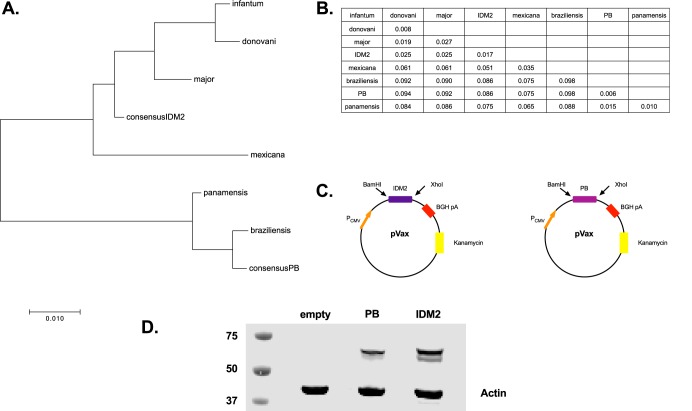
Challenges in Leishmania vaccine development are due in part to the lack of an understanding of the antigens capable of eliciting potent Th1 IFN-γ CD4+ T cell responses. However, recent work by Mou et al. (29) has identified a conserved dominant protein, PEPCK, that elicited strong CD4+ T cell responses. They found that ∼17% of Leishmania-reactive CD4+ T cells were PEPCK specific at the peak of the immune response during L. major infection. Given this robust response, we designed two constructs that encode consensus sequences for PEPCK to maximize the coverage of both Old and New World strains of Leishmania (Fig. 3A and B). The PEPCK genes were inserted into a pGX0001 backbone with a cytomegalovirus (CMV) promoter and an immunoglobulin E (IgE) leader (Fig. 3C). Construct expression in vitro was confirmed using Western blotting (Fig. 3D), to detect binding to PEPCK. Expression of PEPCK was observed in the lysates of transfected cells but was not observed in the supernatant (see Fig. S4 in the supplemental material).
FIG 3.
Diversity among Leishmania parasite and consensus sequences. (A and B) Evolutionary history was inferred by the neighbor-joining method, and all evolutionary distances were computed using the Poisson correction method and are in units of amino acid substitutions per site. All positions with <30% site coverage were eliminated. (C) Map of plasmid construct design for consensus sequences. Each plasmid contains a CMV promoter followed by an IgE leader sequence beside the consensus PEPCK sequence. BGH pA, bovine growth hormone poly(A). (D) HEK293T cells were transfected with either IDM2 or PB consensus plasmids that contained a C-terminal HA tag for detection. Lysates from these cells were used for Western blotting for detection of plasmid expression.
Consensus PEPCK plasmids are immunogenic and induce strong cellular IFN-γ responses following intramuscular vaccination.
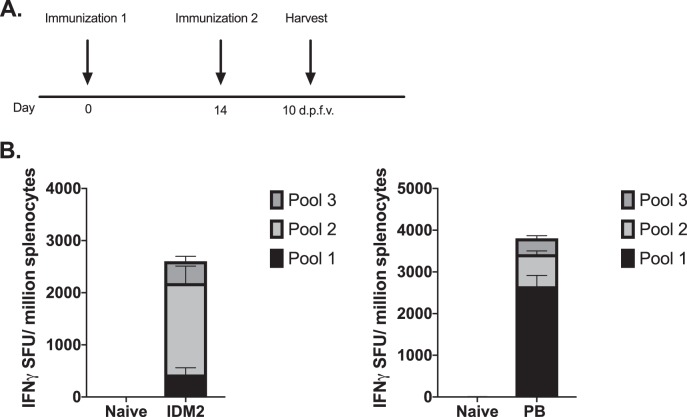
As IDM2 and PB differ by 7.5% in their amino acid sequences, we wanted to verify that both plasmids were immunogenic and to determine whether there might be an advantage to delivering both plasmids in a vaccine strategy. We performed intramuscular DNA delivery of either IDM2 or PB in C57BL/6 mice at a dose of 20 μg each, followed by EP, two times, 2 weeks apart (Fig. 4A). Ten days after the final vaccination, we analyzed the degree of immune responses by isolating splenocytes. A quantitative ELISpot assay was performed to determine the number of PEPCK-specific IFN-γ-secreting T cells that responded to vaccination (Fig. 4B). Both mice vaccinated with IDM2 and those vaccinated with PB mounted a robust immune response, with averages of 2,500 SFU/million and 3,700 SFU/million splenocytes against IDM2 and PB, respectively. Strikingly, there were a much greater number of T cells that responded to peptides in pool 1 of PB than in pool 1 of IDM2, potentially suggesting additional epitopes that the T cells are recognizing. Based on this observation, we decided to combine the two plasmids into one vaccine for the remainder of the studies.
FIG 4.
Delivery of PEPCK IDM2 or PB induces systemic immune responses in the spleen. (A) Immunization schedule for DNA vaccination. C57BL/6 mice were immunized twice, 2 weeks apart (n = 4 to 5 per group), with IDM2 or PB. Spleens were harvested 10 days after the final vaccination to analyze antigen-specific T cell responses. (B) The frequency of PEPCK-specific IFN-γ responses (spot-forming units per million splenocytes) induced after vaccination was determined by an IFN-γ ELISpot assay in response to pooled PEPCK peptides.
Consensus PEPCK plasmids induce a strong systemic immune response following intramuscular and intradermal vaccination.
