Neutrophils are the most abundant circulating leukocytes in humans and are essential for the defense against invading pathogens. Like many other cells of an organism, neutrophils can be highly influenced by the diet. We have previously described that mice fed a high-fat diet rich in polyunsaturated fatty acids (HFD-P) present a higher frequency of neutrophils in bone marrow than mice fed a high-fat diet rich in saturated fatty acids (HFD-S).
KEYWORDS: apoptosis, chemotaxis, dietary fatty acids, neutrophils, polyunsaturated fatty acids
ABSTRACT
Neutrophils are the most abundant circulating leukocytes in humans and are essential for the defense against invading pathogens. Like many other cells of an organism, neutrophils can be highly influenced by the diet. We have previously described that mice fed a high-fat diet rich in polyunsaturated fatty acids (HFD-P) present a higher frequency of neutrophils in bone marrow than mice fed a high-fat diet rich in saturated fatty acids (HFD-S). Interestingly, such an increase correlated with improved survival against bacterium-induced sepsis. In this study, we aimed to investigate the effects of dietary polyunsaturated and saturated fatty acids on neutrophil homeostasis. We found that HFD-P specifically induced the accumulation of neutrophils in the marginal pools of the spleen and liver. The accumulation of neutrophils in the spleen was a result of a dual effect of polyunsaturated fatty acids on neutrophil homeostasis. First, polyunsaturated fatty acids enhanced the recruitment of neutrophils from the circulation into the spleen via chemokine secretion. Second, they delayed neutrophil cell death in the spleen. Interestingly, these effects were not observed in mice fed a diet rich in saturated fatty acids, suggesting that the type of fat rather than the amount of fat mediates the alterations in neutrophil homeostasis. In conclusion, our results show that dietary polyunsaturated fatty acids have a strong modulatory effect on neutrophil homeostasis that may have future clinical applications.
INTRODUCTION
Neutrophils are the most abundant circulating leukocytes in humans and constitute the first cellular line of defense against invading pathogens (1). Their importance is exemplified in patients with neutrophil deficit caused either by diseases, such as congenital neutropenia, or by therapy-induced side effects. These neutropenic patients are highly susceptible to infections by fungal and bacterial pathogens (2, 3).
Neutrophils are rapidly recruited to the site of infection. Once there, they destroy invading microorganisms by direct phagocytosis and through the secretion of specialized antimicrobial molecules (4). However, excessive or prolonged neutrophil activation results in chronic inflammation and collateral damage of host tissues. Neutrophil-induced tissue damage is often observed in diseases such as acute respiratory distress syndrome (5) and arthritis (6). Thus, regulation of neutrophil activation and homeostasis is essential for the balance between the elimination of invading microorganisms and self-damage.
Under homeostatic conditions, neutrophils are produced in the bone marrow from precursor cells via granulocyte colony-stimulating factor (G-CSF)- and granulocyte-macrophage colony-stimulating factor (GM-CSF)-induced granulopoiesis (7, 8). Large quantities of newly synthesized neutrophils rest inside the bone marrow until they are released into the circulation and toward specific organs. Marginal pools of neutrophils can be found in the spleen and the liver (9). The migration of neutrophils from the bone marrow is induced by the C-X-C motif chemokine receptor 2 (CXCR2) and its ligands, whereas the retention of neutrophils in the bone marrow is mediated by the binding of the C-X-C motif chemokine receptor 4 (CXCR4) to its ligand C-X-C motif chemokine ligand 12 (CXCL12) (1). In mice, four ligands for CXCR2 have been characterized to date: keratinocyte chemoattractant/C-X-C motif chemokine ligand 1 (KC/CXCL1), macrophage inflammatory protein 2/C-X-C motif chemokine ligand 2 (MIP-2/CXCL2), lipopolysaccharide-induced CXC chemokine/C-X-C motif chemokine ligand 5 (LIX/CXCL5), and lungkine (10). Increased CXCR4 expression has also been found in aged neutrophils, promoting their homing back to the bone marrow for elimination (11, 12). G-CSF interaction with the neutrophil G-CSF receptor (G-CSFR) also contributes to the induction of neutrophil release from the bone marrow into the circulation (13).
Upon immunological stress, such as an infection, neutrophils are quickly recruited from the bloodstream to the site of inflammation by signals secreted by injured or infected cells (14). These signals vary in nature and range from lipid mediators to chemokines. Neutrophils express a great variety of receptors that sense and integrate signals from damaged tissues (15).
Due to their potential inflammatory properties, an appropriate number of circulating and tissue neutrophils must be maintained through the balance between their production and release from the bone marrow and their destruction in three main organs: the spleen, the liver, and the bone marrow itself (14). Aged or damaged neutrophils are detected, phagocytosed, and eliminated by tissue-resident macrophages (16). Interestingly, recent studies suggest that the spleen may serve as a reservoir of neutrophils and that this cell type exerts important immunomodulatory functions in the spleen (17, 18). Splenic neutrophils induce the differentiation of naive T cells into inflammatory Th1 cells by the secretion of cytokines, such as interleukin-12 (IL-12) (17). Moreover, neutrophils perform antigen presentation to B cells from the spleen marginal zone (18).
In previous studies, we demonstrated how a diet rich in polyunsaturated fatty acids increases survival against Staphylococcus aureus infection as well as the frequency of neutrophils in the bone marrow and blood (19). However, the effects of dietary fatty acids on neutrophil homeostasis still remain largely unknown. Hence, this study aims to further investigate the impact of dietary polyunsaturated and saturated fatty acids on neutrophil homeostasis.
RESULTS
Dietary polyunsaturated fatty acids (PUFA) increase spleen size.
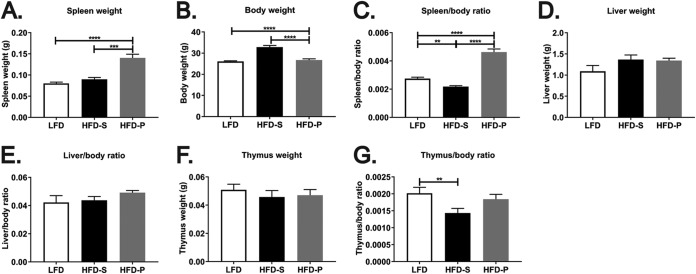
In the course of our investigations, we observed that mice fed a high-fat diet rich in polyunsaturated fatty acids (HFD-P) for 4 weeks had significantly larger spleens (Fig. 1A) than mice fed a high-fat diet rich in saturated fatty acids (HFD-S) (P < 0.001) and a low-fat diet (LFD) (P < 0.0001). After 4 weeks of feeding, mice fed HFD-S had 25% more body weight than mice fed LFD. Mice fed HFD-P (containing the same caloric density as HFD-S) had the same body weight as mice fed LFD (Fig. 1B). Therefore, alterations of body weight caused by the diets do not reflect changes in spleen size. The spleen weight normalized to body weight still showed enlarged spleens in HFD-P-fed mice compared with HFD-S- and LFD-fed mice (Fig. 1C; P < 0.0001) after 4 weeks of diet. After 8 weeks, however, both high-fat diets increased body weight, as we have previously shown (19), but they did it to different extents. Whereas mice fed HFD-S had 40% more body weight than mice fed LFD, mice fed HFD-P had only 15% more body weight than mice fed LFD after 8 weeks of diet. Spleen size normalized to body weight still reflected enlarged spleens in HFD-P-fed mice compared to mice fed the other diets (see Fig. S1A and B in the supplemental material).
FIG 1.
A high-fat diet rich in polyunsaturated fatty acids (HFD-P) increases spleen size. The spleen weight (A), body weight (B), spleen weight normalized to body weight (C), liver weight (D), liver weight normalized to body weight (E), thymus weight (F), and thymus weight normalized to body weight (G) in mice fed LFD, HFD-S, and HFD-P for 4 weeks are shown. Data are for 27 mice per group for panels A, B, and C; 6 mice per group for panels D and E; and 10 mice per group for panels F and G. For panels A and C, data from two different experiments were pooled and the results are shown as the estimated marginal means + SEM. For panels B, D, E, F, and G, the data are shown as the mean + SEM. **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001.
