Abstract
Background:
In chronic heart failure (CHF), several plasma biomarkers identify subjects at risk of death over the midterm. However, their long‐term predictive value in the context of other candidate predictors has never been assessed. This information may prove valuable in the management of a chronic disease with a long natural history, as CHF is today.
Hypothesis:
We aimed to assess the very‐long‐term prognostic power of a set of biomarkers to identify CHF patients at highest risk for all‐cause mortality.
Methods:
A group of 106 consecutive outpatients with CHF (85 male and 21 female, median age 56 y) was followed for 15 years. Echocardiographic tracings and blood samples were collected at study entry to evaluate cardiac function, plasma atrial natriuretic peptide (ANP), aldosterone, and erythropoietin, and plasma renin activity. The relationships between biomarkers, clinical and echocardiographic variables, and mortality were assessed.
Results:
After 15 years, 86 of the 106 patients (81%) had died. Multivariate analysis showed that ANP was the best independent predictor of survival over several clinical, echocardiographic, and humoral variables (hazard ratio: 5.62, 95% confidence interval: 3.37–9.39, P < 0.001 for plasma levels < median value of 71 pg/mL). Plasma renin activity and erythropoietin provided prognostic information in univariate analysis, but lost their predictive power when adjusted for covariates.
Conclusions:
The present study represents the longest available follow‐up of patients with CHF evaluating the prognostic power of multiple biomarkers. It shows that a simple assessment of plasma ANP levels is the strongest long‐term predictor of death in all stages of heart failure. Copyright © 2010 Wiley Periodicals, Inc.
This work was supported by a grant from MIUR 2001068248. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Introduction
During past years, assessment of cardiac and extracardiac endocrine activity gained popularity in risk stratification of patients with chronic heart failure (CHF). Indeed, a number of circulating peptides and neurohormones increase according to disease progression, reflecting the magnitude of cardiac dysfunction.1 Accordingly, plasma levels and activity of several biomarkers including norepinephrine,2 renin,3 endothelin and big‐endothelin,4 erythropoietin (EPO),5 atrial natriuretic peptide (ANP),6 and b‐type natriuretic peptide (BNP)7 have been shown to identify subjects at risk of death or worsening of CHF.
The prognostic value of natriuretic peptides has been studied in patients with CHF and acute and chronic coronary heart disease, and both ANP and BNP have been demonstrated to powerfully predict cardiovascular morbidity and mortality.7, 8, 9, 10, 11 Although previous studies have addressed the prognostic implications of elevated natriuretic peptide plasma levels in CHF over the midterm,6,9,12, 13, 14, 15 their very‐long‐term predictive value as compared with clinical, echocardiographic, and other neurohormonal markers has not been addressed. This information may prove valuable in the management of a chronic disease with a long natural history, as CHF is today.16, 17, 18
The aim of the present prospective follow‐up study was to assess the very‐long‐term prognostic power of a set of biomarkers to identify CHF patients at high risk for all‐cause mortality.
Methods
Study Patients
A total of 106 consecutive patients with CHF were recruited in the outpatient clinic for heart failure at the University Hospital of Naples (Italy) between 1989 and 1990. The experimental protocol was approved by the local ethical committee, and patients gave written, informed consent before entering the study.
The diagnosis of CHF was based on Framingham criteria.19 Post‐ischemic heart failure was diagnosed based on coronary angiography and clinical history of myocardial infarction. Patients were considered to have valve disease‐related CHF after other causes of heart failure were ruled out, and when moderate to severe valve dysfunction had been diagnosed before the onset of left ventricular (LV) dilation, impairment of ejection fraction (EF), or occurrence of CHF symptoms. Patients were considered to have idiopathic dilated cardiomyopathy (IDC) when no underlying causes of CHF, including patent abnormalities of coronary angiograms, could be demonstrated. Patients with unstable angina, end‐stage renal disease, and recent acute cardiac decompensation were excluded from the study.
