Abstract
Background
Coronary heart disease risk increases with advancing age and is further increased in patients with mixed dyslipidemia, characterized by elevated low‐density lipoprotein cholesterol (LDL‐C), low high‐density lipoprotein cholesterol (HDL‐C), and high triglycerides (TG). Combination lipid therapy is an option; however, efficacy and safety data among elderly patients are lacking.
Hypothesis
The combination of rosuvastatin and fenofibric acid (R + FA) results in more comprehensive lipid improvements than corresponding‐dose monotherapies, without additional safety concerns, in elderly patients with mixed dyslipidemia.
Methods
This post‐hoc analysis evaluated data from patients age ≥ 65 years (n = 401) with mixed dyslipidemia (LDL‐C ≥ 130 mg/dL, HDL‐C < 40 mg/dL [men] or < 50 mg/dL [women], and TG ≥ 150 mg/dL) in 2 randomized studies. Patients included in this analysis received either monotherapy (as R 5, 10, or 20 mg or FA 135 mg), or combination therapy with R (5, 10, or 20 mg) + FA 135 mg, for 12 weeks. Data were pooled and analyzed, and mean/median percent changes in multiple lipid parameters and biomarkers were compared.
Results
Combination therapy decreased LDL‐C by 31.8%–47.2% vs 10.6% with FA monotherapy (P < 0.001). Combination therapy also increased HDL‐C by 21.9%–27.0% vs 5.9%–9.9% with R monotherapy (P < 0.001), and decreased TG by 48.3%–53.5% vs 20.7%–32.8% with R monotherapy (P < 0.001). In general, safety profiles were consistent between combination therapy and individual monotherapies.
Conclusions
In these elderly patients with mixed dyslipidemia, R 5, 10, or 20 mg in combination with FA 135 mg improved the overall lipid profile, without new or unexpected safety issues. Copyright © 2010 Wiley Periodicals, Inc.
Financial support for the studies was provided by Abbott and AstraZeneca. Dr. Pepine has served as a consultant for Abbott, Angioblast, Eli Lilly, Forest, Medtelligence, NicOx, Novartis, Sanofi‐Aventis, and SLACK, Inc.; has received research grants from Baxter, Bioheart, Inc., GlaxoSmithKline, and Pfizer; and has received unrestricted educational grants from AstraZeneca, AtCor Medical, Daiichi Sankyo, Eli Lilly, Pfizer, sanofi aventis, and Schering Plough. Dr. Jacobson is a consultant for Abbott, AstraZeneca, GlaxoSmithKline, Merck, and Schering Plough. Dr. Carlson, Dr. Kelly, Ms. Setze, Dr. Stolzenbach, and Dr. Williams are Abbott employees and stockholders. Dr. Gold is an AstraZeneca employee and stockholder.
Introduction
The elderly (age ≥ 65 years) population in the United States is estimated to more than double in the next 40 years to approximately 80 million people.1 Currently, coronary heart disease (CHD) is the leading cause of death in the US elderly.2 CHD risk increases with advancing age, as most first major coronary events occur in persons age ≥ 65 years.3 The specific reasons for these age‐related associations are not completely understood, but may be related in part to progressive exhaustion of capacity for endothelial cell repair.4 Accumulation of these damaged endothelial cells is proatherogenic. Resulting plaque in coronary arteries and elsewhere promotes expansive remodeling of conduit arteries. This condition is associated with continued local lipid accumulation, inflammation, oxidative stress, matrix breakdown, and further atherosclerotic plaque progression.5,6 Additionally, the microvessels are adversely influenced by aging and elevation of plasma lipids, particularly low‐density lipoprotein cholesterol (LDL‐C) and triglycerides (TG).7 Effective management of modifiable conditions contributing to atherosclerosis in the elderly is therefore crucial. However, in elderly patients, therapeutic lifestyle changes may not be effective, control of systolic blood pressure is difficult, and data from studies evaluating effects of drug therapies for risk‐factor control in this population are often lacking.
The association between elevated blood levels of LDL‐C with increased CHD risk is well documented.3 Accumulating evidence indicates that lipid fractions other than elevated LDL‐C, such as elevated non–high‐density lipoprotein cholesterol (non–HDL‐C), elevated TG, and low high‐density lipoprotein cholesterol (HDL‐C), are also independently associated with increased CHD risk.8,9 In addition, the presence of mixed dyslipidemia, defined as elevated LDL‐C and TG with low HDL‐C, has been linked with higher CHD risk than isolated elevated LDL‐C.10,11 Although prevalence estimates for mixed dyslipidemia in the elderly are limited, it has been suggested that a high proportion have elevated LDL‐C, and that the simultaneous presence of elevated TG, with or without decreased HDL‐C, has increased markedly in the elderly in the past decade.12 Furthermore, an evaluation of the National Health and Nutrition Examination Survey 2003–2004 data observed that the percentage of US adults not at recommended individual levels of LDL‐C, non–HDL‐C, or TG increases substantially with advancing age and that only about 30% of those age ≥ 60 years have optimal levels of LDL‐C, HDL‐C, TG, and non–HDL‐C.13
Several studies have observed that lipid therapy is underutilized in elderly patients and patients with mixed dyslipidemia.14, 15, 16, 17 In addition, statin monotherapy often does not adequately treat all the lipid abnormalities associated with mixed dyslipidemia.14,17,18 Although statin treatment in elderly patients is effective in reducing CHD risk,19, 20, 21, 22 many patients with abnormal HDL‐C and TG persisting after statin treatment may continue to be at increased risk for adverse cardiovascular events despite adequately treated LDL‐C.9,23 Consequently, combination therapy with a statin plus another agent is often employed in an attempt to manage multiple lipid abnormalities; however, data demonstrating the efficacy and safety of these therapies among the elderly are limited. The choline salt of fenofibric acid (FA; Trilipix™, Abbott, North Chicago, IL) is approved by the US Food and Drug Administration (FDA) for use in combination with statins for treatment of mixed dyslipidemia in patients with CHD or a CHD risk equivalent who are receiving optimal statin therapy to achieve their LDL‐C goal.24 Accordingly, we conducted this analysis of patients age ≥ 65 years with mixed dyslipidemia by pooling data from 2 randomized studies evaluating FA used in combination with different dosage strengths of rosuvastatin (R; Crestor™, AstraZeneca, Wilmington, DE).
