Little is known about whether pathogen invasion of neural tissue is affected by immune-based mechanisms in endothelial cells. We examined the effects of endothelial cell CD40 on Toxoplasma gondii invasion of the retina and brain, organs seeded hematogenously. T. gondii circulates in the bloodstream within infected leukocytes (including monocytes and dendritic cells) and as extracellular tachyzoites.
KEYWORDS: CD40, autophagy, dendritic cells, endothelial cells, monocytes
ABSTRACT
Little is known about whether pathogen invasion of neural tissue is affected by immune-based mechanisms in endothelial cells. We examined the effects of endothelial cell CD40 on Toxoplasma gondii invasion of the retina and brain, organs seeded hematogenously. T. gondii circulates in the bloodstream within infected leukocytes (including monocytes and dendritic cells) and as extracellular tachyzoites. After T. gondii infection, mice that expressed CD40 restricted to endothelial cells exhibited diminished parasite loads and histopathology in the retina and brain. These mice also had lower parasite loads in the retina and brain after intravenous (i.v.) injection of infected monocytes or dendritic cells. The protective effect of endothelial cell CD40 was not explained by changes in cellular or humoral immunity, reduced transmigration of leukocytes into neural tissue, or reduced invasion by extracellular parasites. Circulating T. gondii-infected leukocytes (dendritic cells used as a model) led to infection of neural endothelial cells. The number of foci of infection in these cells were reduced if endothelial cells expressed CD40. Infected dendritic cells and macrophages expressed membrane-associated inducible Hsp70. Infected leukocytes triggered Hsp70-dependent autophagy in CD40+ endothelial cells and anti-T. gondii activity dependent on ULK1 and beclin 1. Reduced parasite load in the retina and brain not only required CD40 expression in endothelial cells but was also dependent on beclin 1 and the expression of inducible Hsp70 in dendritic cells. These studies suggest that during endothelial cell-leukocyte interaction, CD40 restricts T. gondii invasion of neural tissue through a mechanism that appears mediated by endothelial cell anti-parasitic activity stimulated by Hsp70.
INTRODUCTION
In contrast to information about hematopoietic cells, much less is known about the role that nonhematopoietic cells play in controlling infections. This is an important question since many pathogens infect nonhematopoietic cells and/or traverse biological barriers formed by these cells during their process of dissemination within the host. Endothelial cells (EC) are an important component of biological barriers. Traversal through the EC layer is a key event in the process of invasion of the brain and retina. Lambda interferon (IFN-λ) signaling appears to inhibit West Nile virus infection of the brain by modulating EC tight junctions (1). However, little else is known regarding whether pathogen invasion of the brain and retina is restricted by immune-based mechanisms operative in EC.
CD40 is a tumor necrosis factor (TNF) receptor superfamily member expressed on hematopoietic and various nonhematopoietic cells, including EC. CD154, the main ligand for CD40, is expressed primarily on activated CD4+ T cells and platelets (2, 3). The CD40-CD154 pathway mediates protection against a broad range of pathogens, a response that has been linked to effects of this pathway on hematopoietic cells, such as enhancement of the type 1 cytokine response, maintenance of CD8+ T effector responses, induction of antimicrobial activity, and stimulation of Ig production (2, 3). It is unclear whether CD40 expressed in a nonhematopoietic cell enhances host protection. Studies in a model of Cryptosporidium parvum, a pathogen that infects intestinal epithelial cells, suggested that expression of CD40 in the nonhematopoietic compartment does not promote resistance against infection (4).
Toxoplasma gondii is an obligate intracellular protozoan that infects approximately one-third of the world’s population. The tachyzoite form of the parasite can infect a wide range of mammalian cells. T. gondii causes a chronic infection characterized by the formation of tissue cysts. Encephalitis and retino-choroiditis are the most important clinical manifestations of toxoplasmosis. Studies in knockout mice demonstrated that the CD40-CD154 pathway plays a key role in protection against cerebral and ocular toxoplasmosis (5, 6). Susceptibility to these forms of toxoplasmosis in CD40−/− and CD154−/− mice occurs despite unimpaired IFN-γ production and develops prior to CD8+ T cell exhaustion (5, 6), a mechanism by which the CD40-CD154 pathway enhances control of the chronic phase of infection (7). CD40-CD154 signaling induces toxoplasmacidal activity in macrophages and microglia, a response that likely contributes to protection against cerebral and ocular toxoplasmosis (5, 6).
T. gondii is present in the blood in an intracellular compartment within leukocytes, including CD11b+ monocytes and dendritic cells (DC), as well as extracellular tachyzoites and spreads into the brain and eye through penetration of the blood-brain and blood-retina barriers (8–11). Thus, T. gondii represents an excellent model to study whether molecules of the immune system modulate the barrier function of EC affecting pathogen invasion of neural tissue.
To study whether EC CD40 affects retinal and cerebral spread of T. gondii and development of ocular and cerebral toxoplasmosis, we generated transgenic CD40−/− mice with conditional reconstitution of CD40 expression in EC. Our studies using infection with tissue cysts or intravenous (i.v.) administration of infected CD11b+ cells or DC indicate that expression of CD40 in EC diminishes parasite invasion of the brain and retina. This effect is not mediated by reduced transmigration of infected leukocytes, by reduced invasion by extracellular tachyzoites, or by increased cellular or humoral immunity. Our studies suggest that during interaction with infected leukocytes, EC enhance their barrier function via CD40-dependent induction of autophagy protein-mediated anti-parasitic activity, a process that appears dependent on Hsp70 expressed in leukocytes rather than on CD154.
RESULTS
T. gondii loads in the eye and brain are increased in CD40−/− mice from the early stages of organ involvement.
The parasite load in the eye and brain are higher in CD40−/− mice than in C57BL/6 (B6) mice at 2 and 4 weeks postinfection with a type II strain of T. gondii (6). We examined the effect of CD40 in parasite load at earlier time points. The kinetics of T. gondii dissemination has been studied in mice infected intraperitoneally (i.p.) or orally with type II strains (8, 12–14). Both routes of infection showed rapid parasite dissemination to the spleen, liver, and lung (13, 14), followed by invasion of the brain and eye (8, 12–14). Both routes of infection are suitable to study regulation of hematogenous invasion of the eye and brain since they result in hematogenous seeding of neural tissue with a similar timing of invasion (12). B6 and CD40−/− mice were infected i.p. with ME49 T. gondii tissue cysts. No differences in T. gondii DNA levels in the spleen, liver, and lung of B6 and CD40−/− mice were observed (Table 1). Parasite loads in the brain and eye were detected on day 6 postinfection. In contrast to levels in nonneural organs, T. gondii DNA levels were higher in the eyes and brains of CD40−/− mice on days 6 to 14 postinfection (Table 1). Thus, CD40−/− mice have higher loads of T. gondii in the eye and brain from the early stages after invasion of these organs.
TABLE 1.
T. gondii parasite load in B6 and CD40−/− mice
| Organ | Parasite load (no. of parasites/μg gDNA) by mouse group on:a
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Day 3 p.i. |
Day 6 p.i. |
Day 9 p.i. |
Day 14 p.i. |
|||||
| B6 | CD40−/− | B6 | CD40−/− | B6 | CD40−/− | B6 | CD40−/− | |
| Spleen | 725 ± 119 | 1,277 ± 442 | 31,271 ± 5,341 | 38,346 ± 7,318 | 45,423 ± 11,356 | 54,735 ± 12,124 | 3,126 ± 366 | 4,665 ± 504 |
| Liver | 1,129 ± 256 | 626 ± 214 | 36,462 ± 3,910 | 36,139 ± 5,137 | 13,860 ± 545 | 16,711 ± 4,182 | 838 ± 103 | 1,275 ± 321 |
| Lung | 274 ± 60 | 196 ± 78 | 10,889 ± 2,664 | 8,459 ± 2,483 | 42,131 ± 5,742 | 32,841 ± 1,318 | 14,871 ± 3,004 | 18,672 ± 2,900 |
| Brain | ND | ND | 293 ± 42 | 820 ± 102* | 3,809 ± 607 | 11,104 ± 894* | 11,003 ± 620 | 28,092 ± 3,498* |
| Eye | ND | ND | 117 ± 14 | 391 ± 47* | 762 ± 108 | 2,224 ± 284* | 2,058 ± 247 | 5,785 ± 697* |
B6 and CD40−/− mice were infected i.p. with 10 tissue cysts of the ME49 strain of T. gondii. Mice were euthanized at different times postinfection (p.i.). Genomic DNA was isolated from various organs, and levels of the B1 gene of T. gondii were examined by quantitative PCR. A standard curve of DNA from known numbers of parasites per reaction was used to calculate the number of parasites per microgram of genomic DNA (gDNA) isolated from organs. Results are shown as the means ± standard errors of the means of pooled samples of 9 to 10 mice from 3 independent experiments. ND, not detected. *, P < 0.05 (Student's t test).
Transgenic mice with CD40 expression targeted to EC.
T. gondii traverses the endothelium to reach the eye and brain. Moreover, CD40 is upregulated in cerebral and retinal EC from T. gondii-infected mice (see Fig. S1 in the supplemental material). We generated CD40−/− transgenic mice in which CD40 expression is rescued in EC in order to examine the role of EC CD40 during the development of ocular and cerebral toxoplasmosis. Mice were generated using a binary tetracycline (Tet)-repressible system. The driver line consisted of well-characterized heterozygous mice expressing the tetracycline (Tet)-repressible transactivator (tTA) under the control of the EC promoter Tie1 (Tie1-tTA mice) (15, 16) (Fig. S2A). These mice (B6 background) were backcrossed with CD40−/− mice. The responder line consisted of homozygous CD40−/− mice containing mouse CD40 cloned downstream of the tetracycline operator sequence (TetOS) promoter (17). After the mating of Tie1-tTA mice with TetOS CD40 animals, double-transgenic offspring are predicted to exhibit rescued CD40 expression in EC, while no rescue should occur in single-transgenic mice (carrying either the Tie1-tTA or TetOS CD40). PCR analysis of genomic DNA identified single-transgenic (Trg-Ctr) or double-transgenic (Trg-CD40) mice (Fig. S2B). Trg-CD40 mice expressed CD40 restricted to EC in the retina, choroid, and brain while Trg-Ctr animals lacked CD40 expression (Fig. S2C to F). In these studies, EC were identified as tomato lectin-positive (lectin+) tubular structures or by expression of CD31. Lung CD31+ cells (EC) but not CD31− cells from Trg-CD40 mice expressed CD40 (Fig. S2G). CD40 was not detected on lung cells from Trg-Ctr mice (Fig. S2G). Moreover, flow cytometric analysis confirmed CD40 expression on brain CD31+ cells from Trg-CD40 but not from Trg-Ctr mice (Fig. S2H). Immunostaining of the spleen from B6 mice revealed CD40 expression in EC and parenchymal cells (Fig. S2I). No CD40 was detected in CD40−/− and in Trg-Ctr mice. In contrast, Trg-CD40 mice expressed CD40 in EC (Fig. S2I). While various leukocyte subsets in spleens from B6 mice expressed CD40, no CD40 was detected in Trg-Ctr and Trg-CD40 mice (Fig. S2J). Similarly, brain microglia (Iba-1+ cells) from only B6 mice expressed CD40 (Fig. S2K). Finally, the levels of CD40 in EC from infected B6 and Trg-CD40 mice were similar, indicating that the transgenic mouse system results in physiological expression levels of CD40 during T. gondii infection (Fig. S2L). Thus, Trg-CD40 mice exhibit CD40 rescue in EC.