We have previously reported that mice fed HFD-S have an increased liver size compared with mice fed LFD and HFD-P after 8 weeks of diet (20). However, after 4 weeks of diet, the size of the liver was not altered by either HFD-S or HFD-P (Fig. 1D and E). The size of the thymus remained unaltered by the diets after 4 weeks (Fig. 1F), although HFD-S-fed mice had a decreased thymus weight/body weight ratio compared with LFD-fed mice (Fig. 1G). No differences in thymus size were observed between the different groups after 8 weeks of diet (Fig. S1C and D).
PUFA promote accumulation of innate immune cells in the spleen.
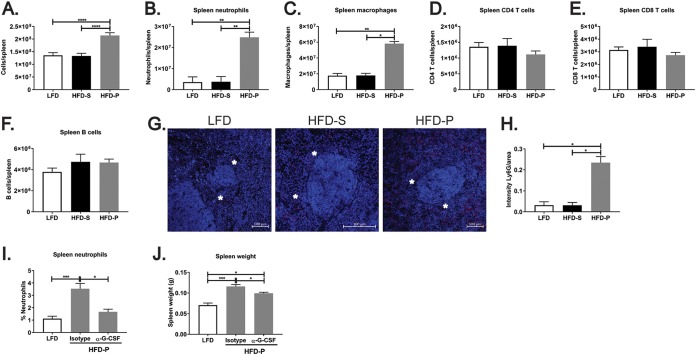
Along with spleen enlargement, an increase in the total number of spleen cells was observed in the spleens from mice fed HFD-P (Fig. 2A) compared with that in the spleens from mice fed HFD-S (P < 0.0001) and LFD (P < 0.0001).
FIG 2.
Dietary polyunsaturated fatty acids (PUFA) promote accumulation of innate immune cells in the spleen. (A) Quantification of the total number of cells in the spleens of mice fed LFD, HFD-S, and HFD-P for 4 weeks. Data are for 17 mice per group. (B to F) Quantification of neutrophils (B), macrophages (C), CD4 T cells (D), CD8 T cells (E), and B cells (F) in the spleens of mice fed the different diets for 4 weeks. Data are for 10 mice per group. (G) Immunofluorescence staining of spleen neutrophils (red) in mice fed the different diets for 4 weeks. Nuclei were stained with DAPI (blue). Asterisks mark the perimarginal zone. (H) Quantification of immunofluorescence staining of spleen neutrophils normalized per area. Data are for 10 mice per group. (I and J) Frequency of spleen neutrophils (I) and spleen weight (J) in mice fed HFD-P upon treatment with anti-G-CSF antibody or an isotype control. Data are for 10 mice per group. For panels A to F, I, and J, data from two different experiments were pooled and the results are shown as the estimated marginal means + SEM. For the rest of the panels, data are shown as the mean + SEM. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.001.
We have previously shown that feeding mice HFD-P induces the accumulation of neutrophils in the bone marrow and increases the frequency of circulating neutrophils (19). These results prompted us to perform neutrophil quantification in the spleens of mice fed the different diets.
Live neutrophils (7-aminoactinomycin D [7-AAD]-negative [7-AAD−] Ly6G-positive [Ly6G+] cells) in the spleens of mice were quantified using flow cytometry after 4 weeks of feeding of LFD, HFD-S, or HFD-P. A 7-fold increase in the number of neutrophils was found in the spleens from mice fed HFD-P (Fig. 2B) compared with those from mice fed LFD (P < 0.001) and HFD-S (P < 0.001). This effect persisted when the diets were prolonged for 8 weeks (Fig. S2A). No changes in the number of neutrophils in the livers of mice fed LFD, HFD-S, and HFD-P for 4 weeks were found (Fig. S2B). However, a significant increase in neutrophil frequency was found in the livers of mice fed HFD-P (P < 0.001) compared with the livers of mice fed the other two diets after 8 weeks of feeding (Fig. S2C).
Multiple types of immune cells from both the innate and the adaptive immune systems converge in the spleen. To clarify whether the increased frequency of neutrophils in mice fed HFD-P was a cell-specific effect or a general activation of the immune system, we also quantified by flow cytometry the number of macrophages/monocytes (7-AAD− F4/80+), CD4 T cells (7-AAD− CD4+), CD8 T cells (7-AAD− CD8+), and B cells (7-AAD− CD19+) in the spleens of mice fed the different diets. We chose to perform our analysis after 4 weeks of diet since that time was enough to induce the increase in spleen size and the accumulation of neutrophils in the HFD-P-fed mice. Our analysis revealed that mice fed HFD-P induced a 3-fold increase in the number of splenic macrophages (Fig. 2C) compared with the number in mice fed HFD-S (P < 0.001) and LFD (P < 0.001). In contrast, there was no effect on the numbers of CD4 (P < 0.05) and CD8 (P < 0.01 and P < 0.001) T cells (Fig. 2D and E, respectively) and of B cells (Fig. 2F) in the spleens from mice fed HFD-P compared with those in the spleens from mice fed the other two diets. Since the spleens from HFD-P-fed mice had a higher total number of cells than spleens from mice fed LFD and HFD-S, we also calculated the percentages of splenic neutrophils, macrophages, CD4 T cells, CD8 T cells, and B cells (Fig. S2D to H). After this normalization, the spleens of mice fed HFD-P still presented higher percentages of both neutrophils and macrophages, whereas the percentages of CD4 and CD8 T cells were decreased in the spleens of HFD-P-fed mice, likely caused by the increase in the percentage of macrophages and neutrophils (Fig. S2F and G). These results indicate that the addition of PUFA into the high-fat diet (HFD) specifically increases the splenic frequencies of innate immune cells, with a more pronounced effect on neutrophils.
The accumulation of neutrophils in the spleens of HFD-P-fed mice was further confirmed by immunofluorescence analysis after 4 weeks of diet (Fig. 2G and H). We observed that splenic neutrophils localized exclusively in the red pulp at the perimarginal zone (peri-MZ) (Fig. 2G). The same distribution was found by immunohistochemistry analysis in mice fed HFD-P and in mice fed LFD and HFD-S for 8 weeks (Fig. S2I). In the liver, neutrophils were found to be distributed evenly throughout the tissue (Fig. S2J).
To test whether the increase in spleen size in mice fed HFD-P was associated with the increased infiltration of neutrophils, we treated mice fed HFD-P with antibodies directed against G-CSF in order to deplete neutrophils. The anti-G-CSF treatment effectively reduced (P < 0.001) the frequency of splenic and blood neutrophils to the level observed in mice fed LFD (Fig. 2I and Fig. S2K, respectively). More importantly, the treatment partially rescued the increase in spleen size (P < 0.01) in mice fed HFD-P compared with the size of the spleens from mice fed the same diet but treated with an isotype control (Fig. 2J).
The increased frequency of splenic neutrophils upon HFD-P is not caused by higher levels of G-CSF or GM-CSF.
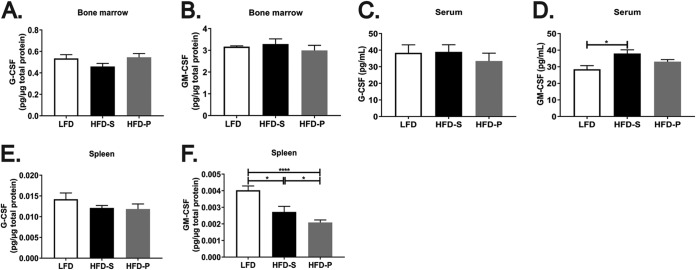
We have previously shown that mice fed HFD-P present a modest increase in the frequency of bone marrow precursors compared with mice fed HFD-S after 8 weeks of diet (19). However, this difference seems to be insufficient to explain the 7-fold increase in splenic neutrophil numbers found in mice fed HFD-P. Therefore, we hypothesized that such an effect may be driven by increased stimulation of the differentiation of precursor cells into mature neutrophils. To test this hypothesis, we analyzed the levels of G-CSF and GM-CSF in the bone marrow, serum, and spleen.