Methods
Patients
The elderly age threshold of ≥ 65 years, as recommended by the FDA and other organizations, was used in this post‐hoc analysis of patients from 2 phase 3, randomized studies comparing efficacy and safety of combination therapy with R 5 mg + FA 135 mg (Study 1/NCT00463606) and R 10 or 20 mg + FA 135 mg (Study 2/NCT00300482) with the efficacy and safety of FA and corresponding‐dose R monotherapies in patients with mixed dyslipidemia.25,26 Patients with mixed dyslipidemia (LDL‐C ≥ 130 mg/dL [≥ 3.37 mmol/L], HDL‐C < 40 mg/dL [<1.04 mmol/L] for men or < 50 mg/dL [<1.30 mmol/L] for women, and TG ≥ 150 mg/dL [≥ 1.70 mmol/L]) after a 6‐week washout of lipid‐altering medications were eligible. Patients with glycated hemoglobin A1c ≤ 10.5% in Study 1 or ≤ 8.5% in Study 2 were included. Use of concomitant antidiabetes medications was allowed. Additional study eligibility criteria are described in detail elsewhere.27
Studies and Analyses
The designs of the studies have been previously presented in detail.25,26 Briefly, both studies consisted of a 6‐week screening period, a 12‐week treatment period, and a 30‐day safety evaluation period. During the screening period, patients stopped lipid‐altering medications and were expected to follow the American Heart Association (AHA) diet.28 Patients included in these analyses were treated with R 5 mg + FA, R 5 mg monotherapy, or FA monotherapy in Study 1; and R 10 mg + FA, R 20 mg + FA, R 10 mg monotherapy, R 20 mg monotherapy, or FA monotherapy in Study 2. All drugs were self‐administered once daily at approximately the same time of day, with or without food. Randomization was stratified by type 2 diabetes (T2DM) status and screening TG level (≤ 250 mg/dL or > 250 mg/dL). Patients and site and sponsor personnel were blinded to lipid results obtained after the baseline visit. Protocols were approved by appropriate ethics committees or institutional review boards at participating institutions, studies were conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki, and all patients provided informed consent prior to enrollment.
Mean percent changes (baseline to final visit) in LDL‐C, HDL‐C, non–HDL‐C, very‐low‐density lipoprotein cholesterol (VLDL‐C), total cholesterol (total‐C), and apolipoprotein B (apo B), and median percent changes in TG and high‐sensitivity C‐reactive protein (hsCRP), were calculated for each treatment group. Statistical comparisons performed in this subanalysis were based on the prespecified comparisons in each study.25,26 For HDL‐C, TG, VLDL‐C, total‐C, apo B, and hsCRP, R + FA was compared with corresponding‐dose R monotherapy (primary comparison); for LDL‐C, the primary comparison was with FA monotherapy. For non–HDL‐C, the primary comparisons were between R + FA and FA, followed by R + FA vs corresponding‐dose R monotherapy.27 An additional analysis for LDL‐C was also performed, comparing R + FA with corresponding‐dose R monotherapy. Efficacy variables were also analyzed in the subgroup with T2DM; however, due to the smaller sample size, data were pooled among all doses of R (5, 10, and 20 mg) monotherapy and R (5, 10, and 20 mg) + FA combination therapy.
Sample‐size considerations and the general analysis plan are described in detail elsewhere.27 Briefly, all statistical comparisons were performed separately for each dose of combination therapy. For efficacy analyses (except TG and hsCRP), percent changes were compared between combination therapy groups and corresponding monotherapy groups using contrast statements within an analysis of covariance (ANCOVA), with the baseline value as the covariate and with effects for treatment group, T2DM status, screening TG level (≤ 250 mg/dL, > 250 mg/dL), and the interaction between T2DM status and screening TG. Due to the distribution of TG and hsCRP values, the van Elteren test with T2DM status and screening TG level as strata was used for comparisons between the combination therapy groups and each corresponding monotherapy group. These analyses included patients with both a baseline and ≥ 1 post‐baseline value, using the last observation carried forward to impute values for patients with missing post‐baseline values. Adverse events were recorded at each visit, coded using the Medical Dictionary for Regulatory Activities, and summarized. Data were analyzed using SAS version 8.2 (SAS Institute, Inc., Cary, NC).
Results
There was a total of 401 patients age ≥ 65 years in this analysis who were treated with either R (5, 10, or 20 mg) monotherapy, FA monotherapy, or R (5, 10, or 20 mg) + FA combination therapy, and their baseline characteristics are summarized in Table 1. Overall mean age was 70.2 years, and the maximum age ranged from 80.0 to 85.0 years among treatment groups. The majority were women and white (Table 1). Approximately 18% had coronary artery disease, 73% hypertension, 34% T2DM, 20% obesity, and 68% metabolic syndrome by the National Cholesterol Education Program Adult Treatment Panel III definition.3 Overall baseline creatinine level was 0.945 ± 0.238 mg/dL (mean ± SD), and estimated glomerular filtration rate (eGFR) was 72.35 ± 16.98 mL/min/1.73 m2, both of which were similar among treatment groups (P = 0.999 for creatinine and P = 0.054 for eGFR, 1‐way analysis of variance).