CD40 expression on EC decreases T. gondii loads in the eye and brain.
Trg-CD40, Trg-Ctr, B6, and CD40−/− mice were infected with T. gondii. Compared to B6 mice, CD40−/− mice exhibited high parasite loads in the eye and brain at 7 and 14 days (Fig. 1A and B). Parasite load in the eyes and brains of Trg-Ctr mice were similar to those in CD40−/− mice (Fig. 1A and B). In contrast, Trg-CD40 mice exhibited reduced parasite loads at 7 and 14 days postinfection (Fig. 1A and B).
FIG 1.
EC expression of CD40 diminishes T. gondii load in the eye and brain and enhances resistance to ocular and cerebral toxoplasmosis. Mice were infected with T. gondii tissue cysts and euthanized at 7 days (A) or 14 days (B to D). (A and B) The T. gondii B1 gene was examined using qPCR. Levels were compared to those of one B6 mouse that was given an arbitrary value of 1. At 14 days postinfection, parasite load in the brain was assessed by counting the number of T. gondii tissue cysts per brain. *, P < 0.05; ***, P < 0.001; ns, nonsignificant (one-way ANOVA). (C) Eyes from infected CD40−/− and Trg-Ctr mice showed disruption of retinal architecture and perivascular and vitreal inflammation. Retina was invaded by retinal pigment epithelial cells (arrows in images located at the far right). H&E staining was used. Magnifications, ×200 (large images) and ×400 (small images). Bar, 50 μm. (D) Brains from CD40−/− and Trg-Ctr mice showed more prominent parenchymal inflammation. PASH staining was used. Original magnification, ×200. Bar, 50 μm. Results are representative of 3 independent experiments. **, P < 0.01.
CD40−/− mice developed more pronounced histopathology in the eye and brain than B6 mice (Fig. 1C and D). Eyes of CD40−/− mice exhibited distortion of the retinal architecture and perivascular and vitreal inflammation as well as invasion of the retina by retinal pigment epithelial cells (Fig. 1C). While brains of B6 mice showed minimal inflammation, brains of CD40−/− mice revealed parenchymal inflammatory foci and perivascular cuffing (Fig. 1D). Histopathology of the eye and brain in Trg-Ctr mice was similar to that in CD40−/− mice (Fig. 1C and D). In contrast, compared to Trg-Ctr mice, Trg-CD40 mice displayed reduced retinal and brain histopathologies that resembled findings in B6 mice (Fig. 1C and D). Altogether, these observations indicate that CD40 expression on EC in Trg-CD40 mice results in protection against ocular and cerebral toxoplasmosis.
CD40 induced resistance against ocular and cerebral toxoplasmosis without increases in cellular and humoral immunity.
Serum levels of interleukin-12 (IL-12) p40, IFN-γ, and TNF-α as well as in vitro secretion of these cytokines were similar between B6 and CD40−/− mice (Fig. S3A and B). B6 and CD40−/− mice also exhibited similar percentages of T. gondii-reactive IFN-γ producing CD4+ and CD8+ T cells (Fig. S3C). Expression of Irgm3 and production of nitric oxide (mediators of protection activated by IFN-γ) were similar in B6 and CD40−/− mice (Fig. S3D and E). Induction levels of systemic type 1 cytokine responses were also comparable in Trg-Ctr and Trg-CD40 mice (Fig. S3F to J). Moreover, serum anti-T. gondii IgG titers were similar in Trg-Ctr and Trg-CD40 mice (log2 antibody titers, 10.44 ± 0.2 versus 11 ± 0.25; n = 5; P > 0.05).
We examined local expression of IL-12 p40, IFN-γ, TNF-α, and nitric oxide synthase 2 (NOS2). mRNA levels of these molecules were not higher in Trg-CD40 mice than in Trg-Ctr mice (Fig. 2). Together, these observations indicate that resistance against ocular and cerebral toxoplasmosis in Trg-CD40 mice occurred without an appreciable increase in local or systemic expression of immune mediators of resistance.
FIG 2.
Effect of CD40 on the expression of IL-12, IFN-γ, TNF-α, and NOS2 in the eye and brain. Mice infected with tissue cysts were euthanized at 7 (A) and 14 (B) days. Levels of IL-12 p40, IFN-γ, TNF-α, and NOS2 mRNAs in eyes and brains were examined using qPCR. Each group contained pooled samples of 10 mice from 3 experiments. Results are shown as the means ± standard errors of the means.
Expression of CD40 in EC restricts invasion of the eye and brain by T. gondii.
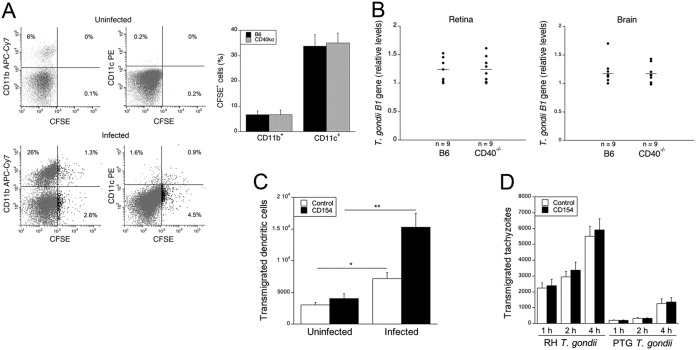
T. gondii disseminates hematogenously to the brain and eye. Parasite loads in blood as assessed by B1 gene quantitative PCR (qPCR) on days 6 and 9 postinfection were similar in B6 and CD40−/− mice (relative load on day 6 for B6, 1 ± 0.23, and for CD40−/−, 1.05 ± 0.20; on day 9 for B6, 1 ± 0.16, and for CD40−/−, 0.95 ± 0.18; P > 0.7). Thus, we examined whether CD40 impairs parasite invasion of the eye and brain. Parasites disseminate within infected CD11b+ and DC (8, 9). We performed experiments in which mice were challenged with purified CD11b+ cells isolated from the blood of T. gondii-infected mice. These experiments were done initially in mice previously infected with T. gondii since invasion of the eye and brain is a delayed event that occurs in animals with ongoing infection; CD40 expression in EC in the eye and brain is enhanced in infected mice, and this upregulation occurs while mice exhibit detectable parasitemia.
Challenge was conducted using strain PTG parasites expressing green fluorescent protein (PTG-GFP)-infected leukocytes injected i.v. into mice infected with ME49 T. gondii (see below). GFP mRNA levels were measured by qPCR to quantitate the load of the challenge parasites since fluorescence microscopy and qPCR to detect GFP in genomic DNA were insensitive in the early stages postchallenge. In vitro studies revealed that GFP mRNA was detected in mammalian cells infected with PTG-GFP tachyzoites but not in those infected with PTG tachyzoites (Fig. 3A and B). Moreover, induction of anti-T. gondii activity reduced the numbers of parasites/100 cells and GFP mRNA levels (Fig. 3C).
FIG 3.
CD40 diminishes T. gondii (Tg) load in the brain and eye after i.v. challenge with T. gondii-infected leukocytes. (A and B) Mouse endothelial cells were infected with PTG or PTG-GFP T. gondii. Quantitative PCR was performed using GFP-specific primers. Endothelial cells were infected with PTG or PTG-GFP at a multiplicity of infection of 1 tachyzoite per endothelial cell. PCR products of the predicted size (101 bp) were detected only in PTG-GFP-infected cells (A). Mouse endothelial cells were infected with tachyzoites at various multiplicities of infection. Samples were subjected to GFP qPCR. Levels were compared to those from the monolayers challenged with the lowest multiplicity of infection, which were given an arbitrary value of 1. The order of the amplification plots (B, from left to right) follows that of the bar graph. No amplification was detected in samples obtained from endothelial cells infected with PTG or left uninfected. (C) Endothelial cells were treated with or without CD154 or IFN-γ/TNF-α and infected with PTG-GFP. The number of tachyzoites per 100 endothelial cells was determined at 24 h. qPCR was run in parallel. Bars are means ± standard errors of the means of 4 samples per group, representative of 3 independent experiments. **, P < 0.01 (one-way ANOVA). (D) Blood CD11b+ cells were purified from mice infected with PTG tachyzoites. Cells were stained with anti-T. gondii Ab followed by Alexa 488-conjugated secondary Ab. Bar, 5 μm. (E) Blood CD11b+ cells were purified from CD40−/− mice infected with PTG-GFP tachyzoites and subjected to GFP qPCR. Shown is a plot of threshold cycle (CT) versus cDNA dilutions obtained from a representative sample collected from a mouse infected with PTG-GFP tachyzoites. (F) Peripheral blood CD11b+ cells were isolated on day 7 after i.p. infection with PTG or PTG-GFP T. gondii. These cells were injected i.v. in B6, CD40−/−, Trg-Ctr, or Trg-CD40 mice that had been infected 9 days before with tissue cysts of ME49 T. gondii. Retinas and BMNC were obtained 2 days after i.v. challenge. GFP mRNA was assessed by qPCR. Levels were compared to those of one B6 mouse that was given an arbitrary value of 1. No GFP mRNA was detected in B6 or CD40−/− mice injected with PTG-infected CD11b+ cells (only data for B6 mice are shown). (G) Mice that had been previously infected with tissue cysts were challenged i.v. with DC infected in vitro with PTG-GFP. Retinas and brains were obtained 1 day after i.v. challenge, and GFP mRNAs were assessed by qPCR. (H) Previously uninfected B6 and CD40−/− mice received T. gondii-infected DC i.v. GFP mRNA levels were examined by qPCR at 1 day postchallenge. (I and J) Previously uninfected mice received 5 × 105 PTG T. gondii-infected DC i.v. Brain sections obtained after 5 days were stained with anti-T. gondii Ab and either anti-CD31 MAb (labels EC) or tomato lectin (labels EC and microglia). Endothelial cells were distinguished from microglia by their tubular structure. Dense clusters of parasites were observed within EC (I) and in the brain parenchyma (J). Images shown were obtained from a representative Trg-Ctr mouse (magnification, ×400). Bar, 10 μm. (K) Numbers of endothelial and parenchymal foci of T. gondii per coronal section. CD40ko, CD40−/−. Bars are means ± standard errors of the means of 12 mice per group from pooled experiments. **, P < 0.01; ***, P < 0.001 (one-way ANOVA).