Given that the majority of vaccines administered today are delivered intramuscularly, we wanted to verify that our PEPCK constructs would be immunogenic whether administered i.m. or i.d. We performed PEPCK (IDM2 and PB) vaccination either i.m. in C57BL/6 mice (n = 5) with a dosage of 40 μg total of DNA (20 μg IDM2 and 20 μg PB) or i.d. (n = 5) with the same dosage, followed by i.m. EP or i.d. SEP, two times, 2 weeks apart (Fig. 5A). The i.d. SEP device is less invasive and targets the epidermis. Ten days after the final vaccination, we analyzed the degree of immune responses by isolating splenocytes. A quantitative ELISpot assay was performed to determine the number of PEPCK-specific IFN-γ-secreting T cells that responded to vaccination (Fig. 5B). Both mice vaccinated i.m. and those vaccinated i.d. mounted robust immune responses, with averages of 1,600 and 1,800 SFU/million splenocytes against IDM2 and 2,500 and 2,200 SFU/million splenocytes against PB, respectively. T cell polyfunctionality has been shown to be protective in some models; thus, we sought to assess the quality of T cells that were responsive to our vaccination. We found that PEPCK-specific CD4+ T cells secreted IFN-γ, IL-2, and TNF-α and that many of these CD4+ T cells secreted all three cytokines (Fig. 5C and D). Furthermore, we observed the humoral response to PEPCK vaccination by an immunofluorescence assay (IFA) (Fig. 5E). Sera from PEPCK-immunized mice were used to detect antibody binding to IDM2/PB-transfected HEK293T cells.
FIG 5.
Delivery of PEPCK DNA to intramuscular or intradermal sites induces systemic immune responses. (A) Immunization schedule for tissue-specific DNA vaccination. C57BL/6 mice were immunized twice, 2 weeks apart (n = 4 to 5 per group), either intramuscularly or intradermally. Spleens were harvested 10 days after the final vaccination to analyze antigen-specific T cell responses. (B) The frequency of PEPCK-specific IFN-γ responses (spot-forming units per million splenocytes) induced after vaccination was determined by an IFN-γ ELISpot assay in response to pooled PEPCK peptides. (C) PEPCK-specific CD4+ T-cell responses to each consensus plasmid determined by intracellular cytokine staining after peptide stimulation. (D) Polyfunctionality of CD4+ T cells in response to PEPCK vaccination in intramuscular and intradermal groups. (E) HEK293T cells transfected with PEPCK plasmids and sera from C57BL/6 mice immunized with the constructs were used to detect antibody binding to PEPCK by an IFA.
Consensus PEPCK plasmids induce a strong cellular response in the skin following intradermal vaccination.
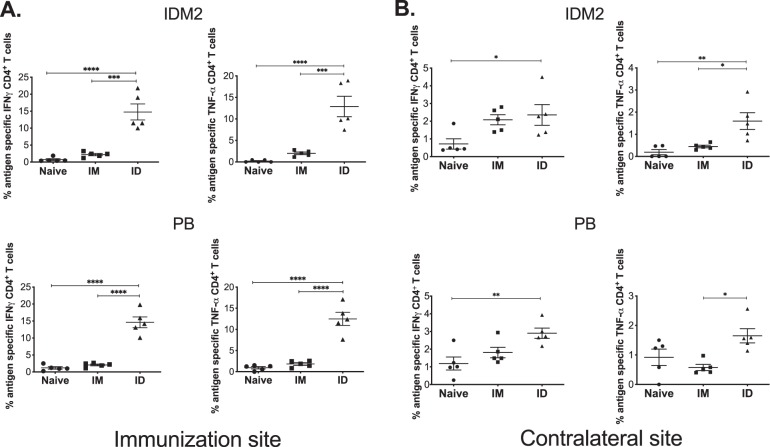
With a high percentage of leishmaniasis cases presenting in the skin, potential therapies for the infection should be able to induce immune responses in this important organ. The vaccine must also be able to induce T cells that are capable of homing to the point of parasitic infection, even when the vaccination site is not in close proximity. To address this, we compared the immune responses mounted in the skin at the site of immunization as well as contralateral sites in response to PEPCK vaccination given by either the i.m. or i.d. route. We found that i.d. immunization resulted in a high frequency of IFN-γ- and TNF-α-secreting PEPCK-specific CD4+ T cells in the skin at the immunization site (Fig. 6A). We did not detect any significant vaccine-induced immune responses in the skin of mice immunized i.m. More importantly, PEPCK-specific CD4+ T cells were found in the skin contralateral to the vaccination site (Fig. 6B), suggesting that this immunization strategy generated T cells that were able to home to noninflamed skin. These responses were primarily found in mice immunized i.d. against both IDM2 and PB.
FIG 6.
Delivery of PEPCK DNA to intradermal sites induces immune responses at the site of vaccination and contralateral sites. C57BL/6 mice were immunized twice, at a 2-week interval (n = 4 to 5 per group), by either i.m. or i.d. EP. Flank or TA skin from vaccination and contralateral sites was harvested 10 days after the final vaccination and analyzed for CD4+ T cell responses. (A) PEPCK-specific responses to each plasmid at the site of vaccination determined by intracellular cytokine staining after peptide stimulation. (B) PEPCK-specific CD4+ T cell responses to each plasmid at the contralateral site determined by intracellular staining after peptide stimulation.
Immune responses to PEPCK DNA vaccination are maintained at memory time points.