Table 1.
Baseline Characteristics
| FA (n = 101) | R 5 mg (n = 46) | R 5 mg + FA (n = 59) | R 10 mg (n = 39) | R 10 mg + FA (n = 55) | R 20 mg (n = 49) | R 20 mg + FA (n = 52) | Total (n = 401) | |
|---|---|---|---|---|---|---|---|---|
| Gender, n (%) | ||||||||
| F | 69 (68.3) | 30 (65.2) | 45 (76.3) | 23 (59.0) | 35 (63.6) | 26 (53.1) | 33 (63.5) | 261 (65.1) |
| M | 32 (31.7) | 16 (34.8) | 14 (23.7) | 16 (41.0) | 20 (36.4) | 23 (46.9) | 19 (36.5) | 140 (34.9) |
| Race, n (%) | ||||||||
| White | 94 (93.1) | 38 (82.6) | 54 (91.5) | 38 (97.4) | 51 (92.7) | 45 (91.8) | 49 (94.2) | 369 (92.0) |
| Black | 1 (1.0) | 5 (10.9) | 2 (3.4) | 1 (2.6) | 3 (5.5) | 4 (8.2) | 2 (3.8) | 18 (4.5) |
| Other | 6 (5.9) | 3 (6.5) | 3 (5.1) | 0 (0.0) | 1 (1.8) | 0 (0.0) | 1 (1.9) | 14 (3.5) |
| Ethnicity, n (%) | ||||||||
| Hispanic | 9 (8.9) | 2 (4.3) | 2 (3.4) | 3 (7.7) | 5 (9.1) | 0 (0.0) | 8 (15.4) | 29 (7.2) |
| Age, y | ||||||||
| Mean (SD) | 70.2 (4.13) | 70.5 (4.85) | 70.8 (5.08) | 68.9 (4.03) | 71.0 (4.76) | 70.2 (4.89) | 69.4 (4.31) | 70.2 (4.57) |
| Medical history, n (%) | ||||||||
| CAD | 21 (20.8) | 4 (8.7) | 12 (20.3) | 5 (12.8) | 8 (14.5) | 13 (26.5) | 7 (13.5) | 70 (17.5) |
| Hypertension | 69 (68.3) | 33 (71.7) | 51 (86.4) | 32 (82.1) | 39 (70.9) | 35 (71.4) | 35 (67.3) | 294 (73.3) |
| T2DM | 29 (28.7) | 16 (34.8) | 29 (49.2) | 15 (38.5) | 18 (32.7) | 14 (28.6) | 17 (32.7) | 138 (34.4) |
| Obesity | 18 (17.8) | 6 (13.0) | 14 (23.7) | 11 (28.2) | 12 (21.8) | 8 (16.3) | 9 (17.3) | 78 (19.5) |
| Metabolic syndromea | 62 (61.4) | 28 (60.9) | 49 (83.1) | 29 (74.4) | 38 (69.1) | 30 (61.2) | 37 (71.2) | 273 (68.1) |
Abbreviations: CAD, coronary artery disease; F, female; FA, fenofibric acid; M, male; R, rosuvastatin; SD, standard deviation; T2DM, type 2 diabetes mellitus.
Determined according to National Cholesterol Education Program Adult Treatment Panel III definition.3
Each dose of R + FA resulted in significantly greater mean percent decreases in LDL‐C, compared with FA monotherapy, and significantly greater mean percent increases in HDL‐C and median percent decreases in TG, compared with corresponding doses of R monotherapy (Figure 1). Additionally, significantly greater mean percent decreases were observed in non–HDL‐C, VLDL‐C, and apo B when comparing R 5 mg + FA with R 5 mg; VLDL‐C and apo B when comparing R 10 mg + FA with R 10 mg; and non–HDL‐C and VLDL‐C when comparing R 20 mg + FA with R 20 mg (Table 2). All other comparisons (including LDL‐C) were not significantly different between R + FA and the corresponding dose of R monotherapy (P > 0.05). Similar changes in LDL‐C, HDL‐C, and TG were observed in the subgroup (n = 135) of elderly patients with T2DM (Figure 2).
Figure 1.
