Abstract
Background
The relationship between HbA1c, blood pressure, and carotid atherosclerosis in nondiabetic patients is not clear.
Hypothesis
HbA1c and blood pressure can affect carotid‐artery atherosclerosis in nondiabetic patients.
Methods
This retrospective cross‐sectional study included 216 patients without diabetes mellitus. A positive carotid ultrasonographic result was defined as intima‐media thickness of the common carotid artery ≥ 0.9 mm, or presence of carotid plaque.
Results
Compared with patients without carotid atherosclerosis, patients with carotid atherosclerosis had significantly higher levels of HbA1c and systolic blood pressure (SBP). Higher levels of HbA1c and SBP were found to be associated with increased carotid atherosclerosis. Given similar SBP levels, higher HbA1c (>5.6%) was also related to increased carotid atherosclerosis. In multiple logistic regression analysis, HbA1c (odds ratio: 4.1, P = 0.009) emerged as the only statistically significant modifiable factor that was associated with carotid atherosclerosis, independent of smoking, body mass index, fasting plasma glucose, 2‐hour plasma glucose, SBP, diastolic blood pressure, low‐density lipoprotein cholesterol, and high‐density lipoprotein cholesterol.
Conclusions
Our study shows that a slight increase of HbA1c may associate with carotid atherosclerosis in nondiabetic patients. Moreover, the coexistence of an elevated SBP level and a slightly increased HbA1c level may have a more significant effect on carotid atherosclerosis. Copyright © 2010 Wiley Periodicals, Inc.
Dr. Zhu and Dr. Sun contributed equally to this work.
The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology (Coats AJ. Ethical authorship and publishing. Int J Cardiol. 2009;131:149–150).
This work was supported by a Chinese National Science Grant to Dr. Yong Li (Grant No. 30873350). The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Introduction
Type 2 diabetes mellitus (DM) is now regarded as a “coronary heart disease (CHD) equivalent.”1 Impaired glucose tolerance (IGT) and impaired fasting glucose (IFG) have also been proved to increase cardiovascular risk.2, 3, 4 Moreover, some research results have implied a linear relationship,5 whereas some others found a threshold effect.6 In a study by Sasso et al, normal postload glucose and glycated hemoglobin A1c (HbA1c) levels have been found to be associated with CHD.7 In fact, fasting or postload glucose may be influenced by stress, diet change, infusion of fluid, and many other factors in clinical practice. Testing for HbA1c is a more accurate way to determine mean glucose levels over a relatively long time period.8
In any hospital cardiovascular department, including the one at Huashan Hospital, a large proportion of patients have multiple risk factors for CHD. Blood pressure > 115/75 mm Hg is continuously associated with cardiovascular risk.9 Furthermore, diabetic hypertensive patients have significantly higher risk of CHD than patients with hypertension (HT) or DM alone.10 However, combined effects of blood pressure and HbA1c on atherosclerosis have not been evaluated in nondiabetic subjects.
Carotid atherosclerosis (AS) has been used as a substitute indicator for AS of cardiac and cerebral vessels.11, 12, 13 Accordingly, carotid ultrasonography was used to evaluate carotid AS in this article. The aim of this study was to investigate the relationship between HbA1c, blood pressure, and carotid AS in nondiabetic patients.
Methods
Patient Characteristics
We enrolled 216 patients from the cardiology inpatient and outpatient department of Huashan Hospital from June 2006 to June 2007. Exclusion criteria: (1) history of type 2 DM, (2) reaching diagnostic criteria of DM in 2‐hour oral glucose tolerance test (further details in Methods), (3) type 1 DM, (4) myocardial infarction within 1 week, (5) severe congestive heart failure (New York Heart Association classes III–IV), (6) renal insufficiency (serum creatinine ≥ 130µmol/L), (7) hyperthyroidism or hypothyroidism, (8) secondary HT, (9) history of malignant tumor, (10) other endocrine disorders or tumors that could affect glucose metabolism, (11) infectious disease, and (12) history of rheumatic disease.
Methods
The study protocol was reviewed and approved by the ethics committees of Huashan Hospital and conducted according to the Declaration of Helsinki. Informed consent was obtained from all participants before they were admitted to the study.