We observed no significant differences in the amounts of G-CSF and GM-CSF in the bone marrow from mice fed the different diets for 8 weeks (Fig. 3A and B, respectively), indicating that mice fed HFD-P do not have enhanced stimulation of the differentiation of precursor cells into neutrophils in the bone marrow. Also, no differences in the levels of G-CSF in the serum from mice fed the different diets for 4 weeks were found (Fig. 3C). We found higher levels of serum GM-CSF in mice fed HFD-S than in mice fed LFD and HFD-P. However, HFD-P-fed mice did not have altered levels of serum GM-CSF (Fig. 3D). Finally, we quantified the G-CSF and GM-CSF concentrations in the spleens of mice fed the different diets for 4 weeks. The G-CSF concentration in the spleen was not altered by any of the diets (Fig. 3E). However, mice fed HFD-P had a significantly lower concentration of GM-CSF (normalized to the number of micrograms of protein) than mice fed LFD (P < 0.001) or HFD-S (P < 0.05), whereas mice fed HFD-S presented an intermediate GM-CSF concentration in the spleen (Fig. 3F). Therefore, the accumulation of neutrophils in the spleens of mice fed HFD-P cannot be explained by the increased levels of G-CSF or GM-CSF in the bone marrow, spleen, or serum.
FIG 3.
The increased frequency of splenic neutrophils upon HFD-P is not caused by higher levels of G-CSF or GM-CSF. (A and B) Quantification of G-CSF protein (A) and GM-CSF protein (B) in bone marrow from mice fed LFD, HFD-S, and HFD-P for 8 weeks. Data are for 16 mice per group. (C and D) Quantification of G-CSF protein (C) and GM-CSF protein (D) in serum from mice fed LFD, HFD-S, and HFD-P for 4 weeks. Data are for 8 to 10 mice per group. (E and F) Quantification of G-CSF protein (E) and GM-CSF protein (F) in the spleens from mice fed LFD, HFD-S, and HFD-P for 4 weeks. Data are for 10 mice per group. Data are shown as the mean + SEM. *, P ≤ 0.05; ****, P ≤ 0.0001.
HFD-P induced the secretion of chemoattractant molecules to recruit neutrophils into the spleen.
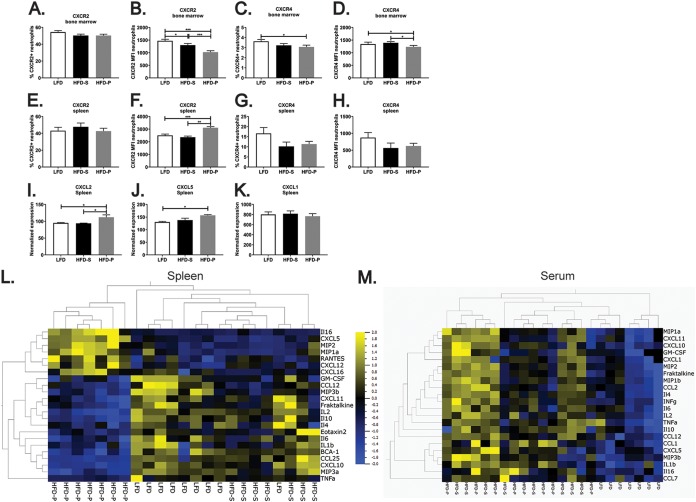
Bone marrow-differentiated neutrophils migrate to the liver and spleen in a process regulated by chemotaxis. Egression of neutrophils from the bone marrow is controlled by the ligand-mediated activation of the neutrophil receptor CXCR2 (7). Senescent neutrophils from the circulation that home back to the bone marrow for destruction present increased expression of the surface receptor CXCR4 (7). We analyzed the surface expression of CXCR2 and CXCR4 by bone marrow neutrophils to assess whether HFD-P may alter chemokine-chemokine receptor activation through the regulation of chemokine receptor expression and, therefore, promote the localization of neutrophils in the marginal pool.
Although mice fed HFD-P presented a frequency of CXCR2-positive neutrophils in the bone marrow similar to that in mice fed LFD or HFD-S (Fig. 4A), the surface expression of CXCR2 by bone marrow neutrophils from mice fed HFD-P was reduced, as indicated by the mean fluorescence intensity (MFI) (Fig. 4B), in comparison with its expression by bone marrow neutrophils from mice fed LFD (P < 0.001) or HFD-S (P < 0.001). Bone marrow from mice fed HFD-P contained fewer CXCR4-positive neutrophils (Fig. 4C) than bone marrow from mice fed LFD (P < 0.05). Additionally, bone marrow neutrophils in mice fed HFD-P expressed less surface CXCR4 (Fig. 4D) than bone marrow neutrophils from mice fed LFD (P < 0.05) and HFD-S (P < 0.05). These data suggest that neutrophil egression from the bone marrow may not be induced in mice fed HFD-P, although the diet could prevent neutrophil homing in the bone marrow to a certain extent by reducing the expression of CXCR4.
FIG 4.
HFD-P induces the secretion of chemoattractant molecules to recruit neutrophils into the spleen. (A to D) Quantification of the percentage of CXCR2+ neutrophils (A), the CXCR2 mean fluorescence intensity (MFI) of neutrophils (B), the percentage of CXCR4+ neutrophils (C) and the CXCR4 MFI of neutrophils (D) in the bone marrow of mice fed LFD, HFD-S, and HFD-P for 8 weeks. Data are for 10 mice per group. (E to H) Quantification of the percentage of CXCR2+ neutrophils (E), the CXCR2 MFI of neutrophils (F), the percentage of CXCR4+ neutrophils (G), and the CXCR4 MFI of neutrophils (H) in the spleens of mice fed LFD, HFD-S, and HFD-P for 4 weeks. Data are for 10 mice per group. (I to K) Gene expression of CXCL2 (I), CXCL5 (J), and CXCL1 (K) in the spleens of mice fed LFD, HFD-S, and HFD-P for 8 weeks normalized to actin expression. Data are for 4 mice. (L and M) Heat map representation of the chemokine and cytokine secretion profile in the spleen (L) and serum (M) of mice fed LFD, HFD-S, and HFD-P for 4 weeks (P ≤ 0.05). Data are for 7 to 9 mice per group. Data are shown as the mean + SEM. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
Next, we analyzed by flow cytometry the expression of CXCR2 and CXCR4 on the surface of neutrophils from the spleens of mice fed the different diets for 4 weeks. We found that the diets did not alter the percentage of CXCR2-positive neutrophils in the spleen (Fig. 4E). However, HFD-P increased the expression of CXCR2 on the surface of spleen neutrophils compared with LFD (P < 0.001) and HFD-S (P < 0.01) (Fig. 4F). The percentage of CXCR4-positive neutrophils and the expression of surface CXCR4 by splenic neutrophils were not affected by the diets (Fig. 4G and H).
We have previously reported the results of a complete microarray analysis of several tissues, including the spleen, from mice fed LFD, HFD-S, and HFD-P (20). When extracting gene expression data corresponding to chemokines or chemokine receptors from that analysis (20), we found no differences in the expression levels of CXCR2 in the spleens from mice fed LFD, HFD-S, and HFD-P (Fig. S3A). However, the mRNA expression of the chemoattractant molecules for neutrophils MIP-2/CXCL2 and LIX/CXCL5 (both CXCR2 ligands) was found to be significantly higher in the spleens from mice fed HFD-P than in the spleens from mice fed the other two diets (Fig. 4I and J, respectively; P < 0.05). Our microarray analysis detected KC/CXCL1 as well, but none of the diets altered its expression (Fig. 4K).
