Abstract
Background:
Safe and effective therapeutic management of refractory coronary artery disease (CAD) in heart patients is critical to enhance cardiovascular function and improve quality of life. Current therapies for refractory CAD are inadequate in ameliorating angina and promoting revascularization of ischemic myocardium.
Hypothesis:
Cardiac shock wave therapy (CSWT) is a safe and effective noninvasive intervention in the management of patients with refractory CAD.
Methods:
The study enrolled 9 male patients age 50 to 70 years (5.11 ± 5.46 years) with a diagnosis of CAD and stent implantation (3.00 ± 2.24 stents). CSWT was carried out for 3 months at 3 intervals during the first week of each month (first, third, and fifth day), for a total of 9 therapies per patient. Dobutamine stress echocardiography and radionuclide angiography identified the myocardial ischemic segments. The effects of CSWT on myocardial perfusion and systolic function were examined. Other outcome measures included myocardial injury enzyme markers, angina scale, nitroglycerin dosage, and cardiopulmonary fitness assessments.
Results:
Improved myocardial blood flow and regional systolic function (stress peak systolic strain rate − 1.10 to − 1.60 s−1, P = 0.002) were detected in patients following CSWT. Reductions in creatine kinase (87.89 ± 36.69 to 86.22 ± 35.96 IU/L, P = 0.046), creatine kinase MB (10.89 ± 5.73 to 10.11 ± 5.93 IU/L, P = 0.008), aspartate transaminase (interquartile range [IQR], 28.00 to 27.00 IU/L, P = 0.034) were also found. Angina (Canadian Cardiovascular Society scale IQR 3.0 to 2.0, P = 0.035) and nitroglycerin dose reduction (IQR 3.0 to 1.0 times/wk, P = 0.038) were reported.
Conclusions:
This study is a preliminary assessment of CSWT in patients with refractory CAD. We report that CSWT is a noninvasive, effective, and safe intervention in the treatment of refractory CAD. Copyright © 2010 Wiley Periodicals, Inc.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
Coronary artery disease (CAD) is recognized as a leading cause of adult mortality worldwide. Current therapies in the treatment of CAD include drug interventions, percutaneous coronary intervention (PCI), coronary artery bypass graft (CABG) surgery, and transmyocardial laser revascularization (TMR). However, these approaches are invasive and often inadequate in the treatment of advanced CAD, and are associated with serious cardiovascular risks and complications.1,2 Elevated patient risk factors including increased age and comorbidities such as hypertension and diabetes may further limit treatment options.3,4 Prognosis for patients diagnosed with end‐stage CAD without indications for PCI or CABG surgery is poor. The use of TMR has demonstrated benefits in patients with diffuse CAD. However, TMR is largely used as an adjunctive therapy to CABG when CABG alone fails to promote complete revascularization.5,6 Furthermore, randomized clinical trials indicate no long‐term reduction in angina in 25% of CAD patients subjected to TMR alone.5,6
Alternative approaches such as gene transfer or stem cell transplantation are promising, yet still remain at the preclinical stage. As with current modes of CAD therapy, both of these methodologies are similarly invasive. Whether either approach results in complete revascularization and/or long‐term angina relief remains to be seen. Thus, it is critical to note that a large number of CAD patients continue to experience angina, decreased exercise tolerance, and the risk of sudden death.
Understandably, there is need for a safe and effective, noninvasive approach toward the treatment of CAD. Cardiac shock wave therapy (CSWT) is a novel, noninvasive intervention that can ameliorate myocardial ischemia and improve cardiac function.7, 8, 9, 10, 11, 12 Early clinical trials showed that CSWT alleviated angina and improved cardiopulmonary fitness in patients with myocardial ischemia.8,11,12 Evidence indicates that CSWT may reduce the ischemic burden and provide angina relief by promoting angiogenesis and revascularization in ischemic myocardium.7, 8, 9 Earlier in vivo animal studies and human clinical studies demonstrated that low‐energy pulse waves produced by CSWT induced a “cavitation effect” (micron‐sized violent bubble collapse within and outside cells), exerting a mechanical shear force on myocardial and vascular endothelial cells. Shock wave treatment promoted angiogenesis in ischemic porcine myocardium by up‐regulating vascular endothelial growth factor messenger RNA and its receptor, fms‐like tyrosine 1.7,13 Furthermore, improved regional myocardial blood flow and capillary density were also observed.7,9 Clinical studies corroborated these findings, as myocardial perfusion in ischemic regions was enhanced following CSWT.