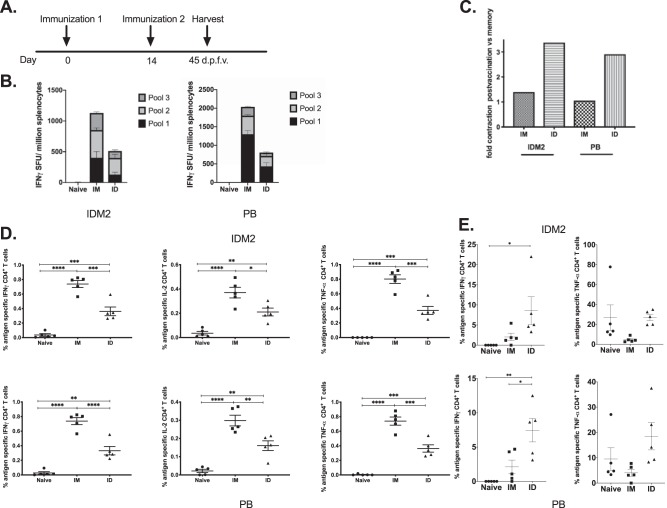
We next sought to test the durability of the immune responses generated by vaccination, so we immunized mice as described above and harvested spleens 45 days after the final vaccination (Fig. 7A). As expected, IFN-γ responses contracted, with averages of 1,200 and 500 SFU/million splenocytes against IDM2 in the i.m. and i.d. groups and 2,000 and 800 SFU/million splenocytes against PB, respectively (Fig. 7B). In the spleen, both i.m. and i.d. groups were able to maintain IFN-γ, IL-2, and TNF-α responses, with the i.m. group maintaining the highest frequency (Fig. 7C). Splenic responses appear to be most affected in the i.d. group, with a 3-fold contraction in IFN-γ ELISpot responses, compared to the i.m. group, which was able to maintain similar numbers of antigen-specific IFN-γ-secreting T cells (Fig. 7D). However, this was not true in the skin. Mice that were immunized i.d. were able to maintain a more significant frequency of IFN-γ-producing CD4+ T cells than were mice immunized i.m. (Fig. 7E).
FIG 7.
Immune responses are maintained at memory time points following intramuscular or intradermal PEPCK DNA vaccination. (A) Immunization schedule for PEPCK DNA vaccination. C57BL/6 mice were immunized twice, 2 weeks apart (n = 4 to 5 per group), either intramuscularly or intradermally. Spleens were harvested 45 days after the final vaccination for analysis of antigen-specific CD4+ T cell responses in the memory phase. (B) The frequency of PEPCK-specific IFN-γ responses (spot-forming units per million splenocytes) induced after vaccination was determined by an IFN-γ ELISpot assay in response to pooled PEPCK peptides. (C) Fold contraction of IFN-γ responses at 10 versus 45 days after the final vaccination. (D) PEPCK-specific CD4+ T cell responses to each consensus plasmid determined by intracellular cytokine staining after peptide stimulation. (E) PEPCK-specific responses to each plasmid at the site of vaccination determined by intracellular cytokine staining after peptide stimulation.
Intradermal vaccination with the PEPCK DNA vaccine leads to lower parasite burden and reduced ear thickness following challenge.
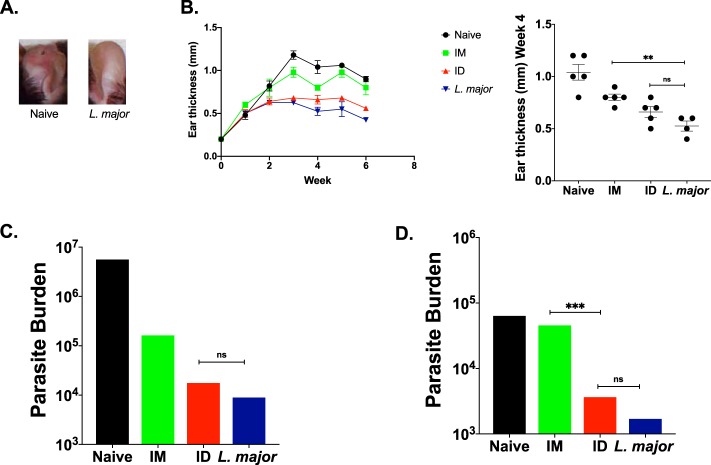
To determine the levels of protection against L. major engendered by the two routes of immunization, vaccinated and control mice were challenged in the ear. The challenged ear from each group was collected to analyze the immune response at the site of challenge. In response to L. major challenge and inflammation, the ear will form parasitic lesions, thicken, and become inflamed (Fig. 8A). Naive mice were unable to clear their infection and as such exhibited thickened ears, whereas i.d. immunized and immune mice maintained a much lower ear thickness, and i.m. immunized mice had an intermediate presentation (Fig. 8B). i.d. immunized mice were able to lower their parasite burden by nearly 3 logs, very similar to the parasite burden seen in immune mice, while i.m. immunized mice lowered their parasite burden by 2 logs (Fig. 8C). More strikingly, at a memory time point, i.d. immunization is able to significantly reduce the parasite burden compared to i.m. immunization (Fig. 8D), suggesting long-lasting protection against challenge. These experiments suggest that i.d. immunization has the potential to generate long-lasting protection against L. major infection, by a simple and less invasive immunization approach.
FIG 8.
Intradermal PEPCK DNA vaccination enhances protection of mice against L. major challenge. (A) Images of L. major-infected ears of naive and immune mice. L. major challenge can cause ear thickening and lesions. (B) Ear thickness curve plotting ear thickness over the span of the challenge. (C) Parasite burden in L. major-challenged ears 55 days after the final vaccination and at 45 days postchallenge. (D) Parasite burden in L. major-challenged ears at the memory time point 90 days after the final vaccination and at 45 days postchallenge.