Laboratory Methods
All blood samples were collected between 8 am and 9 am. Samples were collected on ice and spun immediately; plasma was then separated and frozen until the time of assay. Plasma renin activity (PRA) was measured by radioimmunoassay according to the method described by Menard and Catt20 (sensitivity, 50 pg per tube angiotensin I; intra‐assay and interassay variability coefficients 6% and 10%, respectively). Plasma immunoreactive ANP levels were determined by radioimmunoassay as previously described by our laboratory.21 The radioimmunoassay sensitivity was 1 fmol per tube. Plasma aldosterone concentrations were estimated by a radioimmunoassay using a commercial kit (DPC, Los Angeles, CA). Plasma EPO levels were measured by using a sensitive immunoenzymatic assay with a detection threshold of 1 mU/mL (Amgen Diagnostics, Thousand Oaks, CA). The intra‐assay and interassay coefficients of variation were 2.5% to 10% and 10% to 15%, respectively.22
Statistical Analysis
Baseline characteristics of the patients are presented as percentage for dichotomous variables and median value (range) for continuous variables. Verification of normal distribution of data was accomplished using histograms and Kolmogorov‐Smirnov test. Neurohormonal plasma concentrations showed a logarithmic normal distribution, and were consequently logarithmically transformed. Differences between median levels of plasma neurohormones among New York Heart Association (NYHA) classes and quartiles of EF were assessed using Kruskal‐Wallis test. A Pearson correlation analysis was performed between LV end‐diastolic diameter (LVDd), EF, and log‐neurohormonal plasma concentrations. Survival curves were estimated by means of the Kaplan‐Meier method. Differences in survival were compared using the log‐rank test. To evaluate the significance of predictors of all‐cause mortality, hazard ratios (HR) and 95 percent confidence intervals (CI) were derived from 2 Cox proportional‐hazards regression models (Model 1 and Model 2). Both multivariate models were fitted with baseline covariates associated with mortality by univariate analysis at the 0.1 significance level. Neurohormones where entered as continuous variables in Model 1 and as categorical variables in Model 2 (> vs < median values). A stepwise backward elimination analysis was applied for both models, and the P value for entering and staying in the model was set at < 0.05 and < 0.1, respectively. The proportional‐hazards assumption was checked by plotting Schoenfeld residuals summarized by a loess smoothing line against survival time for continuous variables, and by the log‐minus‐log test of proportionality for categorical variables. Both models were assessed for multicollinearity. All tests were 2‐sided, and a P value of < 0.05 was considered statistically significant. All calculations were generated using SPSS, version 15.0 (SPSS Inc., Chicago, IL).
Results
Table 1 shows the baseline clinical and echocardiographic characteristics, as well as plasma biomarkers, of the 106 patients in the study population.
Table 1.
Baseline Characteristics of the Study Population
| Characteristic | N = 106 |
|---|---|
| Age, y (range) | 56 (28–77) |
| Sex, male, n (%) | 85 (80.2) |
| Etiology of CHF, n (%) | |
| CAD | 63 (59.4) |
| IDC | 32 (30.2) |
| VHD | 11 (10.4) |
| NYHA class, n (%) | |
| I | 28 (26.4) |
| II | 27 (25.5) |
| III | 30 (28.3) |
| IV | 21 (19.8) |
| LVDd, mm, median (range) | 64 (43–87) |
| EF, %, median (range) | 35 (10–56) |
| Heart rate, bpm, median (range) | 72 (50–130) |
| HT, n (%) | 26 (24.5) |
| DM, n (%) | 21 (19.8) |
| Creatinine, mg/dL, median (range) | 1.1 (0.7–1.6) |
| ANP, pg/mL, median (range) | 71 (2–971) |
| EPO, mU/mL, median (range) | 4.4 (1–131) |
| Aldosterone, pg/mL, median (range) | 88 (10–1002) |
| PRA, ng/mL/h, median (range) | 2.6 (0.3–26) |
| CHF treatment, n (%) | |
| ACEIs | 51 (48.1) |
| Cardiac glycosides | 32 (30.2) |
| Diuretics | 64 (60.4) |
| Nitrates | 26 (24.5) |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ANP, atrial natriuretic peptide; CAD, coronary artery disease; CHF, chronic heart failure; DM, diabetes mellitus; EF, ejection fraction; EPO, erythropoietin; HT, hypertension; IDC, idiopathic dilated cardiomyopathy; LVDd, left ventricular end‐diastolic diameter; NYHA, New York Heart Association; PRA, plasma renin activity; VHD, valvular heart disease
All patients were clinically stable and kept on optimal medical treatment for CHF according to the therapeutic options available during the period of follow‐up. Of note, use of β‐blockers was not recommended for the treatment of CHF when the study began and was therefore not recorded at study entry.
