Abstract
Late intracardiac lead perforation is defined as migration and perforation of an implanted lead after 1 month of cardiac electronic device implantation. It is an under‐recognized complication with significant morbidity and mortality, particularly if not recognized early. Two patients with late perforation caused by passive‐fixation leads are reported and the clinical features of their presentation and management are reviewed. We conducted a thorough review of the available English language literature pertaining to this complication to draw relevant conclusions regarding presentation, diagnosis, and management. Early recognition of this complication is important as the indications for and numbers of patients who receive cardiac implantable electronic devices continue to expand. Copyright © 2010 Wiley Periodicals, Inc.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
Perforation by an implanted lead of a cardiac implantable electronic device is a complication with an incidence of < 1%. Late perforation of such leads is defined as the perforation of the lead through the myocardium more than 1 month after implantation.1, 2, 3, 4, 5, 6, 7 Late lead perforation is an often underdiagnosed complication that might entail significant morbidity with potentially catastrophic consequences. We report two representative cases that presented with late perforation of the right ventricle, and we review the available literature specific to late lead perforation to identify important clinical clues and associations that might aid in the early diagnosis of this complication.
Case 1
In 2002, a 75‐year‐old man with hypertension, moderate aortic stenosis, and left ventricular hypertrophy underwent implantation of a dual chamber pacemaker (Vitatron Diamond 3 DDDR Model 840; Medtronic, Minneapolis, MN) for symptomatic complete heart block at an outside hospital. An active fixation lead was implanted in the right atrium (Vitatron Model ICF 09; Medtronic) and a tined passive fixation (Vitatron Model IMK49B; Medtronic) was implanted in the apex of the right ventricle. At the time of implantation, all lead parameters were within normal limits and there was no diaphragmatic stimulation at maximal output.
Two months after implantation the patient developed intermittent chest pain. He presented to the hospital's emergency department where his initial physical examination was unremarkable. A chest x‐ray showed a left pleural effusion (Figure 1A). A pleural tap revealed the effusion to be hemorrhagic in nature: red blood cell count 18400/µl; white blood cell count 2060/µl; and glucose of 76 mg/dL. Computed tomography (CT) without contrast of the chest revealed that the right ventricular lead was outside the cardiac silhouette (Figure 1B). Because the lead had migrated out of the pericardium, the risk of vascular or pulmonary injury upon extraction was felt prohibitive to a transvenous removal. The patient underwent open chest surgical lead extraction that confirmed that the lead had protruded through the right ventricular wall to the pleural cavity. Even though transvenous extraction of a passive fixation lead has been described, we were concerned that the bulky tip of the lead might cause further damage to cardiac, pulmonary, or vascular tissue during removal. Furthermore, surgical lead extraction provides the ability to directly visualize and repair the site of perforation and to deal with injury to the adjacent structures.
Figure 1.
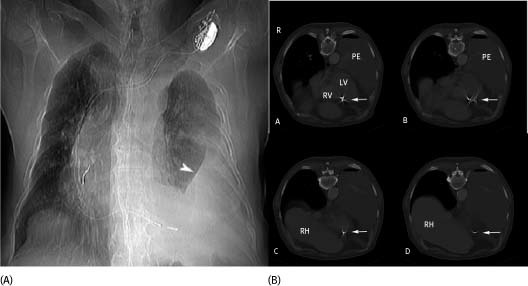
(A) A single view chest x‐ray. A dual lead pacemaker is in situ. There is a large left pleural effusion (arrowhead) with rightward shift of the mediastinum. In this image, it is not clear that the right ventricular lead has perforated the ventricular wall. (B) Shows selected axial images from the CT. In B‐A and B‐B, the right ventricular pacer lead (arrow) appears to extend beyond the right ventricular (RV) cavity. Again seen is the large left pleural effusion (PE). In subsequent sections the right ventricular lead is seen clearly removed from the ventricular cavity (C) and is indeed extracardiac (D). The right hemidiaphragm and liver are now in the section (RH)
The postoperative course was complicated by infection and inability to wean from mechanical ventilation and vasopressor support. After a protracted 5‐month hospital course the patient succumbed to multiorgan failure.