CD40−/− mice were infected with PTG-GFP tachyzoites. CD11b+ cells isolated from blood on day 7 revealed intracellular tachyzoites (Fig. 3D) (0.8% ± 0.2% of infection; n = 5). Moreover, GFP mRNA was readily detectable in these cells (Fig. 3E). A total of 1 × 105 CD11b+ cells from mice infected with PTG or PTG-GFP were injected to CD40−/− mice that had been infected 9 days previously with tissue cysts of ME49 T. gondii. Retinas and brain mononuclear cells (BMNC) obtained 2 days after i.v. challenge revealed GFP mRNA (Fig. 3F). The detection of GFP mRNA was unlikely to be caused by contaminating blood since mice were perfused prior to organ collection and since blood obtained at the time of organ harvest did not reveal detectable GFP mRNA. As additional controls, we examined retinas and BMNC from mice that were challenged with CD11b+ cells isolated from animals infected with the parental strain PTG or from mice that did not receive CD11b+ cells. No GFP mRNA was detected in either case (Fig. 3F). Next, we compared GFP mRNA levels in B6 and CD40−/− mice challenged with CD11b+-PTG-GFP. GFP mRNA was lower in the retinas and BMNC of B6 mice than in those of CD40−/− animals (Fig. 3F). Similar studies performed with Trg-Ctr and Trg-CD40 mice revealed that GFP mRNA levels were lower in Trg-CD40 mice than in Trg-Ctr mice (Fig. 3F).
The experimental setup described above did not allow consistent detection of GFP mRNA prior to day 2 postadministration of infected CD11b+ cells. To improve parasite detection at early time points, we challenged mice with mouse DC that had been infected in vitro with PTG-GFP. DC were chosen as a model since peripheral blood DC from acutely infected mice contain intracellular tachyzoites (10). Indeed, 0.6% ± 0.2% of peripheral blood CD11c+ cells obtained on day 7 postinfection of B6 or transgenic mice with PTG tachyzoites contained intracellular parasites (n = 6). Moreover, DC traffic into the brain of T. gondii-infected mice (18), and infected DC lead to rapid dissemination of infection into the central nervous system (CNS) (9). A total of 1 × 105 DC infected in vitro with PTG-GFP (8% ± 2% of the cells contained intracellular parasites) were injected i.v. into mice that had been previously infected with tissue cysts of ME49 T. gondii. One day after i.v. administration of infected DC, GFP mRNA was detected in the retina and brain. B6 mice had lower expression of GFP mRNA than CD40−/− mice (Fig. 3G). Challenge with PTG-GFP-infected DC resulted in lower GFP mRNA levels in Trg-CD40 than in Trg-Ctr mice (Fig. 3G).
The experiments shown thus far examined parasite invasion of the retina and brain in mice with ongoing toxoplasmosis. We performed studies where previously uninfected animals received T. gondii-infected DC. The parasite load in the retina and brain of B6 mice was lower than that in CD40−/− mice (Fig. 3H). Similar results were obtained when parasite load was examined by qPCR of the T. gondii B1 gene (Fig. S4B). Next, we confirmed the effect of CD40 on parasite invasion by examining T. gondii load using immunohistochemistry. Brains from CD40−/− and Trg-Ctr mice collected 5 days after i.v. injection of 5 × 105 T. gondii-infected DC showed focal areas of parasites in EC and brain parenchyma (Fig. 3I and J). EC contained replicating tachyzoites and evidence suggestive of parasite release from these cells (Fig. 3I). Endothelial cells were identified as tubular structures that were tomato lectin+ or CD31+ (Fig. 3I). Trg-CD40 mice had fewer foci of parasites in EC than Trg-Ctr mice (Fig. 3K). Similar findings were observed when B6 mice were compared to CD40−/− mice. Altogether, the studies indicate that the expression of CD40 in EC diminished invasion of the retina and brain by T. gondii, mainly by reducing the number of foci in infected EC.
Effect of endothelial CD40 on leukocyte recruitment to the eye and brain.
Recruitment of effector cells into the CNS contributes to control of T. gondii (19). However, DC and monocytes also facilitate parasite invasion by carrying intracellular parasites (8, 9). We analyzed subsets of leukocytes in BMNC to determine if enhanced protection in Trg-CD40 mice might be due to changes in leukocyte recruitment. Similar to findings of a previous study in CD154−/− mice (5), at day 14 postinfection there was a significant increase in the number of CD3+ CD4+ T cells in CD40−/− and Trg-Ctr mice compared to levels in B6 and Trg-CD40 mice (Fig. 4A). However, the numbers of cells that were CD3+ CD8+, CD49b+ (NK cells), B220+ (B cells), CD45hi F4/80+ Gr-1− (macrophages; expressing high levels of CD45 [CD45hi]), CD45lo F4/80+ Gr-1− (microglia; expressing low levels of CD45 [CD45lo]), CD11c+ (DC/microglia), and Gr-1+ F4/80− (neutrophils) were not significantly different in B6, CD40−/−, Trg-Ctr, and Trg-CD40 mice (Fig. 4A). PCR was used to examine expression of leukocyte markers in the eye at day 14 postinfection. A tendency for higher mRNA levels for CD4 in the eyes of CD40−/− and Trg-Ctr mice was observed (Fig. 4B). No differences were found in expression levels of CD8, NCR1 (NK cells), F4/80 (macrophage/microglia), CD11c (DC/microglia), and DC-SIGN (DC) among eyes of any groups of mice (Fig. 4B). Similar results were obtained in the brain (Fig. 4B). No differences in the expression levels of leukocyte markers were noted when eyes and brains were examined at day 7 postinfection (Fig. 4C). Together, these results revealed that the resistance against ocular and cerebral toxoplasmosis in Trg-CD40 mice occurred without increased lymphocyte recruitment into the eye and brain or without changes in the expression levels of DC and macrophages/microglia in these organs.
FIG 4.
Effect of CD40 on the expression of leukocyte markers in the eye and brain. (A and B) Mice were euthanized 14 days after infection with tissue cysts. The numbers of BMNC that were CD3+ CD4+, CD3+ CD8+, CD11c+ (dendritic cells/microglia), CD45R+ (B220+, B cells), CD49b+ (NK cells), CD45hi F4/80+ Gr-1− (macrophages), CD45lo F4/80+ Gr-1−(microglia), and CD45+ F4/80− Gr-1+ (granulocytes) were determined using flow cytometry (A). Levels of CD4, CD8, NCR1 (NK cells), F4/80, CD11c, and CD209 (DC-SIGN) mRNAs in eyes and brains of infected B6, CD40−/−, Trg-Ctr, or Trg-CD40 mice were examined by qPCR; levels were measured at day 14 postinfection (B). (C) Levels of mRNAs for leukocyte markers in eyes and brains of mice infected for 7 days. Bars are mean + standard errors of the means of 10 mice per group from 3 pooled experiments. *, P < 0.05; **, P < 0.01 (one-way ANOVA).
CD40 does not impair transmigration of infected leukocytes into the brain or invasion of the brain and retina by extracellular tachyzoites.
Transmigration of infected DC and monocytes across the blood-brain barrier is a mechanism of invasion into the brain. We used a previously described approach to further examine whether CD40 diminishes transmigration of these leukocytes. T. gondii-infected mice were injected with carboxyfluorescein succinimidyl ester (CFSE) i.v., a method to label peripheral blood leukocytes in vivo that does not directly label resident BMNC (8). As reported previously, i.v. injection of CFSE on day 5 postinfection followed by collection of BMNC 2 days afterwards led to detection of CD11b+ and CD11c+ leukocytes in BMNC that were CFSE positive (CFSE+) (Fig. 5A). The percentages of CFSE+ cells among the CD11b+ and CD11c+ populations were similar in B6 and CD40−/− mice (Fig. 5A). These results together with studies shown in Fig. 4 indicate that CD40 does not appreciably restrict the migration of monocytes and DC into neural tissue of T. gondii-infected mice.
FIG 5.
CD40 does not impair transmigration of T. gondii-infected leukocytes into the brain, invasion of the brain and retina by extracellular tachyzoites, or in vitro transmigration of infected leukocytes or extracellular tachyzoites across EC. (A) B6 and CD40−/− mice were injected with CFSE i.v. 5 days after infection with tissue cysts. Total numbers of BMNC per mouse and the percentages of CD11b+ or CD11c+ cells were similar between groups. Expression of CFSE on CD11b+ and CD11c+ cells was examined in BMNC isolated on day 7. Uninfected mice injected with CFSE were used as controls. Dot plots show data from representative B6 mice. Bars are means ± standard errors of the means of 12 mice per group. APC, allophycocyanin; CD4ko, CD40−/−. (B) Mice were injected i.v. with 1 × 106 tachyzoites of PTG-GFP. T. gondii B1 gene expression was examined at 1 day postchallenge. (C and D) mHEVc that express hmCD40 were cultured in Transwell inserts, followed by incubation with or without CD154. A total of 5 × 105 mouse DC infected with RH CPSII−/−-YFP T. gondii tachyzoites were added to the monolayers (C). At 18 h, leukocytes that transmigrated to the lower chamber were counted and analyzed by flow cytometry to identify infected (YFP+) cells. EC incubated with CD154 were challenged with either RH-YFP or PTG-GFP T. gondii tachyzoites (5 × 105) (D). Transmigration of parasites was determined at 4 h. Bars are means ± standard errors of the means of 4 samples per group from a representative experiment of 3. *, P < 0.05; **, P < 0.01 (one-way ANOVA).
Circulating extracellular T. gondii tachyzoites infect EC (10) and have been proposed to transmigrate across endothelial monolayers (20) although circulating extracellular parasites appear less effective than infected leukocytes in invading the brain (21). Administration of tachyzoites i.v. yielded similar parasite loads in the brain and eye regardless of whether mice expressed CD40 (Fig. 5B).
We used an in vitro approach to further examine whether CD40 restricts transmigration of infected leukocytes or extracellular parasites across EC. Mouse high endothelial venule cells (mHEVc) that express a chimera of the extracellular domain of human CD40 and intracytoplasmic domain of mouse CD40 (human-mouse CD40 [hmCD40]) were cultured in the insert of a Transwell plate. These EC were incubated with or without human CD154, followed by addition of mouse DC infected with RH tachyzoites lacking carbamoyl phosphate synthetase II and expressing YFP (RH CPSII−/−-YFP) or PTG-GFP tachyzoites. As reported previously (9), T. gondii-infected DC migrated across the EC layer more readily than noninfected cells (Fig. 5C). EC stimulation with CD154 did not reduce the number of DC that migrated to the lower chamber of the Transwell plate but, indeed, increased this number (Fig. 5C). Similarly, CD40 stimulation of EC did not inhibit transmigration of extracellular tachyzoites across an EC layer (Fig. 5D). DC or tachyzoites did not appear to affect the integrity of the EC monolayers since transendothelial electrical resistance was not decreased (average throughout in vitro coculture for medium, 17.7 ± 2 Ω/cm2; for uninfected DC, 18.1 ± 3 Ω/cm2; for infected DC, 19.4 ± 2 Ω/cm2; for tachyzoites, 19.2 ± 3 Ω/cm2; for Triton X-100, 5.5 ± 1 Ω/cm2). While CD40 restricts invasion of the brain and retina by T. gondii, in vivo and in vitro studies together indicate that it does not impair the transmigration of leukocytes or extracellular tachyzoites and does not diminish invasion of the brain and retina by circulating extracellular parasites.
T. gondii-infected leukocytes induce autophagy protein-dependent anti-T. gondii activity in EC, and expression of the autophagy protein beclin 1 reduces parasite invasion of the retina and brain.