DISCUSSION
In these studies, we provide insight into the potency and durability of intradermal vaccination using the DNA platform in two different models. We observe that i.d. immunization is able to induce systemic immune responses postvaccination as well as maintain immune responses in the skin at memory time points. We demonstrate that i.d. delivery of PEPCK DNA is able to protect mice against challenge at levels similar to those seen in immune mice and superior to those with i.m. injection, both soon after vaccination as well as at later time points. i.d. immunization resulted in reduced inflammation in the ear after L. major challenge and a 3-log reduction in the parasite burden in comparison to naive mice. In contrast, while i.m. immunization generated a strong systemic cellular response, the T cell responses in the skin were limited, and protection against challenge infection was not as robust as that seen following i.d. immunization. We have previously demonstrated that the most optimal protection against Leishmania challenge in mice is likely elicited from a combination of skin-resident and circulating effector T cells (30). With this work, we have shown that while both i.m. and i.d. PEPCK delivery induce similar levels of antigen-specific cellular responses systemically, only i.d. immunized mice are able to significantly drive PEPCK-specific T cells in the skin, resulting in enhanced protection against L. major challenge. These results indicate the superior ability of i.d. DNA vaccination in the Leishmania context to induce skin-resident T cells and protective immune responses and provide practical information needed for translating this vaccine from a mouse model to human study.
Leishmaniasis is an important neglected tropical disease that can cause severe disfiguring lesions or fatal visceral infections (31), and although there have been substantial efforts for decades to develop a leishmanial vaccine, no vaccine exists for this infection. This is in spite of the fact that a primary infection with leishmania usually leads to resistance to reinfection. In order to determine how best to generate effective long-term immunity, we studied infection-induced resistance in mice infected with L. major and found that CD4+ T cells that resided in the skin following a primary infection provided the best protection (4). Thus, after mice had resolved an infection, we were able to find leishmania-specific CD4+ T cells present at skin sites distant from the site of the primary lesion. Furthermore, in skin grafting experiments, we demonstrated that these tissue-resident memory T cells (Trm cells) were maintained in the absence of persistent parasites and could provide significant protection independent of any systemic immune responses (4). Taken together with multiple studies showing the superior protective capacity of tissue-resident memory T cells against many other infections (32), these results indicate that a leishmania vaccine should be targeting Trm cells.
While our studies have identified the appropriate T cells to target in a vaccine, the other major hurdle has been identifying appropriate vaccine candidates that generate effective long-term immunity. A substantial step forward was the identification of a class II-restricted immunodominant antigen (PEPCK) in leishmania. At the peak of infection in mice, over 12% of the CD4+ T cells in the blood recognized PEPCK (29), and vaccination with PEPCK protein, peptides, or DNA could induce significant protection against an L. major challenge. Moreover, PEPCK was found to be a component of many different species of leishmania, including those that cause both cutaneous and visceral disease, making it likely that it could be part of a panleishmania vaccine. Consensus vaccines have the potential to induce cross-protection across species, making it possible to envision a single vaccine that could provide broad protection against a number of Leishmania parasite strains. Therefore, we chose a consensus PEPCK for our studies of how best to induce skin-resident T cells. While intradermal SEP PEPCK vaccination was able to provide protection against L. major challenge and reduce parasite burdens postvaccination and at a memory time point, immune mice still had a lower parasite burden, suggesting room for improvement. As immune mice are whole-parasite experienced, including other antigens besides PEPCK may help improve vaccine-induced immune responses and further reduce parasite burdens as well as lesion size and inflammation.
The notion that T cells primed in a specific site are more likely to return to that site as effector cells is well established, and therefore, it is consistent that the i.d. route may be superior to the i.m. route for generating skin-resident T cells. An excellent example of this concept comes from the earliest vaccine, the use of scarification with vaccinia, which not only generates resident memory T cells at the site of immunization but also leads to the accumulation of Trm cells in sites distant from the immunized skin (3). Similarly, our studies demonstrate that i.d. immunization also generates global immunity in the skin, in a platform that could easily be transferred to human vaccination. Aside from a better ability to generate skin-resident T cells, there may be additional advantages to the i.d. route. As the skin contains more antigen-presenting cells than the muscle, it is believed that i.d. delivery may result in a more efficient vaccination, potentially leading to a dose-sparing effect. This could have a significant impact on the pandemic vaccine field, where there is always a fear of a limited supply of available doses. Data from rabies and influenza clinical trials suggest that i.d. delivery can maintain similar antibody titers while reducing the dose, compared to i.m. delivery (33). Intradermal vaccination is also considered more tolerable than intramuscular vaccination, which can have a positive impact on vaccination rates and adherence, and with the continued development of intradermal devices that reduce the need for needles, reduced needlestick injuries and greater safety may follow (37).
As the climate continues to change, Leishmania, endemic to tropical and subtropical regions, will continue to spread throughout the globe. More therapies are desperately needed to treat leishmaniasis patients, and an effective vaccine could dramatically reduce the burden associated with this disease. Our studies in mice provide the foundation for how best to translate this experimental vaccine into a practical effective vaccine for human leishmaniasis and demonstrate the superior nature of the i.d. route for generating skin-resident Trm cells.
MATERIALS AND METHODS
DNA constructs.
The HIV consensus clade C envelope vaccine used in these studies was previously described (34). Briefly, a consensus sequence of HIV clade C was generated based on the sequences retrieved from HIV databases (http://www.hiv.lanl.gov). To produce a CCR5-tropic version of the HIV-1 envelope, six important amino acids in the V3 loop were designed according to the sequences of early transmitter isolates. Six amino acids in the V1 loop and three amino acids in the V2 loop were deleted. The cytoplasmic tail region was removed to promote higher expression levels of Env protein. The gp120/41 Env cleavage site was incorporated to promote proper folding of the synthetic Env protein. A more efficient leader sequence was added to the N terminus of the gene.
A consensus sequence of Leishmania phosphoenolpyruvate carboxykinase (PEPCK) was generated from sequences obtained from the UniProt database for the Leishmania species L. infantum, L. major, L. mexicana, and L. donovani (accession numbers A4I2Y7, E9ADF9, E9AZ81, and E9BJI0) and is referred to here as IDM2.
The second consensus sequence of Leishmania PEPCK was generated from sequences obtained from the UniProt database for the Leishmania species L. panamensis and L. braziliensis (accession numbers A0A088RTT4 and A4HFV1) and is referred to here as PB.
