Abstract
Background
When human milk is not available for feeding preterm infants, protein hydrolysate, rather than standard cow's milk formulas (with intact proteins), is often used because it is perceived as being tolerated better and less likely to lead to complications. However, protein hydrolysate formulas are more expensive than standard formulas, and concern exists that their use in practice is not supported by high‐quality evidence.
Objectives
To assess the effects of feeding preterm infants hydrolysed formula (vs standard cow's milk formula) on risk of feed intolerance, necrotising enterocolitis, and other morbidity and mortality.
Search methods
We used the standard Cochrane Neonatal search strategy including electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 1), in the Cochrane Library; Ovid MEDLINE (1966 to 28 January 2019); Ovid Embase (1980 to 28 January 2019); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (28 January 2019), as well as conference proceedings and previous reviews.
Selection criteria
Randomised and quasi‐randomised controlled trials that compared feeding preterm infants protein hydrolysate versus standard (non‐hydrolysed) cow's milk formula.
Data collection and analysis
Two review authors assessed trial eligibility and risk of bias and extracted data independently. We analysed treatment effects as described in the individual trials and reported risk ratios and risk differences for dichotomous data, and mean differences for continuous data, with respective 95% confidence intervals (CIs). We used a fixed‐effect model in meta‐analyses and explored potential causes of heterogeneity in sensitivity analyses. We assessed quality of evidence at the outcome level using the GRADE approach.
Main results
We identified 11 trials for inclusion in the review. All trials were small (total participants 665) and had various methodological limitations including uncertainty about methods to ensure allocation concealment and blinding. Most participants were clinically stable preterm infants of less than about 34 weeks' gestational age or with birth weight less than about 1750 g. Fewer participants were extremely preterm, extremely low birth weight, or growth restricted. Most trials found no effects on feed intolerance, assessed variously as mean pre‐feed gastric residual volume, incidence of abdominal distension or other gastrointestinal signs of concern, or time taken to achieve full enteral feeds (meta‐analysis was limited because studies used different measures). Meta‐analysis showed no effect on the risk of necrotising enterocolitis (typical risk ratio 1.10, 95% CI 0.36 to 3.34; risk difference 0.00, 95% CI ‐0.03 to 0.04; 5 trials, 385 infants) (low‐certainty evidence; downgraded for imprecision and design weaknesses).
Authors' conclusions
The identified trials provide only low‐certainty evidence about the effects of feeding preterm infants protein hydrolysate versus standard formula. Existing data do not support conclusions that feeding protein hydrolysate affects the risk of feed intolerance or necrotising enterocolitis. Additional large, pragmatic trials are needed to provide more reliable and precise estimates of effectiveness and cost‐effectiveness.
Plain language summary
Protein hydrolysate versus standard formula for preterm infants
Review question
Does feeding preterm infants cow's milk formula containing predigested (hydrolysed) proteins, rather than whole proteins, improve digestion and reduce the risk of severe bowel problems?
Background
Preterm infants often find cow's milk formula more difficult to digest than human milk, and cow's milk formula may increase the risk of severe bowel problems for preterm (born before their due date) infants. If preterm infants are fed cow's milk formula (when human milk is unavailable), then using a formula in which the protein is already partially digested (called 'hydrolysed') rather than a standard formula (with intact proteins) might reduce the risk of these problems. However, hydrolysed formulas are more expensive than standard formulas, and may have specific side effects not seen with standard formulas. Given these concerns, we have reviewed all available evidence from clinical trials that compared these types of formula for feeding preterm infants.
Study characteristics
In searches of medical databases up to 28 January 2019, we found 11 relevant trials; most were small (involving 665 infants in total) and had methodological weaknesses.
Key results
Currently available evidence suggests that feeding preterm infants hydrolysed formula (rather than standard formula) during their initial hospital admission has no important benefits or harms. However, this finding is not yet conclusive, and larger trials of better quality are needed to provide evidence to help clinicians and families make informed choices about this issue.
Quality of evidence
Data from these trials provide no strong or consistent evidence that feeding preterm infants hydrolysed formula rather than standard formula improved or worsened digestion or changed the risk of severe bowel problems.
Summary of findings
Summary of findings 1. Hydrolysed compared to non‐hydrolysed formula for feeding preterm infants.
| Hydrolysed compared to non‐hydrolysed formula for feeding preterm infants | ||||||
|
Patient or population: feeding preterm infants Setting: neonatal unit Intervention: hydrolysed formula (protein hydrolysate) Comparison: non‐hydrolysed formula | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with non‐hydrolysed formula | Risk with hydrolysed formula | |||||
| Feed intolerance | Study population | RR 2.71 (0.29 to 25.00) | 161 (3 RCTs) | ⊕⊕⊝⊝ Low | Limited data from 3 small RCTs with imprecise estimate of effect size | |
| 13 per 1000 | 34 per 1000 (4 to 316) | |||||
| Necrotising enterocolitis | Study population | RR 1.10 (0.36 to 3.34) | 385 (5 RCTs) | ⊕⊕⊝⊝ Low | Methodological limitations of included trials and imprecise effect size estimate | |
| 32 per 1000 | 35 per 1000 (12 to 107) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
Background
Hydrolysed cow's milk formulas, originally developed for infants with cow's milk protein allergy or intolerance, are used as enteral feeding alternatives for preterm infants for whom human milk is unavailable. These formulas contain hydrolysed, rather than intact, proteins and may also differ from standard cow's milk formulas in carbohydrate, lipid, and micronutrient type and content (Oldaeus 1997). Their use as a sole, or supplemental, enteral feed source for preterm infants has increased since the late 1990s, particularly in high‐income countries, because they are perceived as being tolerated better and less likely to lead to complications than standard cow's milk formulas (Zuppa 2005). However, hydrolysed formulas are more expensive than standard formulas, and concern exists that their use in practice is not supported by high‐certainty evidence (Foucard 2005).
Description of the condition
Human breast milk is recommended as the best form of enteral nutrition for preterm infants (AAP 2012). Breast milk proteins, carbohydrates, fats, and micronutrients have been optimised by evolution for neonatal digestion and absorption. Breast milk contains many non‐nutrient factors including immunoglobulins and lactoferrin that promote intestinal adaptation and maturation, improve enteral feed tolerance, and protect against infection and inflammatory disorders (Agostoni 2010; Arslanoglu 2013).
When sufficient human breast milk is unavailable, cow's milk‐based formulas are used in feeding preterm infants, either as the sole enteral diet or as a supplement to human breast milk (Klingenberg 2012). Feeding preterm infants standard cow's milk formulas, rather than human breast milk, is, however, associated with higher rates of feed intolerance and necrotising enterocolitis (Quigley 2014). Feed intolerance and interruption of enteral feeds are major contributors to cumulative nutrient deficits and postnatal growth restriction in very preterm infants (Cooke 2016; Embleton 2001). Slow postnatal growth is associated with neurodevelopmental impairment in later childhood and with poorer cognitive and educational outcomes (Brandt 2003; Embleton 2013a; Leppanen 2014). Necrotising enterocolitis affects about 5% of very preterm infants. Infants who develop necrotising enterocolitis experience more infections, have lower levels of nutrient intake, grow more slowly, have longer durations of intensive care and hospital stay, and are more likely to die or be disabled than gestation‐comparable infants who do not develop necrotising enterocolitis (Morgan 2011; Pike 2012; Yee 2012).
Description of the intervention
Standard cow's milk formulas can be grouped broadly as 'term' formulas (designed for term infants; nutrient content based on the composition of mature breast milk) and nutrient‐enriched 'preterm' formulas (designed for preterm or low birth weight infants; energy enriched and variably protein and mineral enriched) (Fewtrell 1999). Concern exists that standard cow's milk formulas (both 'term' and 'preterm') are poorly tolerated, especially by very preterm infants, because the immature infant's gastrointestinal tract is less efficient than that of term infants in digesting intact cow's milk proteins and fats (Ewer 1994; Lindberg 1998).
Hydrolysed formulas
'Hydrolysed' protein formulas containing protein digested chemically (acid/alkali) or enzymatically (protease) to oligopeptides are often used in feeding preterm infants, especially infants with feed intolerance or clinical features (such as episodic apnoea, oxygen desaturation, or bradycardia) that are attributed to gastro‐oesophageal reflux, or following gastrointestinal surgery or necrotising enterocolitis (Zuppa 2005).
Several brands of hydrolysed formulas (both 'term' and 'preterm') are available commercially, and these are grouped broadly depending on degree of hydrolysis.
Extensively hydrolysed: residual free amino acids and peptides with molecular weights less than 1.5 kDa to 3.0 kDa.
Partially hydrolysed: residual peptides with molecular weights of 3.0 kDa to 10.0 kDa.
This distinction is mainly relevant to the putative hypo‐allergenic properties of hydrolysed formulas, and limited data show its functional relevance to preterm infants. Formulas also vary by the predominant protein source (casein vs whey‐casein), as well as by carbohydrate (lactose, maltodextrin) and fat (cow, vegetable) type and content (BNFC 2016).
How the intervention might work
Although hydrolysed formulas have been developed as hypo‐allergenic alternatives to standard cow's milk formulas for infants at risk of cow's milk protein intolerance or allergy, evidence for this effect in term infants is very weak (Boyle 2016; Osborn 2017). In preterm infants, hydrolysed formulas are used mostly for their perceived benefits in reducing the risk of feed intolerance and necrotising enterocolitis. When human milk is unavailable, hydrolysed formulas may be used empirically (starter formula) and therapeutically to improve feeding tolerance or reduce gastro‐oesophageal reflux. Possible mechanisms for these effects include accelerated gastric emptying and intestinal transit, more efficient enteric peptide digestion, and stimulation of small intestinal enzymatic and motilin activity (Mihatsch 2001b; Zuppa 2005). If better feed tolerance reduces the time taken to establish full enteral feeding in very preterm infants, this may reduce the adverse infectious or metabolic consequences of prolonged exposure to parenteral nutrition.
