Abstract
Background:
Studies have demonstrated that patients with end‐stage liver disease (ESLD) often have a prolonged corrected QT interval (QTc) with variable changes in the QTc post‐transplant. We sought to characterize the prevalence and degree of QTc prolongation in ESLD patients, identify risk factors for QTc prolongation, and assess changes in QTc following transplant.
Hypothesis:
QTc interval is prolonged in ESLD patients pre‐transplant due to a variety of risk factors and shortens following liver transplantation.
Methods:
We conducted a retrospective, multicenter study utilizing 2 large liver‐transplant databases. QTc intervals were calculated utilizing Bazett's formula. The cutoff used for prolonged QTc was 440 milliseconds for men and 460 milliseconds for women.
Results:
There were 269 patients (169 men, 100 women) included in the final analysis. The mean pre‐transplant QTc was prolonged (449.0 ms), whereas the mean post‐transplant QTc shortened and was within normal limits (416.7 ms) (P < 0.0001). QTc shortened after transplant in 87% of patients. QTc normalized in 70% of patients. Age and Model for End‐Stage Liver Disease (MELD) score were not predictive of prolonged QTc at baseline.
Conclusions:
ESLD patients often have a prolonged QTc, which frequently shortens or normalizes after transplant. Screening for prolonged QTc is warranted if medications known to prolong the QTc interval are used in ESLD patients pre‐transplant. MELD score, age, and sex were not predictive of prolonged QTc at baseline. Copyright © 2010 Wiley Periodicals, Inc.
Ms. Lorenze is a student at the University of Wisconsin whose involvement on this project included, but was not limited to, data collection and analysis and manuscript editing. The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
The QT interval, measured from the onset of the QRS complex to the end of the T wave on a standard 12‐lead electrocardiogram (ECG), measures ventricular electrical repolarization and is typically corrected for heart rate (QTc). Prolongation of the QTc interval is associated with ventricular arrhythmias, namely torsade de pointes, and increased mortality.1, 2, 3, 4 Acquired QT prolongation is known to occur with several classes of medication, including antiarrhythmics, certain antibiotics, and some antidepressants. The QTc is also known to be prolonged in the setting of various medical conditions, such as coronary ischemia, electrolyte abnormalities, inherited genetic mutations causing various ion‐channel disorders, and end‐stage liver disease (ESLD).
More specifically, QTc‐interval prolongation has been found in association with both alcoholic and nonalcoholic liver disease.5, 6, 7 Previous studies have reported that in patients with ESLD the degree of QTc prolongation correlates with the severity of autonomic dysfunction.8 Autonomic dysfunction is common among patients with liver disease, with a similar frequency among patients with alcoholic and nonalcoholic liver disease.9, 10 The presence of autonomic dysfunction is a poor prognostic indicator among those with ESLD, leading to a 25% increase in mortality in 15 to 48 months.7, 11 No clear etiology has been found to account for the development of autonomic dysfunction among these patients, but several mechanisms have been suggested, including an electrolyte imbalance, elevated serum bile acids, increased sympathetic activity, toxic metabolites, and impaired concentrations of circulating neurotransmitters.10
Several prior studies have evaluated the effect of liver transplant on QTc interval; however, most were small and with mixed results.6, 12, 13, 14, 15 One study by Bal and Thuluvath from Johns Hopkins University found that a prolonged QTc interval was common in patients with cirrhosis, with a prevalence of up to 56%. They concluded that age, an alcoholic etiology of cirrhosis, and Child‐Pugh scores were independent predictors of a prolonged QTc.13 Carey and Douglas from the Mayo Clinic in Arizona similarly reported that 54% of their 46 patients with ESLD had a prolonged QTc prior to liver transplant. However, in contrast they found no correlation between QTc prolongation and clinical factors such as Child‐Pugh score, Model for End‐Stage Liver Disease (MELD) score, diabetes mellitus, age, or etiology of liver disease.14 Both studies did find that most patients with ESLD and a prolonged QTc often had a significant improvement in QTc after transplant. Another European study of 94 cirrhotic patients also found that QTc was prolonged in these patients, with a mean QTc of 440 milliseconds. Interestingly, QTc only correlated to Child‐Pugh score and no other clinical factors.7
Based on these reports with somewhat mixed results, we sought to further validate these findings and characterize the QTc interval in a large, multicenter cohort of patients with ESLD who subsequently underwent liver transplant. Thus, the purpose of this study was to determine both the prevalence and degree of QTc prolongation among patients with ESLD and to determine interval QTc changes following liver transplant.