To further investigate the potential benefits of CSWT, we performed a preliminary study of CSWT administered regularly over a 3‐month period in 9 patients with advanced CAD. Patients were clinically assessed before initiation and at 1 month following completion of the CSWT treatment program.
Methods
Patient Study Group
This study was performed in accordance with our institutional review board regulations. Informed written consent was obtained from each patient prior to enrollment. Nine male patients admitted to the cardiology department from August 2008 to January 2009 were enrolled in this study. All patients received standard medical treatment, and the CSWT procedure was explained to each patient. Table 1 shows patient demographics and baseline characteristics. Patients ranged in age from 55 to 70 years (ξ = 64 ± 6 y) and had endured 5.11 ± 5.46 years of CAD diagnosis and stent implantation. All patients took aspirin and at least 1 other medication to manage their disease. The reasons for hospitalization included repeated episodes of chest tightness, shortness of breath, and fatigue after stent implantation for earlier indications of myocardial infarction. Comorbidities included hypertension (n = 8), diabetes mellitus (n = 5), and hyperlipidemia (n = 3). An average of 3.00 ± 2.24 stents were implanted per patient (range, 1–8 stents). On admission, coronary angiography showed coronary intrastent stenosis (n = 1), distal coronary artery disease (n = 2), and diffuse extra‐stent coronary artery disease (n = 6). Echocardiography showed regional wall motion abnormality in all patients. Left ventricular dilation (n = 4) and left ventricular apical aneurysm (n = 4) were also detected.
Table 1.
Patient Baseline Characteristics and Medication Intake
| Case | Age, y | Duration of Disease, y | No. (Duration, y) of Stent | Depth of Left Ventricle, mm | Ventricular Aneurysms | Medications |
|---|---|---|---|---|---|---|
| 1 | 70 | 13 | 8 (10) | 53 | No | ACEI, AP, ASP, BB, CCB, N |
| 2 | 65 | 3 | 2 (2) | 50 | No | ASP, D, N, S |
| 3 | 66 | 2 | 1 (1) | 53 | No | ACEI, ASP, BB, S |
| 4 | 60 | 4 | 2 (1) | 67 | Yes | ACEI, AP, ASP, BB, S |
| 5 | 68 | 3 | 2 (1) | 68 | Yes | AP, ARB, ASP, BB, D, S |
| 6 | 69 | 1 | 1 (1) | 62 | No | ACEI, ASP, BB, S |
| 7 | 55 | 16 | 3 (2) | 50 | Yes | ACEI, AP, ASP, BB, S |
| 8 | 66 | 3 | 5 (2) | 65 | Yes | AP, ASP, BB, D, S |
| 9 | 55 | 1 | 3 (1) | 52 | No | ACEI, AP, ASP, CCB, S |
| Averagea | 63.78 ± 5.74 | 5.11 ± 5.46 | 3.00 ± 2.24 (2.33 ± 2.92) | 57.78 ± 7.58 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AP, antiplatelet agent; ARB, angiotensin II receptor blocker; ASP, aspirin; BB, β‐blocker; CCB, calcium channel blocker; D, diuretic; N, nitrate; S, statin.
Data presented as mean ± SD
Localization of Myocardial Ischemia
Prior to initiation of CSWT, patients received low‐dose dobutamine stress echocardiography (SE) according to the recommended classified drug load exercise program (GE). Baseline cardiac function was assessed at rest and following dobutamine induction (probe frequency, 1.7–3.4 MHz). Two‐dimensional Doppler and tissue Doppler imaging identified regional cardiac dysfunction (Table 2).
Table 2.