The presence of T. gondii-infected DC in circulation led to infection of EC. Of relevance, in vitro and in vivo studies revealed that infected leukocytes could transmit the parasite and infect cells that the leukocytes interact with (22, 23). Moreover, adhesion of infected leukocytes to EC triggers parasite egress that could lead to infection of endothelial cells (24). Given that expression of CD40 in EC reduces the number of parasite foci in endothelial cells in mice challenged i.v. with infected leukocytes, we determined whether the interaction between infected DC and EC results in anti-T. gondii activity in endothelial cells and whether this effect is dependent on CD40. Enhanced green fluorescent protein-positive (EGFP+) EC were infected with T. gondii expressing red fluorescent protein (RFP). This was followed by incubation with uninfected DC or DC previously infected with the T. gondii CPS strain, a nonreverting uracil auxotroph. More than 90% of endothelial cells were EGFP+, allowing for their distinction from DC. Incubation with infected DC induced anti-parasitic activity against RFP-T. gondii in EC that required CD40 expression on EC (Fig. 6A). Similar results were observed in EC incubated with T. gondii-infected mouse macrophage line RAW 264.7 (see Fig. 8F).
FIG 6.
T. gondii-infected DC induce autophagy protein-dependent anti-T. gondii activity in EC, and expression of the autophagy protein beclin 1 reduces parasite invasion of the brain and retina. (A) EGFP+ mouse EC transfected with a plasmid that encodes CD40 or an empty plasmid were infected with RFP-T. gondii. Extracellular tachyzoites were removed by extensive washing. After 2 h, EC were incubated with DC that had been infected 18 h before with CPS T. gondii or with uninfected DC. Vacuoles containing T. gondii-RFP per 100 EC (EGFP+) were counted at 2 and 18 h. (B and C) Mouse EC that express LC3-EGFP and CD40 were incubated for 5 h with uninfected mouse DC or DC infected with CPS T. gondii. DC were incubated with EC at a 1:1 ratio (B) or at increasing ratios (C). Large LC3+ structures in EC were quantitated. (D) Mouse EC that express LC3-EGFP and were transfected with a plasmid that encodes CD40 or an empty plasmid were incubated with mouse DC. (E) Mouse EC that express LC3-EGFP and CD40 were transfected with control or ULK1 siRNA followed by addition of DC. (F) CD40+ EC were infected with RFP-T. gondii followed by incubation with uninfected DC or DC infected with nonfluorescent CPS T. gondii. LC3 expression was examined at 5 h in CD40+ EC that also expressed LC3-EGFP. Arrowheads indicate LC3 accumulation around a vacuole containing a single RFP-expressing tachyzoite within EC. Anti-LAMP-1 MAb was used to examine LAMP-1 expression at 12 h in CD40+ EC not transfected with LC3-EGFP. Arrowheads indicate LAMP-1 accumulation around RFP-T. gondii in EC. In contrast to EC incubated with uninfected DC, RFP-T. gondii in EC exposed to infected DC appeared to exhibit disrupted morphology. Bar, 5 μm. (G and H) EC that express LC3-EGFP and CD40 were transfected with a control, beclin 1, or ULK1 siRNA, followed by infection with RFP-T. gondii. Cells were incubated with DC. The numbers of vacuoles containing T. gondii-RFP per 100 EC (EGFP+) were determined at 18 h. Bars are means ± standard errors of the means of 6 samples per group pooled from 3 experiments. **, P < 0.01; ***, P < 0.001 (one-way ANOVA). (I and J) Becn1+/+ or Becn1+/− mice that had been previously infected with tissue cysts of the ME49 strain of T. gondii (I) or uninfected animals (J) were challenged i.v. with DC infected in vitro with PTG-GFP. GFP mRNAs were assessed by qPCR 1 day after i.v. challenge. (K) Previously uninfected Becn1+/+ or Becn1+/− mice received 5 × 105 T. gondii-infected DC i.v. Numbers of endothelial and parenchymal foci of T. gondii per coronal section were determined. Bars are means ± standard errors of the means of 7 to 9 mice per group from pooled experiments. **, P < 0.01; ***, P < 0.001 (Student's t test).
FIG 8.
Expression of inducible Hsp70 in T. gondii-infected leukocytes appears to trigger autophagy protein-dependent killing of T. gondii in EC and to restrict parasite invasion of the brain and retina. (A) DC were infected with PTG or CPS T. gondii at different multiplicities of infection. Expression levels of inducible Hsp70 and actin were examined in whole-cell lysates at 18 h. Relative density of inducible Hsp70 was obtained by normalization to actin, followed by normalization relative to that of uninfected control samples. Relative density of Hsp70 for uninfected samples was given a value of 1. Densitometry data represent means ± standard errors of the means of 3 independent experiments. (B) Whole-cell lysates (WCL) and plasma membrane (PM) preparations from uninfected DC (U) or dendritic cells infected at 6:1 with CPS T. gondii (I) collected at 18 h postinfection were examined for expression of GAPDH, Na/K ATPase, inducible Hsp70, and T. gondii aldolase. Given that equal amounts of protein were loaded for each sample, the relative density of inducible Hsp70 in plasma membrane preparations from infected cells was compared to the relative density of their respective uninfected control samples. Relative density of Hsp70 for uninfected samples was given a value of 1. Relative density of inducible Hsp70 in whole-cell lysate preparations from infected cells was compared to that of whole-cell lysates from their respective uninfected control samples. The latter samples were also given a value of 1. Densitometry data represent means ± standard errors of the means of 3 independent experiments. (C) DC were incubated with or without CPS-mCherry T. gondii. At 18 h cells were stained with a MAb that detects membrane-associated Hsp70. Cells were not permeabilized. Arrowheads indicate inducible Hsp70. Bar, 5 μm. (D) RAW 264.7 cells were incubated with or without CPS-mCherry T. gondii (Tg). At 18 h cells were stained as described above. DAPI, 4′,6′-diamidino-2-phenylindole. Bar, 5 μm. (E) Mouse EC that express LC3-EGFP with or without CD40 were incubated with mouse DC infected with CPS T. gondii. Large LC3+ structures in EC were quantitated at 5 h. (F) EGFP+ mHEVc that express CD40 were incubated with RFP-T. gondii followed by addition of RAW 264.7 cells previously infected with CPS T. gondii. Vacuoles containing T. gondii-RFP per 100 EC (EGFP+) were counted at 18 h. Bars are means ± standard errors of the means from 2 pooled experiments. (G) DC were transfected with a control siRNA or Hsp70 siRNA. Cells were infected with CPS T. gondii followed by incubation with EC that express LC3-EGFP with or without CD40. Relative density of inducible Hsp70 was obtained by normalization to actin, followed by normalization relative to the level in uninfected samples transfected with an Hsp70 siRNA, which were given a value of 1. Densitometry data represent means ± standard errors of the means of 3 independent experiments. (H) DC transfected with control siRNA of Hsp70 siRNA were infected with CPS T. gondii and added to CD40+ LC3-EGFP+ mHEVc previously infected with RFP-T. gondii. Vacuoles containing T. gondii-RFP per 100 EC (EGFP+) were counted at 18 h. Bars are means ± standard errors of the means and are representative of 6 samples pooled from 3 experiments. (I) CD40− and CD40+ mHEVc were incubated with recombinant Hsp70 (250 nM) or CD154 followed by challenge with RH T. gondii. Vacuoles containing T. gondii were counted at 18 h. (J) CD40+ mHEVc incubated with or without Hsp70 and were infected with RFP-T. gondii. Percentages of EC that accumulated LC3 around the parasites. Bars are means ± standard errors of the means of 2 experiments. (K and L) Previously uninfected B6, CD40−/−, Trg-Ctr, and Trg-CD40 mice were challenged i.v. with DC that had been transfected with control or Hsp70 siRNA followed by in vitro infection with PTG-GFP. GFP mRNA was assessed by qPCR 1 day after i.v. challenge (K), or brains were collected at 5 days to assess parasite foci in endothelial cells (L). Bars are means ± standard errors of the means of 6 to 10 mice per group from pooled experiments. **, P < 0.01; ***, P < 0.001; ns, not significant (one-way ANOVA).
We examined if T. gondii-infected DC stimulate autophagy in EC since CD40 ligation leads to autophagic killing of T. gondii (25). Mouse EC that express CD40 and LC3-EGFP (>90% EGFP+) were incubated with either uninfected mouse DC or DC previously infected with nonreplicating CPS T. gondii. A dose-dependent increase in the number of large LC3+ punctae was noted when EC were incubated with T. gondii-infected DC (Fig. 6B and C). Enhanced formation of LC3+ punctae was dependent on CD40 expression in EC (Fig. 6D). In addition, formation of LC3+ punctae was ablated by knockdown of ULK1, a protein critical for canonical autophagy (Fig. 6E). Addition of CPS-infected DC to EC infected with RFP-T. gondii caused LC3 accumulation around RFP-T. gondii parasites in EC (Fig. 6F). Moreover, CPS-infected DC caused accumulation of the late endosomal/lysosomal marker LAMP-1 around T. gondii parasites in EC (Fig. 6F). At late times postinfection (12 h), tachyzoites encircled by LAMP-1 exhibited morphology that suggested that they might be undergoing degradation (Fig. 6F). Knockdown of the autophagy proteins beclin 1 or ULK1 in EC impaired anti-T. gondii activity (Fig. 6G and H). Thus, interaction of T. gondii-infected DC with EC induced anti-parasitic activity in EC that was dependent on the autophagy machinery.
We used mice deficient in beclin 1 (Becn1+/−) to explore the role of an autophagy protein in T. gondii invasion of the eye and brain. Control and Becn1+/− mice that had been previously infected with tissue cysts of ME49 T. gondii were injected i.v. with DC infected with PTG-GFP T. gondii. Becn1+/− mice had higher GFP mRNA levels than control mice (Fig. 6I). Similar results were obtained following injection of infected DC into previously uninfected mice (Fig. 6J). Moreover, Becn1+/− mice had higher numbers of T. gondii foci within cerebral EC (Fig. 6K). Thus, expression of the autophagy protein beclin 1 diminishes T. gondii invasion of the brain and retina.
CD154 does not play an appreciable role in the induction of anti-T. gondii activity in EC and restriction of parasite invasion of the brain and retina.
CD154, the major ligand for CD40, can be expressed by DC (26). We examined whether CD154 triggered CD40-dependent anti-parasitic activity in EC. A neutralizing anti-CD154 monoclonal antibody (MAb) did not inhibit the anti-T. gondii activity in EC incubated with infected DC (Fig. S4A). In contrast, the anti-CD154 MAb ablated CD154-induced anti-T. gondii activity in EC. Moreover, in vivo studies showed that i.v. administration of T. gondii-infected DC from wild-type (WT) or CD154−/− mice led to similar parasite loads in the retina and brain (Fig. S4B). Thus, it is unlikely that CD154 is a major trigger for induction of anti-T. gondii activity in EC.
Inducible Hsp70 appears to mediate CD40-dependent anti-T. gondii activity in EC and to restrict parasite invasion into the retina and brain.