For both IDM2 and PB, a more efficient immunoglobulin E (IgE) leader sequence was added to the N-terminal region of the gene, as previously described (35). Both inserts were specifically RNA and then codon optimized. The synthesized IDM2 and PB were digested with BamHI and XhoI and cloned into the pGX0001 backbone (GenScript, Piscataway, NJ) under the control of the cytomegalovirus (CMV) immediate early promoter. Endotoxin levels were tested and found to be ≤0.005 endotoxin units (EU)/μg, and no residual RNA or genomic DNA was visible on an agarose gel.
Western blotting.
Transfections were performed using the TurboFectin 8.0 reagent, according to the manufacturer’s protocols (OriGene, Rockville, MD). Briefly, HEK293T cells were grown to 80% confluence in 6-well tissue culture plates and transfected with 2 μg of hemagglutinin (HA)-tagged IDM2 or PB. The cells were collected 2 days after transfection, washed twice with phosphate-buffered saline (PBS), and lysed with cell lysis buffer (Cell Signaling Technology, Danvers, MA). Gradient (4% to 12%) Bis-Tris NuPAGE gels (Life Technologies, Carlsbad, CA) were loaded with transfected cell lysates and transferred to a polyvinylidene difluoride (PDVF) membrane. The membranes were blocked in PBS Odyssey blocking buffer (Li-Cor Biosciences, Lincoln, NE, USA) for 1 h at room temperature. To detect plasmid expression, the anti-HA (clone 5E11D8, catalog number A01244; GenScript) antibody was diluted 1:1,000, and the anti-β-actin antibody was diluted 1:5,000 in Odyssey blocking buffer with 0.2% Tween 20 (Bio-Rad, Hercules, CA) and incubated with the membranes overnight at 4°C. The membranes were washed with PBS-Tween (PBST) and then incubated with the appropriate secondary antibody (goat anti-mouse IRDye680CW; Li-Cor Biosciences) at a 1:15,000 dilution in Odyssey blocking buffer for 1 h at room temperature. After washing, the membranes were imaged on the Odyssey infrared imager (Li-Cor Biosciences).
Immunofluorescence assay.
For the immunofluorescence assay (IFA), HEK293T cells were grown in 6-well tissue culture glass-bottom plates and transfected with 2 μg of the IDM2 or PB vaccine. Two days after transfection, the cells were fixed with 4% paraformaldehyde for 15 min. Nonspecific binding was then blocked with normal goat serum diluted in PBS at room temperature for 1 h. The plates were then washed in PBS for 5 min and subsequently incubated with sera from IDM2- and PB-immunized mice at a 1:100 dilution overnight at 4°C. The plates were washed as described above and incubated with the appropriate secondary antibody (goat anti-mouse IgG–Alexa Fluor 488 [AF488]; Sigma, St. Louis, MO) at 1:200 dilutions at room temperature for 1 h. After washing, DAPI (4′,6-diamidino-2-phenylindole; Millipore Sigma, Burlington, MA) was added to stain the nuclei of all cells according to the manufacturer’s protocol. Wells were washed and maintained in PBS and observed under a microscope (Evos cell imaging systems; Life Technologies).
Animals.
All mice were housed in compliance with the NIH, the University of Pennsylvania School of Veterinary Medicine, and the Wistar Institutional Animal Care and Use Committee (IACUC). Six- to eight-week-old female C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME).
Animal immunizations.
For HIV studies, mice were immunized with 25 μg of DNA in a total volume of 30 μl of water. Mice were injected either intramuscularly (i.m.) in the shaved tibialis anterior (TA) muscle followed by electroporation (EP) using Cellectra 3P or intradermally (i.d.) in the shaved abdominal flank followed by EP using surface electroporation (SEP) (Inovio Pharmaceuticals, Plymouth Meeting, PA), as previously described (36). For PEPCK studies, mice were immunized with 20 μg of IDM2 and 20 μg of PB in 30 μl water delivered either intramuscularly or intradermally as described above. Mice were immunized two times at 2-week intervals.
Electroporation devices.
The epidermis-targeting SEP device consists of an electrode array made up from a 4-by-4 array of gold-plated trocar needles with a 0.43-mm diameter at a 1.5-mm spacing (Inovio Pharmaceuticals). The SEP array was pressed down on the surface of the skin above the intradermal bleb (or wheel) made by Mantoux delivery of a 30-μl plasmid formulation, in a manner in which all electrodes across the array made contact with the surface of the skin. The electrodes did not penetrate the live skin layers. Three individual 100-ms pulses of 10 to 50 V were delivered. The Cellectra 3P EP device (Inovio Pharmaceuticals) was employed to assist in the delivery of the 30-μl plasmid formulation to mouse TA. For each immunization, mice were administered two 0.1-A electric constant current square-wave pulses.
Animal challenge studies.
Mice were challenged with 2 × 106 L. major parasites in the ear 1 week after the final vaccination. L. major (WHO/MHOM/IL/80/Friedlin) parasites were grown in Schneider’s insect medium (Invitrogen) supplemented with 20% heat-inactivated fetal bovine serum (FBS), 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Metacyclic enriched promastigotes were used for infection. Immune mice were previously challenged in the opposite ear 12 weeks before subsequent challenge with the same number of parasites. For memory studies, mice were rested for 6 weeks after the final vaccination before being challenged with 2 × 106 L. major parasites.
Flank skin isolation.
Flank skin was shaved using an electric trimmer equipped with a two-hole precision blade (Wahl). A section of dermis was excised and then minced with a sterile scalpel blade into ∼2-mm sections. Flank sections were incubated in RPMI containing 250 μg/ml Liberase TL for 120 min, with vortexing every 30 min. The resulting solution was passed through a 40-μm cell strainer and resuspended in complete RPMI (cRPMI), which is supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 55 μM 2-mercaptoethanol.