Methods
Study Design and Patients
After institutional review board approval was obtained, we performed a retrospective study evaluating the difference in QTc interval before and after liver transplant at 2 academic institutions. At the University of North Carolina, Chapel Hill, data were collected on 701 consecutive primary liver transplant recipients from 1990 to 2006. At the University of Wisconsin Hospital and Clinics, data were collected on 410 consecutive primary liver transplant recipients from 2002 to 2006. Subjects were identified and data were obtained through a prospectively constructed database with subsequent review of individual electronic and paper medical records. Of the 1111 transplant recipients identified, 269 subjects met the inclusion criteria for the study (Figure 1). Patients included in the final data analysis included men and women with ESLD, age 18–70 years, who were undergoing liver transplant for any indication (Table 1). Patients were excluded if they did not have an interpretable ECG pre‐transplant and/or post‐transplant. The most common reasons for exclusion were absence of a pre‐transplant or post‐transplant ECG, multiple organ transplant, and age <18 years.
Figure 1.
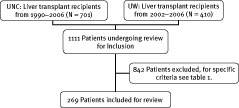
Flow diagram of patient selection.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion Criteria |
| ESLD (EtOH and non‐EtOH) |
| Pre‐transplant 12‐lead ECG at most 1 mo prior to transplant |
| Post‐transplant 12‐lead ECG 1–12 mo following transplant |
| Age 18–70 y |
| Exclusion Criteria |
| History of cardiorespiratory disease |
| Clinically significant valvular heart disease or septal defects |
| Known ischemic cardiomyopathy |
| AF |
| IVCD |
| Evidence of pre‐excitation |
| BBB |
| Paced rhythm |
| Multiple liver transplants |
| Simultaneous multiorgan transplantation |
Abbreviations: AF, atrial fibrillation; BBB, bundle branch block; ECG, electrocardiogram; EtOH, alcohol; ESLD, end‐stage liver disease; IVCD, interventricular conduction delay.
QTc Interval Calculation
The QT interval was measured from the beginning of the QRS complex to the termination of the T wave (defined as the return to the isoelectric line). QTc was calculated manually using Bazett's formula: QTc = QT interval (sec)/ (R‐R interval1/2) (sec). Bazett's formula was chosen because the QTc interval calculated this way has been shown to be a predictor of cardiovascular mortality. Lead II was the first choice for calculating the QTc; however, if lead II could not be used due to poor T‐wave visualization, the lead with the clearest T wave was utilized. A dramatic QTc decrease was defined as a reduction ≥60 ms. A prolonged QTc interval was defined as a QTc >440 ms for men and >460 ms for women.16 Different QTc cutoff values were used based on sex, because women are known to have longer QTc values than men and sex‐specific QTc cutoff values frequently are used when looking at QTc prolongation.17, 18 We wanted to be able to account for baseline QT differences due to gender irrespective of liver disease and other risk factors for QT prolongation.
Variables
To evaluate potential clinical risk factors for prolonged QTc, various patient variables were analyzed: patient characteristics (age at transplant, gender, race, baseline heart rate), liver transplant indication and type of liver transplant performed, serum potassium, MELD score, and medication use (β‐blockers, fluoroquinolones, antifungal agents such as azoles, quinine, and any antiarrhythmic medications) (Table 2). In addition, the change in the QTc interval before and after transplant was calculated to determine the effect of transplant on the QTc interval. Survival status was determined at the end of the follow‐up period. Death was confirmed by searching the Social Security Death Index on November 15, 2007 (http:// ssdi.rootsweb.com).