Patients were relatively young, with a median age of 56 years, and predominantly male. The most common etiology of CHF was coronary artery disease (59.4%), and median left ventricular ejection fraction (LVEF) was 35%. In general, patients with advanced LV dysfunction and more severe symptoms tended to have higher levels of plasma biomarkers than did patients with less‐severe disease. However, whereas PRA, ANP, and EPO showed a clear stepwise increase according to the progression of NYHA class and impairment of EF (Figure 1), aldosterone did not. As shown in Table 2 and Figure 2, ANP levels exhibited the most significant correlation with LVDd and EF.
Figure 1.
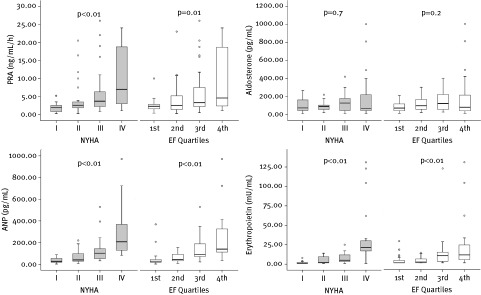
PRA, aldosterone, ANP, and EPO plasma levels according to NYHA class and quartiles of EF (first: > 44%; second: 34%–44%; third: 24%–33%; fourth: < 24%). Significances are by Kruskal‐Wallis test. Abbreviations: ANP, atrial natriuretic peptide; EF, ejection fraction; EPO, erythropoietin; NYHA, New York Heart Association; PRA, plasma renin activity
Table 2.
Correlation Between Echocardiographic Variables and Biomarkers
| Variable | PRA, ln | ANP, ln | Aldosterone, ln | EPO, ln |
|---|---|---|---|---|
| EF (%) | r = −0.36, P = <0.001 | r = −0.60, P = <0.001 | r = −0.11, P = 0.25 | −0.42, P = <0.001 |
| LVDd (mm) | r = 0.18, P = 0.05 | r = 0.42, P = <0.001 | r = 0.03, P = 0.74 | 0.14, P = 0.13 |
Abbreviations: ANP, atrial natriuretic peptide; EF, ejection fraction; EPO, erythropoietin; LVDd, left ventricular end‐diastolic diameter; PRA, plasma renin activity
Figure 2.
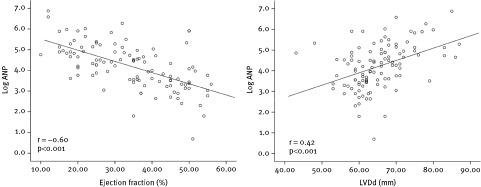
Correlation between plasma ANP levels, LVDd, and EF. Significances are by Pearson correlation. Abbreviations: ANP, atrial natriuretic peptide; EF, ejection fraction; LVDd, left ventricular end‐diastolic diameter
After 15 years of follow‐up, 86 of the 106 patients (81.1%) had died. Detailed information on the causes of death were available for almost all patients. Deaths were attributed to progression of CHF (56.9%), sudden death (11.7%), acute myocardial infarction (6.9%), cardiogenic shock (3.5%), acute pulmonary edema (2.3%), pneumonia (2.3%), end‐stage renal disease (1.2%), stroke (1.2%), pulmonary embolism (1.2%), other noncardiac causes (10.5%), and nondefined causes (2.3%).