Location of Vascular Lesions in Ischemic Myocardium
| Case | Vascular Lesions | Infarction Area | Sections of Ischemic Myocardium |
|---|---|---|---|
| 1 | RCA and distal circumflex artery occlusion | Inferior wall | Middle section of inferior wall, apical section of posterior septum |
| 2 | Proximal RCA and the ostium of first diagonal branch subtotal occlusion | Lateral wall | Apical section of lateral wall |
| 3 | Proximal anterior descending artery occlusion | Inferior wall, anterior septum | Middle sections of anterior septum and inferior wall |
| 4 | Occlusion in the middle section of anterior descending coronary artery, the first diagonal branch and proximal posterior descending branch; 40% of restenosis in LCX and RCA | Inferior wall, anterior septum | Basal and middle sections of anterior septum, middle section of inferior wall |
| 5 | Proximal RCA occlusion; subtotal occlusion in LCX | Inferior wall, right ventricle | Middle sections of anterior septum and inferior wall |
| 6 | The middle section of RCA 50% of stenosis | Posterior wall | Middle sections of inferior wall and posterior wall |
| 7 | The ostium of RCA 85% of stenosis | Anterior septum | Middle section of anterior septum, apical posterior septum, middle section of inferior wall |
| 8 | Distal anterior descending artery 30% of stenosis; distal LCX 50% stenosis | Inferior wall | Middle sections of anterior septum and inferior wall |
| 9 | Distal RCA occlusion | Anterior septum | Middle section of anterior septum, apical section of posterior septum |
Abbreviations: LCX, left circumflex artery; RCA, right coronary artery
All patients underwent technetium sestamibi (99mTc MIBI9) myocardial radionuclide angiography (Symbia T2 SPECT‐CT, Siemens) at resting state and at low‐dose dobutamine stimulation to identify areas of ischemic myocardium. Myocardial wall motion was assessed using the American Society of Echocardiography 16‐segment wall motion score index: 1 = normal, 2 = hypokinetic, 3 = akinetic, and 4 = dyskinetic or aneurysm. Viable myocardial segment was defined by improved contraction of 2 adjacent abnormal segments (decrease ≥ 1).14,15 Myocardial perfusion index (MPI) was determined according to the American Heart Association standards. Segments (n = 17) on the short, horizontal long, and vertical long axes were semiquantitatively assessed and scored (3 = normal intake, 2 = mild reduced intake, 1 = moderately reduced intake, and 0 = severely reduced or no intake).16,17
Cardiac Shock Wave Therapy Procedure and Treatment Program
The CSWT system (Modulith SLC, Storz Medical, Switzerland) was installed with a real‐time ultrasound probe (Aloka SSD‐900). Patients were placed in supine position, resting quietly during the procedure. ECG, blood pressure, breathing, and blood oxygen saturation were concurrently monitored. Target myocardial regions were detected by the ultrasound probe and the water cushion was lowered to contact the chest. Shock waves (150 mm depth; aperture angle 48°), triggered by R wave on ECG, were voluntarily released by the parabolic reflector at the absolute refractory period of electrical activity (Supporting Information Figure 1). Wave energy was gradually increased in all patients (maximum: 0.09 mJ/mm2 in patients exhibiting no chest pain). Regional targeting of shock wave transmission to the myocardium was fine‐tuned by microcontrols. Six control levels (+1, + 2, + 3, 0, − 1, − 2, − 3) allowed for incremental modulation of angle (6°) and distance (2.5 mm) along the treatment area. Each ischemic region was treated at 9 points (−1, 0, + 1 pairs). During treatment, patients were closely monitored for vital signs and symptoms including palpitations, chest pain, breathing difficulty, and dizziness.
Figure 1.