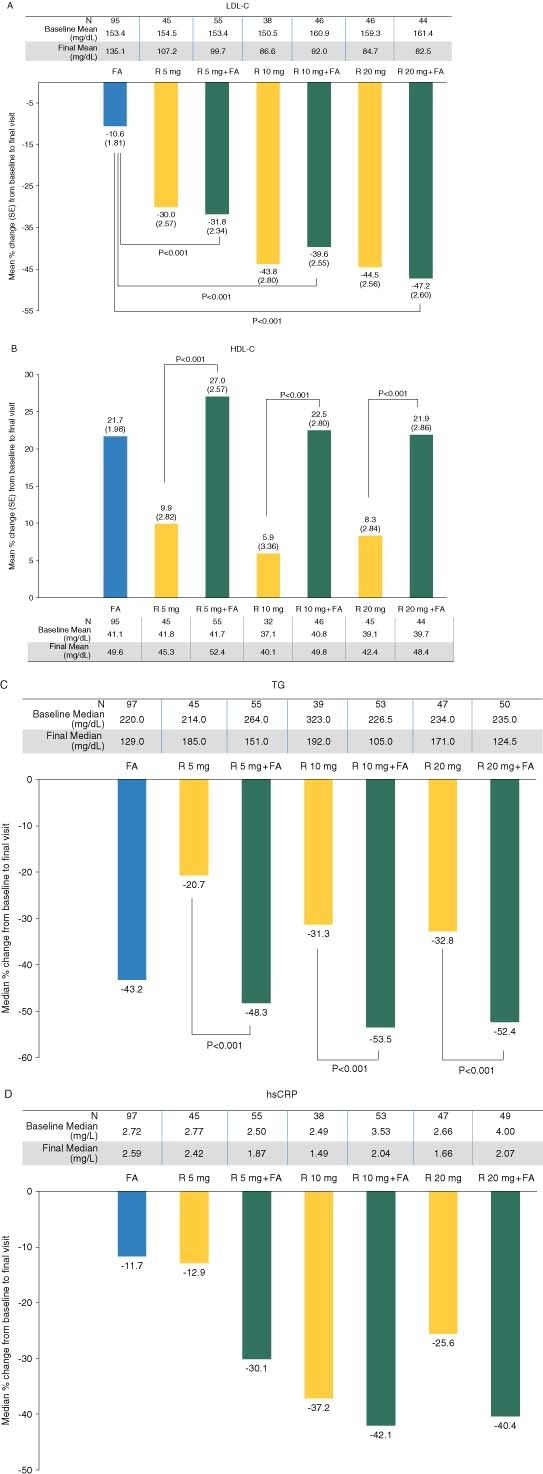
Treatment effect on LDL‐C, HDL‐C, TG, and hsCRP. The mean percent changes (SE) in (A) LDL‐C and (B) HDL‐C, and the median percent changes in (C) TG and (D) hsCRP following 12‐week treatment with rosuvastatin (R) 5, 10, or 20 mg monotherapy, fenofibric acid (FA) 135 mg monotherapy, or combination therapy with R 5 mg + FA, R 10 mg + FA, or R 20 mg + FA are shown, in addition to the baseline and final mean or median values in mg/dL. Abbreviations: FA, fenofibric acid; HDL‐C, high‐density lipoprotein cholesterol; hsCRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; R, rosuvastatin; SE, standard error; TG, triglycerides
Table 2.
Percent Change From Baseline to Final Visit in Additional Lipid Parameters for Monotherapy and Combination Therapy Groups
| FA | R 5 mg | R 5 mg + FA | R 10 mg | R 10 mg + FA | R 20 mg | R 20 mg + FA | |
|---|---|---|---|---|---|---|---|
| Non–HDL‐C | n = 95 | n = 45 | n = 55 | n = 32 | n = 46 | n = 45 | n = 44 |
| Baseline meana | 218.1 | 220.9 | 231.0 | 213.2 | 225.4 | 225.7 | 227.9 |
| Final mean | 168.5 | 146.8 | 131.6 | 123.3 | 115.9 | 117.8 | 103.8 |
| Mean Δ % (SE) | −22.3 (1.42) | −33.1 (2.01) | −40.7 (1.84) | −43.6 (2.38) | −47.5 (1.99) | −46.8 (2.02) | −53.0 (2.04) |
| P value | <0.001b; 0.006c | <0.001b; 0.20c | <0.001b; 0.03c | ||||
| VLDL‐C | n = 96 | n = 45 | n = 55 | n = 37 | n = 51 | n = 45 | n = 46 |
| Baseline mean | 64.7 | 67.0 | 77.1 | 77.8 | 64.9 | 68.7 | 68.9 |
| Final mean | 33.6 | 39.8 | 31.8 | 41.4 | 24.2 | 33.9 | 24.9 |
| Mean Δ % (SE) | −42.5 (2.91) | −30.7 (4.15) | −49.5 (3.79) | −39.6 (4.60) | −58.8 (3.93) | −44.8 (4.17) | −59.4 (4.10) |
| P value | <0.001c | 0.001c | 0.01c | ||||
| Total‐C | n = 97 | n = 45 | n = 55 | n = 39 | n = 53 | n = 47 | n = 50 |
| Baseline mean | 258.0 | 262.9 | 272.6 | 258.7 | 267.1 | 264.8 | 266.7 |
| Final mean | 217.6 | 192.2 | 184.1 | 170.3 | 166.0 | 160.9 | 156.6 |
| Mean Δ % (SE) | −15.6 (1.15) | −26.4 (1.65) | −30.5 (1.50) | −34.8 (1.77) | −36.8 (1.53) | −38.7 (1.62) | −40.3 (1.56) |
| P value | 0.07c | 0.40c | 0.48c | ||||
| Apo B | n = 97 | n = 45 | n = 55 | n = 38 | n = 53 | n = 47 | n = 49 |
| Baseline mean | 138.3 | 138.9 | 137.5 | 146.2 | 151.8 | 148.0 | 149.2 |
| Final mean | 112.1 | 98.6 | 89.0 | 93.3 | 84.5 | 87.8 | 81.9 |
| Mean Δ % (SE) | −19.6 (1.49) | −29.4 (2.14) | −35.9 (1.95) | −35.5 (2.32) | −42.0 (1.99) | −38.4 (2.11) | −43.3 (2.05) |
| P value | 0.03c | 0.03c | 0.10c |
Abbreviations: Apo B, apolipoprotein B; FA, fenofibric acid; non–HDL‐C, non–high‐density lipoprotein cholesterol; R, rosuvastatin; SE, standard error; total‐C, total cholesterol; VLDL‐C, very‐low‐density lipoprotein cholesterol.
To convert non–HDL‐C, VLDL‐C, and total‐C values to mmol/L, multiply by 0.0259.
All means are shown in mg/dL.
Combination therapy vs fenofibric acid monotherapy.
Combination therapy vs corresponding‐dose rosuvastatin monotherapy
Figure 2.