CD40 is reported to have additional ligands besides CD154. In vivo studies linked Hsp70 to CD40 (27, 28). Of relevance, stress-induced mammalian Hsp70 is upregulated in T. gondii-infected cells, and inducible Hsp70 can be expressed on the cell surface (29–32). While some studies reported that mammalian inducible Hsp70 is a ligand for CD40 (33, 34), others could not detect binding between mammalian Hsp70 and CD40 (35–37). These contrasting results may be explained by the likely presence of contaminating proteins (Hsc70) or the source of recombinant Hsp70. To begin to examine the role of inducible Hsp70 in induction of anti T. gondii activity, we first tested the ability of inducible Hsp70 to interact with CD40+ cells. Rather than using Escherichia coli-derived protein, we utilized recombinant baculovirus/Hi5 cell-derived human Hsp70 (baculovirus-derived recombinant proteins are more likely to maintain proper posttranslational processing and protein folding that closely resembles mammalian processes). Recombinant Hsp70 was incubated with CD40+ and CD40− EC. Flow cytometric analysis revealed preferential binding of Hsp70 to CD40+ EC (Fig. 7A). Similar results were obtained using 293T cells that express CD40, and binding of Hsp70 to CD40+ 293T cells was inhibited by a blocking anti-Hsp70 MAb (Fig. 7B). Moreover, binding was also impaired by a blocking anti-CD40 MAb (Fig. 7C). We also examined the effect of Hsp70 on CD40-dependent NF-κB activity using reporter mouse endothelial cells that express an NF-κB response element that drives a luciferase reporter gene. Increased NF-κB activity in response to TNF-α was noted in EC regardless of whether they expressed CD40 (Fig. 7D). In contrast, only CD40+ EC exhibited increased NF-κB activity in response to multimeric CD154 (multimeric CD154 is a more potent inducer of CD40 signaling than monomeric CD154). Recombinant Hsp70 induced NF-κB activity only in CD40+ EC (Fig. 7D). These results suggest that mammalian inducible Hsp70 either directly or indirectly interacts with CD40.
FIG 7.
Mammalian inducible Hsp70 interacts with CD40+ cells. (A) mHEVc (lack endogenous CD40) stably transfected with a CD40-encoding plasmid or parent (empty) vector (PV) were incubated with PE-conjugated anti-CD40 MAb or with His-tagged recombinant baculovirus/Hi5 cell-derived human inducible Hsp70 followed by PE-conjugated anti-His MAb. Histograms show data obtained after incubation with 250 nM Hsp70 (light gray, isotype control or anti-His MAb alone; dark gray, mHEVc-PV; red, mHEVc-CD40). The corrected mean fluorescence intensity (cMFI) was obtained by subtracting fluorescence of the isotype control or anti-His MAb alone from values obtained with anti-CD40 or Hsp70 plus anti-His MAb, respectively. (B) 293T cells (lack endogenous CD40) transduced with a CD40-encoding or parent (empty) retroviral vector (PV) were incubated with His-tagged recombinant human Hsp70 followed by PE-conjugated anti-His MAb as described above. Cells were also incubated with blocking anti-Hsp70 MAb. (C) 293T cells were incubated with recombinant Hsp70 (100 nM) in the presence or absence of a blocking anti-CD40 MAb. (D) mHEVc that express an NF-κB response element that drives transcription of a luciferase reporter plus CD40-encoding plasmid or PV (CD40−) were incubated with recombinant Hsp70 (250 nM), multimeric CD154, or TNF-α (10 ng/ml). Data are expressed as luciferase activity compared to that of cells incubated in culture medium alone (control). Bars are means ± standard errors of the means from a pool of 2 to 4 experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Next, we examined the effects of T. gondii infection on inducible Hsp70 expression. Inducible Hsp70 was expressed at low levels in DC under basal conditions but was upregulated by T. gondii infection (Fig. 8A). Cell membrane preparations from infected and uninfected DC were obtained and were confirmed to be enriched for Na+/K+ ATPase (a cell membrane protein) and to be depleted of glyceraldehyde-3-phosphate dehydrogenase (GAPDH; a cytoplasmic protein) (Fig. 8B). Cell membrane preparations from T. gondii-infected DC expressed inducible Hsp70 (Fig. 8B). T. gondii expresses Hsp70 in its cytoplasm (38). However, plasma membranes from infected DC were devoid of detectable T. gondii aldolase (Fig. 8B). Inducible Hsp70 in the membrane of infected DC was also detected by immunofluorescence using nonpermeabilized cells and a MAb that recognizes Hsp70 in its membrane location (Fig. 8C). Similar results were observed with an infected mouse macrophage line, RAW 264.7 (Fig. 8D).
To continue to examine the functional relevance of membrane Hsp70, T. gondii-infected DC were incubated with a blocking anti-Hsp70 MAb, followed by addition to EC. The upregulation of large LC3+ structures in EC (as seen in Fig. 6B) was inhibited by the anti-Hsp70 MAb (Fig. 8E). Similarly, anti-Hsp70 MAb impaired the ability of CPS T. gondii-infected RAW 264.7 cells from inducing anti-parasitic activity in EC (Fig. 8F). Next, DC were transfected with Hsp70 or a control small interfering RNA (siRNA), followed by infection with T. gondii. Hsp70-deficient DC were unable to increase large LC3+ structures and induce anti-T. gondii activity in CD40+ EC (Fig. 8G and H). Incubation with recombinant Hsp70 induced accumulation of LC3 around T. gondii and parasite killing in CD40+ EC (Fig. 8I and J). Finally, administration of Hsp70-deficient DC led to higher parasite loads in the brain and retina of B6 and Trg-CD40 mice (Fig. 8K) as well as higher numbers of parasite foci in cerebral EC (Fig. 8L). Altogether, these results suggest that inducible Hsp70 in infected leukocytes mediates CD40-dependent anti-T. gondii activity in EC and restricts parasite invasion into the retina and brain.
DISCUSSION
Little is known about the role of host factors in enhancing the barrier function of EC leading to protection against pathogen invasion of neural tissue. Here, we report that CD40 expressed on EC restricts invasion of the retina and brain by T. gondii, diminishing the histopathology in these organs. CD40 expressed in endothelial cells reduced parasite loads in the retina and brain after i.v. injection of infected monocytes or DC. Endothelial CD40 caused a reduction in the number of foci of T. gondii within EC. Upon interaction with infected DC or macrophages, EC acquired anti-T. gondii activity. Infected leukocytes expressed membrane-associated inducible Hsp70. The infected leukocyte-EC interaction triggered the formation of large LC3+ structures, recruitment of LC3 and LAMP-1 around T. gondii parasites, and anti-parasitic activity in EC, all of which were dependent on CD40, autophagy proteins (ULK1 and beclin 1), and Hsp70. The reduction in parasite load within EC and the capacity of these cells to restrict invasion of the retina and brain by T. gondii were not only dependent on their expression of CD40 and the presence of beclin 1 but also appeared to be dependent on the expression of Hsp70 by leukocytes. Given that infected EC are an important portal of entry for T. gondii into the brain parenchyma (10), our studies support a mechanism that stimulates the ability of EC to function as a barrier that protects the brain and retina from invasion by a pathogen.
Prior work on the in vivo role of CD40-CD154 in host protection centered on the effects of this pathway in hematopoietic cells and the regulation of cellular and humoral immunity. Our studies revealed that mice that express CD40 restricted to EC exhibited increased resistance against ocular and cerebral toxoplasmosis, thus revealing that CD40 in nonhematopoietic cells enhances in vivo protection against a pathogen. While Tie1 is a widely used EC promoter, Tie1 promoter activity can be detected in a small fraction of hematopoietic cells (39). However, Tie1 did not drive detectable CD40 expression in hematopoietic cells. Moreover, rescue of CD40 in EC enhanced protection without affecting the following: serum levels of IL-12 p40, IFN-γ, and TNF-α; in vitro production of these cytokines and nitric oxide and IRMG3 expression; mRNA levels of IL-12 p40, IFN-γ, TNF-α, and NOS2 in the eye and brain; expression of CD4+ and CD8+ T cell effector responses; and production of anti-T. gondii antibodies. These findings indicate that CD40 can induce protection against cerebral and ocular toxoplasmosis through a mechanism that does not rely on a type 1 cytokine response, expression of effector molecules downstream of IFN-γ, CD4+ and CD8+ effector responses, and humoral immunity.
Our studies revealed that EC expression of CD40 restricts invasion of the brain and retina, as indicated by the following observations. (i) From the early stages of T. gondii invasion of the retina and brain, parasite loads in these organs were lower in B6 mice than in CD40−/− mice even though both strains of mice had similar loads of T. gondii in peripheral organs and blood. (ii) Mice expressing CD40 in EC (B6 and Trg-CD40) had lower parasite loads in the eye and brain not only after infection with tissue cysts but also after i.v. injection of T. gondii-infected CD11b+ and DC. In contrast to findings in neural tissue, EC expression of CD40 did not affect invasion of the spleen, liver, and lung. This can be explained by the route of invasion. Mice infected with T. gondii tissue cysts exhibit parasite invasion of the spleen and lung that precedes detection of T. gondii in blood (12, 40). Parasitemia in these mice is reported to occur no earlier than day 5 postinfection (8, 41). Thus, it has been considered that T. gondii dissemination to the spleen and lung occurs through lymphatics rather than the bloodstream (40). This is relevant since, in contrast to findings in blood vessels, CD40 has not been observed in lymphatics even under inflammatory conditions (42).
Mechanisms for T. gondii invasion of the CNS include transmigration of infected CD11b+ and CD11c+ cells across the endothelial cell layer (Trojan horse mechanism), paracellular entry whereby extracellular tachyzoites transmigrate through tight junctions between endothelial cells, and transcellular entry that leads to infection of endothelial cells and release of the parasite in the neural parenchyma (8–11, 20). Recent studies support the importance of the last mechanism and the role of infected EC as portals of entry into the CNS (10). CD40 did not restrict T. gondii invasion of the brain and retina by impairing transmigration of leukocytes or invasion by extracellular tachyzoites. We showed that the effect of EC CD40 was to reduce the number of foci of infection in EC. While extracellular tachyzoites circulating in the blood can infect brain endothelial cells prior to invasion of the brain parenchyma (10), our studies indicate that T. gondii-infected DC could also lead to infection of EC, a process that may be dependent on parasite transfer from the infected leukocytes into EC, as reported to occur during other cell-cell interactions involving infected leukocytes (22, 23). Moreover, adhesion of infected leukocytes to EC triggers parasite egress that could lead to infection of endothelial cells (24). While relatively low percentages of monocytes/DC in the blood carry intracellular tachyzoites, only a small number of brain endothelial cells (fewer than 1,000) are infected at the time the parasite invades the brain (10). This may contribute to the ability of infected monocytes/DC to mediate parasite invasion of the CNS. Our studies also showed that CD40 enabled EC to acquire anti-T. gondii activity after interaction with infected DC and macrophages. This process was accompanied by parasite encasement by LC3+ structures and killing dependent on ULK1 and beclin 1. These findings are relevant in vivo since EC expression of CD40 and expression of beclin 1 were required to reduce parasite load in EC and restrict invasion of the brain and retina. Further support for a role of autophagy in restricting T. gondii invasion comes from the demonstration that blockade of epidermal growth factor receptor signaling in EC induces autophagic targeting of T. gondii both in vitro and in vivo and parasite killing and reduces invasion of the CNS (43).