Ear isolation.
For ear preparation, dorsal and ventral layers of the ear were separated and incubated in RPMI (Invitrogen) with 250 μg/ml Liberase TL (Roche, Basel, Switzerland) for 90 min at 37°C in 5% CO2. Ears were dissociated using a 40-μm cell strainer (BD) and resuspended in RPMI medium containing 10% FBS.
The parasite burden from ear was calculated by serial 2-fold dilution in 96-well plates of complete Schneider's medium (CSM) and incubation at 26°C. The number of viable parasites was calculated from the highest dilution at which parasites were observed 7 days into culture.
ELISpot assay.
Anti-IFN-γ-precoated 96-well plates (MabTech, Cincinnati, OH) were used to quantify IFN-γ responses to the vaccine. Spleens were isolated from mice either 1 week after the final vaccination or at 6 weeks postchallenge. Single-cell suspensions of splenocytes were made by homogenizing and processing the spleens through a 40-μm cell strainer. Cells were then resuspended in ACK lysing buffer (Gibco) for 5 min to lyse red blood cells before two washes with PBS and final resuspension in complete RPMI medium (RPMI 1640 plus 10% FBS and 1% penicillin-streptomycin).
For HIV studies, 200,000 splenocytes were added to each well and stimulated overnight at 37°C in 5% CO2 with R10 (negative control), concanavalin A (3 μg/ml; positive control), or specific HIV Env clade C peptides (NIH AIDS Reagent Program). Peptide pools consisted of 15-mer residues overlapping by 11 amino acids, representing the entire protein consensus sequence of HIV-1 clade C, which were obtained from the NIH AIDS Research and Reference Reagent Program. The Env peptides were pooled at a concentration of 2 μg/ml/peptide into 4 pools as antigens for specific stimulation of IFN-γ release.
For PEPCK studies, 200,000 splenocytes were added to each well and stimulated overnight at 37°C in 5% CO2 with R10 (negative control), concanavalin A (3 μg/ml; positive control), or specific IDM2 or PB peptides (GenScript). Peptide pools consisted of 15-mer peptides overlapping by 9 amino acids, representing the entire protein consensus of IDM2 or PB, which were obtained from GenScript. The IDM2 or PB peptides were pooled at a concentration of 1 mg/ml/peptide into 3 pools as antigens for specific stimulation of IFN-γ release.
After 18 h of stimulation, the plates were washed and developed according to the manufacturer’s protocol. The plates were then rinsed with distilled water and dried at room temperature overnight. Spots were counted by an automated ELISpot reader (Cellular Technology Ltd.).
Flow cytometry.
For flow cytometry analysis, 2 million cells were stimulated in 96-well plates with overlapping peptide pools of either IDM2 or PB PEPCK protein for PEPCK studies and HIV Env clade C protein for HIV studies as stated above for ELISpot assays, with medium alone (negative control), and with phorbol 12-myristate 13-acetate (PMA) and ionomycin (BD Biosciences, San Jose, CA) (positive control) for 6 h at 37°C with 5% CO2 in the presence of GolgiPlug and GolgiStop (BD Biosciences). After 6 h, cells were collected and stained in fluorescence-activated cell sorter (FACS) buffer with a panel of surface antibodies containing live/dead eFluor V450, fluorescein isothiocyanate (FITC) anti-CD4, Alexa Fluor 700 anti-CD44, and allophycocyanin (APC)-Cy7 anti-CD8 for 30 min at 4°C. Cells were washed and then fixed with Foxp3/transcription factor fixation/permeabilization buffer (Thermo Fischer Scientific) for 20 min at 4°C. Cells were washed with Perm/Wash buffer before intracellular staining with phycoerythrin (PE)-Cy7 anti-IL-2, peridinin chlorophyll protein (PerCP)-Cy5.5 anti-CD3ε, PE anti-TNF-α, and APC anti-IFN-γ for 1 h at 4°C. Cells were then washed with Perm/Wash buffer before suspension in Perm/Wash buffer and acquisition on a BD LSRII instrument. All results were analyzed using FlowJo v.10.0 (TreeStar).
Statistical analysis.
Statistical analysis was performed using one-way modified analysis of variance (ANOVA) with a Tukey post hoc test for all studies. All analysis was performed using GraphPad Prism software (GraphPad, La Jolla, CA). Horizontal bars represent means, with error bars expressing the standard errors.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Animal Facility staff at the Wistar Institute and University of Pennsylvania School of Veterinary Medicine for providing house and care to the animals. We thank Jeffery Faust and the Flow Cytometry Core at the Wistar Institute for assistance with flow cytometry experiments. We thank the NIH AIDS Research and Reference Reagent Program for reagents.
We thank the NIH and W. W. Smith Charitable Trust for providing funding and support.
D.B.W. has received grant funding, participates in industry collaborations, has received speaking honoraria, and has received fees for consulting, including serving on scientific review committees and board series. Remuneration received by D.B.W. includes direct payments and stock or stock options, and in the interest of disclosure he notes potential conflicts associated with his work with Inovio and possible others.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00227-19.