We next analyzed the protein concentration of secreted chemokines and cytokines in the spleens of mice fed the different diets. Our analysis included the simultaneous quantification of 33 chemokines and cytokines, including KC/CXCL1, MIP-2/CXCL2, and LIX/CXCL5. We found that the concentrations of 23 out of 33 cytokines/chemokines were significantly altered in the spleen by the diets with a P value of <0.05 (Fig. 4L). Interestingly, as shown in the heat map representation, HFD-P was found to be the diet altering the cytokine/chemokine profile in the spleen. Moreover, LIX/CXCL5 and MIP-2/CXCL2 were found among the 7 cytokines/chemokines whose concentrations were exclusively increased (P < 0.05) in the spleens from mice fed HFD-P. KC/CXCL1 was found at a very low concentration in the spleen, and its concentration was not altered by any of the diets (data not shown). Mice fed HFD-P also showed increased splenic concentrations of the chemokines C-C motif chemokine ligand 3 (CCL3)/MIP-1α, CXCL12, CXCL16, and RANTES and of the cytokine IL-16. In contrast, the concentration of chemokines specific for the recruitment of other immune cells and most of the cytokines was reduced in the spleens from mice fed HFD-P.
When the same analysis was applied to serum samples from mice fed the different diets, the result did not resemble our observations for the spleen (Fig. 4M). Of the 33 cytokines/chemokines analyzed, the concentrations of 22 were significantly altered by the diets (P < 0.05). However, in serum, both HFD exerted the same increase in cytokine/chemokine concentrations compared to those in mice fed LFD. These observations suggest that the high concentrations of MIP-2/CXCL2 and LIX/CXCL5 observed in the spleens of HFD-P-fed mice result from local production.
Our data therefore suggest a specific and exclusive enhancement of the recruitment of neutrophils from the circulation into the spleen induced by HFD-P.
Dietary PUFA delays neutrophil cell death in the spleen.
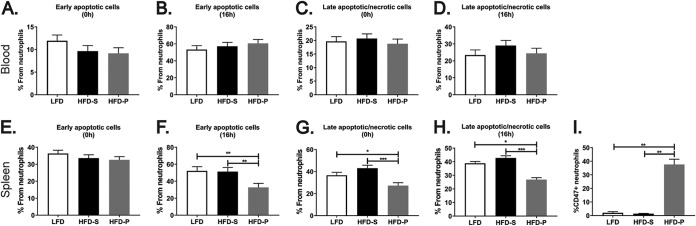
An ultimate factor that could lead to the HFD-P-induced accumulation of neutrophils in the spleen is cell death. Therefore, we next analyzed by flow cytometry the percentage of apoptotic and dead neutrophils using the markers annexin V and 7-AAD. The percentages of early apoptotic and late apoptotic/necrotic neutrophils in the blood and in the spleens of mice fed the different diets were quantified. None of the diets exerted significant changes in the frequency of circulating early apoptotic neutrophils at either the moment of sample extraction (Fig. 5A) or 16 h after sample extraction (Fig. 5B), nor did the diets alter the frequency of late apoptotic/necrotic neutrophils in blood at any of the time points analyzed (Fig. 5C and D).
FIG 5.
Dietary PUFA delays neutrophil cell death in the spleen. (A and B) Percentage of early apoptotic neutrophils in blood analyzed immediately after sample extraction (A) and 16 h after sample extraction (B). (C and D) Percentage of late apoptotic and necrotic neutrophils in blood analyzed immediately after sample extraction (C) and 16 h after sample extraction (D). (E and F) Percentage of early apoptotic neutrophils in the spleen analyzed immediately after sample extraction (E) and 16 h after sample extraction (F). (G and H) Percentage of late apoptotic and necrotic neutrophils in spleen analyzed immediately after sample extraction (G) and 16 h after sample extraction (H). Mice were fed LFD, HFD-S, and HFD-P for 8 weeks (blood) or 4 weeks (spleen). (I) Quantification of CD47+ neutrophils in the spleens of mice fed the different diets for 4 weeks. Data are for 20 mice per group for panels A and C; 10 mice per group for panels B, D, and I; and 20 to 25 mice per group for panels E, F, G, and H. Data are shown as the mean + SEM. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
The same frequency of early apoptotic neutrophils was found in the spleens of mice fed HFD-P and those of mice fed LFD or HFD-S (Fig. 5E) when analyzed at the time of sample extraction. However, at 16 h after extraction, mice fed HFD-P were found to have a significantly lower frequency of early apoptotic spleen neutrophils (Fig. 5F) than mice fed HFD-S (P < 0.01) and LFD (P < 0.01). Mice fed HFD-P had a decreased frequency of late apoptotic/necrotic spleen neutrophils after sample extraction (Fig. 5G) and after 16 h (Fig. 5H) compared with mice fed HFD-S (P < 0.01) and LFD (P < 0.05).
The presence of phosphatidylserine in the external surface of the cellular membrane of neutrophils (here detected by annexin V staining) serves as one of the signals that promote their phagocytosis by macrophages (21). On the other hand, cells block being phagocytized by macrophages by expressing CD47 on their surface (22). We found that neutrophils from the spleens of mice fed HFD-P had increased surface expression of CD47 compared to neutrophils from the spleens of mice fed LFD or HFD-S (Fig. 5I).
Therefore, our results suggest that HFD-P induces an antiapoptotic environment in the spleen, contributing to the prolonged survival of spleen neutrophils.
DISCUSSION
Neutrophil homeostasis is altered by the addition of polyunsaturated fatty acids to HFD and not by the increase in dietary fat.
In the present study, we investigated the effects of high-fat diets rich in saturated or polyunsaturated fatty acids on neutrophil homeostasis under noninflammatory conditions. We performed different analyses with bone marrow, blood, liver, and spleen. Interestingly, we found that most of the alterations in neutrophil homeostasis induced by the high-fat diets occurred in the spleen and not in the other tissues. Moreover, these alterations were mainly caused by HFD-P, whereas the neutrophil homeostasis in the spleens of HFD-S-fed mice was comparable to that in LFD-fed mice for most of the parameters analyzed. This suggests that such alterations were caused by the addition of PUFA into the diet and not by the increase in fat content, since both HFD-S and HFD-P contain the same percentage of fat.
This pattern is in accordance with the findings of our previous microarray analysis performed in the spleen, bone marrow, skeletal muscle, white adipose tissue, and brown adipose tissue from mice fed LFD, HFD-S, or HFD-P (20). In such an analysis, bone marrow and skeletal muscle were relatively unchanged by the diets, whereas both white and brown adipose tissues were similarly affected by HFD-S and HFD-P. However, changes in the spleen transcriptomic profile were exerted exclusively by HFD-P, in line with our observations presented in this study.
Neutrophil accumulation in the spleens of mice fed HFD-P results in spleen enlargement.
We found that mice fed a diet rich in PUFA had an increased frequency of neutrophils in the spleen and the liver, suggesting a general increase in neutrophil frequency in the marginal pool organs. Spleen neutrophils were located in the perimarginal zone (peri-MZ) of the red pulp surrounding the white pulp. Recently, it has been shown that neutrophils colonize the peri-MZ both under inflammatory conditions and in the absence of infection or other inflammatory stimuli (18). This location is fundamental for neutrophil-mediated antigenic presentation to B cells in the spleen. This indicates that the increase in neutrophils induced by HFD-P could be a mechanism to enhance antigenic presentation to B cells.