Blood samples were collected after 12‐hour overnight fasting. All samples were analyzed for plasma total cholesterol, high‐density lipoprotein cholesterol (HDL‐C), low‐density lipoprotein cholesterol (LDL‐C), triglycerides, and blood glucose by enzymatic methods with an automatic analyzer (Hitachi 7060; Hitachi, Japan). Results of a 75‐g oral glucose tolerance test (OGTT) were recorded as well.
Demographic data and vascular risk factors were recorded, including age, gender, weight, height, body mass index (BMI), systolic blood pressure (SBP), and diastolic blood pressure (DBP). Height in stocking feet and weight in light clothing were measured, and BMI was calculated as weight in kg divided by height in meters squared. Blood pressure was measured 3 times in the seated position after a rest of 5 minutes using a mercury sphygmomanometer; the average of the last 2 readings was taken as the analyzed blood pressure level. Medical history and the status of alcohol intake and cigarette smoking were obtained from all subjects with a standardized questionnaire.
DM was diagnosed according to the 2006 American Diabetes Association (ADA) guidelines of diabetes care as fasting plasma glucose (FPG) ≥ 7.0 mmol/L or 2‐hour glucose with OGTT ≥ 11.1 mmol/L. IFG was diagnosed as FPG 5.6 to 6.9 mmol/L, and IGT as 2‐hour glucose 7.8 to 11.1 mmol/L. HT was defined as average SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg for > 2 weeks or history of HT, with or without treatment.
Carotid arteries were evaluated with high‐resolution B‐mode ultrasonography and a 7.5–10 MHz transducer. Measurements were taken of the maximum common carotid artery intima‐media thickness (IMT) and the IMT at 1 cm distal and proximal to the maximum IMT; the maximum of the 3 measurements was used as the IMT value. Carotid AS was defined as IMT ≥ 0.9 mm and/or presence of atherosclerotic plaque in the carotid artery. Carotid ultrasonography was conducted by doctors from the ultrasound room who were blinded to the patients' plasma glucose and blood pressure status.
Statistical Analysis
The statistical analysis was performed using SPSS 13.0 (SPSS Inc., Chicago, IL). Differences of proportions between groups were compared using the χ2 test for categorical variables. Quantitative variables were reported as mean ± SD for normal distribution data, and median (25th percentile, 75th percentile) for non‐normal distribution data. Differences between groups were compared using an independent samples t test for normal distribution data and the Mann‐Whitney test for non‐normal distribution data. Logistic regression was used to assess risk factors for carotid atherosclerosis. P < 0.05 was considered to be statistically significant, and all reported P values are 2‐sided.
Results
In total, 216 eligible patients were enrolled in the study. Of these, 154 (71.3%) were male, and the median age was 74 years (61, 81). History of HT was reported by 143 patients (66.2%), and 92 (42.6%) had history of CHD. According to the 2006 ADA guidelines, 121 of the patients (56.0%) had normal glucose levels, 25 (11.6%) had IFG, 46 (21.3%) had IGT, and 24 (11.1%) had IFG + IGT. As for medical therapy, 40.9% of the patients were taking an angiotensin‐converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB), 40.2% were taking β‐receptor blockers, 41.6% were taking calcium channel blockers, and 13.6% were on diuretics. Of the patients, 3.8%, 1.5%, 0.8%, and 0.8% were taking biguanides, α‐glucosidase inhibitors, glinides, and sulfonylureas, respectively. In addition, 37.9% of the patients were taking 3‐hydroxy‐3‐methylglutaryl‐coenzyme A (HMG‐CoA) reductase inhibitors and 4.5% were taking fibrates.
As shown in Table 1, compared with subjects without carotid AS, age, SBP, male gender, HT, and CHD were significantly higher in patients with carotid AS. Mean HbA1c was also significantly elevated in patients with carotid AS (5.7 ± 0.4 vs 5.5 ± 0.4, P = 0.009).
Table 1.