Table 2.
Baseline Patient Characteristics
| Patient Characteristics (N = 269) | Cases |
|---|---|
| Age at transplant (mean y) | 50.9 |
| Gender (% M/F) | 62/38 |
| Race (% Caucasian) | 85.5 |
| Donor type (% orthotopic) | 94 |
| Post‐transplant mortality at end of follow‐up (%) | 22.7 |
| Etiology of liver disease | |
| EtOH alone % | 26 |
| EtOH involvement % | 34.8 |
| Non‐EtOH % | 65.2 |
| MELD score (median) | 19 |
| Pre‐transplant medications (n) | |
| β‐Blockers | 98 |
| Fluoroquinolones | 52 |
| Antifungals (azoles) | 5 |
| Quinine | 15 |
| Antiarrhythmics | 7 |
Abbreviations: EtOH, alcohol; F, female; M, male; MELD, Model for End‐Stage‐Liver Disease; n, number of patients.
Statistical Methods
The difference in QTc interval before and after liver transplant and the effect of gender and MELD score on QTc were assessed using t tests. Age, gender, potassium level, use of quinolones, MELD, and donor type were considered as possible predictor variables. Their effects on the difference between the pre‐transplant QTc and post‐transplant QTc interval were assessed using multiple linear regression. The effect of the predictor variables on the response variables of prolonged preoperative QTc interval, normal preoperative QTc interval, postoperative normalized QTc interval, and whether the patient survived the surgery was assessed using multiple logistic regression. We used t tests for 2 sample comparisons of continuous variables.
Results
Patient Characteristics
There were 1111 primary liver transplants performed at the participating institutions between 1990 and 2006. Of those, 269 met inclusion criteria for data analysis. The baseline pre‐transplant characteristics of the 269 patients are summarized in Table 2. The mean age at the time of transplant was 51 years. The majority of subjects were Caucasian and male (169 men, 100 women; 230 Caucasian, 26 African American, 13 Hispanic). Most patients received an orthotopic transplant (94%). Alcohol alone was the most common sole etiology of liver disease and was identified in 26% of the transplant recipients, whereas alcohol involvement was identified 35% of the time. The mean MELD score of all patients at the time of transplant was 20.7 (median 19). For spontaneous bacterial peritonitis prophylaxis 19% of patients were on a fluoroquinolone, a medication class known to prolong the QTc interval.
Pre‐Transplant QTc Data Analysis
The overall mean baseline QTc interval was 449 milliseconds (range, 320–554 ms). There was no difference in baseline QTc between men and women. Of the 269 included subjects, 50% (n = 135) had a prolonged QTc interval pre‐transplant. Of the clinical predictors of prolonged QTc that we considered, only sex was found to be a possible predictor; men were more likely than women to have a prolonged QTc interval pre‐transplant (31% vs 19%; odds ratio: 2.3, P = 0.001). However, this was using the longer QTc of 460 milliseconds as the definition of prolonged QTc for women; men were more likely to have a QTc >440 milliseconds than women were to have a QTc >460 milliseconds.
Post‐Transplant QTc Data Analysis
The overall mean QTc interval normalized post‐transplant, with a decrease in QTc interval from 449 milliseconds to 417 milliseconds (P < 0.001). Both men and women had a significant reduction in mean QTc interval post‐transplant compared with baseline (P < 0.001). However, women did have a longer post‐transplant mean QTc than men (421 ms vs 414 ms), which corresponds to a gender difference that is typically seen in healthy noncirrhotic adults.
Of the 135 patients (84 men, 51 women) with baseline prolonged QTc, 87% shortened, but not necessarily normalized, their QTc interval post‐transplant. Of the 135 subjects with initial prolongation of their QTc, 70% (n = 95; 67 men, 28 women) had corrected their prolongation and were now in normal range, while 30% (n = 40; 17 men, 23 women) continued to have a prolonged QTc post‐transplant, as based on our prespecified definition (Table 3). Of those who did shorten their QTc interval, 23% experienced a dramatic decrease (ie, >60 ms). These large decreases were fairly similar for both sexes (25% of men and 19% of women).