Mortality rates were significantly higher in patients with baseline ANP and EPO levels above the median value (71 pg/mL for ANP and 4.4 mU/mL for erythropoietin), with clear and early separation of curves and marked stepwise increase in the cumulative incidence of mortality (Figure 3C,D). On the contrary, baseline PRA and aldosterone did not stratify long‐term prognosis (Figure 3A,B).
Figure 3.
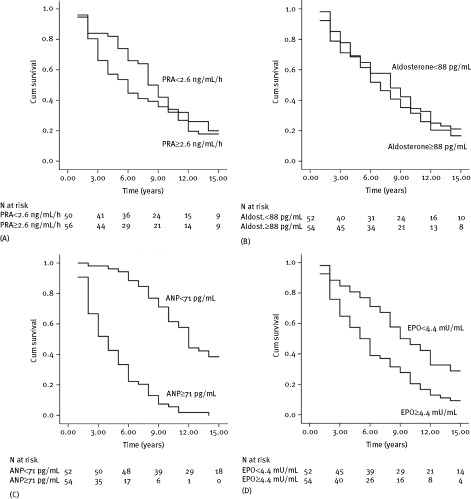
Overall survival among patients with CHF according to median plasma levels of (A) PRA, (B) aldosterone, (C) ANP, and (D) EPO. P < 0.001 for ANP and P < 0.01 for EPO by the log rank test. Abbreviations: Aldost., aldosterone; ANP, atrial natriuretic peptide; CHF, chronic heart failure; Cum, cumulative; EPO, erythropoietin; PRA, plasma renin activity
Among all of the available clinical variables, age, NYHA class, LVEF, LVDd, heart rate, use of diuretics at study entry, PRA (as continuous variable), EPO, and ANP (both as continuous and categorical variables) emerged as predictors of death by univariate analysis (Table 3).
Table 3.
Hazard Ratios for All‐Cause Mortality in Univariate and Multivariate Analyses
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variable | Unadjusted HR (95% CI) | P Value | Model 1, Adjusted HR (95% CI) | P Value | Model 2, Adjusted HR (95% CI) | P Value |
| Age | 1.06 (1.03–1.09) | <0.001 | 1.02 (1.0–1.05) | 0.03 | 1.05 (1.02–1.08) | <0.001 |
| DMa | 1.65 (0.99–2.73) | 0.05 | … | NS | … | NS |
| CHF etiologyb | 1.49 (0.95–2.33) | 0.07 | … | NS | … | NS |
| CHF severity (NYHA)c | 2.94 (1.89–4.56) | <0.001 | 1.73 (1.05–2.85)d | NS | 1.79 (1.12–2.87) | 0.01 |
| LVEF | 0.95 (0.93–0.97) | <0.001 | 0.96 (0.94–0.98)d | <0.01 | 0.96 (0.94–0.98)d | NS |
| LVDd | 1.06 (1.03–1.09) | <0.001 | NA | NA | NS | |
| Heart rate | 1.03 (1.01–1.04) | 0.002 | … | NS | … | NS |
| Use of diureticse | 2.78 (1.73–4.44) | <0.001 | … | NS | … | NS |
| Log ANP | 4.15 (2.60–6.61) | <0.001 | 3.15 (2.34–4.25) | <0.001 | NA | |
| Log EPO | 1.46 (1.22–1.74) | <0.001 | d | NS | NA | |
| Log PRA | 1.23 (0.96–1.56) | 0.08 | … | NS | NA | |
| ANP (categorical)f | 6.23 (3.80–10.20) | <0.001 | NA | 5.62 (3.37–9.39) | <0.001 | |
| EPO (categorical)g | 1.97 (1.28–3.04) | <0.01 | NA | d | NS | |
Abbreviations: ANP, atrial natriuretic peptide; CI, confidence interval; DM, diabetes mellitus; EF, ejection fraction; EPO, erythropoietin; HR, hazard ratio; LVDd, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; NA, not applicable; NS, not significant; NYHA, New York Heart Association; PRA, plasma renin activity.
Presence vs absence of diabetes.
Ischemic vs nonischemic etiology.