Although activated CD4+ T cells express CD154 and may contribute to CD40 stimulation of EC, CD40-induced anti-T. gondii activity would be more effective if it were to take place during leukocyte-endothelial cell interaction. While DC can express CD154 (26), our in vitro and in vivo studies suggest that Hsp70 rather than CD154 mediates induction of anti-T. gondii activity in EC. Despite the fact that Hsp70 lacks the transmembrane domain and consensus signal required for secretion by the endoplasmic reticulum (ER)-Golgi pathway, the association of Hsp70 with lipid rafts and other alternative transport pathways likely explains the multiple lines of evidence indicating that inducible Hsp70 can localize to the cell membrane (44). Cancer cells and cells subjected to stress, including viral infection, express Hsp70 in their cell membranes (29–32). Our studies using cell membrane preparations and/or immunofluorescence indicated that infected DC and macrophages expressed inducible Hsp70 associated with the cell membrane.
Various receptors for Hsp70 have been described (45), and these include CD40 (33, 34). The evidence that more than one receptor coexists in cells (46) may explain, in part, the discrepant results observed in studies that examined the role of surface proteins as receptors for Hsp70. There are likely additional factors that could contribute to the contrasting results on the ability of Hsp70 to bind CD40. Preparations that likely contained Hsc70 were used in some studies that could not detect binding of Hsp70 to CD40 (35, 36). In addition, the use of E. coli-derived recombinant mammalian Hsp70 (37) may also affect the results. Mammalian inducible Hsp70 undergoes posttranslational modifications (47), events that may not be properly reproduced in bacterial expression systems. Using mammalian Hsp70 of baculovirus origin, our studies revealed preferential binding of Hsp70 to CD40+ cells that was inhibited by an anti-CD40 MAb. The potential explanation for the partial effect of the anti-CD40 MAb is that this MAb impairs CD40-CD154 interaction, and the regions of CD40 that interact with CD154 or Hsp70 may be different (48). Our studies do not rule out an indirect interaction between Hsp70 and CD40 and/or the cooperative role of other surface molecules. Regardless of whether there is a direct or indirect interaction between Hsp70 and CD40, our studies support a link between these molecules since they show (i) that a blocking anti-Hsp70 MAb inhibited CD40-dependent autophagy and autophagic killing of T. gondii in EC; (ii) that knockdown of Hsp70 in infected DC had similar in vitro effects; and (iii) that knockdown of Hsp70 in infected DC increased the number of foci of infection in EC in vivo and enhanced parasite invasion of neural tissue. These findings suggest a model whereby Hsp70 associated with the membrane of infected leukocytes interacts either directly or indirectly with CD40 expressed on brain and retinal EC, inducing autophagy in these cells. These cells would acquire the capacity to kill T. gondii via autophagy, thus reducing the number of foci of infected EC. This would then restrict invasion of brain and retinal parenchyma (Fig. 9). While it would appear logical that Hsp70 associated with the cell membrane is of mammalian origin, it is possible that T. gondii Hsp70, which has been found in the parasitophorous vacuole (38), could contribute to membrane Hsp70. Regardless of the source of Hsp70, this protein triggers anti-T. gondii activity in EC. Of relevance, using a MAb that detects mammalian Hsp70 indicated that expression of this protein is associated with increased in vivo protection against toxoplasmosis (49).
FIG 9.
Proposed model of CD40- and inducible Hsp70-mediated restriction of invasion of the CNS by T. gondii. Circulating T. gondii-infected monocytes and dendritic cells upregulate inducible Hsp70 on their cell membranes. Inducible Hsp70 interacts directly or indirectly with CD40 expressed on endothelial cells, triggering CD40 signaling and stimulation of autophagy. Endothelial cells become infected in the early stages of CNS invasion by T. gondii. It is possible that this may occur as a result of parasite egress from infected leukocytes during their interaction with endothelial cells (24). CD40-driven stimulation of autophagy in endothelial cells would result in entrapment of T. gondii by autophagosomes and autophagic killing of the parasite, reducing invasion of the CNS parenchyma. Our studies do not rule out the possibility that other infected leukocytes besides monocytes and dendritic cells could trigger CD40-Hsp70-mediated restriction of CNS invasion by T. gondii.
In summary, we report that the function of EC as a biological barrier that restricts T. gondii access into the brain and retina can be enhanced by a mechanism dependent on an interaction between EC and leukocytes involving CD40 and Hsp70 and the induction of autophagy protein-dependent anti-parasitic activity in EC. This mechanism of protection against CNS invasion may apply to not only T. gondii but also other organisms including flaviviruses and Mycobacterium tuberculosis, pathogens that reach the CNS within infected leukocytes, infect EC, are controlled by CD40 and autophagy, and may induce Hsp70. Further elucidation of the molecular events that regulate this process may lead to therapeutic approaches to impair pathogen dissemination into neural tissue.
MATERIALS AND METHODS
Animals.
TetOS-CD40 transgenic mice were reported previously (17). Homozygous TetOS-CD40 (responder) and heterozygous Tie1-tTA (driver) transgenic mice (15) (both B6) were backcrossed onto a CD40−/− background. Both lines of transgenic mice were bred, and offspring were identified by PCR analysis of genomic DNA using the following primers: Tie1-tTA forward, 5′-CTCACTTTTGCCCTTTAGAA-3′, and reverse, 5′-GCTGTACGCGGACCCACTTT-3′; TetOSCD40 forward, 5′-GCAACGTGCTGGTTATTGTG-3′, and reverse: 5′-CCGGGACTTTAAACCACAGA-3′. Littermates that inherited only one transgene (single transgenic and nonexpressing) served as controls (Trg-Ctr) for double-transgenic animals (Trg-CD40; expressing Tie1 promoter-specific CD40 expression). C57BL/6 (B6), CD40−/−, CD154−/−, and Becn1+/− mice (both B6 background) were bred at the Animal Resource Center (Case Western Reserve University). Female mice (6 to 12 weeks old) were used for this study. Littermates of the same sex were randomly assigned to experimental groups. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (50). The protocol was approved by the Institutional Animal Care and Use Committee of Case Western Reserve University School of Medicine (protocol number 2015-0130).
Mammalian cells.
Lungs and brains collected from perfused animals were digested with collagenase I (1 mg/ml) plus DNase (100 μg/ml; Worthington Biochemical, Lakewood, NJ) using Gentle Max C tubes (Miltenyi Biotec, Auburn). Homogenates were passed through a 40-μm-pore-size cell strainer. Enrichment for EC was performed using anti-CD146 magnetic beads (Miltenyi Biotec). Blood CD11b+ and CD11c+ cells were purified using immunomagnetic beads (Miltenyi Biotec). Brain mononuclear cells and mouse bone marrow-derived DC were isolated as described previously (6, 51). Murine high endothelial venule cells (mHEVc; lack endogenous CD40) stably transfected with a plasmid encoding the extracellular domain of human CD40 with the intracellular domain of mouse CD40 (hmCD40) or with empty vector (pRSV.5), with or without an LC3-GFP plasmid, were previously described (25). hmCD40 mHEVc or control (CD40−) mHEVc were also transfected with a pGL4.32luc2P/NF-κB-RE/Hygro vector (Promega Corporation, Madison, WI), a plasmid that encodes an NF-κB response element that drives transcription of the luciferase reporter gene luc2P (Photinus pyralis), as previously reported (52). 293T cells were transduced with previously described MIEG3 or MIEG3-human CD40 retroviral vectors (52). The mouse DC line DC2.4 (gift from Kenneth Rock, University of Massachusetts) and RAW 264.7 cells were also infected with T. gondii.
T. gondii.
Mice were infected i.p. with 10 cysts of the ME49 strain of T. gondii. In certain experiments, mice were infected i.p. with 1 × 105 tachyzoites of PTG or PTG-GFP T. gondii. Blood was collected from these animals after 7 days to purify CD11b+ and CD11c+ cells. A total of 1 × 105 CD11b+ cells was injected to mice i.v. as indicated. DC were also infected with tachyzoites of the PTG or PTG-GFP strains of T. gondii. Monolayers were washed thoroughly at 1 h and 18 h to remove extracellular parasites prior to i.v. injection into mice. Parasite load in mice was assessed by real-time PCR to examine expression of the T. gondii B1 gene or GFP (see below), as well as by counting tissue cysts in brain homogenates. Tachyzoites of the PTG strain of T. gondii, PTG-GFP, the RH strains of T. gondii that express either cytoplasmic YFP or RFP, the RH strain lacking carbamoyl phosphate synthetase II and expressing YFP (RH CPSII−/−-YFP), and CPS (including CPS-mCherry), a nonreverting uracil auxotroph mutant of T. gondii based on disruption of the orotidine-5′-monophosphate decarboxylase (ΔOMPDC) (53), were maintained in human foreskin fibroblasts. Mammalian cells were challenged with T. gondii tachyzoites, and the number of tachyzoites or vacuoles per 100 cells was determined by either light or fluorescence microscopy.
Transmigration of extracellular tachyzoites was assessed as described previously (20). hmCD40-mHEVc were plated on 24-well Transwell inserts and incubated with or without human CD154 (hCD154 supernatant secreted from CHO cells; gift from Richard Kornbluth, University of California San Diego) for 18 h. Freshly egressed RH-YFP or PTG-GFP tachyzoites (0.5 × 106 to 1 × 106) were added to the upper chamber. After 1 to 4 h, the number of tachyzoites that traversed to the lower chamber was determined by using a fluorescence microscope. Transmigration of DC was performed as described previously (9). Mouse DC were infected with RH CPSII−/−-YFP or PTG-GFP. After 6 h, cells were washed three times at 80 × g to remove extracellular parasites. DC that had been incubated previously with or without T. gondii were added to the inserts of the Transwell plates. Cells that migrated to the lower chamber were counted at 18 h. Transendothelial electrical resistance (TEER) was calculated by measuring resistance values of EC monolayers minus those of blank wells. Values are expressed as number of ohms/centimeter squared. Triton X-100 was used as a positive control.
Histopathology and immunohistochemistry.
Four 5-μm sections at four different areas of brain and eye were stained with periodic acid-Schiff hematoxylin (PASH) or hematoxylin and eosin (H&E) stain, respectively, and used to score histopathologic changes as described previously (6). Immunohistochemistry to detect T. gondii parasites was performed using anti-T. gondii antibody (Ab) (BioGenex Laboratories, Fremont, CA) and a secondary Ab conjugated with Alexa Fluor 568 (Jackson ImmunoResearch Laboratories, West Grove, PA). Sections were also incubated with tomato lectin-DyLight 488 (labels EC and microglia in rodents [Vector Laboratories, Burlingame, CA]). Four coronal sections per mouse at the septo-diencephalic region were examined at a magnification of ×400, and the numbers of clusters of T. gondii parasites per section were determined. Endothelial foci of infection were identified by the presence of parasites within tubular structures that were tomato lectin+ (endothelial cells). In addition, zinc-fixed brain, eye, and spleen sections were incubated with biotinylated anti-CD40 Ab (3/23; BioLegend, San Diego, CA), followed by incubation with streptavidin-Alexa Fluor 647 (Jackson ImmunoResearch Laboratories). This was followed by incubation with tomato lectin-DyLight 488, and in the case of brain sections, also by incubation with anti-Iba-1 (Wako Chemicals, Richmond, VA) plus Alexa Fluor 488-conjugated secondary Ab. Slides were analyzed using an Olympus BX-60 upright microscope and a Leica DMI 6000B epifluorescence microscope.