Comparison Between Groups With and Without Carotid AS
| With Carotid AS | With Carotid AS | P Value | |
|---|---|---|---|
| n | 132 | 84 | |
| Ma | 103 (78.0) | 51 (60.7) | 0.006 |
| Age, y | 78 (73, 82) | 61 (47, 73) | 0.000 |
| BMIb | 23.6±3.1 | 24.2±3.3 | 0.172 |
| Smoking | 16 (12.1) | 16 (19.0) | 0.688 |
| HT | 97 (73.5) | 46 (54.8) | 0.005 |
| CHD | 65 (49.2) | 26 (31.0) | 0.008 |
| SBP (mm Hg) | 131.5±15.5 | 122.7±17.2 | 0.000 |
| DBP (mm Hg) | 74.4±9.8 | 76.5±11.9 | 0.162 |
| FPG (mmol/L) | 5.2 (4.9, 5.6) | 5.2 (4.8, 5.5) | 0.428 |
| 2‐h oral glucose (mmol/L) | 7.2 (6.3, 9.0) | 6.6 (5.8, 8.1) | 0.091 |
| HbA1c (%) | 5.7±0.4 | 5.5±0.4 | 0.009 |
| LDL‐C (mmol/L) | 2.91 (2.39, 3.38) | 2.70 (2.26, 3.43) | 0.756 |
| HDL‐C (mmol/L) | 1.13 (0.96, 1.32) | 1.16 (0.97, 1.36) | 0.358 |
Abbreviations: AS, atherosclerosis; BMI, body mass index; CHD, coronary heart disease; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; HT, hypertension; LDL‐C, low‐density lipoprotein cholesterol; M, male; n, number of patients; SBP, systolic blood pressure.
Categorical data is presented as n (%), and P values were results of a χ2 test.
Quantitative data is reported as the mean±SD for normal distribution data, and median (25th percentile, 75th percentile) for non‐normal distribution data. P values were results of an independent samples t test for normal distribution data and the Mann‐Whitney test for non‐normal distribution data
To investigate the relationship between HbA1c and carotid AS, we divided the patients into 4 groups according to HbA1c quartiles. For the first, second, third, and fourth quartiles, respectively, the number of patients was 53, 63, 50, and 50; the proportion of male gender was 73.5%, 65.1%, 72.0%, and 76.0%; and the median age was 67.0 (49.5, 77.0), 73.0 (57.0, 82.0), 77.0 (71.0, 81.0), and 77.5 (66.8, 81.0). As HbA1c level increased, the proportion of patients with carotid AS increased, with 26 (49.1%), 37 (58.7%), 30 (60.0%), and 39 (78.0%) atherosclerotic patients in the 4 quartiles. Significant differences were found between the first and fourth quartiles (P = 0.050), the second and fourth quartiles (P = 0.030), and the third and fourth quartiles (P = 0.002)(Figure 1A).
Figure 1.
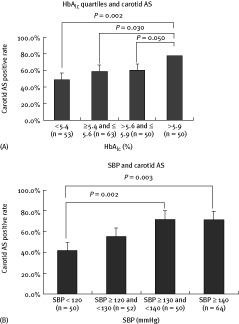
(A) Glycated hemoglobin A1c quartiles and carotid atherosclerosis. (B) Relationship of systolic blood pressure and carotid atherosclerosis. Abbreviations: AS, atherosclerosis; HbA1c, glycated hemoglobin A1c; SBP, systolic blood pressure
To study the relationship between SBP and carotid AS, the patients were then divided according to SBP levels into 4 groups: the SBP < 120 mm Hg group, the SBP ≥ 120 mm Hg and < 130 mm Hg group, the SBP ≥ 130 mm Hg and < 140 mm Hg group, and the SBP ≥ 140 mm Hg group. These 4 groups had median age of 67.0 (51.0, 78.0), 70.0 (57.0, 77.0), 78.0 (69.0, 83.0), and 77.0 (68.5, 82.0), and male gender proportions of 63.3%, 66.7%, 81.6%, and 71.3%, respectively. The proportion of subjects with carotid AS increased along with SBP elevation, with 21 (42.9%), 29 (56.9%), 36 (73.5%), and 46 (68.7%) atherosclerotic patients in the 4 groups, respectively. There were significant differences in the proportion of carotid AS between the SBP < 120 mm Hg group and the SBP ≥ 130 mm Hg and < 140 mm Hg group (P = 0.002); and between the SBP < 120 mm Hg group and the SBP ≥ 140 mm Hg group (P = 0.003)(Figure 1B).