Several potential adverse effects of hydrolysed formulas are recognised. Osmolality is increased when protein is hydrolysed into smaller peptides, and these higher‐osmolarity fluids delivered to the small intestine may increase the risk of necrotising enterocolitis. Furthermore, if bioactive proteins such as immunoglobulin or lactoferrin are hydrolysed, this may reduce their putative benefits in reducing the risk of infection or necrotising enterocolitis. It is possible that some peptides created by artificial hydrolysis may have diminished or harmful functional activities (Embleton 2013b). Concern about micronutrient bioavailability in hydrolysed formulas exists, particularly regarding whether bone minerals are well absorbed in the absence of intact casein proteins (Zuppa 2005). Another theoretical long‐term risk is that the high levels of "advanced glycation end‐products" in most hydrolysed formulas might be associated with development of chronic inflammatory, metabolic, or neurodegenerative diseases (Uribarri 2015).
Why it is important to do this review
Given the potential for protein hydrolysate formulas (rather than standard cow's milk formulas) to improve enteral feed tolerance and prevent adverse outcomes in preterm infants, we undertook a systematic review of the randomised trial data to help to inform practice and research.
Objectives
To assess the effects of feeding preterm infants hydrolysed formula (vs standard cow's milk formula) on risk of feed intolerance, necrotising enterocolitis, and other morbidity and mortality.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised controlled trials, including cluster‐randomised controlled trials.
Types of participants
Preterm (less than 37 weeks' gestation) newborn infants who received cow's milk formula as their sole or supplemental enteral diet.
Types of interventions
Hydrolysed cow's milk formula versus standard (non‐hydrolysed) cow’s milk formula or another type of hydrolysed cow's milk formula. Formula was to be allocated as at least 20% of the intended enteral diet for at least two weeks to discern measurable effects on growth rates and episodes of feed intolerance. Trials should have compared formulas with similar energy and protein levels (i.e. hydrolysed 'preterm' formula vs non‐hydrolysed 'preterm' formula, or hydrolysed 'term' formula vs non‐hydrolysed 'term' formula).
We planned separate comparisons of trials that assessed:
empirical use of hydrolysed formulas; and
indicated (therapeutic) use of hydrolysed formulas to treat infants with feed intolerance or gastro‐oesophageal reflux (and associated apnoea, desaturation, or bradycardia), or following gastrointestinal surgery or necrotising enterocolitis (as defined by the primary investigators).
Types of outcome measures
Primary outcomes
Infants with at least one episode of feed intolerance that resulted in cessation of, or reduction in, enteral feeding (enteral feeds were reduced or ceased for longer than four hours), or mean number of episodes of feed intolerance during the trial period, or both
Infants with at least one episode of necrotising enterocolitis (modified Bell stage 2/3) (Walsh 1986) (unless indicated for use following necrotising enterocolitis)
Secondary outcomes
Time to full enteral feeding independent of parenteral fluids (days)
Growth: time to regain birth weight, and subsequent rates of weight (grams/kilogram/d), length (millimetre/week), and head growth (millimetre/week) during hospital admission
Duration of hospital admission (days)
-
Measures of bone mineralisation
Serum alkaline phosphatase level at 36 to 40 weeks' postmenstrual age or
Bone mineral content assessed post term by dual energy X‐ray absorptiometry (DEXA) or
Clinical or radiological evidence of rickets on long‐term follow‐up
Late‐onset invasive infection diagnosed more than 72 hours after birth, as determined by culture from a normally sterile site: cerebrospinal fluid, blood, bone or joint, peritoneum, pleural space, or central venous line tip; or findings on autopsy examination consistent with invasive microbial infection
Mortality: all‐cause until 28 days and during hospital admission
Neurodevelopmental outcomes assessed by a validated test after 12 months post term: neurological evaluations, developmental scores, and classifications of disability, including auditory and visual disability
Allergy or atopy diagnosed after 12 months post term: asthma, eczema, allergic rhinitis or conjunctivitis, food allergy, allergic sensitisation (skin prick, or specific or total immunoglobulin E level) (Boyle 2016)
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for the specialised register).
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 1), in the Cochrane Library; Ovid MEDLINE (1946 to 28 January 2019); Ovid Embase (1974 to 28 January 2019); Ovid Maternity & Infant Care Database (1971 to January 2019); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to 28 January 2019) using a combination of the following text words and medical subject heading (MeSH) terms as described in Appendix 1. We limited search outputs by using relevant search filters for clinical trials, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We did not apply language restrictions.
We searched ClinicalTrials.gov and the World Health Organization's International Trials Registry and Platform (www.who.int/ictrp/en/) for completed and ongoing trials.
Searching other resources
We examined reference lists in previous reviews and included studies. We searched the proceedings of the annual meetings of the Pediatric Academic Societies (1993 to 29 January 2019), the European Society for Paediatric Research (1995 to 28 January 2019), the Royal College of Paediatrics and Child Health (2000 to 28 January 2019), and the Perinatal Society of Australia and New Zealand (2000 to 28 January 2019). Trials reported only as abstracts were eligible if sufficient information was available from the report, or from contact with study authors, to fulfil the inclusion criteria.
Data collection and analysis
We used the standard methods of Cochrane Neonatal.
Selection of studies
We screened the title and abstract of all studies identified by the search strategy, and two review authors independently assessed the full articles for all potentially relevant trials. We excluded those studies that did not meet all of the inclusion criteria, and we stated the reasons for exclusion. We discussed any disagreements until consensus was achieved.
Data extraction and management
Two review authors (DN and WM) extracted data independently using a data collection form to aid extraction of information on design, methods, participants, interventions, outcomes, and treatment effects from each included study. We discussed any disagreements until we reached a consensus. If data from the trial reports were insufficient, we contacted the triallists for further information.
Assessment of risk of bias in included studies
Two review authors (DN and JK) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool for the following domains (Higgins 2011).
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Any other bias.
Any disagreements were resolved by discussion or by consultation with a third assessor (WM). See Appendix 2 for a more detailed description of risk of bias for each domain. We requested additional information from the trial authors to clarify methods and results when necessary. We did not exclude trials on the basis of risk of bias, but we did plan to conduct sensitivity analyses if applicable to explore the consequences of synthesising evidence of variable quality (Higgins 2011).
Measures of treatment effect
We analysed treatment effects in individual trials using Review Manager 5 (RevMan 2014), and we reported risk ratio (RR) and risk difference (RD) for dichotomous data, and mean difference (MD) for continuous data, with respective 95% confidence intervals (CIs). We determined the number needed to treat for an additional beneficial outcome (NNTB) or an additional harmful outcome (NNTH) for analyses with a statistically significant difference in the RD.
Unit of analysis issues
The unit of analysis was the participating infant for individually randomised trials, and the neonatal unit (or subunit) for cluster‐randomised trials. For cluster‐randomised trials, we planned to undertake analyses at the level of the participant while accounting for clustering of data using the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
Where data were missing and could not be derived as described, we approached the analysis of missing data as follows.
We contacted the original study investigators to request the missing data.
Where possible, we imputed missing standard deviations (SDs) using the coefficient of variation (CV) or calculated from other available statistics including standard errors, CIs, t values, and P values.
If data were assumed to be missing at random, we analysed the data without imputing any missing values.
If this could not be assumed, then we planned to impute the missing outcomes with replacement values, assuming all to have a poor outcome. We planned sensitivity analyses to assess any changes in the direction or magnitude of effect resulting from data imputation.
Assessment of heterogeneity
Two review authors assessed clinical heterogeneity, with a meta‐analysis conducted only when both authors agreed that study participants, interventions, and outcomes were sufficiently similar.
We examined treatment effects of individual trials and heterogeneity between trial results by inspecting the forest plots. We calculated the I² statistic for each analysis to quantify inconsistency across studies, and we described the percentage of variability in effect estimates that may be due to heterogeneity rather than to sampling error. If we detected moderate or high heterogeneity (I² > 50%), we planned to explore the possible causes (e.g. differences in study design, participants, interventions, or completeness of outcome assessments).
Assessment of reporting biases
If more than 10 trials were included in a meta‐analysis, we planned to examine a funnel plot for asymmetry.
Data synthesis
We used the fixed‐effect model in Review Manager 5 for meta‐analyses (as per Cochrane Neonatal recommendations) (RevMan 2014). Where moderate or high heterogeneity existed, we planned to examine the potential causes in subgroup and sensitivity analyses.
Quality of evidence
We used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: feed tolerance and incidence of necrotising enterocolitis.
Two review authors (DN and JK) independently assessed the quality of evidence for each of the outcomes above. We considered evidence from randomised controlled trials (RCTs) as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of evidence, precision of estimates, and presence of publication bias. We used the GRADEpro Guideline Development Tool to create Summary of findings table 1 to report the quality of evidence (GRADEpro GDT).
The GRADE approach yields an assessment of the quality of a body of evidence using one of four grades.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses by:
gestational age at birth: very preterm (less than 32 weeks' gestation) infants versus infants born at 32 weeks' gestation or later;
indication (for therapeutic use): post surgery versus post necrotising enterocolitis versus feeding intolerance or gastro‐oesophageal reflux; and
extent of protein hydrolysis (as defined by manufacturers): extensively versus partially hydrolysed formula.
Sensitivity analysis
We planned to perform sensitivity analyses to determine if findings were affected by including only studies using adequate methods (low risk of bias), defined as adequate randomisation and allocation concealment, blinding of intervention and measurement, and less than 10% loss to follow‐up.
Results
Description of studies
Results of the search
See Figure 1.
1.
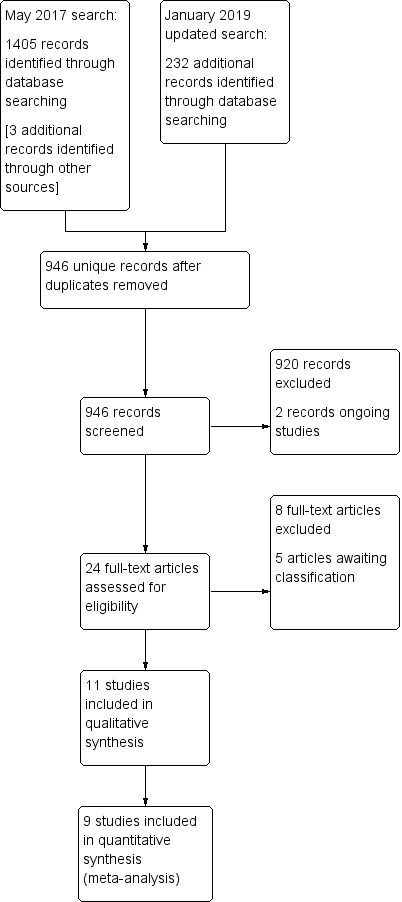
Study flow diagram (updated January 2019).