Real-time quantitative PCR.
To analyze expression of the T. gondii B1 gene, genomic DNA was subjected to PCR using SYBR green PCR Master Mix (Applied Biosystems, Waltham, MA) (6). Samples were run against a standard curve of DNA from 1 to 105 ME49 tachyzoites per reaction volume to calculate parasite load. Each sample was run in triplicate. RNA was reverse transcribed to cDNA with Super-Script III reverse transcriptase (Invitrogen, Carlsbad, CA). cDNA was used as the template for reverse transcription-PCR (RT-PCR) using SYBR green PCR Master Mix and primers as described previously (6). GFP primers were 5′-GTCCACACAATCTGCCCTTT-3′ (forward) and 5′-CATCCATGCCATGTGTAATC-3′ (reverse). Gene expression was assessed using a StepOne Real Time PCR system (Applied Biosystems). Each cDNA sample was run in triplicate. Samples were normalized according to the content of 18S rRNA.
Flow cytometry.
CD40 expression was examined in cell suspensions from lungs or EC isolated from lungs and brains by staining cells with anti-CD40 and either anti-ICAM-2 (eBiosciences, San Diego, CA) or anti-CD31 (eBiosciences) MAbs. Splenocytes and brain mononuclear cells isolated as described previously (6) were stained with anti-CD3, anti-CD4, anti-CD8, anti-CD11b, anti-CD11c, anti-CD40, anti-CD45R, anti-CD49b (DX5), anti-F4/80, anti-Gr-1, or control MAb (all from eBiosciences). For detection of intracellular cytokines, splenocytes were incubated with or without anti-CD3 plus brefeldin A (eBiosciences). Cells were stained with anti-CD3, anti-CD4, or anti-CD8, permeabilized, and stained with anti-IFN-γ MAb (eBiosciences). To assess binding of Hsp70 to mammalian cells, CD40+ and CD40− mHEVc or 293T cells were incubated with His-tagged recombinant baculovirus/Hi5 cell-derived human Hsp70 (StressMarq Biosciences, Victoria, BC, Canada), followed by addition of phycoerythrin (PE)-conjugated anti-His MAb (Miltenyi Biotec). A blocking anti-CD40 MAb (5C3; BioLegend) was added in some experiments. Cells were analyzed with an LSR II flow cytometer (BD Biosciences, San Jose, CA).
ELISA and nitric oxide assays.
Serum was used to measure concentrations of IL-12 p40, IFN-γ, and TNF-α by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN). Splenocytes were incubated with T. gondii lysate antigens. Supernatants were collected at 24 h for detection of IL-12 p40 and TNF-α or were collected at 72 h for measurement of IFN-γ. Supernatants collected at 72 h were used to measure nitrite concentrations using a Griess reaction (Promega Corporation). Data are expressed as micromolars of nitrite. Anti-T. gondii IgG was detected by ELISA, and antibody titer was calculated by determining the highest dilution of serum that yielded a reading higher than the mean plus 2 standard deviations (SD) of the reading in sera from uninfected mice.
Transfections.
Cells were transfected with beclin 1 siRNA (Dharmacon, Lafayette, CO), ULK1 siRNA (Life Technologies, Carlsbad, CA), Hsp70 siRNA (Santa Cruz Biotechnologies), control siRNA, or pGL4.32luc2P/NF-κB-RE/Hygro using Lipofectamine 2000 (Life Technologies) according to instructions provided by the manufacturer. siRNAs (20 pmol each) were used, and cells were utilized 48 h after transfection with siRNAs.
Isolation of cell membrane preparations.
Isolation of cell membranes was accomplished as previously described (54). Cells were washed in ice-cold buffer (125 mM NaCl, 5 mM KCl, 1 mM MgCl2, 25 mM HEPES) and resuspended in cold lysis buffer (0.1 M KCl, 5 mM NaCl, 50 mM HEPES, 3 mM MgCl2, 1 mM dithiothreitol). Lysates were obtained by N2 cavitation (20-min incubation on ice at 400 lb/in2 of N2) and centrifuged for 20 min at 400 × g at 4°C. The supernatant was collected and centrifuged for 20 min at 20,000 × g at 4°C. The resulting pellet was resuspended in lysis buffer, followed by a second centrifugation at 20,000 × g for 20 min at 4°C.
Immunoblotting.
Membranes were probed with antibody to inducible Hsp70 (C92F3A; StressMarq Biosciences), Na/K ATPase (Cell Signaling, Danvers, MA), GAPDH (Cell Signaling), T. gondii aldolase (gift from David Sibley, Washington University), beclin 1 (BD Bioscience), ULK1 (Cell Signaling), and Irgm3 or actin (both from Santa Cruz Biotechnologies). Band density was assessed using ImageJ (NIH).
Immunofluorescence.
Mouse DC were incubated with or without or nonreverting uracil auxotroph tachyzoites. After overnight incubation, DC were washed extensively and incubated with CD40+ or CD40− mHEVc with or without LC3-EGFP. Slides were analyzed by fluorescence microscopy for distinct LC3-positive structures that measure at least 1 μm in diameter. For assessment of accumulation of LC3 or LAMP-1 around the parasite, experiments were set up as described above, using mHEVc infected with RFP-T. gondii. LC3 or LAMP-1 accumulation around RFP-T. gondii parasites was assessed at 5 h or 12 h, respectively, by examining for the presence of rings surrounding parasites. The numbers of RFP-T. gondii-containing vacuoles in mHEVc (EGFP+) were determined at 18 h. To examine expression of membrane-associated inducible Hsp70, nonpermeabilized cells were incubated with anti-Hsp70 MAb (catalog no. 1H11; StressMarq), followed by a secondary Ab conjugated with Cy2 (Jackson ImmunoResearch Laboratories).
Statistics.
A D’Agostino-Pearson omnibus test was used to confirm normal distribution of the data (GraphPad Prism). Statistical significance was assessed by a parametric paired Student's t test and analysis of variance (ANOVA). Differences were considered significant at a P value of <0.05.
Supplementary Material
ACKNOWLEDGMENTS
We thank Scott Howell and Catherine Doller from the Visual Sciences Research Center for image analysis and for tissue processing, respectively.
This work was supported by grants from the National Institutes of Health: R01 EY018341 and R01 EY019250 to C.S.S.; REY018341B to Y.L.C.; R01 HL05636 and R01 AI08313 to Z.T.; R21 AI099494 to R.E.R.; and P30 EY011373.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00868-18.
REFERENCES
- 1.Lazear HM, Daniels BP, Pinto AK, Huang AC, Vick SC, Doyle SE, Gale M Jr, Klein RS, Diamond MS. 2015. Interferon-lambda restricts West Nile virus neuroinvasion by tightening the blood-brain barrier. Sci Transl Med 7:284ra59. doi: 10.1126/scitranslmed.aaa4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Kooten C, Banchereau J. 1997. Functions of CD40 on B cells, dendritic cells and other cells. Curr Opin Immunol 9:330–337. doi: 10.1016/S0952-7915(97)80078-7. [DOI] [PubMed] [Google Scholar]
- 3.Grewal IS, Flavell RA. 1998. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol 16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 4.Hayward AR, Cosyns M, Jones M, Ponnuraj EM. 2001. Marrow-derived CD40 positive cells are required for mice to clear a Cryptosporidium parvum infection. Infect Immun 69:1630–1634. doi: 10.1128/IAI.69.3.1630-1634.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reichmann G, Walker W, Villegas EN, Craig L, Cai G, Alexander J, Hunter CA. 2000. The CD40/CD40 ligand interaction is required for resistance to toxoplasmic encephalitis. Infect Immun 68:1312–1318. doi: 10.1128/iai.68.3.1312-1318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Portillo J-A, Okenka G, Reed E, Subauste A, Van Grol J, Gentil K, Komatsu M, Tanaka K, Landreth G, Levine B, Subauste CS. 2010. The CD40-autophagy pathway is needed for host protection despite IFN-γ-dependent immunity and CD40 induces autophagy via control of p21 levels. PLoS One 5:e14472. doi: 10.1371/journal.pone.0014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhadra R, Gigley JP, Khan IA. 2011. Cutting edge: CD40-CD40 ligand pathway plays a critical CD8-intrinsic and -extrinsic role during rescue of exhausted CD8 T cells. J Immunol 187:4421–4425. doi: 10.4049/jimmunol.1102319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Courret N, Darche S, Sonigo P, Milon G, Buzoni-Gâtel D, Tardieux I. 2006. CD11c- and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood 107:309–316. doi: 10.1182/blood-2005-02-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambert H, Hitziger N, Dellacasa I, Svensson M, Barragan A. 2006. Induction of dendritic cell migration upon Toxoplasma gondii infection potentiates parasite dissemination. Cell Microbiol 8:1611–1623. doi: 10.1111/j.1462-5822.2006.00735.x. [DOI] [PubMed] [Google Scholar]
- 10.Konradt C, Ueno N, Christian DA, Delong JH, Pritchard GH, Herz J, Bzik DJ, Koshy AA, McGavern DB, Lodoen MB, Hunter CA. 2016. Endothelial cells are a replicative niche for entry of Toxoplasma gondii to the central nervous system. Nat Microbiol 1:16001. doi: 10.1038/nmicrobiol.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendez OA, Koshy AA. 2017. Toxoplasma gondii: Entry, association, and physiological influence on the central nervous system. PLoS Pathog 13:e1006351. doi: 10.1371/journal.ppat.1006351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derouin F, Garin Y. 1991. Toxoplasma gondii: Blood and tissue kinetics during acute and chronic infections in mice. Exp Parasitol 73:460–468. doi: 10.1016/0014-4894(91)90070-D. [DOI] [PubMed] [Google Scholar]
- 13.Mordue DG, Monroy F, La Regina M, Dinarello CA, Sibley LD. 2001. Acute toxoplasmosis leads to lethal overproduction of Th1 cytokines. J Immunol 167:4574–4584. doi: 10.4049/jimmunol.167.8.4574. [DOI] [PubMed] [Google Scholar]
- 14.Hitziger N, Dellacasa I, Albiger B, Barragan A. 2005. Dissemination of Toxoplasma gondii to immunoprivileged organs and role of Toll/interleukin-1 receptor signalling for host resistance assessed by in vivo bioluminescence imaging. Cell Microbiol 7:837–848. doi: 10.1111/j.1462-5822.2005.00517.x. [DOI] [PubMed] [Google Scholar]
- 15.Sarao R, Dumont DJ. 1998. Conditional transgene expression in endothelial cells. Transgenic Res 7:421–427. doi: 10.1023/A:1008837410485. [DOI] [PubMed] [Google Scholar]