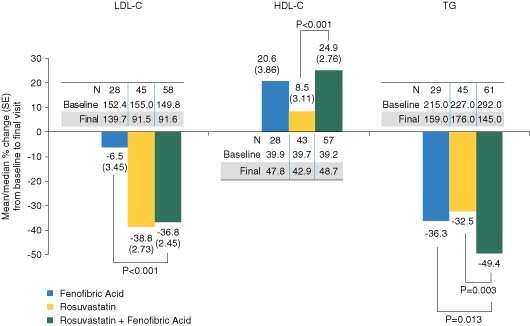
Efficacy in elderly patients with T2DM. The mean percent changes (SE) in LDL‐C and HDL‐C, and the median percent changes in TG following 12‐week treatment are shown for the subgroup of patients with T2DM, in addition to the baseline and final mean or median values in mg/dL. Data were pooled among the doses of R (5, 10, and 20 mg) monotherapy and R (5, 10, and 20 mg) + FA combination therapy. Abbreviations: FA, fenofibric acid; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; R, rosuvastatin; SE, standard error; T2DM, type 2 diabetes mellitus; TG, triglycerides
An ANCOVA with the corresponding baseline lipid value as the covariate and with effects for treatment group, age, and the treatment group by age interaction was performed with the overall population data from Study 1 and Study 2, and no statistically significant interactions between treatment and age were observed for mean percent change in the efficacy parameters. All 3 doses of combination therapy generally resulted in a greater treatment effect among patients ≥ 65 years of age than patients < 65 years of age, though the differences in the mean percent changes were small.
In general, the safety profile of R + FA combination therapy was consistent with the known safety profiles of the individual monotherapies (Table 3). In the higher‐dose groups of R + FA combination therapy and R monotherapy, more patients experienced treatment‐emergent adverse events and discontinued due to adverse events. The incidence of increases in creatine phosphokinase (CK), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and creatinine to clinically relevant thresholds was low, and similar between combination therapy and corresponding‐dose monotherapies. One investigator‐reported case of rhabdomyolysis occurred in the R 5 mg + FA group. This 76‐year‐old man reported leg pain and body ache, with the patient's peak CK 3308 U/L. However, this event was not associated with any increase in serum creatinine or evidence of myoglobinuria and is most consistent with the definition of “myositis” based on AHA/American College of Cardiology definitions for muscle events.29 This patient's symptoms resolved, and CK values returned to normal after therapy was discontinued. Other than this case, no other occurrence of rhabdomyolysis or myositis was reported.
Table 3.
Summary of Patient Disposition, Treatment‐Emergent Adverse Events, and Laboratory Tests of Special Interest
| FA | R 5 mg | R 5 mg + FA | R 10 mg | R 10 mg + FA | R 20 mg | R 20 mg + FA | |
|---|---|---|---|---|---|---|---|
| Patient Disposition | |||||||
| Randomized | 101 | 46 | 59 | 41 | 55 | 49 | 53 |
| Treated | 101 | 46 | 59 | 39 | 55 | 49 | 52 |
| Completed | 87 | 43 | 47 | 35 | 43 | 44 | 40 |
| Discontinueda | 14 (13.9) | 3 (6.5) | 12 (20.3) | 6 (15.4) | 12 (21.8) | 5 (10.2) | 13 (25.0) |
| No. (%) of patients with: | |||||||
| Any treatment‐emergent AE | 63 (62.4) | 24 (52.2) | 35 (59.3) | 26 (66.7) | 39 (70.9) | 34 (69.4) | 45 (86.5)b |
| Discontinuations due to AE | 10 (9.9) | 2 (4.3) | 6 (10.2) | 2 (5.1) | 9 (16.4) | 3 (6.1) | 9 (17.3) |
| Any special‐interest AEsc | 17 (16.8) | 2 (4.3) | 7 (11.9) | 6 (15.4) | 8 (14.5) | 1 (2.0) | 8 (15.4)d |
| Muscle, n/N (%) | |||||||
| CK >10 × ULN on any visit | 0/99 | 0/46 | 1/58 (1.7) | 0/39 | 0/53 | 0/48 | 0/50 |
| CK >5 × ULN on any visit | 0/99 | 0/46 | 1/58 (1.7) | 0/39 | 0/53 | 0/48 | 0/50 |
| Hepatic, n/N (%) | |||||||
| ALT >3 × ULN on 2 consecutive visits | 2/99 (2.0) | 0/46 | 0/58 | 0/39 | 0/53 | 0/48 | 0/50 |
| ALT >5 × ULN on single visit | 1/99 (1.0) | 1/46 (2.2) | 1/58 (1.7) | 0/39 | 1/53 (1.9) | 0/48 | 0/50 |
| AST >3 × ULN on 2 consecutive visits | 1/99 (1.0) | 0/46 | 0/58 | 0/39 | 0/53 | 0/48 | 0/50 |
| AST >5 × ULN on single visit | 0/99 | 1/46 (2.2) | 0/58 | 0/39 | 0/53 | 0/48 | 0/50 |
| Renal, n/N (%) | |||||||
| Creatinine ≥50% increase from BL and >ULN on single visit | 7/99 (7.1) | 1/46 (2.2) | 2/58 (3.4) | 2/39 (5.1) | 0/53 | 1/48 (2.1) | 4/50 (8.0) |
| Creatinine ≥100% increase from BL | 0/99 | 1/46 (2.2) | 0/58 | 0/39 | 0/53 | 0/48 | 0/50 |
| Creatinine >2 mg/dL | 6/99 (6.1) | 1/46 (2.2) | 0/58 | 0/39 | 2/53 (3.8) | 0/48 | 0/50 |
Abbreviations: AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BL, baseline (defined as last value before start of study drug); CK, creatine phosphokinase; FA, fenofibric acid; R, rosuvastatin; ULN, upper limit of normal.
Patients could have ≥1 reason for discontinuation. Percentages reflect proportion of treated patients.
P < 0.001 vs FA, and P = 0.019 vs R 20 mg.
Investigator‐reported AEs related to muscle, hepatic, or renal function were considered special‐interest AEs.