We screened the title and abstract (if available) of 946 unique records identified by the searches.
We identified two ongoing trials (ACTRN12613000481774; Yin 2015).
We excluded 920 articles outright and screened the full text of 24 study reports.
We included 11 trials ‐ Characteristics of included studies ‐ and excluded eight full‐text reports ‐ Characteristics of excluded studies.
Five reports await classification. One is awaiting further data (del Moral 2017), and four reports await full text and English language translation to allow assessment of eligibility for inclusion (Dobryanskyy 2015; Gu 2017; Kwinta 2002; Luo 2016).
Included studies
We included 11 trials (Baldassarre 2017; Florendo 2009; Huston 1992; Maggio 2005; Mihatsch 2002; Pauls 1996; Picaud 2001; Raupp 1995; Riezzo 2001; Schweizer 1993; Szajewska 2004). Most of the included trials were undertaken during the 1990s and 2000s by investigators in neonatal units in Europe (mainly Germany and Italy) and North America. For further details, see the Characteristics of included studies table.
Participants
In total, 665 infants participated in the included trials. Most participants were clinically stable preterm infants of gestational age less than about 34 weeks' gestation or birth weight less than about 1750 g. Few participants were extremely preterm, extremely low birth weight, or growth restricted. Most of the trials specifically excluded infants with congenital anomalies or gastrointestinal or neurological problems.
Interventions
All trials assessed the empirical use of protein hydrolysate formulas; none assessed indicated use.
Trials varied according to the brand of formula studied. All trials except one assessed a "preterm" (nutrient‐enriched) hydrolysed formula; Schweizer 1993 assessed a "term" hydrolysed formula. Most trials used a whey‐casein‐based hydrolysate. Two trials used a predominantly casein‐based hydrolysate (Huston 1992; Riezzo 2001). Most studies assessed a partially hydrolysed formula. Three trials used an extensively hydrolysed formula (Baldassarre 2017; Mihatsch 2002; Schweizer 1993). One (three‐arm) trial randomly allocated infants to receive a partially hydrolysed formula, an extensively hydrolysed formula, or a standard preterm formula (Szajewska 2004). Control diets were preterm non‐hydrolysed formulas in all except Riezzo 2001, where the control diet was a standard term formula.
No trials compared hydrolysed cow's milk formula versus another type of hydrolysed cow's milk formula.
Trial participants received the intervention or control formulas on commencing enteral feeds either as a sole diet or as a supplement when mother's own milk was not available or was insufficient. One trial specifically excluded participants post hoc if mother's own milk formed more than 10% of enteral intake (Mihatsch 2002). In general, trial feeds were allocated for several weeks (at least two weeks), or until participating infants reached a specified weight (typically about 1.8 kg).
Outcomes
Outcomes reported most commonly were feed intolerance (reported in various ways but often without accompanying numerical data), growth parameters during the study period or until hospital discharge, and adverse events (including mortality and necrotising enterocolitis). None of the trials reported long‐term growth and neurodevelopmental outcomes.
Excluded studies
We excluded eight studies (Agosti 2003; Corvaglia 2013; Logarajaha 2015; Mihatsch 1999; Mihatsch 2001a; Rigo 1994; Rigo 1995; Yu 2014). Reasons for exclusion are described in the Characteristics of excluded studies table.
Risk of bias in included studies
Quality assessments are detailed in the Characteristics of included studies table and are summarised in Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Three trials reported adequate allocation concealment methods (sealed, numbered envelopes; central randomisation in blocks) and were at low risk of bias (Florendo 2009; Maggio 2005; Szajewska 2004). None of the remaining trials reported sufficient details for assessment of whether or how allocation concealment was achieved.
Blinding
Four trials reported blinding of investigators and carers or parents (Baldassarre 2017; Florendo 2009; Maggio 2005; Schweizer 1993). It is probable that the other trials were not blinded as the reports did not describe any methods that might achieve this.
Incomplete outcome data
Most trials were likely to be at low risk of bias because of incomplete assessment of the trial cohort. In one trial, investigators recruited 129 infants initially, then excluded 42 participants post hoc because they had received more than 10% of their enteral intake as human milk (Mihatsch 2002).
Selective reporting
We were unable to assess reliably whether selective reporting occurred as we did not have protocols or other indicators of prespecified outcomes for any of the trials.
Other potential sources of bias
We did not identify any other potential sources of bias in the reports.
Effects of interventions
See: Table 1
Empirical use of protein hydrolysate versus standard formula (Comparison 1)
1. Feed intolerance (Outcome 1.1)
Three trials reported numerical data on the incidence of feed intolerance (Baldassarre 2017; Florendo 2009; Maggio 2005). Meta‐analysis found no statistically significant effect (typical risk ratio (RR) 2.71, 95% confidence interval (CI) 0.29 to 25.00; typical risk difference (RD) 0.02, 95% CI ‐0.03 to 0.08) (I² not applicable) (Analysis 1.1).
1.1. Analysis.

Comparison 1: Hydrolysed versus non‐hydrolysed formula, Outcome 1: Feed intolerance
The other trials did not report any numerical data but described their findings in a narrative format. These trials found no differences in measures of gastric residual volumes (Mihatsch 2002; Pauls 1996), frequency of regurgitation (Riezzo 2001), or vomiting or diarrhoea (Szajewska 2004). Raupp 1995 reported that "both formulas were well tolerated". The remaining trials did not report any measures of feed intolerance (Huston 1992; Picaud 2001; Schweizer 1993).
2. Incidence of necrotising enterocolitis (Outcome 1.2)
Meta‐analysis of data from five trials (385 infants) revealed no differences (typical RR 1.10, 95% CI 0.36 to 3.34; typical RD 0.00, 95% CI ‐0.03 to 0.04; I² = 0%) (Analysis 1.2; Figure 3).
1.2. Analysis.

Comparison 1: Hydrolysed versus non‐hydrolysed formula, Outcome 2: Necrotising enterocolitis
3.

Forest plot of comparison: 1 Hydrolysed versus non‐hydrolysed formula, outcome: 1.2 Necrotising enterocolitis.
The other trials did not report this outcome, although in most, it seems likely that none of the participants developed necrotising enterocolitis.
The quality of evidence for the primary outcomes was low because of methodological limitations of the included trials (including uncertainty about allocation concealment and blinding) and imprecision of effect size estimates (Table 1).
3. Time to full enteral feeding (Outcome 1.3)
Most trials did not report time to full enteral feeds (Florendo 2009; Huston 1992; Maggio 2005; Raupp 1995; Riezzo 2001; Szajewska 2004).
Mihatsch 2002 reported that the median time to full enteral feeding was shorter in the intervention group (10 days vs 12 days in the control group).
Four trials reported no difference.
Schweizer 1993: 24 days versus 25 days (standard deviation (SD) not reported).
Pauls 1996; no data reported.
Picaud 2001: 16 (SD 8) days versus 17 (SD 8) days (mean difference (MD) ‐1.00 days, 95% CI ‐8.36 to 6.36).
Baldassarre 2017: 11 days versus 10 days (SD not reported).
4. Growth: time to regain birth weight, and subsequent rates of growth during hospital admission (Outcomes 1.4 to 1.6)
Four trials did not report any growth data (Baldassarre 2017; Pauls 1996; Riezzo 2001; Szajewska 2004). The other trials reported some data on growth parameters during the study period or until hospital discharge, but most did not provide sufficient data for inclusion in the meta‐analysis (Huston 1992; Mihatsch 2002; Raupp 1995; Schweizer 1993).
Time to regain birth weight
One trial reported days to regain birth weight (Schweizer 1993). This trial found no difference (10 days in the intervention group vs 9 days in the control group; SD not reported).
Weight gain
Three trials reported rates of weight gain over the study period or until hospital discharge (Florendo 2009; Maggio 2005; Picaud 2001). Meta‐analysis showed that weight gain was slower among infants fed hydrolysed formula (MD ‐3.02 g/kg/d, 95% CI ‐4.66 to ‐1.38) (Analysis 1.4; Figure 4).
1.4. Analysis.

Comparison 1: Hydrolysed versus non‐hydrolysed formula, Outcome 4: Weight gain (g/kg/day)
4.

Forest plot of comparison: 1 Hydrolysed versus non‐hydrolysed formula, outcome: 1.4 Weight gain (g/kg/d).
Length change
Meta‐analysis of data from two trials (97 infants) showed no difference in length change (MD ‐0.04 mm/week, 95% CI ‐1.24 to 1.15) (Analysis 1.5).
1.5. Analysis.

Comparison 1: Hydrolysed versus non‐hydrolysed formula, Outcome 5: Length gain (mm/week)
Head circumference growth
Meta‐analysis of data from two trials (97 infants) revealed no difference in head circumference growth (MD 0.27 mm/week, 95% CI ‐0.39 to 0.94) (Analysis 1.6).
1.6. Analysis.

Comparison 1: Hydrolysed versus non‐hydrolysed formula, Outcome 6: Head circumference growth (mm/week)
5. Duration of hospital admission
No trials reported the duration of hospital admission.
6. Measures of bone mineralisation (Outcome 1.7)
Two trials reported measures of bone mineralisation (Florendo 2009; Raupp 1995). Neither trial nor a meta‐analysis of data from both trials showed a difference in serum alkaline phosphatase level at 36 to 40 weeks' postmenstrual age (MD 16.6 IU/L, 95% CI ‐34.1 to 67.4) (Analysis 1.7; Figure 5). None of the trials reported bone mineral content assessed post term nor clinical or radiological evidence of rickets on long‐term follow‐up.
1.7. Analysis.

Comparison 1: Hydrolysed versus non‐hydrolysed formula, Outcome 7: Serum alkaline phosphatase (IU/L)
5.

Forest plot of comparison: 1 Hydrolysed versus non‐hydrolysed formula, outcome: 1.7 Serum alkaline phosphatase (IU/L).