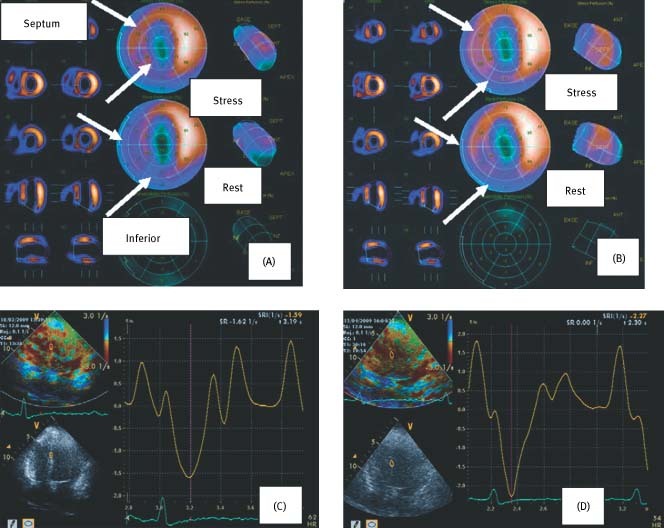
Upper images indicate myocardial perfusion by SPECT analysis before (A) and after (B) CSWT treatment at resting and stress states in patient 4. Perfusion was diminished prior to CSWT (A) (septum: stress score 2, rest score 1; inferior wall: stress score 1; rest score 0) and improved following CSWT (B) (septum: stress score 3, rest score 2; inferior wall: stress score 2; rest score 2). Lower images indicate PSSR (1/s) in septal segments pre‐CSWT (C) and post‐CSWT (D). Increased PSSR was evident post‐CSWT (SRI = − 2.27) vs. pre‐CSWT (SRI = − 1.59). Abbreviations: CSWT, cardiac shock wave therapy; PSSR, peak systolic strain rate; SPECT; single‐photon emission computed tomography; SRI, strain rate imaging
The therapy schedule was devised according to the recommendations of the Cardiovascular Center of Munich, Germany and the Cardiovascular Center at Kyushu University Graduate School of Medical Sciences, Fukuoka, Japan.8,11 The CSWT regimen was performed over a 3‐month period at 3‐week intervals. CSWT sessions were administered during the first week of the month on the first, third, and fifth day for 3 months, for a total of 9 therapies per patient. Clinical assessments of cardiac function and myocardial perfusion were performed in patients at 1 month following the CSWT schedule.
Clinical Assessment Parameters
Enzyme markers of myocardial injury (creatine kinase [CK], creatine kinase MB [CK‐MB], cardiac troponin I, alanine aminotransferase [ALT], and aspartate aminotransferase [AST]) were measured prior to CSWT and at 1 month following completion of CSWT. The efficacy of CSWT was assessed using the Canada Cardiovascular Society (CCS) angina scale, New York Heart Association (NYHA) class, Seattle Angina Questionnaire (SAQ) scale, and nitroglycerin dose.18,19 Single‐photon emission computed tomography (SPECT) image analysis of myocardial perfusion was conducted using the 4‐point MPI scoring system for semiquantitative evaluation of ischemia at resting and stress states. Stress echocardiography (SE) was also performed to quantitatively assess cardiac systolic function by determining peak systolic strain rate (PSSR).
Statistical Analysis
Data were presented as the mean ± SD. Nonparametric data were represented as the median (interquartile range). Pretreatment and post‐treatment values were compared using paired t test or Wilcoxon signed rank test. Two‐tailed analyses of variance were made using a 95% confidence interval for statistical significance. Statistical analyses were performed using SPSS 15.0 statistics software (SPSS Inc., Chicago, IL).
Results
Stress SPECT and SE identified a total of 19 viable ischemic myocardial segments in the 9 patients. Supporting Information Figures 2 and 3 illustrate typical examples of vascular stenosis and myocardial localization in shock wave treatment. During the CSWT procedure, 4 patients had occasional ventricular premature heart beats without any impact on blood pressure, heart rate, and blood oxygen saturation. Three patients subjected to CSWT on the apical segment of the posterior septum and 2 subjected to CSWT on the middle segment of the anterior septum complained of chest pain; however, chest pain disappeared when wave energy was reduced. Patients did not experience any serious cardiovascular health complications (heart failure, bleeding, thrombosis, shock, or death) either during or after CSWT. Furthermore, none of the patients experienced any pulmonary complications either during or after CSWT.
Plasma levels of enzyme markers measured before and after CSWT are shown in Table S1. At 1 month post‐treatment, significant decreases were detected in levels of CK (from 87.89 ± 36.69 to 86.22 ± 35.96, P = 0.046), CK‐MB (from 10.89 ± 5.73 to 10.11 ± 5.93, P = 0.008), and AST (from 28.00 to 27.00, P = 0.034) compared with pretreatment levels. ALT levels were unchanged (from 26.33 ± 6.52 to 26.56 ± 6.33, P = 0.169). These findings indicate that CSWT did not induce further damage to the liver or heart in our patient group.