Feeding mice HFD-P induced an enlargement of the spleen, but this effect was not extended to other organs where marginal pool neutrophils accumulate (liver), nor was it a general effect on immune organs, since the thymus size also remained unaltered. Additionally, the increase in spleen size was not caused by a proportional increase in overall body weight, since mice fed HFD-P have a lower body weight than mice fed HFD-S (19). HFD-P-induced spleen enlargement correlated with an increase in spleen cellularity. Spleens from mice fed HFD-P contained approximately 75 million more cells than those from mice fed LFD or HFD-S. However, the increase in the numbers of neutrophils could account for only approximately 30% of the increase in spleen cellularity. We found that the number of macrophages in the spleens from mice fed HFD-P was also significantly higher than that in the spleens from mice fed the other diets, which accounted for most of the remaining spleen hypercellularity in mice fed HFD-P. However, we cannot exclude the possibility that the frequency of other splenic cell types not included in our analysis may also be increased by HFD-P.
Spleen enlargement and hypercellularity have previously been linked to increased numbers of neutrophils in the bone marrow, blood, peritoneum, and spleen in a mouse model lacking macrophages in the splenic marginal zone, the bone marrow, and the peritoneum (16). In that context, treatment of mice with a neutralizing antibody against G-CSF effectively restored both neutrophil accumulation and the spleen size to basal levels. In agreement with the results described above, we found that treatment of mice with anti-G-CSF antibody produced a splenic neutrophil frequency in HFD-P-fed mice comparable to that in mice fed LFD. However, a complete rescue of the spleen size was not obtained, suggesting that other factors, such as the increase in spleen macrophage frequency, contribute to spleen enlargement in mice fed HFD-P. The simultaneous depletion of both spleen neutrophils and macrophages should be performed to test this hypothesis.
The effect of dietary PUFA on the spleen immune populations was specific for cells of the innate branch, as we found the total number of CD4+ and CD8+ T cells and B cells to be unaltered by the PUFA-rich diet. The relative frequencies of these populations were slightly reduced by HFD-P, which was likely a consequence of the increase in neutrophils and macrophages rather than a reduction of the total level of CD4+ and CD8+ T cells and B cells in mice fed HFD-P. Of note, the fact that HFD-P did not alter the number of T cells and B cells in the spleen suggests that the observed spleen enlargement was not caused by an autoimmune disorder.
No effect of PUFA on the differentiation of cells from the bone marrow into neutrophils.
In order to explain the accumulation of neutrophils in the spleen caused by HFD-P, we studied the levels of G-CSF and GM-CSF in the bone marrow, blood, and spleen as a readout for granulopoiesis. G-CSF and GM-CSF production in the bone marrow and G-CSF levels in serum were unaltered by the diets. Although we have previously shown an increased frequency of precursor cells in the bone marrow of mice fed HFD-P (19), it seems insufficient to explain the 7-fold increase in the absolute number of neutrophils in the spleens of those mice, even with the same concentration of G-CSF. Local spleen granulopoiesis has previously been detected under specific conditions, such as inflammation or anemia (23). In our model, the concentration of GM-CSF in the spleen was actually reduced in mice fed HFD-P, whereas the levels of spleen G-CSF were unaltered by the diets, confirming that there is no increased medullary or extramedullary granulopoiesis induced by HFD-P. In fact, decreased levels of GM-CSF could be a negative regulatory mechanism caused by the increased number of neutrophils in the marginal pools (24).
Neutrophils are actively recruited into the spleen in mice fed HFD-P.
The release of neutrophils into the circulation and their retention in the bone marrow are processes regulated by the activation of two receptors present in neutrophils: CXCR2 and CXCR4, respectively (25). We analyzed the surface expression of CXCR2 by bone marrow neutrophils from mice fed the different diets, and we found that although the percentage of neutrophils expressing CXCR2 remained unchanged, CXCR2 expression per neutrophil was significantly reduced by the PUFA-rich diet. Therefore, HFD-P did not induce an enhanced release of neutrophils from the bone marrow by a CXCR2-dependent mechanism. In contrast, the expression of CXCR2 by spleen neutrophils was increased in mice fed HFD-P compared with that by neutrophils from the spleens of mice fed the other diets, suggesting that dietary PUFA promote the recruitment of neutrophils into this organ.
In contraposition to CXCR2, activation of neutrophil CXCR4 promotes the retention of neutrophils inside the bone marrow (13). We found that the percentage of CXCR4+ neutrophils in the bone marrow from mice fed HFD-P was reduced and that neutrophils from mice fed HFD-P expressed less surface CXCR4 than those from mice fed the other diets. These results suggest that the retention of differentiated neutrophils in the bone marrow could be partially prevented by dietary PUFA.
Since neutrophils released from the bone marrow do not excessively accumulate in the circulation of mice fed HFD-P (19) but, rather, accumulate in the spleen, we next analyzed the chemoattractant molecules secreted from this tissue. Our quantification of secreted spleen chemokines and cytokines showed that dietary PUFA induced the secretion of the chemokines MIP-2/CXCL2 and LIX/CXCL5, both of which are ligands for the neutrophil receptor CXCR2, indicating an active recruitment of neutrophils by splenocytes. The HFD-P-induced increase in MIP-2/CXCL2 and LIX/CXCL5 was regulated at the transcriptional level, as confirmed by our microarray analysis.
Besides MIP-2/CXCL2 and LIX/CXCL5, MIP-1α/CCL3, CXCL12, CXCL16, RANTES, and IL-16 were also found among the 7 cytokines/chemokines upregulated by HFD-P in the spleen. Although MIP-1α/CCL3 is classically known as a chemoattractant molecule for mononuclear cells (26), it can also recruit and activate neutrophils (27, 28). CXCL12 has recently been characterized to be one of the main factors inducing extramedullary hematopoiesis for the production of neutrophils in the spleen (29). The roles of CXCL16 and IL-16 in the spleen have not yet been explored; however, CXCL16 acts as a chemoattractant specifically for neutrophils and macrophages into injured muscle (30), whereas IL-16 controls neutrophil recruitment to the lung in the pristane-induced lung inflammation model (31). RANTES stimulates the secretion of the neutrophil chemokines KC/CXCL1 and MIP-2/CXCL2 by dendritic cells (DCs) (32) and promotes the accumulation of antigen-presenting cells (APCs) in the marginal zone of the spleen (33). Further experiments are required to decipher the different mechanisms behind the regulation of the production of the above-mentioned chemokines by HFD-P in the spleen.
The fact that the concentrations of MIP-2/CXCL2, LIX/CXCL5, MIP-1α/CCL3, CXCL12, CXCL16, RANTES, and IL-16 were not increased in the blood of HFD-P-fed mice compared with the blood of mice fed the other diets indicates that their secretion was locally regulated rather than systemically regulated.
Interestingly, neutrophils are not only the target but also the main cells producing and secreting KC/CXCL1, MIP-2/CXCL2, and MIP-1α/CCL3 (34) in order to expand the inflammatory response. Further investigations will be required to discern whether the increase in these chemokines is a consequence or a cause of neutrophil accumulation in the spleens of mice fed HFD-P. The production and secretion of KC/CXCL1 and MIP-2/CXCL2 by neutrophils is induced by multiple factors, such as the cytokines tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), interleukin-15 (IL-15), GM-CSF, and the lipid-mediator leukotriene B4 (35). We found reduced levels of TNF-α, IL-1β, and GM-CSF in the spleens of mice fed HFD-P, excluding the possibility that they are the initial inducers of neutrophil accumulation. The synthesis of leukotrienes B4 and B5 is dependent on the fatty acid cellular composition (36). A possible mechanism for the induced splenic recruitment of neutrophils could be the increased production of leukotriene B4, a lipid mediator derived from PUFA (37). Neutrophils initially recruited by leukotriene B4 could be responsible for the secretion of MIP-2/CXCL2 and the subsequent expansion of the neutrophil population in the spleen (the lipid-cytokine-chemokine cascade hypothesis). Which cell type is responsible for the initial leukotriene B4 secretion and what kind of stimulus triggers such secretion will be the subject of future investigations.