To investigate the combined effects of HbA1c and SBP on carotid AS, we divided the patients according to HbA1c and SBP levels into 4 groups: the SBP < 130 mm Hg and HbA1c% ≤ 5.6 group, the SBP < 130 mm Hg and HbA1c% > 5.6 group, the SBP ≥ 130 mm Hg and HbA1c% ≤ 5.6 group, and the SBP ≥ 130 mm Hg and HbA1c% > 5.6 group. The median age in the 4 groups was 60.5 years (48.0, 73.0), 74.5 (62.5, 79.3), 75.5 (62.0, 82.0), and 79.0 (73.5, 82.5), respectively. Male gender represented 64.8%, 65.2%, 72.6%, and 81.5% of patients in the 4 groups, respectively. When SBP levels were similar, the proportion of subjects with carotid AS increased with HbA1c elevation. Significant difference was found between the SBP < 130 mm Hg and HbA1c% ≤ 5.6 group and the SBP ≥ 130 mm Hg and HbA1c% ≤ 5.6 group (P = 0.018), as well as between the SBP < 130 mm Hg and HbA1c% ≤ 5.6 group and the SBP ≥ 130 mm Hg and HbA1c% > 5.6 group (P = 0.000)(Figure 2).
Figure 2.
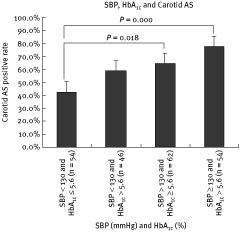
Combined effects of glycated hemoglobin A1c and systolic blood pressure on carotid atherosclerosis. Abbreviations: AS, atherosclerosis; HbA1c, glycated hemoglobin A1c; SBP, systolic blood pressure
In univariate logistic regression, we found that carotid AS was associated with age, gender, the presence or absence of HT, 2‐hour glucose, HbA1c, and SBP (Table 2). Among these are nonmodifiable risk factors such as age and gender, but also the modifiable risk factors of 2‐hour glucose, HbA1c, and SBP.
Table 2.
Univariate Association of Carotid AS With Multiple Risk Factors
| Risk Factor | OR | P Value |
|---|---|---|
| Age (per y) | 1.093 | 0.000 |
| Gender (F vs M) | 0.435 | 0.007 |
| BMI (per unit) | 0.940 | 0.173 |
| Smoking (yes vs no) | 1.178 | 0.688 |
| HT (yes vs no) | 2.289 | 0.005 |
| SBP (per mm Hg) | 1.035 | 0.000 |
| DBP (per mm Hg) | 0.981 | 0.163 |
| FPG (per mmol/L) | 1.339 | 0.269 |
| 2‐h oral glucose (per mmol/L) | 1.183 | 0.047 |
| HbA1c (per 1%) | 2.557 | 0.010 |
| LDL‐C (per mmol/L) | 0.961 | 0.822 |
| HDL‐C (per mmol/L) | 0.709 | 0.434 |
Abbreviations: AS, atherosclerosis; BMI, body mass index; DBP, diastolic blood pressure; F, female; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; HT, hypertension; LDL‐C, low‐density lipoprotein cholesterol; M, male; OR, odds ratio; SBP, systolic blood pressure
To discriminate the most important risk factors associated with carotid AS in the study, we constructed multiple logistic regression model 1, and all the risk factors that were found to be significant among the univariate logistic regression—age, gender, the presence or absence of HT, 2‐hour glucose, HbA1c, and SBP—were included as independent variables. Age (odds ratio [OR]: 1.090, P = 0.000) and gender (OR: 0.445, P = 0.023) were proved to be significant risk factors in this multivariate model (Table 3).
Table 3.
Multivariate Logistic Regression Models 1 and 2
| Risk Factor | OR 1a | P Value 1a | OR 2b | P Value 2b |
|---|---|---|---|---|
| Age (per y) | 1.090 | 0.000 | ||
| Gender (F vs M) | 0.445 | 0.023 | ||
| BMI (per unit) | NSc | NS | ||
| Smoking (yes vs no) | NS | NS | ||
| HT (yes vs no) | NS | NS | ||
| SBP (per mm Hg) | NS | NS | NS | NS |
| DBP (per mm Hg) | NS | NS | ||
| FPG (per mmol/L) | NS | NS | ||
| 2‐h oral glucose (per mmol/L) | NS | NS | NS | NS |
| HbA1c (per 1%) | NS | NS | 4.053 | 0.009 |
| LDL‐C (per mmol/L) | NS | NS | ||
| HDL‐C (per mmol/L) | NS | NS |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; F, female; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; HT, hypertension; LDL‐C, low‐density lipoprotein cholesterol; M, male; OR, odds ratio; SBP, systolic blood pressure.