P = 0.032 vs R 20 mg
Discussion
This is the first report evaluating combination lipid therapy with a fibrate and statin in a cohort of elderly patients with mixed dyslipidemia. Each of the 3 doses of combination therapy with R + FA resulted in significantly greater improvements in HDL‐C, TG, and VLDL‐C, compared with corresponding‐dose R monotherapy, and significantly greater improvements in LDL‐C and non–HDL‐C, compared with FA monotherapy. Results are consistent with the findings in the overall population of each study,25,26 suggesting that the effect of the combination of R + FA on lipid parameters is not diminished in the elderly. On the contrary, the effect of combination therapy on several lipid parameters including LDL‐C, HDL‐C, and non–HDL‐C was numerically greater in the elderly subgroup, compared with the overall population.
Levels of non–HDL‐C and/or apo B are believed to better reflect total atherogenic particle number than levels of LDL‐C, and when elevated, are strongly associated with increased CHD risk.30 In addition, a recent meta‐analysis of clinical studies shows a strong correlation between non–HDL‐C reduction and CHD event reduction.31 In this elderly cohort, the combination of R + FA resulted in a significantly greater or similar decrease in non–HDL‐C and apo B, compared with the corresponding dose of R monotherapy, which is consistent with results in the overall population of each study.25,26 Recent studies have also suggested an association between elevated levels of the inflammatory marker hsCRP and increased risk for cardiovascular events in patients age > 65 years.32,33 In the current analysis, median percent changes in hsCRP following treatment with R 5 mg + FA, R 10 mg + FA, and R 20 mg + FA were − 30.1% (interquartile range [IQR], − 43.1% to 0.0%), − 42.1% (IQR, − 62.1% to − 19.0%), and − 40.4% (IQR, − 54.3% to − 6.4%), respectively (Figure 1D). These improvements in hsCRP were numerically larger, but not significantly different, than the corresponding dose of R monotherapy. Taken together, the treatment effects on multiple cardiovascular risk factors may be an advantage of R + FA combination therapy, resulting in more comprehensive improvements vs monotherapy with either agent.
In this elderly patient population, all 3 doses of R + FA combination therapy were generally well tolerated. However, the incidence of discontinuation due to adverse events was numerically higher than that observed in the overall population.25 Previous studies have demonstrated that a higher risk of skeletal muscle effects such as myopathy and rhabdomyolysis has been associated with high‐dose statins as well as the combination of a statin with a fibrate (in particular gemfibrozil).34 In the current analysis, only 1 patient in the R 5 mg + FA group had an elevation in CK to clinically relevant thresholds, and the incidence of increases in ALT or AST to predefined thresholds was similar to that observed in the overall population.25 However, increases in creatinine to predefined thresholds generally occurred more frequently in the elderly cohort than in the overall population.25 These differences are likely not related to differences in metabolism, as pharmacokinetic data with the individual FA and R agents is similar between elderly and younger adults.24,35 Elderly patients are known to have a higher prevalence of renal impairment; however, the baseline eGFR and serum creatinine levels of the elderly population in this analysis did not classify them as renally impaired.
Advancing age is also associated with higher prevalence of hypertension, kidney disease, CHD, heart failure, atrial fibrillation, and osteoarthritis. Studies have shown that the incidence of T2DM, considered a CHD risk equivalent, also increases with age, although the cumulative lifetime risk for T2DM tends to plateau around 70 years of age.36 Available prevalence estimates and large clinical outcomes studies21,37 indicate that the presence of ≥ 1 lipid abnormality is very common among the elderly, which likely contributes to increased CHD risk. In this analysis of elderly patients with mixed dyslipidemia, comorbidities including hypertension and metabolic syndrome were present in the majority of patients, and hypertension, coronary artery disease, and T2DM were present in higher proportion than observed in the overall population.25 In addition, efficacy results were similar in the overall cohort and the subgroup of patients with T2DM (34.4% of the cohort). Thus, the current results suggest that R + FA combination therapy may be effective in improving lipid levels in elderly patients with multiple comorbidities, although the impact on cardiovascular events and mortality rates is not known, as these studies were not designed to have sufficient power or long‐term follow‐up to evaluate adverse cardiovascular outcomes.
Clinical evidence of the reduction in cardiovascular events with use of statin and fibrate combination is limited. The recent Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial evaluating patients with T2DM demonstrated that the combination of fenofibrate and simvastatin did not significantly reduce the rate of major cardiovascular events compared with simvastatin alone, and similar results were observed comparing patients < 65 years of age (n = 3660) vs those ≥ 65 years (n = 1858).38 However, the baseline mean LDL‐C was only 100.6 mg/dL and median TG was 162 mg/dL vs 156.7 mg/dL and 279.5 mg/dL, respectively, in our patients. Of note, the ACCORD protocol did not require washout of any pre‐existing statin therapy that could have lowered baseline levels of LDL‐C and TG. In the subgroup of ACCORD patients (regardless of age) with high baseline TG (≥ 204 mg/dL) and low baseline HDL‐C (≤ 34 mg/dL), fenofibrate + simvastatin reduced the relative risk of events by 31%. In the overall ACCORD population, LDL‐C declined by 18.9% to a mean final value of 81.1 mg/dL, HDL‐C increased by 8.4% to a mean final value of 41.2 mg/dL, and TG declined by 22.2% to a median final value of 147.0 mg/dL. Larger percent changes in these parameters were observed in our patients with T2DM (Figure 2), likely related to differences in patient characteristics and baseline lipid levels.