7. Late‐onset invasive infection (Outcome 1.8)
Only one trial reported the incidence of late‐onset invasive infection (Baldassarre 2017), describing no difference in the incidence of microbiologically confirmed bacteraemia (typical RR 1.50, 95% CI 0.27 to 8.34; typical RD ‐0.03, 95% CI ‐0.11 to 0.17) (Analysis 1.8).
1.8. Analysis.

Comparison 1: Hydrolysed versus non‐hydrolysed formula, Outcome 8: Late‐onset invasive infection
8. Mortality
No trials reported the incidence of mortality.
9. Neurodevelopmental outcomes
No trials reported neurodevelopmental outcomes.
10. Allergy or atopy diagnosed after 12 months post term (Outcome 1.8)
One trial assessed allergy or atopy (Szajewska 2004). This trial found no difference in the incidence of "any allergic disease" (atopic dermatitis, gastrointestinal symptoms, wheezing) at 12 months (RR 0.62, 95% CI 0.27 to 1.42; RD ‐0.13, 95% CI ‐0.36 to 0.10) (Analysis 1.9).
1.9. Analysis.

Comparison 1: Hydrolysed versus non‐hydrolysed formula, Outcome 9: Any allergic disease
Subgroup analyses
Gestational age at birth: very preterm (less than 32 weeks) infants versus infants born at 32 weeks or later: subgroup data not available
Indication (for therapeutic use): post surgery versus post necrotising enterocolitis versus feeding intolerance or gastro‐oesophageal reflux: not applicable as all trials assessed empirical use
Extent of protein hydrolysis (as defined by manufacturers): data for subgroup analysis sufficient for necrotising enterocolitis (Outcome 1.2) only. Three trials used a partially hydrolysed preterm formula (Florendo 2009; Pauls 1996; Raupp 1995). Two trials used an extensively hydrolysed formula (Baldassarre 2017; Mihatsch 1999). Meta‐analysis showed no evidence of a subgroup effect (test for subgroup differences: Chi² = 0.75, df = 1 (P = 0.39), I² = 0%) (Figure 3).
Indicated use of protein hydrolysate versus standard formula (Comparison 2)
We found no trials comparing protein hydrolysate versus standard formula.
Discussion
Summary of main results
Data from 11 small randomised controlled trials provided only low‐certainty evidence about how feeding preterm infants (typically stable infants less than 34 weeks' gestation at birth) protein hydrolysate rather than standard cow's milk formula affects the risk of feed intolerance, necrotising enterocolitis, or other adverse outcomes. Limited data did not indicate any important effects on growth, although a meta‐analysis of data from three trials suggested that weight gain was slower among infants fed protein hydrolysate compared with isocaloric preterm formula. Currently no data are available to assess effects on growth and neurodevelopmental outcomes beyond the initial hospital admission.
Overall completeness and applicability of evidence
These findings should be interpreted and applied cautiously. The primary outcome, feed intolerance, was reported in various ways, and together with the paucity of numerical data, this precluded meta‐analysis. Trials generally reported that feeding with protein hydrolysate did not affect measures such as the pre‐feed gastric residual volume or the need to cease enteral feeding. Similarly, few trials reported the impact of the intervention on time to achieve full enteral feeding, and trials that did report this outcome found no statistically significant or clinically important effects.
Although a meta‐analysis of five trials (385 participants) found no effect on the risk of necrotising enterocolitis, data were insufficient to exclude a more modest but still important effect size. The lower bound of the 95% confidence interval (CI) was consistent with a 3% absolute risk reduction (i.e. one fewer infant developing necrotising enterocolitis for every 33 infants who received protein hydrolysate formula). Because necrotising enterocolitis is a relatively rare outcome, affecting about 5% of very preterm infants, much larger trials would be needed to obtain a more precise estimate of the effect of feeding with protein hydrolysate versus standard formula (Yee 2012).
Data on growth parameters are limited, as are data on other adverse outcomes. Furthermore, uncertainty remains about longer‐term impact on growth or development. As concerns exist that hydrolysed proteins may be utilised less efficiently than intact proteins by preterm infants, and that concomitant mineral uptake may be lower, trials that assess effects on both short‐ and long‐term growth and body composition (including bone health) may help to inform policy and practice (Senterre 2016).
Another major applicability limitation of this review is that all included trials were undertaken at healthcare facilities in high‐income countries, and none in low‐income countries. Therefore, this evidence may be of limited applicability to practices in resource‐limited settings, where, globally, most preterm and low birth weight infants are cared for (Imdad 2013).
All included trials assessed the effects of empirical (primary) use of protein hydrolysate for feeding preterm infants. We found no trials that assessed the indicated use of protein hydrolysate versus standard formula for preterm infants with feed intolerance or gastro‐oesophageal reflux (and associated apnoea, desaturation, or bradycardia), or following gastrointestinal surgery or necrotising enterocolitis. Although indicated use of protein hydrolysate is common, based on perceptions that formulas with intact proteins may be tolerated poorly by infants with intestinal trauma or compromise, trials have provided no evidence to inform this practice (Lapillonne 2016).
Quality of the evidence
The GRADE assessments indicated that the quality of evidence for the primary outcomes was 'low' because of methodological limitations of the included trials (including uncertainty about allocation concealment and blinding) and imprecision of effect size estimates (Table 1).
Most of the included trials were funded or supported by the manufacturers of the formulas being assessed, but the funders were not involved in trial design or analysis. However, there remains some concern that formula manufacturers may promote study findings of trials of specialist formulas selectively as part of a marketing strategy that subverts UNICEF Baby Friendly Initiative regulations (Cleminson 2015).
Potential biases in the review process
It is possible that our findings were subject to publication and other reporting biases. We attempted to minimise this by screening the reference lists of included trials and related reviews and by searching the proceedings of major international perinatal conferences to identify trial reports that were not (or were not yet) published in full form in academic journals. The meta‐analyses that we performed did not include sufficient trials to explore symmetry of funnel plots as a means of identifying possible publication or reporting bias.
Authors' conclusions
Implications for practice.
This review provides only low‐certainty evidence regarding any benefits or harms of feeding preterm infants with protein hydrolysate versus standard formula. Although no trial data suggest an effect on the risk of feed intolerance or necrotising enterocolitis, the total number of infants studied was small (665 infants), and the data that could be abstracted from published studies for inclusion in meta‐analyses were limited.
Implications for research.
Further high‐quality randomised controlled trials are needed to assess the benefits and safety of protein hydrolysate versus standard cow's milk formulas for feeding very preterm infants when maternal breast milk is insufficient or is not available. Trials could assess primary (empirical) use and secondary (indicated) use in infants with feed intolerance or gastro‐oesophageal reflux, or following gastrointestinal surgery or necrotising enterocolitis. Trials should aim to ensure the participation of extremely preterm, extremely low birth weight, or growth‐restricted infants, so that subgroup analyses can be planned for these infants at higher risk of necrotising enterocolitis. Given that protein hydrolysate preterm formula is more expensive than standard preterm formula, trials could justifiably include a cost‐benefit analysis.
What's new
| Date | Event | Description |
|---|---|---|
| 8 July 2020 | Amended | Typo corrected in Declarations of interest section |
History
Protocol first published: Issue 10, 2016 Review first published: Issue 10, 2017
| Date | Event | Description |
|---|---|---|
| 7 August 2019 | Amended | Declaration of interest updated for Dr. Nicholas D Embleton. |
| 26 February 2019 | New citation required but conclusions have not changed | Conclusions remain consistent with the previously published version of the review (Ng 2017) |
| 29 January 2019 | New search has been performed | Search has been updated ‐ no new trials are included |
Acknowledgements
We thank the corresponding authors of the included trials for providing further information on methods and outcomes.
We thank Kath Wright for developing and running the electronic searches and managing the database of study reports.
The methods section of this protocol is based on a standard template used by Cochrane Neonatal.