Pre‐CSWT and post‐CSWT assessments of angina (NYHA class, CCS scale, SAQ), cardiopulmonary functional capacity (6‐minute walking test), and angina medication intake (nitroglycerin dose) are presented in Table 3. Significant reductions in CCS angina scale (from 3.0 to 2.0, P = 0.035) and nitroglycerin intake (from 3.0 to 1.0, P = 0.038) were observed post‐CSWT. Image analysis results following SPECT and SE to determine myocardial perfusion and PSSR, respectively, are shown in Table 4. MPI values indicated no significant difference in myocardial perfusion before and after treatment at resting and stress states. Local systolic function, assessed by PSSR, was significantly improved after 1 month of treatment (PSSR at stress from − 1.10 to − 1.60, P = 0.002). Representative SPECT and SE images taken before and after CWST, depicted in the Figure 1, clearly indicate improved myocardial blood flow within 1 month following CSWT despite the absence of detectable increases in MPI.
Table 3.
Clinical Parameters Before (Pre) and 1 Month After (Post) CSWTa
| Case | NYHA Class | CCS Angina Scale | SAQ | 6MWT, m | Nitroglycerin Dose, Times/Wk | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| 1 | 2 | 1 | 3 | 2 | 75 | 80 | 108 | 342 | 3 | 2 |
| 2 | 4 | 4 | 4 | 3 | 59 | 60 | 162 | 162 | 4 | 3 |
| 3 | 3 | 2 | 1 | 1 | 52 | 72 | 216 | 378 | 0 | 0 |
| 4 | 2 | 1 | 4 | 3 | 52 | 66 | 360 | 324 | 4 | 2 |
| 5 | 2 | 1 | 3 | 2 | 70 | 77 | 342 | 450 | 0 | 0 |
| 6 | 2 | 2 | 2 | 1 | 76 | 80 | 540 | 540 | 2 | 0 |
| 7 | 3 | 1 | 3 | 2 | 67 | 80 | 324 | 396 | 1 | 0 |
| 8 | 2 | 3 | 3 | 4 | 76 | 60 | 234 | 306 | 3 | 3 |
| 9 | 2 | 1 | 3 | 1 | 67 | 80 | 378 | 360 | 3 | 1 |
| Overallb | ||||||||||
| Before | 2.0 (2.0, 3.0) | 3.0 (2.5, 3.5) | 67.0 (55.5, 75.5) | 324.0 (189.0, 369.0) | 3.0 (0.0, 3.5) | |||||
| After | 1.0 (1.0, 2.5) | 2.0 (1.0, 3.0) | 77.0 (63.0, 80.0) | 360.0 (315.0, 423.0) | 1.0 (0.0, 2.5) | |||||
| P value | 0.058 | 0.035c | 0.086 | 0.063 | 0.038c | |||||
Abbreviations: 6MWT, six‐minute walking test; CCS, Canadian Cardiovascular Society; CSWT, cardiac shock wave therapy; NYHA, New York Heart Association; SAQ, Seattle Angina Questionnaire.
Wilcoxon signed rank test was used to compare the difference between pretreatment and post‐treatment.
Data presented as median (interquartile range).
P < 0.05 indicates statistical significance
Table 4.
Myocardial Perfusion and Systolic Function in Ischemic Segments (n = 19)
| Index | Before Treatment | 1 Mo After Treatment | P Value |
|---|---|---|---|
| MPIa | |||
| Rest | 2 (1, 2) | 2 (1, 3) | 0.157 |
| Stress | 0 (0, 1) | 1 (0, 1) | 0.059 |
| PSSR (s−1) | |||
| Restb | −0.92 ± 0.46 | −1.05 ± 0.33 | 0.222 |
| Stressa | −1.10 (−1.50, −0.89) | −1.60 (−2.11, −1.40) | 0.002c |
Abbreviations: MPI, myocardial perfusion index; PSSR, peak systolic strain rate.
Data presented as median (interquartile range); Wilcoxon signed rank test was used to compare the difference between pretreatment and post‐treatment.
Data presented as mean ± SD; paired t test was used to compare the difference between pretreatment and post‐treatment.