OR 1 and P value 1 refer to the OR and P value in model 1.
OR 2 and P value 2 refer to the OR and P value in model 2.
NS indicates risk factors tested as independent variables that failed to show any significance in one of the models
However, though important, both age and gender are nonmodifiable risk factors. In order to select the most important risk factors among the modifiable ones, we constructed multiple logistic regression model 2, with all the modifiable risk factors as independent variables—including smoking, BMI, SBP, DBP, FPG, 2‐hour glucose, HbA1c, LDL‐C, and HDL‐C (Table 3). Finally, HbA1c was found to be the only significant modifiable risk factor in this multivariate model.
Discussion
The present study reveals a strong relationship between HbA1c and carotid AS, and found that the relationship was enhanced by elevated SBP.
As described in the Methods section, we strictly excluded diabetic patients. All patients went through an OGTT test, and anyone who reached diagnostic criteria for DM was excluded from the study. As a result, 76.9% of the study population was found to be within a normal HbA1c level of < 6.0% (data not shown).
Despite the normal glucose level, our data still showed an association between HbA1c and carotid AS. Previous studies implied that elevated HbA1c may contribute to carotid AS and cardiovascular risk. The Atherosclerosis Risk in Communities (ARIC) study14 suggested HbA1c to be an independent indicator of CHD risk both in the diabetic and nondiabetic population. In addition, the EPIC‐Norfolk study15 (European Prospective Investigation of Cancer–Norfolk, UK) found a linear relationship between HbA1c and CHD risk. The SHARE study (Survey of Health, Ageing and Retirement in Europe) showed that the degree of AS was related to HbA1c, irrespective of diabetes status.16 All these data suggest that elevated HbA1c is a strong indicator of atherosclerotic disease.
Moreover, in the OPTIMAAL trial17 (Optimal Trial in Myocardial Infarction with the Angiotensin II Antagonist Losartan), the results supported that HbA1c level was a potent predictor of mortality in post–myocardial infarction patients who had no DM history. Consistent with our study, OPTIMAAL found that in a high‐risk population, a slightly elevated HbA1c level (1% absolute increase) could be very important for patient prognosis (resulted in a 24% increase in mortality).
Our data also suggested a combined effect of SBP and HbA1c on carotid AS. In patients with SBP ≥ 130 mm Hg, a slight elevation of HbA1c was associated with increased carotid AS rate. This indicates that a slightly increased SBP level, working together with a slightly increased HbA1c level, could have significant effects on AS.
Nowadays, many patients in a hospital cardiology department, especially the high‐risk patients who are more likely to go to a hospital such as ours, are well treated, often with several kinds of medications which have been proven to be very helpful in reducing future cardiovascular risk and improving prognosis. However, even with optimal therapy—antiplatelet agents, ACEI/ARBs, β‐blockers, statins, or other agents—residual risk is still significant. Our study results indicated that HbA1c may be an independent and modifiable risk factor for carotid AS.
This study had several limitations. First, we enrolled patients from the cardiology department, which was different from population‐based research or studies including patients with a single category of disease, so caution should be taken when applying these results to other populations. Second, medication history was not adjusted. Third, we used a binary result of carotid ultrasonography, positive or negative, which might have diminished our ability to detect the relationship between HbA1c and the severity of carotid AS. Finally, as this was a cross‐sectional study, we need further prospective research to confirm our results.
Conclusion
In conclusion, our study shows that a slight increase in HbA1c may be associated with carotid AS in nondiabetic subjects. Moreover, the coexistence of elevated SBP and slightly increased HbA1c may have a more significant effect on carotid AS.