Table 3.
QTc Interval Data for Overall Cohort, Men and Women, Both Pre‐Transplant and Post‐transplant
| QTc Analysis (N = Overall 269, M 169, F 100) | P Value | |
|---|---|---|
| Overall QTc pre‐transplant (mean ms) | 449 | |
| M | 449 | |
| F | 449 | |
| Overall QTc post‐transplant (mean ms) | 417 | <0.001 |
| M | 414 | <0.001 |
| F | 421 | <0.001 |
| Overall QTc difference pre‐transplant vs post‐transplant (mean ms) | 32 | <0.001 |
| Prolonged QTc pre‐transplant (n = 135)/ post‐transplant (n = 40) (% all patients) | 50/15 | <0.001 |
| Prolonged QTc pre‐transplant (n = 135)/ post‐transplant (n = 40); (% M/F) | 62/38; 43/57 | |
| Normalized post‐transplant QTc (overall/M/F) | 95/67/28 | |
| Dramatic decrease in QTc (% M/F) | 22/20 |
Abbreviations: F, female; M, male; n, number of patients.
Additionally, number of patients with prolonged pre‐transplant QTc and number of patients with normalized QTc following transplantation are included.
As of 2006, 80% of men and 73% of women were still alive and being actively followed in transplant clinics. There was no difference in baseline QTc between those who died and those who were still alive at the end of the study.
Analysis of Predictors and Comparative Data
Clinical variables including age, gender, serum potassium, quinine use, etiology of liver disease, MELD score, medications, and donor‐liver type were collected and evaluated for being potential predictors of prolonged QTc interval in cirrhotic patients awaiting transplant (Table 2). Of these predictors, only gender was significantly related to prolonged QTc interval; however, this depended on the definition of prolonged QTc in women that was used. When 440 milliseconds was used as a cutoff value for both sexes, the difference in QTc at baseline was not significantly different and male gender was no longer a predictor of QT prolongation.
Discussion
QTc interval prolongation has been documented previously in patients with chronic liver disease. However, most of these reports were small, single‐center studies. Our study represents a large, multicenter cohort consisting of 269 patients undergoing liver transplant. Overall, our results showing a 50% prevalence of prolonged QTc in patients with severe liver disease are consistent with and further validate prior studies. Most studies reviewed found a prevalence of prolonged QTc in ESLD patients ranging from 36% to 60%.7, 13, 14, 15, 19 However, 1 study conducted in Madrid, Spain with 37 subjects did not have any enrollees with a QTc >440 milliseconds, and conversely, the prevalence of prolonged QTc (>440 ms) in a study from Birmingham, England was 87%.6, 12 Our data showing that the QTc interval significantly shortens post–liver transplant also correlates with these prior studies that showed QT shortening anywhere from 55% to 95% of the time following liver transplant.
Gender Differences
We were surprised to find that men and women with ESLD have a similar mean QTc interval pre‐transplant as well as post‐transplant. Women tend to have longer QTc intervals than men in healthy cohorts. Gender differences in the incidence of ventricular arrhythmias have been reported, and torsades de pointes associated with prolonged QTc is more common in women than in men.20, 21 To our knowledge, there are only 2 other studies in the literature that looked at the interaction of gender and QTc in ESLD subjects. They found, as we did, essentially equal QTc values between men and women.7, 19 Adigun and colleagues showed that there was no statistically significant difference between females and males both before and after transplant.22 Bernardi et alin Italy also showed that there was no gender difference following liver transplantation; however, unfortunately, they only looked at gender‐related QTc differences post‐transplant.7 One possible explanation for the loss of gender difference regarding the QTc may be due to the hormonal abnormalities present in cirrhotic patients. Adigun and colleagues demonstrated that cirrhotic men have profoundly diminished levels of testosterone, whereas cirrhotic women have normal levels.22 Although they felt that this should translate into men having a QTc greater than females, it may very well be the reason why the males with ESLD “catch up” to the women in terms of QTc duration, rather than continue to have shorter QTc intervals as seen in healthy populations.