In addition to the higher prevalence of comorbidities, the treatment of elderly patients poses unique challenges, such as increased numbers of prescribed medications (ie, polypharmacy) and lower patient adherence to therapy. Adherence to statin therapy is known to be low in elderly patients.39,40 The asymptomatic course of lipid abnormalities, in addition to the increased numbers of prescribed medications and doses, may be expected to adversely affect patient adherence to therapy. Development of combination therapies (eg, polypill) could potentially increase patient adherence by reducing total pill burden.41
Limitations of this subgroup analysis are that randomization was not stratified by age and patients with renal impairment were excluded from the studies. Additionally, this was a relatively short‐term evaluation of the safety and efficacy; however, in long‐term open‐label extension studies with the overall population, efficacy was maintained up to 2 years and there was no evidence of cumulative toxicity or late‐onset adverse events.42,43
Conclusion
The efficacy and safety of lipid‐modifying agents in the elderly are becoming increasingly important as this population continues to grow and the burden of CHD continues to increase. The combination of R and FA may represent a useful therapeutic option for modification of lipids in elderly patients with mixed dyslipidemia. Further evaluation of this population with a cardiovascular outcomes trial may be warranted.
Acknowledgements
The authors would like to thank Hsiaoming Sun, MS, and Aditya Lele, MS, of Abbott for providing assistance with statistical analyses; Noreen Travers, RN, MSN, and Lura Morris, BA, of Abbott for providing assistance with clinical study management; and Erin Blondell, PhD, of Abbott for providing assistance with the writing and preparation of this manuscript.
References
- 1. US Bureau of the Census , Hobbs FB. Population Profile of the United States: The Elderly Population. http://www.census.gov/population/www/pop‐profile/elderpop.html. Accessed December 31, 2009.
- 2. American Heart Association . Heart Disease and Stroke Statistics—2008 Update. Dallas, TX: American Heart Association; 2008. [Google Scholar]
- 3. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106: 3143–3421. [PubMed] [Google Scholar]
- 4. Thorin E, Thorin‐Trescases N. Vascular endothelial ageing, heartbeat after heartbeat. Cardiovasc Res 2009; 84: 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grundy SM. Age as a risk factor: you are as old as your arteries. Am J Cardiol 1999; 83: 1455–1457. [DOI] [PubMed] [Google Scholar]
- 6. Grundy SM, Cleeman JI, Rifkind BM, et al. Cholesterol lowering in the elderly population. Coordinating Committee of the National Cholesterol Education Program. Arch Intern Med 1999; 159: 1670–1678. [DOI] [PubMed] [Google Scholar]
- 7. Wang L, Jerosch‐Herold M, Jacobs DR Jr, et al. Coronary risk factors and myocardial perfusion in asymptomatic adults: the Multi‐Ethnic Study of Atherosclerosis (MESA). J Am Coll Cardiol 2006; 47: 565–572. [DOI] [PubMed] [Google Scholar]
- 8. Arsenault BJ, Rana JS, Stroes ES, et al. Beyond low‐density lipoprotein cholesterol: respective contributions of non‐high‐density lipoprotein cholesterol levels, triglycerides, and the total cholesterol/high‐density lipoprotein cholesterol ratio to coronary heart disease risk in apparently healthy men and women. J Am Coll Cardiol 2010; 55: 35–41. [DOI] [PubMed] [Google Scholar]
- 9. Fruchart JC, Sacks F, Hermans MP, et al. The Residual Risk Reduction Initiative: a call to action to reduce residual vascular risk in patients with dyslipidemia. Am J Cardiol 2008; 102(10 suppl): 1K–34K. [DOI] [PubMed] [Google Scholar]
- 10. Stanek EJ, Sarawate C, Willey VJ, et al. Risk of cardiovascular events in patients at optimal values for combined lipid parameters. Curr Med Res Opin 2007; 23: 553–563. [DOI] [PubMed] [Google Scholar]
- 11. Manninen V, Tenkanen L, Koskinen P, et al. Joint effects of serum triglyceride and LDL cholesterol and HDL cholesterol concentrations on coronary heart disease risk in the Helsinki Heart Study: implications for treatment. Circulation 1992; 85: 37–45. [DOI] [PubMed] [Google Scholar]
- 12. Cohen JD, Cziraky MJ, Jacobson TA, et al. Changes in the prevalence of abnormal lipid fractions among US adults: results from the National Health and Nutrition Examination Survey II, III and 1999–2006 [abstract 1189]. Circulation 2008; 118: S1081–S1082. [Google Scholar]
- 13. Ghandehari H, Kamal‐Bahl S, Wong ND. Prevalence and extent of dyslipidemia and recommended lipid levels in US adults with and without cardiovascular comorbidities: the National Health and Nutrition Examination Survey 2003–2004. Am Heart J 2008; 156: 112–119. [DOI] [PubMed] [Google Scholar]
- 14. Alsheikh‐Ali AA, Lin JL, Abourjaily P, et al. Extent to which accepted serum lipid goals are achieved in a contemporary general medical population with coronary heart disease risk equivalents. Am J Cardiol 2006; 98: 1231–1233. [DOI] [PubMed] [Google Scholar]
- 15. Aronow WS. Underutilization of lipid‐lowering drugs in older persons with prior myocardial infarction and a serum low‐density lipoprotein cholesterol > 125 mg/dL. Am J Cardiol 1998; 82: 668–669. [DOI] [PubMed] [Google Scholar]
- 16. Sueta CA, Chowdhury M, Boccuzzi SJ, et al. Analysis of the degree of undertreatment of hyperlipidemia and congestive heart failure secondary to coronary artery disease. Am J Cardiol 1999; 83: 1303–1307. [DOI] [PubMed] [Google Scholar]