Appendices
Appendix 1. Electronic search strategy
Database: CENTRAL
Searched via Cochrane Library 28 January 2019
437 records identified
ID Search
#1 MeSH descriptor: [Infant, Newborn] explode all trees
#2 MeSH descriptor: [Premature Birth] explode all trees
#3 neonat*:ti,ab,kw (Word variations have been searched)
#4 neo‐nat*:ti,ab,kw (Word variations have been searched)
#5 newborn or new born* or newly born*:ti,ab,kw (Word variations have been searched)
#6 preterm or preterms or (pre term) or (pre terms):ti,ab,kw (Word variations have been searched)
#7 preemie* or premie or preemies:ti,ab,kw (Word variations have been searched)
#8 prematur* near/3 (birth* or born or deliver*):ti,ab,kw (Word variations have been searched)
#9 low near/3 (birthweight* or birth weight*):ti,ab,kw (Word variations have been searched)
#10 lbw or vlbw or elbw:ti,ab,kw (Word variations have been searched)
#11 infan* or baby or babies:ti,ab,kw (Word variations have been searched)
#12 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11
#13 (hydroly* near/3 (formula* or milk or protein* or whey)):ti,ab,kw (Word variations have been searched)
#14 (hypoallergen* near/3 (formula* or milk or protein* or whey)):ti,ab,kw (Word variations have been searched)
#15 (Nutramigen or Nutriprem or Pregestamil or Profylac or Nan or Aptamil Pepti or Pepti‐Junior or Pepdite or Infatrini or Similac or Gold Prem Pro or Alimentum):ti,ab,kw (Word variations have been searched)
#16 #13 or #14 or #15
#17 #12 and #16
CINAHL Complete
Searched via EBSCO, 28 January 2019, 210 records identified
| Search ID# | Search Terms | Search Options |
| S1 | (MH "Infant, Newborn+") | Search modes ‐ Boolean/Phrase |
| S2 | TX ( (neonat* or neo nat*) ) OR TX ( (newborn* or new born* or newly born*) ) OR TX ( (preterm or preterms or pre term or pre terms) ) OR TX ( (preemie$ or premie or premies) ) OR TX ( (prematur* N3 (birth* or born or deliver*)) ) OR TX ( (low N3 (birthweight* or birth weight*)) ) OR TX ( (lbw or vlbw or elbw) ) OR TX infan* OR TX ( (baby or babies) ) | Search modes ‐ Boolean/Phrase |
| S3 | S1 OR S2 | Search modes ‐ Boolean/Phrase |
| S4 | TX ( (hydroly* N3 (formula* or milk or protein* or whey)) ) OR TX ( (hypoallergen* N3 (formula* or milk or protein* or whey)) ) OR TX ( (Nutramigen or Nutriprem or Pregestamil or Profylac or Nan or Aptamil Pepti or Pepti‐Junior or Pepdite or Infatrini or Similac or Gold Prem Pro or Alimentum) ) | Search modes ‐ Boolean/Phrase |
| S5 | S3 AND S4 | Search modes ‐ Boolean/Phrase |
| S6 | S3 AND S4 |
Limiters ‐ Clinical Queries: Therapy ‐ High Sensitivity Search modes ‐ Boolean/Phrase (210 records) |
Embase
Searched via OVID 28 January 2019 443 records
Database: Embase <1974 to 2019 January 25>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp Infant, Newborn/ (494224)
2 Premature Birth/ (48011)
3 (neonat$ or neo nat$).ti,ab. (314056)
4 (newborn$ or new born$ or newly born$).ti,ab. (179767)
5 (preterm or preterms or pre term or pre terms).ti,ab. (93664)
6 (preemie$ or premie or premies).ti,ab. (234)
7 (prematur$ adj3 (birth$ or born or deliver$)).ti,ab. (19781)
8 (low adj3 (birthweight$ or birth weight$)).ti,ab. (40166)
9 (lbw or vlbw or elbw).ti,ab. (10334)
10 infan$.ti,ab. (459564)
11 (baby or babies).ti,ab. (89325)
12 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 (1048188)
13 (hydroly$ adj3 (formula$ or milk or protein$ or whey)).ti,ab. (9398)
14 (hypoallergen$ adj3 (formula$ or milk or protein$ or whey)).ti,ab. (463)
15 (Nutramigen or Nutriprem or Pregestamil or Profylac or Nan or Aptamil Pepti or Pepti‐Junior or Pepdite or Infatrini or Similac or Gold Prem Pro or Alimentum).ti,ab. (1811)
16 13 or 14 or 15 (11365)
17 clinical trial/ (953847)
18 randomized controlled trial/ (533609)
19 randomization/ (81009)
20 single blind procedure/ (33779)
21 double blind procedure/ (157592)
22 crossover procedure/ (58047)
23 placebo/ (329701)
24 randomi?ed controlled trial$.tw. (195584)
25 rct.tw. (31133)
26 random allocation.tw. (1905)
27 randomly allocated.tw. (31798)
28 allocated randomly.tw. (2419)
29 (allocated adj2 random).tw. (879)
30 single blind$.tw. (22298)
31 double blind$.tw. (194704)
32 ((treble or triple) adj blind$).tw. (912)
33 placebo$.tw. (284340)
34 prospective study/ (498156)
35 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 (1999977)
36 case study/ (58844)
37 case report.tw. (372753)
38 abstract report/ or letter/ (1093127)
39 36 or 37 or 38 (1515434)
40 35 not 39 (1949209)
41 12 and 16 and 40 (443)
Maternity and Infant Care
Searched via Ovid 28 January 2019 22 records identified
Database: Maternity & Infant Care Database (MIDIRS) <1971 to December 2018FF
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 (neonat$ or neo nat$).ti,ab. (43123)
2 (newborn$ or new born$ or newly born$).ti,ab. (19566)
3 (preterm or preterms or pre term or pre terms).ti,ab. (25419)
4 (preemie$ or premie or premies).ti,ab. (53)
5 (prematur$ adj3 (birth$ or born or deliver$)).ti,ab. (3927)
6 (low adj3 (birthweight$ or birth weight$)).ti,ab. (10548)
7 (lbw or vlbw or elbw).ti,ab. (2974)
8 infan$.ti,ab. (62846)
9 (baby or babies).ti,ab. (28609)
10 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 (116422)
11 (hydroly$ adj3 (formula$ or milk or protein$ or whey)).ti,ab. (157)
12 (hypoallergen$ adj3 (formula$ or milk or protein$ or whey)).ti,ab. (32)
13 (Nutramigen or Nutriprem or Pregestamil or Profylac or Nan or Aptamil Pepti or Pepti‐Junior or Pepdite or Infatrini or Similac or Gold Prem Pro or Alimentum).ti,ab. (36)
14 11 or 12 or 13 (205)
15 10 and 14 (195)
16 limit 15 to randomised controlled trial (22)
MEDLINE searched 28 January 2019 465 records identified
Database: Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present>
Database: Ovid MEDLINE(R) ALL <1946 to January 25, 2019>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp Infant, Newborn/ (577694)
2 Premature Birth/ (11711)
3 (neonat$ or neo nat$).ti,ab. (245408)
4 (newborn$ or new born$ or newly born$).ti,ab. (156950)
5 (preterm or preterms or pre term or pre terms).ti,ab. (67233)
6 (preemie$ or premie or premies).ti,ab. (155)
7 (prematur$ adj3 (birth$ or born or deliver$)).ti,ab. (14660)
8 (low adj3 (birthweight$ or birth weight$)).ti,ab. (32141)
9 (lbw or vlbw or elbw).ti,ab. (7634)
10 infan$.ti,ab. (409379)
11 (baby or babies).ti,ab. (65510)
12 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 (996498)
13 (hydroly$ adj3 (formula$ or milk or protein$ or whey)).ti,ab. (8524)
14 (hypoallergen$ adj3 (formula$ or milk or protein$ or whey)).ti,ab. (287)
15 (Nutramigen or Nutriprem or Pregestamil or Profylac or Nan or Aptamil Pepti or Pepti‐Junior or Pepdite or Infatrini or Similac or Gold Prem Pro or Alimentum).ti,ab. (1851)
16 13 or 14 or 15 (10495)
17 randomized controlled trial.pt. (475348)
18 controlled clinical trial.pt. (92893)
19 randomized.ab. (433287)
20 placebo.ab. (195102)
21 drug therapy.fs. (2078948)
22 randomly.ab. (304542)
23 trial.ab. (452332)
24 groups.ab. (1875742)
25 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 (4366018)
26 exp animals/ not humans.sh. (4541052)
27 25 not 26 (3775428)
28 12 and 16 and 27 (465)
Appendix 2. Risk of bias
‘Risk of bias’ tool
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
· low risk (any truly random process e.g. random number table; computer random number generator);
· high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number); or
· unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
· low risk (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
· high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
· unclear risk.
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or classes of outcomes. We categorised the methods as:
· low risk, high risk, or unclear risk for participants; and
· low risk, high risk, or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or classes of outcomes. We categorised the methods as:
· low risk for outcome assessors;
· high risk for outcome assessors; or
· unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised the methods as:
· low risk (< 20% missing data);
· high risk (≥ 20% missing data); or
· unclear risk.
6. Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies for which study protocols were published in advance, we compared prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
· low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
· high risk (where not all of the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
· unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design, whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
· low risk;
· high risk; or
· unclear risk.
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. Hydrolysed versus non‐hydrolysed formula.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Feed intolerance | 3 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.29, 25.00] |
| 1.2 Necrotising enterocolitis | 5 | 385 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.03, 0.04] |
| 1.2.1 Partially hydrolysed | 3 | 238 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.03, 0.06] |
| 1.2.2 Extensively hydrolysed | 2 | 147 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.07, 0.04] |
| 1.3 Time to full enteral feeding | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐1.00 [‐8.36, 6.36] |
| 1.4 Weight gain (g/kg/day) | 3 | 113 | Mean Difference (IV, Fixed, 95% CI) | ‐3.02 [‐4.66, ‐1.38] |
| 1.5 Length gain (mm/week) | 2 | 97 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐1.24, 1.15] |
| 1.6 Head circumference growth (mm/week) | 2 | 97 | Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐0.39, 0.94] |
| 1.7 Serum alkaline phosphatase (IU/L) | 2 | 88 | Mean Difference (IV, Fixed, 95% CI) | 16.61 [‐34.15, 67.37] |
| 1.8 Late‐onset invasive infection | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.27, 8.34] |
| 1.9 Any allergic disease | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.27, 1.42] |