QTc and MELD Score
The lack of association between pre‐transplant QTc interval and various clinical variables has previously been shown in other retrospective studies. However, these studies had fewer patients and could possibly have been underpowered to detect this association.6, 7, 14, 15 An association between Child‐Pugh or MELD score and the QTc interval has been reported in some prior studies, but the majority did not show any correlation. Our study confirms the lack of association between MELD score and the QTc interval. Perhaps this is explained by a lack of association between these various clinical variables and autonomic function.
QTc and Mortality
In our study, there did not appear to be a difference in post‐transplant mortality based on baseline QTc prolongation. However, autonomic dysfunction is known to worsen overall prognosis and increase mortality in patients with chronic conditions such as heart failure and diabetes. In addition, increased mortality, predominantly from cardiovascular causes, in chronic alcohol abusers with vagal neuropathy has been reported.23 There is also 1 study in liver disease patients where autonomic dysfunction does portend an increased mortality11; however, this dataset was in less‐sick patients who were stable clinically and therefore not listed for transplant. A few studies have looked at the baseline pre‐transplant QT prolongation and autonomic neuropathy and its potential influence on post‐transplant mortality. They did not see any relationship between QTc and autonomic neuropathy on mortality.6, 13 Since the QTc prolongation and the autonomic neuropathy reverse in the majority of patients after transplant, we believe that the liver transplant and subsequent reverse remodeling of autonomic tone reverse the increased mortality risk that is present pre‐transplant. We hypothesize that a lack of reversal in QTc and autonomic tone post‐transplant would have a worse prognosis, but longer‐term data may be needed to assess this adequately. Finally, since cause of death was not a specific variable included in this study, it is also difficult to know for certain whether patients died due to a cardiac or noncardiac cause.
QTc After Liver Transplant
A large majority of patients had reductions and even normalization in their QTc interval following transplant. Chronic liver disease is known to impair cardiovascular autonomic reflexes.17 It has been hypothesized by other authors that alterations in autonomic function play an important role in reversing this impairment. We believe that one of the major mechanisms of QTc normalization is the reversal of autonomic dysfunction. There are reports in the literature that severity of autonomic dysfunction increases with worsening liver dysfunction. There are also data that hepatic dysfunction severity correlates to QTc prolongation severity, although our dataset did not show this. It is not understood how liver failure causes autonomic neuropathy, but it may be related to abnormal lipid metabolism or abnormal immunoglobulin deposition, both of which would return to normal post‐transplant. However, since not all patients normalized their QTc post‐transplant, there is probably a multifactorial cause of the QTc interval, with the absence of liver disease being the major component.
In addition to, and possibly synergistic with, autonomic dysfunction, alteration of the sympathetic nervous and renin‐angiotensin‐aldosterone systems can prolong the action potential duration, which can affect the QT interval. The degree of action potential increase may be different from patient to patient. Michael et aldiscuss a phenomenon known as repolarization reserve, in which certain ion channels can increase function or compensate when other ion channels have reduced functionality. This is affected by cardiac disease states such as heart failure and atrial fibrillation, and we wonder whether the cardiac sequelae of liver disease can include altered functioning of various ion channels. Genetic susceptibility to ion‐channel dysfunction may affect the improvement in QT post‐transplant, where some patients with reduced reserve do not normalize their QT as most patients can.24 Additionally, an entity known as cirrhotic cardiomyopathy has been previously described, albeit mostly in the gastroenterology literature. This condition consists of 4 common features: (1) baseline increased cardiac output, (2) attenuated systolic contraction and diastolic relaxation, (3) electrophysiologic abnormalities including repolarization change, and (4) reduced response of the heart to direct β‐stimulation (β‐incompetence).25 While patients may exhibit some or all of these features, it is not well understood how these features reverse after liver transplant and why some patients would reverse less than others, or never normalize.