Neutrophil cell death is reduced in spleens from mice fed PUFA.
Neutrophil homeostasis is regulated by their production in the bone marrow, their migration toward marginal pools, and, ultimately, their death and subsequent elimination. The spleen has, in fact, been characterized to be the main organ for the clearance of neutrophils under noninflammatory conditions (16). Gordy et al. demonstrated that depletion of macrophages results in an increased number of neutrophils in the bone marrow, the spleen, and the blood under noninflammatory conditions (16). Further, the spleen marginal zone macrophages and not the liver Kupffer cells were pointed out to be the cells responsible for homeostatic neutrophil elimination (16).
However, our investigations showed a concomitant increase in both spleen macrophages/monocytes and neutrophils in mice fed HFD-P. This effect could be explained by the fact that dietary PUFA reduce the percentage of spleen neutrophils that show apoptotic and necrotic markers which are recognized by macrophages and which induce the phagocytosis of dead cells (21). Moreover, HFD-P induced a marked increase in the expression of the surface receptor CD47, known as a “do not eat me” signal that prevents phagocytosis of those cells expressing it by macrophages (22). In combination with the increased recruitment of neutrophils, the PUFA-mediated extension of neutrophil viability contributes to the accumulation of neutrophils initially observed in the spleens of mice fed HFD-P.
In summary, our study demonstrates that a high-fat diet rich in PUFA enhances the accumulation of viable neutrophils in the spleen as a result of increased chemokine-mediated attraction toward the spleen, in combination with the delay of neutrophil death by PUFA. Moreover, these effects were specifically caused by the addition of PUFA and not by a general increase of the percentage of fat in the diet, since mice fed HFD-S did not have alterations of neutrophil homeostasis compared to mice fed LFD.
MATERIALS AND METHODS
Experimental protocol.
Seven-week-old male C57BL/6 mice were obtained from Janvier Labs (France) or Harland (The Netherlands). The mice were housed under standard conditions of light and temperature at the animal facility of the Laboratory for Experimental Biomedicine at the University of Gothenburg (Gothenburg, Sweden). Water and food were provided ad libitum. From 8 weeks of age, the mice were fed a low-fat diet (LFD), a high-fat diet rich in saturated fatty acids (HFD-S), or a high-fat diet rich in polyunsaturated fatty acids (HFD-P) as previously described (19). LFD (catalog number D12450B; Research Diets, USA) has a caloric density of 3.9 kcal/g, from which 10% of kilocalories comes from fat, 20% of kilocalories comes from protein, and 70% of kilocalories comes from carbohydrate. HFD-S (catalog number D12492; Research Diets, USA) has a caloric density of 5.2 kcal/g, from which 60% of kilocalories comes from fat, 20% of kilocalories comes from protein, and 20% of kilocalories comes from carbohydrate. HFD-P (catalog number D09020505; Research Diets, USA) has the same caloric content and the same proportion of nutrients as HFD-S, but 69% of the lard (a saturated fat) was exchanged for menhaden oil (a polyunsaturated fat). A more detailed list of the composition of the diets can be found in our previous publications (20). Food was exchanged three times per week for a total duration of 4 or 8 weeks. After 4 or 8 weeks on diet, the mice were euthanized according to ethical protocols and tissues were harvested for subsequent analysis. All animal experiments were done in accordance with the guidelines of the local welfare committee in Gothenburg, Sweden.
Anti-G-CSF treatment.
Mice fed HFD-P were intraperitoneally (i.p.) injected with either 10 μg antibody against G-CSF (Bio-Techne, USA) or 10 μg isotype control IgG (Bio-Techne) 2 times per week for 4 weeks. The mice were euthanized 2 days after the last dose, and blood and spleens were extracted. Blood samples were collected into tubes containing EDTA and analyzed for the granulocyte count using a Vetscan analyzer (Abaxis).
Flow cytometry analysis of immune populations in the spleen and liver and expression of surface receptors by splenic neutrophils.
Spleen and liver were homogenized by mechanical disruption through a 40-μm-mesh-size cell strainer (BD Biosciences, USA) in sterile phosphate-buffered saline (PBS). To isolate bone marrow, one femur was cleared of surrounding tissue and flushed with 2% formaldehyde–PBS. Homogenized samples were kept on ice for the duration of the procedure unless stated otherwise. Red blood cells (RBC) were lysed with RBC lysis buffer (BD Biosciences) according to the manufacturer’s instructions. After washing, cells were resuspended in 3% bovine serum albumin (BSA)–PBS and counted. One million cells per sample were used for each experiment. Fc receptors were blocked prior to antibody incubation with anti-mouse CD16/CD32 antibody (BD Biosciences) to avoid unspecific binding of antibodies. For the analysis of the frequency of immune populations in the liver and spleen, the following antibodies were used: anti-mouse Ly6G-phycoerythrin (PE) (Miltenyi, Germany), anti-mouse Ly6G-fluorescein isothiocyanate (FITC) (BD Biosciences), anti-mouse F4/80-FITC (Miltenyi), anti-mouse CD4-peridinin chlorophyll protein-Cy5.5 (BD Biosciences), anti-mouse CD8-PE (BD Biosciences), and anti-mouse CD19-FITC (BD Biosciences). For the analysis of splenic neutrophil surface receptors, the antibodies anti-mouse CXCR4-allophycocyanin (R&D Systems, USA), anti-mouse CXCR2-PE (R&D Systems), and anti-mouse CD47-PE (BD Biosciences) were used. All antibody incubations were performed at 4°C for 20 min. Additionally, 7-AAD (Invitrogen, USA) was added to the samples to exclude dead cells from the analysis of the frequency of immune populations. Data were acquired with a FACSCanto II cytometer (BD Biosciences) and FACSDiva software (BD Biosciences). Analysis was performed with FlowJo software (FlowJo, LLC, USA). Cells were gated according to size and morphology in the forward scatter (FSC)/side scatter plot, and only single cells were selected for analysis according to the FSC area/FSC height plot. The gating strategies are described in Fig. S4 in the supplemental material. The absolute quantity of immune cells in the spleen was calculated as the product of the immune cell frequency and the total number of cells in the spleen.
Immunostaining of neutrophils in spleen and liver.
The spleens and livers from mice were perfused with 4% formaldehyde, incubated in 30% sucrose solution in phosphate buffer for 48 h for dehydration, and then frozen in cryo-embedding medium (OCT). Ten-micrometer sections were cut with a cryostat for analysis. Before staining, sections were rehydrated with PBS. For immunofluorescence staining, sections were blocked with Fc blocker (BD Biosciences) for 30 min at room temperature (RT) and then incubated with anti-mouse Ly6G-PE (Miltenyi) and DAPI (4′,6-diamidino-2-phenylindole) for 1 h at RT, followed by extensive washing. PE isotype antibody staining was used as a negative control. For immunoperoxidase staining, following rehydration with PBS, endogenous peroxidase was blocked using 0.6% H2O2 for 15 min. Next, avidin-biotin blocking was performed (Vector Laboratories, USA) and samples were incubated with 10% rabbit serum for 30 min. Sections were incubated with rat anti-mouse Ly6G antibody (BD Biosciences) or an isotype control antibody. Staining was detected with an ABC alkaline phosphatase kit (Vector Laboratories) and, subsequently, diaminobenzidine (Vector Laboratories).
Multiplex analysis of chemokines and cytokines in serum, spleen, and bone marrow.