REFERENCES
- 1.Gaide O, Emerson RO, Jiang X, Gulati N, Nizza S, Desmarais C, Robins H, Krueger JG, Clark RA, Kupper TS. 2015. Common clonal origin of central and resident memory T cells following skin immunization. Nat Med 21:647–653. doi: 10.1038/nm.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN, Heath WR, Carbone FR, Gebhardt T. 2012. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci U S A 109:7037–7042. doi: 10.1073/pnas.1202288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. 2012. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature 483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glennie ND, Yeramilli VA, Beiting DP, Volk SW, Weaver CT, Scott P. 2015. Skin-resident memory CD4+ T cells enhance protection against Leishmania major infection. J Exp Med 212:1405–1414. doi: 10.1084/jem.20142101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu L, Zhong Q, Tian T, Dubin K, Athale SK, Kupper TS. 2010. Physical disruption of skin during poxvirus immunization is critical for the generation of highly protective T cell-mediated immunity. Nat Med 16:224–227. doi: 10.1038/nm.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levin C, Perrin H, Combadiere B. 2015. Tailored immunity by skin antigen-presenting cells. Hum Vaccin Immunother 11:27–36. doi: 10.4161/hv.34299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray JI, Westerhof LM, MacLeod MKL. 2018. The roles of resident, central and effector memory CD4 T‐cells in protective immunity following infection or vaccination. Immunology 154:574–581. doi: 10.1111/imm.12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iborra S, Martínez-López M, Khouili SC, Enamorado M, Cueto FJ, Conde-Garrosa R, del Fresno C, Sancho D. 2016. Optimal generation of tissue-resident but not circulating memory T cells during viral infection requires crosspriming by DNGR-1+ dendritic cells. Immunity 45:847–860. doi: 10.1016/j.immuni.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takamura S. 2018. Niches for the long-term maintenance of tissue-resident memory T cells. Front Immunol 9:1214. doi: 10.3389/fimmu.2018.01214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosa FD, Gebhardt T. 2016. Bone marrow T cells and the integrated functions of recirculating and tissue-resident memory T cells. Front Immunol 7:51. doi: 10.3389/fimmu.2016.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.PLoS Neglected Tropical Diseases Staff. 2016. Correction: A historical overview of the classification, evolution, and dispersion of Leishmania parasites and sandflies. PLoS Negl Trop Dis 10:e0004770. doi: 10.1371/journal.pntd.0004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ponte-Sucre A, Gamarro F, Dujardin J-C, Barrett MP, López-Vélez R, García-Hernández R, Pountain AW, Mwenechanya R, Papadopoulou B. 2017. Drug resistance and treatment failure in leishmaniasis: a 21st century challenge. PLoS Negl Trop Dis 11:e0006052. doi: 10.1371/journal.pntd.0006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sundar S, Singh B. 2014. Identifying vaccine targets for anti-leishmanial vaccine development. Expert Rev Vaccines 13:489–505. doi: 10.1586/14760584.2014.894467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sunyoto T, Potet J, Boelaert M. 2018. Why miltefosine—a life-saving drug for leishmaniasis—is unavailable to people who need it the most. BMJ Glob Health 3:e000709. doi: 10.1136/bmjgh-2018-000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.den Boer M, Argaw D, Jannin J, Alvar J. 2011. Leishmaniasis impact and treatment access. Clin Microbiol Infect 17:1471–1477. doi: 10.1111/j.1469-0691.2011.03635.x. [DOI] [PubMed] [Google Scholar]
- 16.Bush JT, Wasunna M, Alves F, Alvar J, Olliaro PL, Otieno M, Sibley CH, Strub Wourgaft N, Guerin PJ. 2017. Systematic review of clinical trials assessing the therapeutic efficacy of visceral leishmaniasis treatments: a first step to assess the feasibility of establishing an individual patient data sharing platform. PLoS Negl Trop Dis 11:e0005781. doi: 10.1371/journal.pntd.0005781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.González U, Pinart M, Reveiz L, Rengifo-Pardo M, Tweed J, Macaya A, Alvar J. 2010. Designing and reporting clinical trials on treatments for cutaneous leishmaniasis. Clin Infect Dis 51:409–419. doi: 10.1086/655134. [DOI] [PubMed] [Google Scholar]
- 18.López‐Carvajal L, Vélez I, Arbeláez MP, Olliaro P. 2018. Eligibility criteria and outcome measures adopted in clinical trials of treatments of cutaneous leishmaniasis: systematic literature review covering the period 1991–2015. Trop Med Int Health 23:448–475. doi: 10.1111/tmi.13048. [DOI] [PubMed] [Google Scholar]
- 19.Khalil E, Hassan A, Zijlstra E, Mukhtar M, Ghalib H, Musa B, Ibrahim M, Kamil AA, Elsheikh M, Babiker A, Modabber F. 2000. Autoclaved Leishmania major vaccine for prevention of visceral leishmaniasis: a randomised, double-blind, BCG-controlled trial in Sudan. Lancet 356:1565–1569. doi: 10.1016/S0140-6736(00)03128-7. [DOI] [PubMed] [Google Scholar]
- 20.Bahar K, Dowlati Y, Shidani B, Alimohammadian MH, Khamesipour A, Ehsasi S, Hashemi-Fesharki R, Ale-Agha S, Modabber F. 1996. Comparative safety and immunogenicity trial of two killed Leishmania major vaccines with or without BCG in human volunteers. Clin Dermatol 14:489–495. doi: 10.1016/0738-081X(96)00071-5. [DOI] [PubMed] [Google Scholar]