- 16.Chang L, Noseda M, Higginson M, Ly M, Patenaude A, Fuller M, Kyle AH, Minchinton AI, Puri MC, Dumont DJ, Karsan A. 2012. Differentiation of vascular smooth muscle cells from local precursors during embryonic and adult aryeriogenesis requires Notch signaling. Proc Natl Acad Sci U S A 109:6993–6998. doi: 10.1073/pnas.1118512109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Portillo J-A, Lopez Corcino Y, Miao Y, Tang J, Sheibani N, Kern TS, Dubyak GR, Subauste CS. 2017. CD40 in retinal Muller cells induces P2X7-dependent cytokine expression in macrophages/microglia in diabetic mice and development of early experimental diabetic retinopathy in mice. Diabetes 66:483–493. doi: 10.2337/db16-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.John B, Ricart B, Tait Wojno ED, Harris TH, Randall LM, Christian DA, Gregg B, De Almeida DM, Weninger W, Hammer DA, Hunter CA. 2011. Analysis of behavior and trafficking of dendritic cells within the brain during toxoplasmic encephalitis. PLoS Pathog 7:e1002246. doi: 10.1371/journal.ppat.1002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlüter D, Hein A, Dörries R, Deckert-Schlüter M. 1995. Different subsets of T cells in conjunction with natural killer cells, macrophages, and activated microglia participate in the intracerebral immune response to Toxoplasma gondii in athymic nude and immunocompetent rats. Am J Pathol 146:999–1007. [PMC free article] [PubMed] [Google Scholar]
- 20.Barragan A, Sibley LD. 2002. Transepithelial migration of Toxoplasma gondii is linked to parasite motility and virulence. J Exp Med 195:1625–1633. doi: 10.1084/jem.20020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Unno A, Suzuki K, Xuan X, Nishikawa Y, Kitoh K, Takashima Y. 2008. Dissemination of extracellular and intracellular Toxoplasma gondii tachyzoites in the blood flow. Parasitol Int 57:515–518. doi: 10.1016/j.parint.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Chtanova T, Han S-J, Schaeffer M, van Dooren GG, Herzmark P, Striepen B, Robey EA. 2009. Dynamics of T cell, antigen-presenting cell, and pathogen interactions during recall responses in the lymph node. Immunity 31:342–355. doi: 10.1016/j.immuni.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Persson EK, Agnarson AM, Lambert H, Hitziger N, Yagita H, Chambers BJ, Barragan A, Grandien A. 2007. Death receptor ligation or exposure to perforin trigger rapid egress of the intracellular parasite Toxoplasma gondii. J Immunol 179:8357–8365. doi: 10.4049/jimmunol.179.12.8357. [DOI] [PubMed] [Google Scholar]
- 24.Baba M, Batanova T, Kitoh K, Takashima Y. 2017. Adhesion of Toxoplasma gondii tachyzoite-infected vehicle leukocytes to capillary endothelial cells triggers timely parasite egression. Sci Rep 7:5675. doi: 10.1038/s41598-017-05956-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Grol J, Muniz-Feliciano L, Portillo J-A, Bonilha VL, Subauste CS. 2013. CD40 induces anti-Toxoplasma gondii activity in non-hematopoietic cells dependent on autophagy proteins. Infect Immun 81:2002–2011. doi: 10.1128/IAI.01145-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson S, Zhan Y, Sutherland RM, Mount AM, Bedoui S, Brady JL, Carrington EM, Brown LE, Belz GT, Heath WR, Lew AM. 2009. Selected Toll-like receptor ligands and viruses promote helper-independent cytotoxic T cell priming by upregulating CD40L on dendritic cells. Immunity 30:218–227. doi: 10.1016/j.immuni.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazarevic V, Myers AJ, Scanga CA, Flynn JL. 2003. CD40, but not CD40L, is required for the optimal priming of T cells and control of aerosol M. tuberculosis infection. Immunity 19:823–835. doi: 10.1016/S1074-7613(03)00324-8. [DOI] [PubMed] [Google Scholar]
- 28.Millar DG, Garza KM, Odermatt B, Elford AR, Ono N, Li Z, Ohashi PS. 2003. Hsp70 promotes antigen-presenting cell function and converts T-cell tolerance to autoimmunity in vivo. Nat Med 9:1469–1476. doi: 10.1038/nm962. [DOI] [PubMed] [Google Scholar]
- 29.Multhoff G, Botzler C, Wiesnet M, Muller E, Meier T, Wilmanns W, Issels RD. 1995. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer 61:272–279. doi: 10.1002/ijc.2910610222. [DOI] [PubMed] [Google Scholar]
- 30.Brown G, Rixon HW, Steel J, McDonald TP, Pitt AR, Graham S, Sugrue RJ. 2005. Evidence for an association between heat shock protein 70 and the respiratory syncytial virus polymerase complex within lipid-raft membranes during virus infection. Virology 338:69–80. doi: 10.1016/j.virol.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Martin CA, Kurkowski DL, Valentino AM, Santiago-Schwarz F. 2009. Increased intracellular, cell surface, and secreted inducible heat shock protein 70 responses are triggered during the monocyte to dendritic cell (DC) transition by cytokines independently of heat stress and infection and may positively regulate DC growth. J Immunol 183:388–399. doi: 10.4049/jimmunol.0802688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Seidl T, Whittall T, Babaahmady K, Lehner T. 2010. Stress-activated dendritic cells interact with CD4+ T cells to elicit homeostatic memory. Eur J Immunol 40:1628–1638. doi: 10.1002/eji.200940251. [DOI] [PubMed] [Google Scholar]
- 33.Becker T, Hartl FU, Wieland F. 2002. CD40, an extracellular receptor for binding and uptake of Hsp70-peptide complexes. J Cell Biol 158:1277–1285. doi: 10.1083/jcb.200208083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shevtsov MA, Yakovleva LY, Nikolaev BP, Marchenko YY, Dobrodumov AV, Onokhin KV, Onokhina YS, Selkov SA, Mikhrina AL, Guzhova IV, Martynova MG, Bystrova OA, Ischenko AM, Margulis BA. 2014. Tumor targeting using magnetic nanoparticle Hsp70 conjugate in a model of C6 glioma. Neuro Oncol 16:38–49. doi: 10.1093/neuonc/not141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Theriault JR, Mambula SS, Sawamura T, Stevenson MA, Calderwood SK. 2005. Extracellular HSP70 binding to surface receptors present on antigen presenting cells and endothelial/epithelial cells. FEBS Lett 579:1951–1960. doi: 10.1016/j.febslet.2005.02.046. [DOI] [PubMed] [Google Scholar]
- 36.Binder RJ. 2009. CD40-independent engagement of mammalian hsp70 by antigen-presenting cells. J Immunol 182:6844–6850. doi: 10.4049/jimmunol.0900026. [DOI] [PubMed] [Google Scholar]
- 37.Tao Y, Nomura M, Kitabatake N, Tani F. 2007. Mouse CD40-transfected cell lines cannot exhibit the binding and RANTES-stimulating activity of exogenous heat shock protein 70. Mol Immunol 44:1262–1273. doi: 10.1016/j.molimm.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Weiss LM, Ma YF, Takvorian PM, Tanowitz HB, Wittner M. 1998. Bradyzoite development in Toxoplasma gondii and the hsp70 stress response. Infect Immun 66:3295–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gustafsson E, Brakebusch C, Hietanen K, Fassler R. 2000. Tie-1-directed expression of Cre recombinase in endothelial cells of embryoid bodies and transgenic mice. J Cell Sci 114:671–676. [DOI] [PubMed] [Google Scholar]
- 40.Sumyuen MH, Garin YJF, Derouin F. 1995. Early kinetics of Toxoplasma gondii infection in mice infected orally with cysts of an avirulent strain. J Parasitol 81:327–329. doi: 10.2307/3283948. [DOI] [PubMed] [Google Scholar]
- 41.Norose K, Naoi K, Fang H, Yano A. 2008. In vivo study of toxoplasmic parasitemia using interferon-g-deficient mice: absolute cell number of leukocytes, parasite load and cell susceptibility. Parasitol Int 57:447–453. doi: 10.1016/j.parint.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Pammer J, Weninger W, Mazal PR, Horvat R, Tschachler E. 1996. Expression of the CD40 antigen on normal endothelial cells and in benign and malignant tumours of vascular origin. Histopathology 29:517–524. doi: 10.1046/j.1365-2559.1996.d01-531.x. [DOI] [PubMed] [Google Scholar]
- 43.Lopez Corcino Y, Portillo J-A, Subauste CS. 2019. Epidermal growth factor receptor promotes cerebral and retinal invasion by Toxoplasma gondii. Sci Rep 9:669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Maio A. 2011. Extracellular heat shock proteins, cellular export vesicles, and the Stress Observation System: a form of communication during injury, infection, and cell damage. Cell Stress Chaperones 16:235–249. doi: 10.1007/s12192-010-0236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Asea A. 2005. Stress proteins and initiation of immune response: chaperokine activity of hsp72. Exerc Immunol Rev 11:34–45. [PMC free article] [PubMed] [Google Scholar]
- 46.Zitzler S, Hellwig A, Hartl FU, Wieland F, Diestelkotter-Bachert P. 2008. Distinct binding sites for the ATPase and substrate-binding domain of human Hsp70 on the cell surface of antigen presenting cells. Mol Immunol 45:3974–3983. doi: 10.1016/j.molimm.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 47.Cloutier P, Coulombe B. 2013. Regulation of molecular chaperones through post-translational modifications: decrypting the chaperone code. Biochim Biophys Acta 1829:443–454. doi: 10.1016/j.bbagrm.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lehner T, Wang Y, Whittall T, McGowan E, Kelly CG, Singh M. 2004. Functional domains of HSP70 stimulate generation of cytokines and chemokines, maturation of dendritic cells and adjuvanticity. Biochem Soc Trans 32:629–632. doi: 10.1042/BST0320629. [DOI] [PubMed] [Google Scholar]
- 49.Nagasawa H, Oka M, Maeda K, Jian-Guo C, Hisaeda H, Ito Y, Good RA, Himeno K. 1992. Induction of heat shock protein closely correlates with protection against Toxoplasma gondii infection. Proc Natl Acad Sci U S A 89:3155–3158. doi: 10.1073/pnas.89.7.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC. [Google Scholar]
- 51.Liu E, van Grol J, Subauste CS. 2015. Atg5 but not Atg7 in dendritic cells enhance IL-2 and IFN-γ production by Toxoplasma gondii-reactive CD4+ T cells. Microbes Infect 17:275–284. doi: 10.1016/j.micinf.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Portillo J-A, Greene JA, Schwartz I, Subauste MC, Subauste CS. 2015. Blockade of CD40-TRAF2,3 or CD40-TRAF6 interactions is sufficient to impair pro-inflammatory responses in human aortic endothelial cells and human aortic smooth muscle cells. Immunology 144:21–33. doi: 10.1111/imm.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fox BA, Bzik DJ. 2010. Avirulent uracil auxotrophs based on disruption of orotidine-5'-monophosphate decarboxylase elicit protective immunity to Toxoplasma gondii. Infect Immun 78:3744–3752. doi: 10.1128/IAI.00287-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cowen DS, Baker B, Dubyak GR. 1990. Pertussis toxin produces differential inhibitory effects on basal, P2-purinergic, and chemotactic peptide-stimulated inositol phospholipid breakdown in HL-60 cells and HL-60 cell membranes. J Biol Chem 265:16181–16189. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.