Bone marrow and spleen homogenates were resuspended in Bio-Plex lysis buffer (Bio-Rad, USA). The total protein content was quantified using a bicinchoninic acid protein assay kit (Sigma-Aldrich, USA). Serum was obtained after centrifugation of blood extracted transcardially from the mice and diluted in Bio-Plex lysis buffer. A Bio-Plex Pro 33-plex mouse chemokine panel was used to quantify 33 different chemokines and cytokines (including GM-CSF) in bone marrow, spleen, and serum samples. Data from the bone marrow and spleen were normalized to the total protein content. The Bio-Plex Pro mouse cytokine G-CSF set (Bio-Rad) was used for the quantification of G-CSF in the bone marrow, spleen, and serum. Data from the bone marrow and spleen were normalized to the total protein content. A heat map representation of the multiplex results was performed using Qlucore Omics Explorer software (Qlucore AB). Only cytokines that were found to be regulated by the diets with a P value of <0.05 were included in the heat map.
Flow cytometry analysis of neutrophil cell death.
Blood samples were collected transcardially and stored in heparin-containing tubes on ice until the apoptosis analysis was performed. Murine neutrophils were isolated by Percoll gradient centrifugation. In short, samples were diluted in Hanks balanced salt solution (HBSS), a 3-layer Percoll gradient of 78%, 63%, and 50% Percoll, respectively, diluted in HBSS (100% Percoll = 9 parts of Percoll and 1 part of 10× HBSS) was overlaid, and the samples were centrifuged at 500 × g for 30 min at 4°C. The neutrophils were harvested from the 63%-78% interface after carefully removing the cells from the upper phases. After washing the neutrophils, the remaining red cells in the neutrophil fraction were eliminated by hypotonic lysis (3 ml of water for 30 s and 1 ml of 2.4% NaCl). The step was repeated until no red blood cells remained. Isolated neutrophils (95 to 98% purity) were then pelleted by centrifugation and resuspended in RPMI 1640 supplemented with 10% fetal bovine serum (FBS).
Spleen homogenates were diluted in PBS, and red blood cell lysis was performed using RBC lysis buffer (BD Biosciences) according to the manufacturer’s instructions. The cells were then washed and resuspended in 3% BSA–PBS.
For both blood and spleen samples, 1 million cells per sample were used. The analysis of neutrophil cell death was performed immediately after extraction (0 h) or after 16 h of incubation at 37°C in 5% CO2. Samples for the 16-h time point were left in RPMI 1640 supplemented with 10% FBS during the incubation.
Cells were stained with anti-mouse Ly6G antibodies for 20 min at 4°C. After washing out the excess antibody, cells were resuspended in annexin V buffer (10 mM HEPES, 140 mM NaCl, 2.5mM CaCl2, pH 7.4). 7-AAD (Invitrogen) and annexin V (Life Technologies) were added, and the cells were incubated for 15 min at room temperature with protection from light. Samples were acquired with a FACSCanto II cytometer (BD Biosciences) and FACSDiva software (BD Biosciences). Analysis was performed with FlowJo software (FlowJo, LLC). Early apoptotic neutrophils were identified as Ly6G+, annexin V-positive (annexin V+), and 7-AAD− cells, and late apoptotic and necrotic neutrophils were identified as Ly6G+, annexin V+, and 7-AAD+ cells.
RNA isolation and microarray analysis.
Total RNA was isolated from the spleen using an RNeasy lipid tissue minikit (Qiagen Nordic, Sweden) according to the manufacturer’s instruction. The total RNA concentration was measured with a NanoDrop spectrophotometer (Thermo Fisher Scientific, USA). The quality of the RNA was evaluated by use of an RNA 6000 Nano LabChip for the Agilent 2100 bioanalyzer (Agilent Technologies, USA). The RNA was labeled and hybridized to GeneChip Mouse Gene 1× ST arrays (Affymetrix, USA) at the Genomics Core Facility, Swegene Centre for Integrative Biology at Lund University (Lund, Sweden). Data were analyzed as previously described (20).
Statistical analysis.
Differences between samples were analyzed using the Kruskal-Wallis test followed by Dunn's multiple-comparison test. Data are shown as the mean + the standard error of the mean (SEM). For the analysis of the spleen weight and the absolute quantification of immune cells in the spleen, data from different experimental days were pooled. In this case, two-way analysis of variance with the experimental day as a nuisance factor was used. Then, the data were expressed as the estimated marginal mean + SEM. The number of mice used per experiment and other specific statistical details are stated in the figure legends. Qlucore Omics Explorer software (Qlucore AB) was used to create heat map representations of the data.
Accession number(s).
All microarray data files are available at the Gene Expression Omnibus (GEO) database under accession number GSE79434.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Britt Gabrielsson for critically reviewing the manuscript.
This work was supported by grants from the Swedish Research Council (2017-02186 [to M.E.J.] and K2013-54X-09894-19-3 [to J.-O.J.]); the Swedish Society of Medicine (to M.E.J.); the Magnus Bergvall Foundation (to M.E.J.); the Längmanska Kulturfonden (to M.E.J.); the Stiftelsen Gamla Trotjänarinnor (to M.E.J.); the Lars Hiertas Foundation (to M.E.J.); the Åke Wiberg Foundation (to M.E.J.); the Stiftelsen Tornspiran (to S.L.S.); the W. and M. Lundgren Foundation (to S.L.S. and M.A.U.); the Kungliga Vetenskaps- och Vitterhetssamhället i Göteborg (to S.G.); the Adlerbertska Stiftelsen (to S.L.S.); the Sahlgrenska Center for Cardiovascular, Metabolic Research (CMR; no. A305: 188), which is supported by the Swedish Strategic Foundation (to J.-O.J.); and EC FP7 funding (Full4Health FP7-KBBE-2010-4-266408 [to J.-O.J.]).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00270-19.
REFERENCES
- 1.de Oliveira S, Rosowski EE, Huttenlocher A. 2016. Neutrophil migration in infection, and wound repair: going forward in reverse. Nat Rev Immunol 16:378–391. doi: 10.1038/nri.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donadieu J, Fenneteau O, Beaupain B, Mahlaoui N, Chantelot CB. 2011. Congenital neutropenia: diagnosis, molecular bases and patient management. Orphanet J Rare Dis 6:26. doi: 10.1186/1750-1172-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manus MM, Lamborn K, Khan W, Varghese A, Graef L, Knox S. 1997. Radiotherapy-associated neutropenia and thrombocytopenia: analysis of risk factors and development of a predictive model. Blood 89:2303–2310. [PubMed] [Google Scholar]
- 4.Mantovani A, Costantini C, Cassatella MA, Jaillon S. 2011. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 5.Windsor AC, Mullen PG, Fowler AA, Sugerman HJ. 1993. Role of the neutrophil in adult respiratory distress syndrome. Br J Surg 80:10–17. doi: 10.1002/bjs.1800800106. [DOI] [PubMed] [Google Scholar]
- 6.Fattori V, Amaral FA, Verri WA Jr. 2016. Neutrophils and arthritis: role in disease and pharmacological perspectives. Pharmacol Res 112:84–98. doi: 10.1016/j.phrs.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 7.Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. 2010. Neutrophil kinetics in health and disease. Trends Immunol 31:318–324. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Theilgaard-Monch K, Jacobsen LC, Borup R, Rasmussen T, Bjerregaard MD, Nielsen FC, Cowland JB, Borregaard N. 2005. The transcriptional program of terminal granulocytic differentiation. Blood 105:1785–1796. doi: 10.1182/blood-2004-08-3346. [DOI] [PubMed] [Google Scholar]