NYHA classes III and IV vs NYHA classes I and II.
Hazard ratios are given for Model 1 and Model 2 not incorporating ANP as a covariate (high collinearity with EPO).
Presence vs absence of diuretics in drug therapy.
ANP > vs ≤ median value (71 pg/mL).
EPO > vs ≤ median value (4.4 mU/mL)
Independent predictors of all‐cause mortality at 15 years were calculated from 2 different Cox regression models. As ANP and EPO showed high correlation (r = 0.54, P < 0.01), they were considered mutually exclusive and separately entered into the models. Although EF showed significant correlation with ANP plasma levels (r = − 0.60, P < 0.001), we decided to keep the variable in the model, as EF is a well‐known predictor of death in CHF.
When the first Cox regression model (Model 1) was fitted with all of the covariates predictive of mortality at the 0.1 significance level by univariate analysis, only plasma ANP and age independently predicted long‐term survival, with ANP emerging as by far the most powerful independent predictor of all‐cause mortality (Table 3). ANP and age independently predicted death even when the neurohormone was entered into the second multivariable model (Model 2) as a dichotomous variable, with NYHA class emerging as a significant independent predictor of all‐cause mortality (Table 3). After exclusion of ANP, EPO did not independently predict survival, whereas EF emerged as a significant independent predictor in both models (Table 3). Excluding from the analysis patients with atrial fibrillation (n = 13), which has an independent influence on ANP plasma levels, did not substantially affect ANP HRs (3.28, 95% CI: 2.4–4.49, P < 0.001 in Model 1, and 3.79, 95% CI: 2.00–7.20, P < 0.001 in Model 2).
Discussion
The main finding in our study is that baseline ANP provides the strongest long‐term prognostic information on all‐cause mortality in patients with mild to severe CHF. Indeed, while other biomarkers did not predict survival (aldosterone) or failed to provide prognostic information when adjusted for other covariates (PRA and EPO), ANP predicted death from any cause independently from other well‐known prognosticators.
Among a number of studies aimed to determine the prognostic value of various biomarkers in CHF, ANP consistently was shown to predict survival over the midterm.6,9,12,14,15 However, this is the first time that a biomarker is reported to predict survival in CHF better than any other clinical or functional predictors over such a long observational period. In a follow‐up study on a large population of CHF patients, Berger et al15 found the N‐terminal fragment N‐ANP to be the best independent predictor of a combined endpoint of death and urgent cardiac transplantation at 2 and 3 years. Of note, when the full spectrum of CHF severity was considered, N‐ANP showed to be superior to BNP in predicting the outcome. These findings are consistent with those of Selvais et al,23 who reported that N‐proANP was better than BNP in predicting 3‐year mortality. In a direct comparison of N‐ANP, N‐BNP, and BNP, Hülsmann et al6 reported that mortality‐risk estimation was similar for the 3 peptides. However, mortality after 43 months was 0 in patients with normal N‐ANP levels, being 4% and 6% in patients with normal values of N‐BNP and BNP, respectively.6
In general, comparison among biomarkers is difficult due to differences in study design and populations. Therefore, single biomarkers may be ranked differently according to the clinical stage of the patients and the length of the follow‐up.15 This likely reflects differences in the pathophysiology of each biomarker. Plasma ANP mostly reflects atrial‐wall stretch, but its secretion from the ventricles increases according to the progression of CHF. On the contrary, BNP is secreted from the ventricles.24 With progression of CHF, LV dysfunction couples with additional factors, such as mitral regurgitation and worsening of diastolic dysfunction, that may increase more atrial than ventricular wall stress. This may explain why BNP powerfully predicts the short‐term risk of sudden death in milder stages of CHF, whereas N‐ANP takes over in predicting death owing to progressive heart failure in more diseased subjects and in longer follow‐up. In our study population, the rate of sudden death is lower than expected based on other studies in CHF patients.25, 26, 27 However, our population comprises a considerable number of patients with severe symptoms, who are known to be more prone to die from progressive heart failure rather than sudden death.28,29
In 1994, we first reported that plasma EPO increases according to NYHA class progression in patients with heart failure.22 In 2 previous analyses on small populations, plasma EPO independently predicted survival in CHF patients over 2 to 3 years.5,30 In our study, erythropoietin predicted survival only in univariate analysis, losing its prognostic power when adjusted for other covariates. Marked difference in the length of follow‐up is the most convincing explanation for this discrepancy.