1.3. Analysis.

Comparison 1: Hydrolysed versus non‐hydrolysed formula, Outcome 3: Time to full enteral feeding
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Baldassarre 2017.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Preterm infants (28 to 33 weeks' gestational age; birth weight 700 to 1750 g and appropriate to gestational age) within 24 hours of first enteral feeding (and whose mother did not plan to exclusively breast feed) | |
| Interventions | Extensively hydrolysed casein infant formula (n = 33) Standard cow's milk‐based preterm infant formula (n = 35) |
|
| Outcomes | Enteral intake (mL/kg/d) during first 14 days after birth Feed intolerance measures (abdominal distension, regurgitation/emesis, feedings withheld ≥ 4 hours, or bloody stools) Necrotising enterocolitis Invasive infection |
|
| Notes | University of Bari‐Policlinico Hospital, Neonatology and Neonatal Intensive Care Unit, Department of Biomedical Science and Human Oncology, Bari, Italy Trial dates: 2014 to 2016 Trial registration: clinicaltrials.gov/ct2/show/NCT01987154 Further information provided by investigators (August 2017) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 60/68 enrolled infants completed trial and contributed to outcome analysis |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Funded by Mead Johnson Nutrition |
Florendo 2009.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Preterm infants (≤ 32 weeks' gestational age, ≤ 1750 g at birth) receiving ≤ 25% breast milk as total enteral intake | |
| Interventions | Empirical use of partially hydrolysed whey‐casein preterm formula (n = 42) Intact preterm formula (n = 38) |
|
| Outcomes | Feed intolerance (interruption of enteral feeds) Necrotising enterocolitis |
|
| Notes | Division of Neonatology, University of Tennessee Center for Health Sciences, Memphis, TN, USA Trial dates: 2004 to 2005 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Sequentially labelled, sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐blind"; ready‐to‐feed, colour‐coded cartons |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete outcome data for 74/80 participants 1 infant in the control group developed sepsis; 1 infant from the hydrolysed formula group developed necrotising enterocolitis and was withdrawn |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Funded by Nestle (manufacturer of the trial formula) |
Huston 1992.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Preterm very low birth weight infants | |
| Interventions | Empirical use of partially hydrolysed casein hydrolysate formula (with 40% or 60% medium‐chain triglyceride) Non‐hydrolysed preterm formula Total N = 60 |
|
| Outcomes | Food tolerance Growth rates |
|
| Notes | Department of Pediatrics, Emanuel Children's Health Care Centre, Portland, OR, USA Trial date: early 1990s Reported as abstract only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information ‐ only abstract available |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information ‐ only abstract available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely to be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unlikely to be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all participants |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Funder: Mead Johnson Nutritional Group |
Maggio 2005.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Preterm infants (≤ 34 weeks' gestational age, ≤ 1750 g at birth) | |
| Interventions | Empirical use of partially hydrolysed whey‐based formula* (n = 10) Conventional preterm formula* (n = 11) |
|
| Outcomes | Growth rates from inclusion until hospital discharge Feed intolerance (no infants had enteral feeds interrupted) |
|
| Notes | Division of Neonatology, Department of Paediatrics, Catholic University of the Sacred Heart, Rome, Italy Trial dates: 1998 to 2000 * Energy content of both formulas: 75 kcal/100 mL |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised schedule generated ‐ not specified how |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study and control formulas identical in colour and smell |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study and control formulas identical in colour and smell |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all participants |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Funded by Humana (manufacturer of the trial formula) |
Mihatsch 2002.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Very low birth weight (< 1500 g) infants | |
| Interventions | Empirical use of extensively hydrolysed (whey‐casein) preterm formula* (n = 41) Standard preterm formula* (n = 46) |
|
| Outcomes | Necrotising enterocolitis Proportion of enteral feeds with gastric residual volumes > 5 mL/kg birth weight |
|
| Notes | Division of Neonatology and Pediatric Critical Care, Department of Pediatrics, Ulm University, 89070 Ulm, Germany Trial dates: 1999 to 2001 * Energy content of both formulas: 80 kcal/100 mL |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes ‐ unclear whether opaque |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Double‐blind"; same appearance, but investigators acknowledged different tastes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 129 infants recruited initially, then 42 excluded post hoc because they received > 10% of enteral intake as human milk |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Funder: Milupa GmbH, Germany (manufacturer of the trial formula) |
Pauls 1996.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Very low birth weight (< 1500 g) infants | |
| Interventions | Empirical use of partially hydrolysed whey‐casein formula* (n = 25) Non‐hydrolysed protein formula* (n = 25) |
|
| Outcomes | Mean gastric residual volume (% of intake) Time to full enteral feeds Necrotising enterocolitis |
|
| Notes | Kinderklinik, Freie Universitat Berlin, Germany Trial date: early 1990s Reported as an abstract only * Energy content of both formulas: 80 kcal/100 mL; protein content: hydrolysed formula 2.9 g/100 mL vs non‐hydrolysed formula 2.7 g/100 mL |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information ‐ only abstract available |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information ‐ only abstract available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely to be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unlikely to be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all participants |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Funder: not stated |
Picaud 2001.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Preterm newborns with birth weight < 1500 g and aged < 15 days when enteral feeds commenced | |
| Interventions | Empirical use of partially hydrolysed formula* (n = 9) Standard preterm formula* (n = 7) Until 40 weeks' postmenstrual age |
|
| Outcomes | Rate of weight gain during initial hospital admission Nitrogen balance studies |
|
| Notes | Edouard Herriot Hospital, Claude Bernard University, Lyon, France Trial date: late 1990s * Energy content of both formulas: 80 kcal/100 mL, but nitrogen content 10% higher in standard preterm formula |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes ‐ unclear whether opaque |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Investigators unaware of formula; unclear if carers or parents aware |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Investigators unaware of formula; unclear if carers or parents aware |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All infants assessed for primary outcomes |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Funder: Nestle (manufacturer of the trial formula) |
Raupp 1995.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Neonates; body weight 1000 to 1799 g | |
| Interventions | Empirical use of partially hydrolysed whey‐casein formula* (n = 56) Non‐hydrolysed preterm formula* (n = 52) |
|
| Outcomes | Biochemistry Bone mineralisation Blood/serum Necrotising enterocolitis |
|
| Notes | University Children's Hospital of Düsseldorf * Energy content of both formulas: 80 kcal/100 mL |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All infants assessed for primary outcomes |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Funder: Nestle (manufacturer of the trial formula) |
Riezzo 2001.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Preterm infants (n = 36) | |
| Interventions | Partially hydrolysed casein preterm formula* (n = 18) Standard (whey‐casein) formula* (n = 18) |
|
| Outcomes | Proportion of infants who had > 1 episode of regurgitation or vomiting per day | |
| Notes | Department of Pediatrics, Neonatology Section, University of Bari, Bari, Italy Trial date: 2000 Energy content of hydrolysed formula (80 kcal/100 mL) higher than control standard term formula (68 kcal/100 mL). Because trial did not report growth rates (the reason for specifying similar energy levels in comparison formulas), we made a consensus decision to include the trial |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear evidence provided ‐ stated only that infants were randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | Unclear evidence provided ‐ stated only that infants were randomly assigned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All infants assessed for primary outcomes |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Funder: not stated |
Schweizer 1993.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Preterm infants (formula fed) | |
| Interventions | Extensively hydrolysed whey‐casein term formula (Alfare)* (n = 26) Non‐hydrolysed preterm formula (Prematil)* (n = 26) |
|
| Outcomes | Time to regain birth weight Time to full enteral feeding Mean number of high gastric residual volumes per day |
|
| Notes | Kinderklinik der Stadt, Klinlken, Dortmund Trial dates: 1991 to 1993 * Energy content of hydrolysed formula (70 kcal/100 mL) lower than control standard preterm formula (80 kcal/100 mL). Because trial did not report growth rates (the reason for specifying similar energy levels in comparison formulas), we made a consensus decision to include the trial |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information ‐ only abstract available |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information ‐ only abstract available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Double‐blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all participants |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Funder: not stated |
Szajewska 2004.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Preterm infants, body weight < 2500 g with ≥ 1 first‐degree relative with atopy | |
| Interventions | Extensively (n = 26) or partially hydrolysed whey‐casein preterm formula* (n = 32) Standard preterm formula* (n = 32) |
|
| Outcomes | Allergic disease in infancy Feed intolerance |
|
| Notes | Primary aim: to assess effects on allergy and atopic disease In‐hospital feed tolerance, growth, and adverse outcomes not reported. We contacted corresponding author in December 2016 to seek these data * Energy content of both formulas: 80 kcal/100 mL 33% "dropout" before assessment at 4 to 5 months post term |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised schedule generated ‐ unspecified how |
| Allocation concealment (selection bias) | Low risk | Sealed numbered envelopes ‐ not stated whether opaque, but codes concealed from investigators until trial completed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Double‐blind" but study and control formulas not identical in texture and smell |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Double‐blind" but study and control formulas not identical in texture and smell |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all participants |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Funded by Ovita Nutricia Research Foundation |
n: number of infants. RCT: randomised controlled trial.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agosti 2003 | Not an RCT |
| Corvaglia 2013 | Cross‐over RCT with cross‐over at each enteral feed |
| Logarajaha 2015 | Cross‐over RCT with cross‐over at 24 hours |
| Mihatsch 1999 | Cross‐over RCT with initial formula allocation for 5 days only |
| Mihatsch 2001a | Cross‐over RCT with initial formula allocation for 5 days only |
| Rigo 1994 | 5‐Arm RCT with term infants receiving different types of hydrolysed formula (3 different whey hydrolysate formulas, a soy‐collagen hydrolysate formula, or a whey‐casein hydrolysate formula) |
| Rigo 1995 | Not an RCT |
| Yu 2014 | Cross‐over study with initial formula allocation for 10 days, and cross‐over occurring only if feeds not well tolerated Unclear whether randomised |
RCT: randomised controlled trial.
Characteristics of studies awaiting classification [ordered by study ID]
del Moral 2017.
| Methods | RCT (double blind) Randomisation sequence generated by computer, allocation by sealed envelopes |
| Participants | Very low birth weight or very preterm infants (stratified by 2 birth weight categories (500 to 1000 g and 1001 to 1500 g)) who survived > 3 days after birth and for whom breast milk was not available or was insufficient for requirements |
| Interventions | Empirical use of 100% whey protein partially hydrolysed preterm formula (n = 62) vs intact preterm formula (n = 73) Breast milk allowed if available; different formulas given to supplement when no breast milk available (postrandomisation exclusion if breast milk > 25% of total enteral intake) |
| Outcomes | Time to achieve full feeds Number of days from initiating oral feeds to achieving full feeds Mortality Necrotising enterocolitis |
| Notes | Principal investigator: Teresa del Moral, Department of Pediatrics, Miller School of Medicine, University of Miami, FL, USA Contacted tdelmoral@miami.edu in July 2017 seeking data Trial dates: 2004 to 2005 Funded by Nestle Study discontinued because the increasingly common use of breast milk meant recruitment was much slower than planned (remains unreported June 2019) |
Dobryanskyy 2015.
| Methods | RCT |
| Participants | Very low birth weight (< 1500 g) infants |
| Interventions | Hydrolysed formula (n = 35) vs standard preterm formula (n = 25) |
| Outcomes | Feed intolerance Time to full enteral feeding Necrotising enterocolitis 9 additional infants (originally randomised) who died were excluded |
| Notes | Article in Ukrainian; awaiting translation |
Gu 2017.
| Methods | Unclear whether RCT or observational study |
| Participants | Very low birth weight infants |
| Interventions | Extensively hydrolysed formula (n = 187) vs standard preterm formula (n = 188) |
| Outcomes | Feed intolerance Time to full enteral feeding Time to complete meconium excretion Number of spontaneous bowel movements Growth and development Motilin level at 4 days and 10 days after feeding Incidence rate of infection |
| Notes | Article in Chinese language; awaiting full text and translation |
Kwinta 2002.
| Methods | RCT |
| Participants | Very low birth weight infants |
| Interventions | Hydrolysed protein formula vs preterm formula |
| Outcomes | Feed intolerance Gram‐negative sepsis Weight gain Duration of parenteral nutrition |
| Notes | Article in Polish; awaiting full text and translation Author contacted January 2019 (kwintap@mp.pl) |
Luo 2016.
| Methods | RCT |
| Participants | "Very/extremely" low birth weight infants |
| Interventions | Hydrolysed protein formula vs preterm formula |
| Outcomes | Feed intolerance Growth rates |
| Notes | Article in Chinese language; awaiting full text and translation |
RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
ACTRN12613000481774.
| Study name | Effects of a New Hydrolyzed Powdered Formula on Feeding Tolerance in Preterm Neonates: A Randomised Placebo‐Controlled Study |
| Methods | RCT |
| Participants | 60 newborns with birth weight < 1500 g |
| Interventions | Powdered hydrolysed formula vs standard preterm formula |
| Outcomes | Time to reach full enteral feeding (120 kcal/kg/d) |
| Starting date | 2013 |
| Contact information | Professor Gianluca Terrin, University of Rome "La Sapienza", Italy |
| Notes | Trial has not yet started due to lack of funding (personal communication from Professor Terrin) |
RCT: randomised controlled trial.