- 21.Noazin S, Khamesipour A, Moulton LH, Tanner M, Nasseri K, Modabber F, Sharifi I, Khalil EA, Bernal ID, Antunes CM, Smith PG. 2009. Efficacy of killed whole-parasite vaccines in the prevention of leishmaniasis—a meta-analysis. Vaccine 27:4747–4753. doi: 10.1016/j.vaccine.2009.05.084. [DOI] [PubMed] [Google Scholar]
- 22.Vélez ID, Gilchrist K, Arbelaez MP, Rojas CA, Puerta JA, Antunes CMF, Zicker F, Modabber F. 2005. Failure of a killed Leishmania amazonensis vaccine against American cutaneous leishmaniasis in Colombia. Trans R Soc Trop Med Hyg 99:593–598. doi: 10.1016/j.trstmh.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Armijos RX, Weigel MM, Aviles H, Maldonado R, Racines J. 1998. Field trial of a vaccine against New World cutaneous leishmaniasis in an at‐risk child population: safety, immunogenicity, and efficacy during the first 12 months of follow‐up. J Infect Dis 177:1352–1357. doi: 10.1086/515265. [DOI] [PubMed] [Google Scholar]
- 24.Sharifi I, Fekri AR, Aflatonian M-R, Khamesipour A, Nadim A, Mousavi M-RA, Momeni AZ, Dowlati Y, Godal T, Zicker F, Smith PG, Modabber F. 1998. Randomised vaccine trial of single dose of killed Leishmania major plus BCG against anthroponotic cutaneous leishmaniasis in Bam, Iran. Lancet 351:1540–1543. doi: 10.1016/S0140-6736(98)09552-X. [DOI] [PubMed] [Google Scholar]
- 25.Armijos RX, Weigel MM, Calvopina M, Hidalgo A, Cevallos W, Correa J. 2004. Safety, immunogenecity [sic], and efficacy of an autoclaved Leishmania amazonensis vaccine plus BCG adjuvant against New World cutaneous leishmaniasis. Vaccine 22:1320–1326. doi: 10.1016/j.vaccine.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Nadim A, Javadian E, Mohebali M. 1997. The experience of leishmanization in the Islamic Republic of Iran. East Mediterr Health J 3:284–289. [Google Scholar]
- 27.Gafurov IM. 1999. Experience in controlling and preventing zoonotic cutaneous leishmaniasis in Uzbekistan. Med Parazitol (Mosk) 1999:58–59. (In Russian.) [PubMed] [Google Scholar]
- 28.Khamesipour A, Dowlati Y, Asilian A, Hashemifesharki R, Javadi A, Noazin S, Modabber F. 2005. Leishmanization: use of an old method for evaluation of candidate vaccines against leishmaniasis. Vaccine 23:3642–3648. doi: 10.1016/j.vaccine.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 29.Mou Z, Li J, Boussoffara T, Kishi H, Hamana H, Ezzati P, Hu C, Yi W, Liu D, Khadem F, Okwor I, Jia P, Shitaoka K, Wang S, Ndao M, Petersen C, Chen J, Rafati S, Louzir H, Muraguchi A, Wilkins JA, Uzonna JE. 2015. Identification of broadly conserved cross-species protective Leishmania antigen and its responding CD4+ T cells. Sci Transl Med 7:310ra167. doi: 10.1126/scitranslmed.aac5477. [DOI] [PubMed] [Google Scholar]
- 30.Glennie N, Volk SW, Scott P. 2017. Skin-resident CD4 T cells protect against Leishmania major by recruiting and activating inflammatory monocytes. PLoS Pathog 13:e1006349. doi: 10.1371/journal.ppat.1006349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott P, Novais FO. 2016. Cutaneous leishmaniasis: immune responses in protection and pathogenesis. Nat Rev Immunol 16:581–592. doi: 10.1038/nri.2016.72. [DOI] [PubMed] [Google Scholar]
- 32.Muruganandah V, Sathkumara HD, Navarro S, Kupz A. 2018. A systematic review: the role of resident memory T cells in infectious diseases and their relevance for vaccine development. Front Immunol 9:1574. doi: 10.3389/fimmu.2018.01574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laurent PE, Bourhy H, Fantino M, Alchas P, Mikszta JA. 2010. Safety and efficacy of novel dermal and epidermal microneedle delivery systems for rabies vaccination in healthy adults. Vaccine 28:5850–5856. doi: 10.1016/j.vaccine.2010.06.062. [DOI] [PubMed] [Google Scholar]
- 34.Yan J, Corbitt N, Pankhong P, Shin T, Khan A, Sardesai NY, Weiner DB. 2011. Immunogenicity of a novel engineered HIV-1 clade C synthetic consensus-based envelope DNA vaccine. Vaccine 29:7173–7181. doi: 10.1016/j.vaccine.2011.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muthumani K, Griffin BD, Agarwal S, Kudchodkar SB, Reuschel EL, Choi H, Kraynyak KA, Duperret EK, Keaton AA, Chung C, Kim YK, Booth SA, Racine T, Yan J, Morrow MP, Jiang J, Lee B, Ramos S, Broderick KE, Reed CC, Khan AS, Humeau L, Ugen KE, Park YK, Maslow JN, Sardesai NY, Kim JJ, Kobinger GP, Weiner DB. 2016. In vivo protection against ZIKV infection and pathogenesis through passive antibody transfer and active immunisation with a prMEnv DNA vaccine. NPJ Vaccines 1:16021. doi: 10.1038/npjvaccines.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith TRF, Schultheis K, Morrow MP, Kraynyak KA, McCoy JR, Yim KC, Muthumani K, Humeau L, Weiner DB, Sardesai NY, Broderick KE. 2017. Development of an intradermal DNA vaccine delivery strategy to achieve single-dose immunity against respiratory syncytial virus. Vaccine 35:2840–2847. doi: 10.1016/j.vaccine.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Resik S, Tejeda A, Mas Lago P, Diaz M, Carmenates A, Sarmiento L, Alemañi N, Galindo B, Burton A, Friede M, Landaverde M, Sutter RW. 2010. Randomized controlled clinical trial of fractional doses of inactivated poliovirus vaccine administered intradermally by needle-free device in Cuba. J Infect Dis 201:1344–1352. doi: 10.1086/651611. [DOI] [PubMed] [Google Scholar]
- 38.Sangaré L, Manhart L, Zehrung D, Wang CC. 2009. Intradermal hepatitis B vaccination: a systematic review and meta-analysis. Vaccine 27:1777–1786. doi: 10.1016/j.vaccine.2009.01.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.