P < 0.05 indicates statistical significance
Discussion
The present investigation was a preliminary assessment of the efficacy and safety of CSWT in the treatment of refractory CAD. We report that CSWT improved regional cardiac systolic function and that imaging studies demonstrated increased myocardial blood flow in ischemic myocardium, supporting the notion of CSWT‐mediated promotion of angiogenesis. Other reports indicate that CSWT also increases endothelial nitric oxide synthase activity and reduces the expression of inflammatory cytokines inhibiting ventricular remodeling processes toward the development of heart failure.20,21,22
All 9 patients enrolled in our study experienced repeated angina pectoris and a diminished quality of life despite undergoing standard medical treatment and interventional stent therapy. The CSWT procedure was well tolerated, performed without anesthesia, and allowed for concurrent monitoring of ECG, blood pressure, and blood oxygen saturation. Ischemic segments were determined in both left ventricular long and short axes as well as in apical four‐chamber views. No hemodynamic complications, myocardial damage, thrombosis, or bleeding resulted from the procedure. All patients reported relief of chest pain and fatigue symptoms following CSWT. Enzyme markers of myocardial injury were significantly reduced following CSWT. This reduction may not necessarily indicate that CSWT lessened myocardial or liver injury, but more likely demonstrates that CSWT did not promote cardiac or liver damage. CCS angina scale and nitroglycerin dose were significantly reduced in patients following CSWT; these changes were accompanied by improvements in exercise tolerance and overall quality of life. Improvements in myocardial perfusion and local systolic function following CSWT were evident in SPECT and SE analyses.
In a recent study by Kikuchi et al,23 8 patients who had undergone CABG or PCI and were still symptomatic underwent CSWT for 3 months. As in our study, nitroglycerin use decreased significantly and some signs of improved left ventricular function were seen.
Conclusion
Our results confirm the efficacy and safety of CSWT in the treatment of refractory CAD. The limitations of the present investigation are the small patient population and the short follow‐up period. In our study, follow‐up assessments were performed at 1 month after 3 treatments, and not after the 9 treatments as reported in other studies where patients were assessed at 3 months following 9 CSWT treatments.23 CSWT is an angiogenesis therapy. The growth of the vascular bed to supply the myocardium is a gradual, chronic process. Therefore, the resulting changes in MPI may not reach statistical significance after only 3 applications of therapy. Regardless, we were still able to demonstrate clinical benefit of CSWT in patients with refractory CAD within a relatively short time period. Larger patient numbers investigated in previous studies by German and Japanese groups restricted their analyses to patients with refractory CAD who had recurrent angina and decreased quality of life despite treatment with coronary stents and/or CABG on the basis of drug treatment.23 While the findings from these earlier studies are valid, it must be kept in mind that China remains an underdeveloped country with a large number of CAD patients for whom treatment is ineffective or extremely limited by outdated concepts. Additionally, stent or bypass treatment may be financially inaccessible for many patients. Because for these reasons many patients in China are not treated with stents, we included both CAD patients who received stent implantation as well as CAD patients experiencing angina who had not received stent implantation. Our inclusion criteria contrasts that reported in the earlier studies and represents a unique feature of the current study. Our future studies of CSWT will examine a larger patient population and post‐treatment follow‐up for 24 months. Currently, follow‐up assessments have been completed in 50 patients and studies are underway to investigate any potential placebo effect of CSWT by comparing CSWT‐treated CAD patients to drug‐treated CAD patients.