REFERENCES
- 1. Juutilainen A, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Type 2 diabetes as a “coronary heart disease equivalent”: an 18‐year prospective population‐based study in Finnish subjects. Diabetes Care 2005; 28: 2901–2907. [DOI] [PubMed] [Google Scholar]
- 2. Jarrett RJ. The cardiovascular risk associated with impaired glucose tolerance. Diabet Med 1996; 13(3 suppl2): S15–S19. [PubMed] [Google Scholar]
- 3. Saydah SH, Loria CM, Eberhardt MS, Brancati FL. Subclinical states of glucose intolerance and risk of death in the U.S. Diabetes Care 2001; 24: 447–453. [DOI] [PubMed] [Google Scholar]
- 4. Barr EL, Zimmet PZ, Welborn TA, et al. Risk of cardiovascular and all‐cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (Ausdiab). Circulation 2007; 116: 151–157. [DOI] [PubMed] [Google Scholar]
- 5. Gerstein HC. Glucose: a continuous risk factor for cardiovascular disease. Diabet Med 1997; (14 suppl 3): S25–S31. [DOI] [PubMed] [Google Scholar]
- 6. Balkau B, Shipley M, Jarrett RJ, et al. High blood glucose concentration is a risk factor for mortality in middle‐aged nondiabetic men: 20‐year follow‐up in the Whitehall Study, the Paris Prospective Study, and the Helsinki Policemen Study. Diabetes Care 1998; 21: 360–373. [DOI] [PubMed] [Google Scholar]
- 7. Sasso FC, Carbonara O, Nasti R, et al. Glucose metabolism and coronary heart disease in patients with normal glucose tolerance. JAMA 2004; 291: 1857–1863. [DOI] [PubMed] [Google Scholar]
- 8. Avignon A, Radauceanu A, Monnier L. Nonfasting plasma glucose is a better marker of diabetic control than fasting plasma glucose in type 2 diabetes. Diabetes Care 1997; 20: 1822–1826. [DOI] [PubMed] [Google Scholar]
- 9. Victor RG. Arterial hypertension In: Goldman L, Ausiello D, eds. Cecil Medicine. 23rd ed. Philadelphia, PA: Saunders Elsevier; 2007; 430–450. [Google Scholar]
- 10. Grossman E, Messerli FH. Hypertension and diabetes. Adv Cardiol 2008; 45: 82–106. [DOI] [PubMed] [Google Scholar]
- 11. Akosah KO, McHugh VL, Barnhart SI, et al. Carotid ultrasound for risk clarification in young to middle‐aged adults undergoing elective coronary angiography. Am J Hypertens 2006; 19: 1256–1261. [DOI] [PubMed] [Google Scholar]
- 12. Brook RD, Bard RL, Patel S, et al. A negative carotid plaque area test is superior to other noninvasive atherosclerosis studies for reducing the likelihood of having underlying significant coronary artery disease. Arterioscler Thromb Vasc Biol 2006; 26: 656–662. [DOI] [PubMed] [Google Scholar]
- 13. O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr; Cardiovascular Health Study Collaborative Research Group. Carotid‐artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med 1999; 340: 14–22. [DOI] [PubMed] [Google Scholar]
- 14. Selvin E, Coresh J, Golden SH, Brancati FL, Folsom AR, Steffes MW. Glycemic control and coronary heart disease risk in persons with and without diabetes: the atherosclerosis risk in communities study. Arch Intern Med 2005; 165: 1910–1916. [DOI] [PubMed] [Google Scholar]
- 15. Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European Prospective Investigation into Cancer in Norfolk. Ann Intern Med 2004; 141: 413–420. [DOI] [PubMed] [Google Scholar]
- 16. Gerstein HC, Anand S, Yi QL, et al. The relationship between disglycemia and atherosclerosis in South Asian, Chinese, and European individuals in Canada: a randomly sampled cross‐sectional study. Diabetes Care 2003; 26: 144–149. [DOI] [PubMed] [Google Scholar]
- 17. Gustafsson I, Kistorp CN, James MK, Faber JO, Dickstein K, Hildebrandt PR; OPTIMAAL Study Group . Unrecognized glycometabolic disturbance as measured by hemoglobin A1c is associated with a poor outcome after acute myocardial infarction. Am Heart J 2007; 154: 470–476. [DOI] [PubMed] [Google Scholar]