As far as the length of the observational period is concerned, data on the very‐long‐term prognostic value of biomarkers in CHF are sparse. Alehagen et al31 reported that N‐ANP, N‐BNP, and BNP provide long‐term (ie, 6 yrs) prognostic information on the risk of cardiovascular death in a population of subjects with “possible” heart failure. Unlike our present analysis, < 50% of patients in that study had either systolic or diastolic dysfunction according to Doppler echocardiography.31 In one of the few long‐term follow‐up studies on neurohormonal prognosticators, Van Beneden et al4 found that big endothelin‐1 and endothelin‐1 were superior to natriuretic peptides in predicting survival over a 7‐year follow‐up. However, this was true only for a small subgroup of selected patients with severe CHF (NYHA classes III and IV).
Different studies have reported an improvement in survival from CHF over the past decades.32, 33, 34, 35 As the natural history of CHF is progressively prolonging, its epidemiological impact is gradually increasing. In this view, the availability of biomarkers that may help long‐term prognostic stratification will certainly assist the complex clinical approach and management of the disease.
Study Limitations
Some limits of our study need to be mentioned. First, as a reflection of the length of the observational period, treatment standards for CHF have been markedly changing throughout the time course of our study, and patient therapy has been gradually updated with the introduction of β‐blockers and aldosterone antagonists, up‐titration of angiotensin‐converting enzyme inhibitors, as well as the introduction of cardiac resynchronization therapy and implantable cardioverter‐defibrillators.
Second, we assessed plasma levels of the C‐terminal fragment ANP and not N‐ANP. However, the correlation between ANP and N‐ANP is very high, and ANP has been reported to lose its prognostic power in multivariate models only after adjustment for N‐ANP.36
Finally, we did not assess BNP, because at the end of the 1980s the scientific community was not clearly aware of the role of BNP in the pathophysiology of CHF. However, ANP and BNP act differently in specific disease states24,37,38 and provide independent prognostic information according to disease progression and mode of death.9,12,13,23,39
Conclusion
This study represents the longest available prospective follow‐up of patients with CHF assessing the prognostic implications of a set of biomarkers in comparison with multiple well‐known predictors of death. It brings up the evidence that a simple assessment of ANP plasma levels is the strongest predictor of death in all stages of heart failure over the very long term.
References
- 1. Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med 1999;341:577–585. [DOI] [PubMed] [Google Scholar]
- 2. Madsen BK, Keller N, Christiansen E, et al. Prognostic value of plasma catecholamines, plasma renin activity, and plasma atrial natriuretic peptide at rest and during exercise in congestive heart failure: comparison with clinical evaluation, ejection fraction, and exercise capacity. J Card Fail 1995;1:207–216. [DOI] [PubMed] [Google Scholar]
- 3. Latini R, Masson S, Anand I, et al. The comparative prognostic value of plasma neurohormones at baseline in patients with heart failure enrolled in Val‐HeFT. Eur Heart J 2004;25:292–299. [DOI] [PubMed] [Google Scholar]
- 4. Van Beneden R, Gurné O, Selvais PL, et al. Superiority of big endothelin‐1 and endothelin‐1 over natriuretic peptides in predicting survival in severe congestive heart failure: a 7‐year follow‐up study. J Card Fail 2004;10:490–495. [DOI] [PubMed] [Google Scholar]
- 5. George J, Patal S, Wexler D, et al. Circulating erythropoietin levels and prognosis in patients with congestive heart failure: comparison with neurohormonal and inflammatory markers. Arch Intern Med 2005;165:1304–1309. [DOI] [PubMed] [Google Scholar]