- 9.Athens JW, Haab OP, Raab SO, Mauer AM, Ashenbrucker H, Cartwright GE, Wintrobe MM. 1961. Leukokinetic studies. IV. The total blood, circulating and marginal granulocyte pools and the granulocyte turnover rate in normal subjects. J Clin Invest 40:989–995. doi: 10.1172/JCI104338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tateda K, Moore TA, Newstead MW, Tsai WC, Zeng X, Deng JC, Chen G, Reddy R, Yamaguchi K, Standiford TJ. 2001. Chemokine-dependent neutrophil recruitment in a murine model of Legionella pneumonia: potential role of neutrophils as immunoregulatory cells. Infect Immun 69:2017–2024. doi: 10.1128/IAI.69.4.2017-2024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartl D, Krauss-Etschmann S, Koller B, Hordijk PL, Kuijpers TW, Hoffmann F, Hector A, Eber E, Marcos V, Bittmann I, Eickelberg O, Griese M, Roos D. 2008. Infiltrated neutrophils acquire novel chemokine receptor expression and chemokine responsiveness in chronic inflammatory lung diseases. J Immunol 181:8053–8067. doi: 10.4049/jimmunol.181.11.8053. [DOI] [PubMed] [Google Scholar]
- 12.Furze RC, Rankin SM. 2008. The role of the bone marrow in neutrophil clearance under homeostatic conditions in the mouse. FASEB J 22:3111–3119. doi: 10.1096/fj.08-109876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eash KJ, Means JM, White DW, Link DC. 2009. CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions. Blood 113:4711–4719. doi: 10.1182/blood-2008-09-177287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolaczkowska E, Kubes P. 2013. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 15.Futosi K, Fodor S, Mocsai A. 2013. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol 17:638–650. doi: 10.1016/j.intimp.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordy C, Pua H, Sempowski GD, He YW. 2011. Regulation of steady-state neutrophil homeostasis by macrophages. Blood 117:618–629. doi: 10.1182/blood-2010-01-265959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deniset JF, Surewaard BG, Lee W-Y, Kubes P. 2017. Splenic Ly6Ghigh mature and Ly6Gint immature neutrophils contribute to eradication of S. pneumoniae. J Exp Med 214:1333–1350. doi: 10.1084/jem.20161621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puga I, Cols M, Barra CM, He B, Cassis L, Gentile M, Comerma L, Chorny A, Shan M, Xu W, Magri G, Knowles DM, Tam W, Chiu A, Bussel JB, Serrano S, Lorente JA, Bellosillo B, Lloreta J, Juanpere N, Alameda F, Baro T, de Heredia CD, Toran N, Catala A, Torrebadell M, Fortuny C, Cusi V, Carreras C, Diaz GA, Blander JM, Farber CM, Silvestri G, Cunningham-Rundles C, Calvillo M, Dufour C, Notarangelo LD, Lougaris V, Plebani A, Casanova JL, Ganal SC, Diefenbach A, Arostegui JI, Juan M, Yague J, Mahlaoui N, Donadieu J, Chen K, Cerutti A. 2011. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol 13:170–180. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Svahn SL, Grahnemo L, Palsdottir V, Nookaew I, Wendt K, Gabrielsson B, Schele E, Benrick A, Andersson N, Nilsson S, Johansson ME, Jansson JO. 2015. Dietary polyunsaturated fatty acids increase survival and decrease bacterial load during septic Staphylococcus aureus infection and improve neutrophil function in mice. Infect Immun 83:514–521. doi: 10.1128/IAI.02349-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Svahn SL, Varemo L, Gabrielsson BG, Peris E, Nookaew I, Grahnemo L, Sandberg AS, Wernstedt Asterholm I, Jansson JO, Nielsen J, Johansson ME. 2016. Six tissue transcriptomics reveals specific immune suppression in spleen by dietary polyunsaturated fatty acids. PLoS One 11:e0155099. doi: 10.1371/journal.pone.0155099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Y, Tibrewal N, Birge RB. 2006. Phosphatidylserine recognition by phagocytes: a view to a kill. Trends Cell Biol 16:189–197. doi: 10.1016/j.tcb.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Barrera L, Montes-Servín E, Hernandez-Martinez J-M, García-Vicente MÁ, Montes-Servín E, Herrera-Martínez M, Crispín JC, Borbolla-Escoboza JR, Arrieta O. 2017. CD47 overexpression is associated with decreased neutrophil apoptosis/phagocytosis and poor prognosis in non-small-cell lung cancer patients. Br J Cancer 117:385–397. doi: 10.1038/bjc.2017.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oda A, Tezuka T, Ueno Y, Hosoda S, Amemiya Y, Notsu C, Kasahara T, Nishiyama C, Goitsuka R. 2018. Niche-induced extramedullary hematopoiesis in the spleen is regulated by the transcription factor Tlx1. Sci Rep 8:8308. doi: 10.1038/s41598-018-26693-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borregaard N. 2010. Neutrophils, from marrow to microbes. Immunity 33:657–670. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. 2003. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity 19:583–593. doi: 10.1016/S1074-7613(03)00263-2. [DOI] [PubMed] [Google Scholar]
- 26.Rot A, von Andrian UH. 2004. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol 22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- 27.Ramos CD, Canetti C, Souto JT, Silva JS, Hogaboam CM, Ferreira SH, Cunha FQ. 2005. MIP-1alpha[CCL3] acting on the CCR1 receptor mediates neutrophil migration in immune inflammation via sequential release of TNF-alpha and LTB4. J Leukoc Biol 78:167–177. doi: 10.1189/jlb.0404237. [DOI] [PubMed] [Google Scholar]
- 28.Reichel CA, Puhr-Westerheide D, Zuchtriegel G, Uhl B, Berberich N, Zahler S, Wymann MP, Luckow B, Krombach F. 2012. C-C motif chemokine CCL3 and canonical neutrophil attractants promote neutrophil extravasation through common and distinct mechanisms. Blood 120:880–890. doi: 10.1182/blood-2012-01-402164. [DOI] [PubMed] [Google Scholar]
- 29.Inra CN, Zhou BO, Acar M, Murphy MM, Richardson J, Zhao Z, Morrison SJ. 2015. A perisinusoidal niche for extramedullary haematopoiesis in the spleen. Nature 527:466–471. doi: 10.1038/nature15530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, Ran L, Garcia GE, Wang XH, Han S, Du J, Mitch WE. 2009. Chemokine CXCL16 regulates neutrophil and macrophage infiltration into injured muscle, promoting muscle regeneration. Am J Pathol 175:2518–2527. doi: 10.2353/ajpath.2009.090275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith S, Wu PW, Seo JJ, Fernando T, Jin M, Contreras J, Montano EN, Gabhann JN, Cunningham K, Widaa A, McCarthy EM, Molloy ES, Kearns G, Murphy CC, Kong W, Bjorkbacka H, Kornfeld H, Forbess L, Venuturupalli S, Ishimori M, Wallace D, Weisman MH, Jefferies CA. 2018. IL-16/miR-125a axis controls neutrophil recruitment in pristane-induced lung inflammation. JCI Insight 3:120798. doi: 10.1172/jci.insight.120798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischer FR, Luo Y, Luo M, Santambrogio L, Dorf ME. 2001. RANTES-induced chemokine cascade in dendritic cells. J Immunol 167:1637–1643. doi: 10.4049/jimmunol.167.3.1637. [DOI] [PubMed] [Google Scholar]
- 33.Faunce DE, Stein-Streilein J. 2002. NKT cell-derived RANTES recruits APCs and CD8+ T cells to the spleen during the generation of regulatory T cells in tolerance. J Immunol 169:31–38. doi: 10.4049/jimmunol.169.1.31. [DOI] [PubMed] [Google Scholar]
- 34.Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. 2000. The neutrophil as a cellular source of chemokines. Immunol Rev 177:195–203. doi: 10.1034/j.1600-065X.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 35.Cassatella MA. 1999. Neutrophil-derived proteins: selling cytokines by the pound. Adv Immunol 73:369–509. doi: 10.1016/S0065-2776(08)60791-9. [DOI] [PubMed] [Google Scholar]
- 36.Calder PC. 2008. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot Essent Fatty Acids 79:101–108. doi: 10.1016/j.plefa.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 37.Sadik CD, Luster AD. 2012. Lipid-cytokine-chemokine cascades orchestrate leukocyte recruitment in inflammation. J Leukoc Biol 91:207–215. doi: 10.1189/jlb.0811402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.