Differences between protocol and review
Two trials compared formulas with different energy densities (Riezzo 2001; Schweizer 1993). These trials did not report growth rates (the reason for prespecified similar energy levels), so we made a consensus decision to include them in the review.
Contributions of authors
All authors developed the protocol.
DN and WM: screened search outputs, assessed study eligibility, and extracted and synthesised data.
DN and JK assessed risk of bias across key domains and undertook the GRADE assessment with WM.
All review authors revised the final review.
Sources of support
Internal sources
University of York, UK
External sources
-
National Institute for Health Research, UK
This report is independent research funded by a UK National Institute for Health Research Grant (NIHR) Cochrane Programme Grant (16/114/03). The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the NIHR, or the UK Department of Health.
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
-
The Gerber Foundation, USA
Editorial support for this review, as part of a suite of preterm nutrition reviews, has been provided by a grant from The Gerber Foundation. The Gerber Foundation is a separately endowed, private, 501(c)(3) foundation not related to Gerber Products Company in any way.
Declarations of interest
NDE declares the following: receiving a research grant award for an RCT of breast milk products by Prolacta Bioscience, 2017; receiving a grant from Danone Early Life Nutrition to support a study on feeding in late and moderately preterm infants, 2018; receiving a grant from Nestle Nutrition for transcriptomic analyses of gut tissue, 2016; lectures with Wyeth Nutrition in 2017, Nestle Nutrition Institute in 2017 and 2018, Philipps in 2017, and Fresenius, 2017.
DN has no interest to declare.
JK has no interest to declare.
WM has no interest to declare.
Core editorial and administrative support for this review has been provided by a grant from The Gerber Foundation. The Gerber Foundation is a separately endowed, private foundation, independent from the Gerber Products Company. The grantor has no input on the content of the review or the editorial process (see Sources of support).
To maintain the utmost editorial independence for this Cochrane Review, an editor outside of the Cochrane Neonatal core editorial team who is not receiving any financial remuneration from the grant, Jann Foster, was the Sign‐off Editor for this review. Additionally, a Senior Editor from the Cochrane Children and Families Network, Robert Boyle, assessed and signed off on this Cochrane Review.
Edited (no change to conclusions)
References
References to studies included in this review
Baldassarre 2017 {published and unpublished data}
- Baldassarre ME, Di Mauro A, Fanelli M, Montagna O, Wampler J, Cooper T, et al. Shorter time to full enteral feedings among infants fed an intact protein (IP) vs an extensively hydrolyzed (EH) formula does not appear to be related to differences in gastric emptying. In: ESPGHAN. Vol. 64. 2017:920-1.
Florendo 2009 {published data only}
- Florendo KN, Bellflower B, Zwol A, Cooke RJ. Growth in preterm infants fed either a partially hydrolyzed whey or an intact casein/whey preterm infant formula. Journal of Perinatology 2009;29(2):106-11. [DOI: 10.1038/jp.2008.124] [PMID: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Huston 1992 {published data only}
- Huston RK, Murphy DL, Jelen BJ. Preliminary evaluation of casein hydrolysate containing formulas in low birthweight preterm infants. Pediatric Research 1992;31:289A. [Google Scholar]
Maggio 2005 {published data only}
- Maggio L, Zuppa AA, Sawatzki G, Valsasina R, Schubert W, Tortorolo G. Higher urinary excretion of essential amino acids in preterm infants fed protein hydrolysates. Acta Paediatrica 2005;94(1):75-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mihatsch 2002 {published data only}
- Mihatsch WA, Franz AR, Hogel J, Pohlandt F. Hydrolyzed protein accelerates feeding advancement in very low birth weight infants. Pediatrics 2002;110(6):1199-203. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pauls 1996 {published data only}
- Pauls J, Bauer K, Versmold H. Randomized controlled trial of formulas with hydrolyzed versus non-hydrolyzed protein for starting enteral feedings in preterm infants < 1500g body weight. Journal of Pediatric Gastroenterology and Nutrition 1996;22(4):450. [Google Scholar]
Picaud 2001 {published data only}
- Picaud JC, Rigo J, Normand S, Lapillonne A, Reygrobellet B, Claris O, et al. Nutritional efficacy of preterm formula with a partially hydrolyzed protein source: a randomized pilot study. Journal of Pediatric Gastroenterology and Nutrition 2001;32(5):555-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Raupp 1995 {published data only}
- Raupp P, Von Kries R, Gobel C, Fox A, Lemburg P, Schmidt E, et al. Bone density and biochemical parameters of bone mineral metabolism and acid load in preterm infants. Cow's milk formula versus hydrolysed protein preparation. Monatsschrift fur Kinderheilkunde 1995;143(7 Suppl 2):S140-6. [Google Scholar]
Riezzo 2001 {published data only}
- Riezzo G, Indrio F, Montagna O, Tripaldi C, Laforgia N, Chiloiro M, et al. Gastric electrical activity and gastric emptying in preterm newborns fed standard and hydrolysate formulas. Journal of Pediatric Gastroenterology and Nutrition 2001;33(3):290-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schweizer 1993 {published data only}
- Schweizer P, Stock GJ, Diekmann L, Heitmann F, Kalhoff H, Manz F. Build up nutrition in premature babies: premature baby milk nutrition vs protein hydrolysate [Nährungsaufbau bei Frühgeborenen (FG; GG 750 - 1250 g): FG milchahrung vs proteinhydrolysat]. Monatsschrift Kinderheilkunde 1993;141:S8. [Google Scholar]
Szajewska 2004 {published data only}
- Szajewska H, Mrukowicz JZ, Stoinska B, Prochowska A. Extensively and partially hydrolysed preterm formulas in the prevention of allergic diseases in preterm infants: a randomized, double-blind trial. Acta Paediatrica 2004;93(9):1159-65. [PMID: ] [PubMed] [Google Scholar]
References to studies excluded from this review
Agosti 2003 {published data only}
- Agosti M, Pugni L, Ramenghi LA, Mosca F, Marini A. Hydrolysed proteins in preterm formula: influence on plasma aminoacids, blood fatty acids and insulinaemia. Acta Paediatrica Supplement 2003;91(441):34-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Corvaglia 2013 {published data only}
- Corvaglia L, Mariani E, Aceti A, Galletti S, Faldella G. Extensively hydrolyzed protein formula reduces acid gastro-esophageal reflux in symptomatic preterm infants. Early Human Development 2013;89(7):453-5. [DOI: 10.1016/j.earlhumdev.2013.04.003] [PMID: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Logarajaha 2015 {published data only}
- Logarajaha V, Onga C, Jayagobib PA, Khoob PC, Heina M, Fanga H, et al. PP-15: the effect of extensively hydrolyzed protein formula in preterm infants with symptomatic gastro-oesophageal reflux. Journal of Pediatric Gastroenterology and Nutrition 2015;61(4):526. [DOI: 10.1097/01.mpg.0000472243.59979.4a] [PMID: 26439580] [DOI] [Google Scholar]
Mihatsch 1999 {published data only}
- Mihatsch WA, Pohlandt F. Protein hydrolysate formula maintains homeostasis of plasma amino acids in preterm infants. Journal of Pediatric Gastroenterology and Nutrition 1999;29(4):406-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mihatsch 2001a {published data only}
- Mihatsch WA, Hogel J, Pohlandt F. Hydrolysed protein accelerates the gastrointestinal transport of formula in preterm infants. Acta Paediatrica 2001;90(2):196-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rigo 1994 {published data only}
- Rigo J, Salle BL, Putet G, Senterre J. Nutritional evaluation of various protein hydrolysate formulae in term infants during the first month of life. Acta Paediatrica Supplement 1994;402:100-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Rigo J, Senterre J. Metabolic balance studies and plasma amino acid concentrations in preterm infants fed experimental protein hydrolysate preterm formulas. Acta Paediatrica 1994;405:98-104. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rigo 1995 {published data only}
- Rigo J, Salle BL, Picaud JC, Putet G, Senterre J. Nutritional evaluation of protein hydrolysate formulas. European Journal of Clinical Nutrition 1995;49(Suppl 1):S26-38. [PMID: ] [PubMed] [Google Scholar]
Yu 2014 {published data only}
- Yu MX, Zhuang SQ, Wang DH, Zhou XY, Liu XH, Shi LP, et al. Effects of extensively hydrolyzed protein formula on feeding and growth in preterm infants: a multicenter controlled clinical study. Zhongguo Dang Dai er Ke Za Zhi = Chinese Journal of Contemporary Pediatrics 2014;16(7):684-90. [PMID: ] [PubMed] [Google Scholar]
References to studies awaiting assessment
del Moral 2017 {published data only}
- https://clinicaltrialsgov/show/NCT01156493.