- 6. Hülsmann M, Berger R, Mörtl D, et al. Incidence of normal values of natriuretic peptides in patients with chronic heart failure and impact on survival: a direct comparison of N‐terminal atrial natriuretic peptide, N‐terminal brain natriuretic peptide and brain natriuretic peptide. Eur J Heart Fail 2005;7:552–556. [DOI] [PubMed] [Google Scholar]
- 7. Doust JA, Pietrzak E, Dobson A, et al. How well does B‐type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review. BMJ 2005;330:625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. James SK, Lindahl B, Siegbahn A, et al. N‐terminal pro‐brain natriuretic peptide and other risk markers for the separate prediction of mortality and subsequent myocardial infarction in patients with unstable coronary artery disease: a Global Utilization of Strategies To Open occluded arteries (GUSTO)‐IV substudy. Circulation 2003;108:275–281. [DOI] [PubMed] [Google Scholar]
- 9. Berger R, Hülsmann M, Strecker K, et al. Neurohormonal risk stratification for sudden death and death owing to progressive heart failure in chronic heart failure. Eur J Clin Invest 2005;35:24–31. [DOI] [PubMed] [Google Scholar]
- 10. Kragelund C, Grønning B, Køber L, et al. N‐terminal pro‐B‐type natriuretic peptide and long‐term mortality in stable coronary heart disease. N Engl J Med 2005;352:666–675. [DOI] [PubMed] [Google Scholar]
- 11. Suzuki S, Yoshimura M, Nakayama M, et al. Plasma level of B‐type natriuretic peptide as a prognostic marker after acute myocardial infarction: a long‐term follow‐up analysis. Circulation 2004;110:1387–1391. [DOI] [PubMed] [Google Scholar]
- 12. Hülsmann M, Berger R, Sturm B, et al. Prediction of outcome by neurohumoral activation, the six‐minute walk test and the Minnesota Living with Heart Failure Questionnaire in an outpatient cohort with congestive heart failure. Eur Heart J 2002;23:886–891. [DOI] [PubMed] [Google Scholar]
- 13. Berger R, Hülsmann M, Strecker K, et al. B‐type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation 2002;105:2392–2397. [DOI] [PubMed] [Google Scholar]
- 14. Yan RT, White M, Yan AT, et al; Randomized Evaluation of Strategies for Left Ventricular Dysfunction (RESOLVD) Investigators. Usefulness of temporal changes in neurohormones as markers of ventricular remodeling and prognosis in patients with left ventricular systolic dysfunction and heart failure receiving either candesartan or enalapril or both. Am J Cardiol 2005;96:698–704. [DOI] [PubMed] [Google Scholar]
- 15. Berger R, Strecker K, Hülsmann M, et al. Prognostic power of neurohumoral parameters in chronic heart failure depends on clinical stage and observation period. J Heart Lung Transplant 2003;22:1037–1045. [DOI] [PubMed] [Google Scholar]
- 16. Kannel WB. Incidence and epidemiology of heart failure. Heart Fail Rev 2000;5:167–173. [DOI] [PubMed] [Google Scholar]
- 17. Barker WH, Mullooly JP, Getchell W. Changing incidence and survival for heart failure in a well‐defined older population, 1970–1974 and 1990–1994. Circulation 2006;113:799–805. [DOI] [PubMed] [Google Scholar]
- 18. Djoussé L, Kochar J, Gaziano JM. Secular trends of heart failure among US male physicians. Am Heart J 2007;154:855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971;285:1441–1446. [DOI] [PubMed] [Google Scholar]
- 20. Preibisz JJ, Sealey JE, Aceto RM, et al. Plasma renin activity measurements: an update. Cardiovasc Rev Rep 1982;5:787–804. [Google Scholar]
- 21. Indolfi C, Piscione F, Volpe M, et al. Cardiac effects of atrial natriuretic peptide in subjects with normal left ventricular function. Am J Cardiol 1989;63:353–357. [DOI] [PubMed] [Google Scholar]
- 22. Volpe M, Tritto C, Testa U, et al. Blood levels of erythropoietin in congestive heart failure and correlation with clinical, hemodynamic, and hormonal profiles. Am J Cardiol 1994;74:468–473. [DOI] [PubMed] [Google Scholar]