- 17. Yan AT, Yan RT, Tan M, et al. Contemporary management of dyslipidemia in high‐risk patients: targets still not met. Am J Med 2006; 119: 676–683. [DOI] [PubMed] [Google Scholar]
- 18. Stacy TA, Egger A. Results of retrospective chart review to determine improvement in lipid goal attainment in patients treated by high‐volume prescribers of lipid‐modifying drugs. J Manag Care Pharm 2006; 12: 745–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lemaitre RN, Psaty BM, Heckbert SR, et al. Therapy with hydroxymethylglutaryl coenzyme a reductase inhibitors (statins) and associated risk of incident cardiovascular events in older adults: evidence from the Cardiovascular Health Study. Arch Intern Med 2002; 162: 1395–1400. [DOI] [PubMed] [Google Scholar]
- 20. Miettinen TA, Pyörälä K, Olsson AG, et al. Cholesterol‐lowering therapy in women and elderly patients with myocardial infarction or angina pectoris: findings from the Scandinavian Simvastatin Survival Study (4S). Circulation 1997; 96: 4211–4218. [DOI] [PubMed] [Google Scholar]
- 21. Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002; 360: 1623–1630. [DOI] [PubMed] [Google Scholar]
- 22. Williams MA, Fleg JL, Ades PA, et al. Secondary prevention of coronary heart disease in the elderly (with emphasis on patients > or = 75 years of age): an American Heart Association scientific statement from the Council on Clinical Cardiology Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention. Circulation 2002; 105: 1735–1743. [DOI] [PubMed] [Google Scholar]
- 23. Alagona P Jr. Beyond LDL cholesterol: the role of elevated triglycerides and low HDL cholesterol in residual CVD risk remaining after statin therapy. Am J Manag Care 2009; 15(3 suppl): S65–S73. [PubMed] [Google Scholar]
- 24. Trilipix [package insert] North Chicago, IL: Abbott. [Google Scholar]
- 25. Jones PH, Davidson MH, Kashyap ML, et al. Efficacy and safety of ABT‐335 (fenofibric acid) in combination with rosuvastatin in patients with mixed dyslipidemia: a phase 3 study. Atherosclerosis 2009; 204: 208–215. [DOI] [PubMed] [Google Scholar]
- 26. Roth EM, Rosenson RS, Carlson DM, et al. A phase III study evaluating the efficacy and safety of 135 mg fenofibric acid (ABT‐335) in combination with 5 mg rosuvastatin in patients with atherogenic dyslipidemia [abstract 1021‐85]. J Am Coll Cardiol 2009; 53(suppl A): A208. [Google Scholar]
- 27. Jones PH, Bays HE, Davidson MH, et al. Evaluation of a new formulation of fenofibric acid, ABT‐335, co‐administered with statins. Clin Drug Investig 2008; 28: 625–634. [DOI] [PubMed] [Google Scholar]
- 28. American Heart Association . An Eating Plan for Healthy Americans: Our American Heart Association Diet. Dallas, TX: American Heart Association; 2004. AHA publication 50‐1481A. [Google Scholar]
- 29. Pasternak RC, Smith SC Jr, Bairey‐Merz CN, et al. ACC/AHA/ NHLBI Clinical Advisory on the Use and Safety of Statins. Circulation 2002; 106: 1024–1028. [DOI] [PubMed] [Google Scholar]
- 30. Jones PH. Fenofibric acid plus statin combination therapy for the treatment of mixed dyslipidemia. Clin Lipidol 2009; 4: 699–711. [Google Scholar]
- 31. Robinson JG, Wang S, Smith BJ, et al. Meta‐analysis of the relationship between non‐high‐density lipoprotein cholesterol reduction and coronary heart disease risk. J Am Coll Cardiol 2009; 53: 316–322. [DOI] [PubMed] [Google Scholar]
- 32. Libby P, Ridker PM. Inflammation and atherosclerosis: role of C‐reactive protein in risk assessment. Am J Med 2004; 116(suppl 6A): S9–S16. [DOI] [PubMed] [Google Scholar]
- 33. Tracy RP, Lemaitre RN, Psaty BM, et al. Relationship of C‐reactive protein to risk of cardiovascular disease in the elderly: results from the Cardiovascular Health Study and the Rural Health Promotion Project. Arterioscler Thromb Vasc Biol 1997; 17: 1121–1127. [DOI] [PubMed] [Google Scholar]
- 34. Shek A, Ferrill MJ. Statin‐fibrate combination therapy. Ann Pharmacother 2001; 35: 908–917. [DOI] [PubMed] [Google Scholar]
- 35. Crestor [package insert] Wilmington, DE: AstraZeneca Pharmaceuticals LP. [Google Scholar]
- 36. Narayan KM, Boyle JP, Thompson TJ, et al. Lifetime risk for diabetes mellitus in the United States. JAMA 2003; 290: 1884–1890. [DOI] [PubMed] [Google Scholar]
- 37. Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358: 1887–1898. [DOI] [PubMed] [Google Scholar]
- 38. The ACCORD Study Group . Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010; 362: 1563–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Benner JS, Glynn RJ, Mogun H, et al. Long‐term persistence in use of statin therapy in elderly patients. JAMA 2002; 288: 455–461. [DOI] [PubMed] [Google Scholar]
- 40. Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 2002; 288: 462–467. [DOI] [PubMed] [Google Scholar]
- 41. Sica DA. Rationale for fixed‐dose combinations in the treatment of hypertension: the cycle repeats. Drugs 2002; 62: 443–462. [DOI] [PubMed] [Google Scholar]
- 42. Bays HE, Jones PH, Mohiuddin SM, et al. Long‐term safety and efficacy of fenofibric acid in combination with statin therapy for the treatment of patients with mixed dyslipidemia. J Clin Lipidol 2008; 2: 426–435. [DOI] [PubMed] [Google Scholar]
- 43. Kipnes MS, Roth EM, Rhyne JM, et al. Year two assessment of fenofibric acid and moderate‐dose statin combination: a phase 3, open‐label, extension study. Clin Drug Investig 2010; 30: 51–61. [DOI] [PubMed] [Google Scholar]