Use of QTc‐Prolonging Drugs
Several medications are known to increase QTc interval, including quinine and certain antibiotics that are frequently used in ESLD patients. Use of these medications needs to be carefully considered, because they will likely cause further QTc interval prolongation in addition to that from the underlying liver disease. Interestingly, our model did not find that use of β‐blockers, fluoroquinolones, antifungals (azoles), or quinine was statistically associated with an increased probability of QTc interval prolongation prior to liver transplant. However, their utilization should still be considered carefully in this at‐risk population, and at a minimum the ECG should be monitored for changes in QTc interval.
Study Limitations
Our study has several limitations. We recognize that our preoperative ECGs were obtained during an arbitrarily defined time period. Given the significant electrolyte alterations typically encountered in the ESLD patient population, it is difficult to account for this possible confounding factor. Furthermore, postoperative ECGs were arbitrarily obtained 1 to 12 months following liver transplant. This extended time interval certainly permitted a larger cohort for evaluation purposes; however, because there was no prespecified a priori time frame for obtaining postoperative ECG evaluations, we realize this permits sampling bias. In addition, it is possible, although not really known, that 1 month is insufficient time to allow the QT to reach its new baseline following liver transplant.
Conclusion
We believe that cautious medication administration in this patient population is warranted, particularly in those patients with QTc intervals markedly >440 milliseconds. Baseline and serial ECG monitoring seems warranted perioperatively, and even potentially up to 1 year postoperatively, to evaluate for QTc interval change. We also feel that more research needs to be done to further elucidate the underlying etiology of QTc interval prolongation in this patient population, as well as the long‐term effects of prolonged QTc in these patients. A prospective trial with pre‐transplant and post‐transplant testing of autonomic function might help to further elucidate the mechanism of QTc interval prolongation in these patients and assess for any relationship between QTc prolongation and post‐transplant mortality, particularly sudden cardiac death.
In summary, our data show that the QTc interval is often prolonged in ESLD patients awaiting transplant and that it shortens significantly following liver transplant in most patients. The degree of QTc prolongation is independent of age, severity of liver disease as determined by MELD score, or etiology of liver failure. Approximately 50% of patients receiving liver transplant will have a prolonged QTc interval while waiting on the transplant list, potentially putting them at increased risk of ventricular arrhythmias. Medications known to prolong QTc interval should be used with caution in this select subset of patients, and baseline as well as serial ECGs should be obtained to evaluate for this irregularity.
REFERENCES
- 1. Schwartz PJ, Periti M, Malliani A. The long Q‐T syndrome. Am Heart J 1975; 89: 378–390. [DOI] [PubMed] [Google Scholar]
- 2. Surawicz B. The QT interval and cardiac arrhythmias. Annu Rev Med 1987; 38: 81–90. [DOI] [PubMed] [Google Scholar]
- 3. Schwartz PJ, Wolf S. QT interval prolongation as predictor of sudden death in patients with myocardial infarction. Circulation 1978; 57: 1074–1077. [DOI] [PubMed] [Google Scholar]
- 4. Kahn JK, Sisson JC, Vinik AI. QT interval prolongation and sudden death in diabetic autonomic neuropathy. J Clin Endocrinol Metab 1987; 64: 751–754. [DOI] [PubMed] [Google Scholar]
- 5. Day CP, James OF, Butler TJ, et al. QT prolongation and sudden cardiac death in patients with alcoholic liver disease. Lancet 1993; 341: 1423–1428. [DOI] [PubMed] [Google Scholar]