Dobryanskyy 2015 {published data only}
- Dobryanskyy D, Borysiuk O, Novikova O, Dubrovna Y, Salabay Z, Detsyk O. Clinical effectiveness of hydrolysed protein preterm formula in prevention of feeding intolerance in very low birth weight infants. Journal of Pediatric and Neonatal Individualized Medicine 2015;4(2):e040210. [DOI: 10.7363/040210] [DOI] [Google Scholar]
Gu 2017 {published data only}
- Gu CY, Jiang HF, Wang JX. Effect of extensively hydrolyzed formula on growth and development of infants with very/extremely low birth weight. Zhongguo Dang Dai Er Ke Za Zhi [Chinese Journal of Contemporary Pediatrics] 2017;19(8):852-5. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kwinta 2002 {published data only}
- Kwinta P, Mitkowska Z, Kruczek P, Tomasik T, Pietrzyk JJ. Influence of the lactose free and lactose containing diet on prevalence of gram-negative sepsis and feeding intolerance in very low birth weight infants: double-blind randomized trial [Wplyw rodzaju stosowanej diety na tolerancje karmienia i czestosc posocznicy Gram ujemnej u noworodkow z bardzo niska urodzeniowa masa ciala]. Przeglad Lekarski 2002;59 Suppl 1:63-6. [PMID: ] [PubMed] [Google Scholar]
- Kwinta P, Sawiec P, Klimek M, Lis G, Cichocka-Jarosz E, Pietrzyk JJ. Correlation between early neonatal diet and atopic symptoms up to 5-7 years of age in very low birth weight infants: follow-up of randomized, double-blind study. Pediatric Allergy and Immunology: official publication of the European Society of Pediatric Allergy and Immunology 2009;20(5):458-66. [DOI] [PubMed] [Google Scholar]
Luo 2016 {published data only}
- Luo Z, Wang Y, Wang l. A study on the effects of extensively hydrolyzed formula for very/extremely low birth weight infants. Chinese Journal of Neonatology 2016;31(2):110-4. [Google Scholar]
References to ongoing studies
ACTRN12613000481774 {unpublished data only}
- ACTRN12613000481774. Improvement of feeding tolerance by using a new formula in preterm neonates [Effects of a new hydrolyzed powdered formula on feeding tolerance in preterm neonates: a randomized placebo-controlled study]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=363880 (first received 30 April 2013).
Additional references
AAP 2012
- American Academy of Pediatrics (AAP) Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics 2012;129(3):e827-41. [DOI: 10.1542/peds.2011-3552] [PMID: ] [DOI] [PubMed] [Google Scholar]
Agostoni 2010
- Agostoni C, Buonocore G, Carnielli VP, De Curtis M, Darmaun D, Decsi T, et al. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. Journal of Pediatric Gastroenterology and Nutrition 2010;50(1):85-91. [DOI: 10.1097/MPG.0b013e3181adaee0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Arslanoglu 2013
- Arslanoglu S, Corpeleijn W, Moro G, Braegger C, Campoy C, Colomb V, et al. Donor human milk for preterm infants: current evidence and research directions. Journal of Pediatric Gastroenterology and Nutrition 2013;57(4):535-42. [DOI: 10.1097/MPG.0b013e3182a3af0a] [PMID: ] [DOI] [PubMed] [Google Scholar]
BNFC 2016
- Paediatric Formulary Committee. www.medicinescomplete. London (UK): British Medical Association, Royal Pharmaceutical Society, the Royal College of Paediatrics and Child Health, and the Neonatal and Paediatric Pharmacists Group, 2016. [Google Scholar]
Boyle 2016
- Boyle RJ, Ierodiakonou D, Khan T, Chivinge J, Robinson Z, Geoghegan N, et al. Hydrolysed formula and risk of allergic or autoimmune disease: systematic review and meta-analysis. BMJ 2016;352:i974. [DOI: 10.1136/bmj.i974] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brandt 2003
- Brandt I, Sticker EJ, Lentze MJ. Catch-up growth of head circumference of very low birth weight, small for gestational age preterm infants and mental development to adulthood. Journal of Pediatrics 2003;142(5):463-8. [DOI: 10.1067/mpd.2003.149] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cleminson 2015
- Cleminson J, Oddie S, Renfrew MJ, McGuire W. Being baby friendly: evidence-based breastfeeding support. Archives of Disease in Childhood. Fetal and Neonatal Edition 2015;100(2):F173-8. [DOI: 10.1136/archdischild-2013-304873] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cooke 2016
- Cooke RJ. Improving growth in preterm infants during initial hospital stay: principles into practice. Archives of Disease in Childhood. Fetal and Neonatal Edition 2016;101(4):F366-70. [DOI: 10.1136/archdischild-2015-310097] [PMID: ] [DOI] [PubMed] [Google Scholar]
Embleton 2001
- Embleton NE, Pang N, Cooke RJ. Postnatal malnutrition and growth retardation: an inevitable consequence of current recommendations in preterm infants? Pediatrics 2001;107(2):270-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Embleton 2013a
- Embleton ND. Early nutrition and later outcomes in preterm infants. World Review of Nutrition and Dietetics 2013;106:26-32. [DOI: 10.1159/000342553] [PMID: ] [DOI] [PubMed] [Google Scholar]
Embleton 2013b
- Embleton ND, Berrington JE, McGuire W, Stewart CJ, Cummings SP. Lactoferrin: antimicrobial activity and therapeutic potential. Seminars in Fetal & Neonatal Medicine 2013;18(3):143-9. [DOI: 10.1016/j.siny.2013.02.001] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ewer 1994
- Ewer AK, Durbin GM, Morgan ME, Booth IW. Gastric emptying in preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 1994;71(1):F24-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fewtrell 1999
- Fewtrell M, Lucas A. Nutritional physiology: dietary requirements of term and preterm infants. In: Rennie JM, Roberton NRC, editors(s). Textbook of Neonatology. 3rd edition. Edinburgh: Churchill Livingstone, Inc., 1999:305-25. [Google Scholar]
Foucard 2005
- Foucard T. Is there any role for protein hydrolysates to premature newborns? Acta Paediatrica 2005;94(1):20-2. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 26 April 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2014.Available at gradepro.org.
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Imdad 2013
- Imdad A, Bhutta ZA. Nutritional management of the low birth weight/preterm infant in community settings: a perspective from the developing world. Journal of Pediatrics 2013;162(3 Suppl):S107-14. [DOI: 10.1016/j.jpeds.2012.11.060] [PMID: ] [DOI] [PubMed] [Google Scholar]
Klingenberg 2012
- Klingenberg C, Embleton ND, Jacobs SE, O'Connell LA, Kuschel CA. Enteral feeding practices in very preterm infants: an international survey. Archives of Disease in Childhood. Fetal and Neonatal Edition 2012;97(1):F56-61. [DOI: 10.1136/adc.2010.204123] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lapillonne 2016
- Lapillonne A, Matar M, Adleff A, Chbihi M, Kermorvant-Duchemin E, Campeotto F. Use of extensively hydrolysed formula for refeeding neonates postnecrotising enterocolitis: a nationwide survey-based, cross-sectional study. BMJ Open 2016;6(7):e008613. [DOI: 10.1136/bmjopen-2015-008613] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leppanen 2014
- Leppanen M, Lapinleimu H, Lind A, Matomaki J, Lehtonen L, Haataja L, et al, PIPARI Study Group. Antenatal and postnatal growth and 5-year cognitive outcome in very preterm infants. Pediatrics 2014;133(1):63-70. [DOI: 10.1542/peds.2013-1187] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lindberg 1998
- Lindberg T, Engberg S, Sjoberg LB, Lonnerdal B. In vitro digestion of proteins in human milk fortifiers and in preterm formula. Journal of Pediatric Gastroenterology and Nutrition 1998;27(1):30-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mihatsch 2001b
- Mihatsch WA, Hogel J, Pohlandt F. Hydrolysed protein accelerates the gastrointestinal transport of formula in preterm infants. Acta Paediatrica 2001;90(2):196-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Morgan 2011
- Morgan JA, Young L, McGuire W. Pathogenesis and prevention of necrotizing enterocolitis. Current Opinion in Infectious Diseases 2011;24(3):183-9. [DOI: 10.1097/QCO.0b013e328345d5b5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Oldaeus 1997
- Oldaeus G, Anjou K, Bjorksten B, Moran JR, Kjellman NI. Extensively and partially hydrolysed infant formulas for allergy prophylaxis. Archives of Disease in Childhood 1997;77(1):4-10. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Osborn 2017
- Osborn DA, Sinn JK, Jones LJ. Infant formulas containing hydrolysed protein for prevention of allergic disease and food allergy. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD003664.pub4] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pike 2012
- Pike K, Brocklehurst P, Jones D, Kenyon S, Salt A, Taylor D, et al. Outcomes at 7 years for babies who developed neonatal necrotising enterocolitis: the ORACLE Children Study. Archives of Disease in Childhood. Fetal and Neonatal Edition 2012;97(5):F318-22. [DOI: 10.1136/fetalneonatal-2011-300244] [PMID: ] [DOI] [PubMed] [Google Scholar]
Quigley 2014
- Quigley M, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD002971.pub3] [PMID: ] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). GRADE handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.gradepro.org/app/handbook/handbook.html..
Senterre 2016
- Senterre T, Rigo J. Hydrolyzed proteins in preterm infants. Nestle Nutrition Institute Workshop Series 2016;86:39-49. [DOI: 10.1159/000444199] [PMID: ] [DOI] [PubMed] [Google Scholar]
Uribarri 2015
- Uribarri J, Castillo MD, la Maza MP, Filip R, Gugliucci A, Luevano-Contreras C, et al. Dietary advanced glycation end products and their role in health and disease. Advances in Nutrition 2015;6(4):461-73. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 1986
- Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatric Clinics of North America 1986;33(1):179-201. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yee 2012
- Yee WH, Soraisham AS, Shah VS, Aziz K, Yoon W, Lee SK, Canadian Neonatal Network. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics 2012;129(2):e298-304. [DOI: 10.1542/peds.2011-2022] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zuppa 2005
- Zuppa AA, Visintini F, Cota F, Maggio L, Romagnoli C, Tortorolo G. Hydrolysed milk in preterm infants: an open problem. Acta Paediatrica 2005;94(449):84-6. [DOI: 10.1080/08035320510043600] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ng 2017
- Ng DH, Klassen J, Embleton ND, McGuire W. Protein hydrolysate versus standard formula for preterm infants. Cochrane Database of Systematic Reviews 2017, Issue 10. [DOI: 10.1002/14651858.CD012412] [DOI] [PMC free article] [PubMed] [Google Scholar]