- 23. Selvais PL, Robert A, Ahn S, et al. Direct comparison between endothelin‐1, N‐terminal proatrial natriuretic factor, and brain natriuretic peptide as prognostic markers of survival in congestive heart failure. J Card Fail 2000;6:201–207. [DOI] [PubMed] [Google Scholar]
- 24. Yasue H, Yoshimura M, Sumida H, et al. Localization and mechanism of secretion of B‐type natriuretic peptide in comparison with those of A‐type natriuretic peptide in normal subjects and patients with heart failure. Circulation 1994;90:195–203. [DOI] [PubMed] [Google Scholar]
- 25. Yap YG, Duong T, Bland M, et al. Temporal trends on the risk of arrhythmic vs non‐arrhythmic deaths in high‐risk patients after myocardial infarction: a combined analysis from multicentre trials. Eur Heart J 2005;26:1385–1393. [DOI] [PubMed] [Google Scholar]
- 26. Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med 1996;335:1933–1940. [DOI] [PubMed] [Google Scholar]
- 27. Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced Titcolor [[check]] ejection fraction. Multicenter Automatic Defibrillator Implantation Trial II Investigators. N Engl J Med 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 28. Bardy GH, Lee KL, Mark DB, et al; Sudden Cardiac Death in Heart Failure Trial (SCD‐HeFT) Investigators. Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure [published correction appears in N Engl J Med. 2005;352:2146]. N Engl J Med 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 29. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT‐HF). Lancet 1999;353:2001–2007. [PubMed] [Google Scholar]
- 30. Van der Meer P, Voors AA, Lipsic E, et al. Prognostic value of plasma erythropoietin on mortality in patients with chronic heart failure. J Am Coll Cardiol 2004;44:63–67. [DOI] [PubMed] [Google Scholar]
- 31. Alehagen U, Svensson E, Dahlström U. Natriuretic peptide biomarkers as information indicators in elderly patients with possible heart failure followed over six years: a head‐to‐head comparison of four cardiac natriuretic peptides. J Card Fail 2007;13:452–461. [DOI] [PubMed] [Google Scholar]
- 32. Levy D, Kenchaiah S, Larson MG, et al. Long‐term trends in the incidence of and survival with heart failure. N Engl J Med 2002;347:1397–1402. [DOI] [PubMed] [Google Scholar]
- 33. Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community‐based population. JAMA 2004;292:344–350. [DOI] [PubMed] [Google Scholar]
- 34. MacIntyre K, Capewell S, Stewart S, et al. Evidence of improving prognosis in heart failure: trends in case fatality in 66 547 patients hospitalized between 1986 and 1995. Circulation 2000;102:1126–1131. [DOI] [PubMed] [Google Scholar]
- 35. Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251–259. [DOI] [PubMed] [Google Scholar]
- 36. Omland T, Bonarjee VV, Nilsen DW, et al. Prognostic significance of N‐terminal pro‐atrial natriuretic factor (1‐98) in acute myocardial infarction: comparison with atrial natriuretic factor (99‐126) and clinical evaluation. Br Heart J 1993;70:409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dzimiri N, Moorji A, Afrane B, et al. Differential regulation of atrial and brain natriuretic peptides and its implications for the management of left ventricular volume overload. Eur J Clin Invest 2002;32:563–569. [DOI] [PubMed] [Google Scholar]
- 38. Yoshimura M, Yasue H, Okumura K, et al. Different secretion patterns of atrial natriuretic peptide and brain natriuretic peptide in patients with congestive heart failure. Circulation 1993;87:464–469. [DOI] [PubMed] [Google Scholar]
- 39. Stanek B, Frey B, Hülsmann M, et al. Prognostic evaluation of neurohumoral plasma levels before and during beta‐blocker therapy in advanced left ventricular dysfunction. J Am Coll Cardiol 2001;38:436–442. [DOI] [PubMed] [Google Scholar]