References
- 1. Doyle BJ, Rihal CS, Gastineau DA, et al. Bleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: implications for contemporary practice. J Am Coll Cardiol 2009;53:2019–2027. [DOI] [PubMed] [Google Scholar]
- 2. Wann S, Balkhy H. Evaluation of patients after coronary artery bypass grafting. Cardiol Rev 2009;17:176–180. [DOI] [PubMed] [Google Scholar]
- 3. Rihal CS, Raco DL, Gersh BJ, et al. Indications for coronary artery bypass surgery and percutaneous coronary intervention in chronic stable angina: review of the evidence and methodological considerations. Circulation 2003;108:2439–2445. [DOI] [PubMed] [Google Scholar]
- 4. Singh M, Rihal CS, Roger VL, et al. Comorbid conditions and outcomes after percutaneous coronary intervention. Heart 2008;94:1424–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Horvath KA. Transmyocardial laser revascularization. J Card Surg 2008;23:266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Allen KB, Kelly J, Borkon AM, et al. Transmyocardial revascularization: from randomized trials to clinical practice. A review of techniques, evidence‐based outcomes, and future directions. Anesthesiol Clin 2008;26:501–519. [DOI] [PubMed] [Google Scholar]
- 7. Nishida T, Shimokawa H, Oi K, et al. Extracorporeal cardiac shock wave therapy ameliorates ischemia‐induced myocardial dysfunction in pigs in vivo. Circulation 2004;110:3055–3061. [DOI] [PubMed] [Google Scholar]
- 8. Fukumoto Y, Ito A, Uwatoku T, et al. Extracorporeal cardiac shock wave therapy ameliorates myocardial ischemia in patients with severe coronary artery disease. Coron Artery Dis 2006;17:63–70. [DOI] [PubMed] [Google Scholar]
- 9. Uwatoku T, Ito K, Abe K, et al. Extracorporeal cardiac shock wave therapy improves left ventricular remodeling after acute myocardial infarction in pigs. Coron Artery Dis 2007;18:397–404. [DOI] [PubMed] [Google Scholar]
- 10. Shimokawa H, Ito K, Fukumoto Y, et al. Extracorporeal cardiac shock wave therapy for ischemic heart disease. Shock Waves 2008;17:449–455. [Google Scholar]
- 11. Khattab AA, Brodersen B, Schürmann‐Kuchenbrandt D, et al. Extracorporeal cardiac shock wave therapy: first experience in the everyday practice for treatment of chronic refractory angina pectoris. Int J Cardiol 2007;121:84–85. [DOI] [PubMed] [Google Scholar]
- 12. Prinz C, Lindner O, Bitter T, et al. Extracorporeal cardiac shock wave therapy ameliorates clinical symptoms and improves regional myocardial blood flow in a patient with severe coronary artery disease and refractory angina. Case Report Med 2009;2009:639594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gutersohn A, Gaspari G. Shock waves upregulate vascular endothelial growth factor m‐RNA in human umbilical vascular endothelial cells. Circulation 2000;102((suppl):): 18. [Google Scholar]
- 14. Zhu TG. Quantitative dobutamine stress echocardiography [in Chinese]. Chinese J Ultrasound Imaging 2003;12:468–469. [Google Scholar]
- 15. Schiller NB, Shah PM, Crawford M, et al. Recommendation for quantitation of the left ventricle by two‐dimensional echocardiography. J Am Soc Echocardiogr 1989;2:358–367. [DOI] [PubMed] [Google Scholar]
- 16. Klocke FJ, Baird MG, Lorell BH, et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). J Am Coll Cardiol 2003;42:1318–1333. [DOI] [PubMed] [Google Scholar]
- 17. Cerqueira MD, Weissman NJ, Dilsizian V, et al; American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the cardiac imaging committee of the Council on Clinical Cardiology of the American Heart Association. J Nucl Cardiol 2002;9:240–245. [DOI] [PubMed] [Google Scholar]
- 18. Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol 1995;25:333–341. [DOI] [PubMed] [Google Scholar]
- 19.The Cardiovascular Section of the Chinese Medical Association. The diagnosis and treatment guidelines of chronic stable angina pectoris [in Chinese]. Chinese J Cardiovascular Dis 2007;35:195–206. [Google Scholar]
- 20. Mariotto S, Cavalieri E, Amelio E, et al. Extracorporeal shock waves: from lithotripsy to anti‐inflammatory action by NO production. Nitric Oxide 2005;12:89–96. [DOI] [PubMed] [Google Scholar]
- 21. Ciampa AR, de Prati AC, Amelio E, et al. Nitric oxide mediates anti‐inflammatory action of extracorporeal shock waves. FEBS Lett 2005;579:6839–6845. [DOI] [PubMed] [Google Scholar]
- 22. Fisher AB, Chien S, Barakat AI, et al. Endothelial cellular response to altered shear stress. Am J Physiol Lung Cell Mol Physiol 2001;281:L529–L533. [DOI] [PubMed] [Google Scholar]
- 23. Kikuchi Y, Ito K, Ito Y, et al. Double‐blind and placebo‐controlled study of the effectiveness and safety of extracorporeal cardiac shock wave therapy for severe angina pectoris. Circ J 2010;74:589–591. [DOI] [PubMed] [Google Scholar]