- 6. Mohamed R, Forsey PR, Davies MK, et al. Effect of liver transplantation on QT interval prolongation and autonomic dysfunction in end stage liver disease. Hepatology 1996; 23: 1128–1134. [DOI] [PubMed] [Google Scholar]
- 7. Bernardi M, Calandra S, Colantoni A, et al. Q‐T interval prolongation in cirrhosis: prevalence, relationship with severity and etiology of the disease and possible pathogenetic factors. Hepatology 1998; 27: 28–34. [DOI] [PubMed] [Google Scholar]
- 8. Puthumana L, Chaudhry V, Thuluvath PJ. Prolonged QTc interval and its relationship to autonomic cardiovascular reflexes in patients with cirrhosis. J Hepatol 2001; 35: 733–738. [DOI] [PubMed] [Google Scholar]
- 9. Thuluvath PJ, Triger DR. Autonomic neuropathy in chronic liver disease. Q J Med 1989; 72: 737–747. [PubMed] [Google Scholar]
- 10. MacGilchrist AJ, Reid JL. Impairment of autonomic reflexes in cirrhosis. Am J Gastroenterol 1990; 85: 288–292. [PubMed] [Google Scholar]
- 11. Hendrickse MT, Thuluvath PJ, Triger DR. Natural history of autonomic neuropathy in chronic liver disease. Lancet 1992; 339: 1462–1464. [DOI] [PubMed] [Google Scholar]
- 12. García González M, Hernandez‐Madrid A, Lopez‐Sanromán A, et al. Reversal of QT interval electrocardiographic alterations in cirrhotic patients undergoing liver transplantation. Transplant Proc 1999; 31: 2366–2367. [DOI] [PubMed] [Google Scholar]
- 13. Bal JS, Thuluvath PJ. Prolongation of QTc interval: relationship with etiology and severity of liver disease, mortality and liver transplantation. Liver Int 2003; 23: 243–248. [DOI] [PubMed] [Google Scholar]
- 14. Carey EJ, Douglas DD. Effects of orthotopic liver transplantation on the corrected QT interval in patients with end‐stage liver disease. Dig Dis Sci 2005; 50: 320–323. [DOI] [PubMed] [Google Scholar]
- 15. Finucci G, Lunardi F, Sacerdoti D, et al. Q‐T interval prolongation in liver cirrhosis: reversibility after orthotopic liver transplantation. Jpn Heart J 1998; 39: 321–329. [DOI] [PubMed] [Google Scholar]
- 16. Goldenberg I, Moss AJ, Zareba W. QT interval: how to measure it and what is “normal.” J Cardiovasc Electrophysiol 2006; 17: 333–336. [DOI] [PubMed] [Google Scholar]
- 17. Lunzer MR, Newman SP, Bernard AG, et al. Impaired cardiovascular responsiveness in liver disease. Lancet 1975; 2: 382–385. [DOI] [PubMed] [Google Scholar]
- 18. Locati ET. QT Interval duration remains a major risk factor in long QT syndrome patients. J Am Coll Cardiol 2006; 48: 1053–1055. [DOI] [PubMed] [Google Scholar]
- 19. Merri M, Benhorin J, Alberti M, et al. Electrocardiographic quantitation of ventricular repolarization. Circulation 1989; 80: 1301–1308. [DOI] [PubMed] [Google Scholar]
- 20. Larsen JA, Kadish AH. Effects of gender on cardiac arrhythmias. J Cardiovasc Electrophysiol 1998; 9: 655–664. [DOI] [PubMed] [Google Scholar]
- 21. Locati EH, Zareba W, Moss AJ, et al. Age‐ and sex‐related differences in clinical manifestations in patients with congenital long‐QT syndrome: findings from the International LQTS Registry. Circulation 1998; 97: 2237–2244. [DOI] [PubMed] [Google Scholar]
- 22. Adigun AQ, Pinto AG, Flockhart DA, et al. Effect of cirrhosis and liver transplantation on the gender difference in QT interval. Am J Cardiol 2005; 95: 691–694. [DOI] [PubMed] [Google Scholar]
- 23. Johnson RH, Robinson BJ. Mortality in alcoholics with autonomic neuropathy. J Neurol Neurosurg Psychiatry 1988;51: 476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Michael G, Xiao L, Qi XY, et al. Remodelling of cardiac repolarization: how homeostatic responses can lead to arrhythmogenesis. Cardiovasc Res 2009; 81: 491–499. [DOI] [PubMed] [Google Scholar]
- 25. Liu H, Song D, Lee SS. Cirrhotic cardiomyopathy. Gastroenterol Clin Biol 2002; 26: 842–847. [PubMed] [Google Scholar]