Case 2
In 2008, 3 years after receiving a cardiac resynchronization therapy capable implantable cardioverter defibrillator for primary prophylaxis against sudden cardiac death at an outside hospital, a 60‐year‐old female with ischemic cardiomyopathy (ejection fraction, 25%; left ventricular end diastolic diameter, 7.4 cm) presented to the device clinic for a routine checkup. Episodes of noise were noted on the right ventricular electrogram obtained through the tined, integrated, defibrillator lead (Endotak Model 0125; Guidant Corp., St Paul, MN). The patient was asymptomatic at that time. The episodes of noise could not be reproduced by valsalva or pocket manipulation, but they reproducibly appeared, albeit transiently, on deep inspiration (Figure 2A). In retrospect, right ventricular perforation was evident on a CT scan that she had received 9 months prior for an episode of diverticulitis (Figure 2B). The lead was extracted transvenously with transesophageal echocardiographic (TEE) monitoring. A new right ventricular lead was reimplanted in the same setting with no complications.
Figure 2.
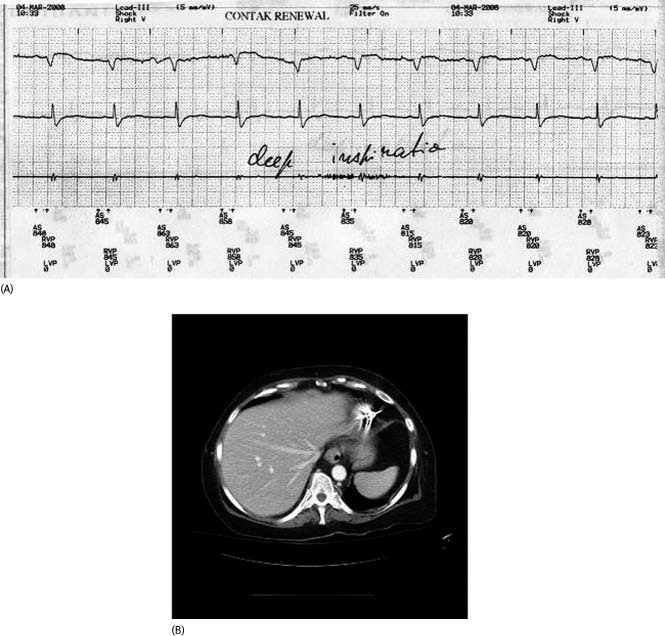
(A) This shows episodes of noise on the right ventricular lead that appeared on deep inspiration in a patient with a late lead perforation. (B) Shown is a computerized tomography scan (sagittal view) of the heart confirming perforation of the right ventricular apex with the right ventricular lead tip
Methods
Literature Review
We searched for case reports, case series, and review articles in MEDLINE from 1980 to 2009 as primary references using the Medical Subject Headings terms “lead perforation,” “pacemakers,” “defibrillators,” and “defibrillators, implantable.” Although most perforations are recognized during or shortly after implantation, we focused our search on late lead perforation (detected more than 1 month after implant). Subsequently, we identified secondary references from the primary references.
Results
We searched PubMed and found 51 reports that would fit the definition of late lead perforation. Table 1 summarizes relevant reported cases with presentation, type, and characteristics of the offending lead and timing from lead implant.
Table 1.
Peer‐Reviewed Studies Evaluating Published Cases of Late Lead Perforation
| Author | Patient Characteristics and Presentation | Indication, Time From Implant, Type of Implant, and Lead Mechanism | Key Findings and Investigationsa | Offending Lead, Intervention |
|---|---|---|---|---|
| Mortensen et al11 | 66‐year‐old female, dizziness, chest pain, and dyspnea | Sick sinus syndrome, 6 weeks, dual chamber pacemaker, active fixation | ECG, CXR (Left‐sided hemothorax), Cath lab fluoroscopy, echocardiogram | Ventricular lead, surgically removed |
| Haq et al1 | 86‐year‐old male, palpitations | Syncope, paroxysmal atrial fibrillation, and symptomatic bradycardia; 16 months; pacemaker; active fixation | Device interrogation (failure of the ventricular lead to capture and sense), CXR, echocardiogram (pericardial effusion), chest CT (pleural effusion) | Ventricular lead surgically removed via an open sternotomy |
| Krivan et al12 | 47‐year‐old female, asymptomatic | LQT syndrome and history of ventricular fibrillation, between 30th and 58th days, ICD, active fixation | Device interrogation, CXR, echocardiogram, and chest CT | Sternotomy and surgical removal of the right ventricular ICD lead |
| Fisher et al13 | 71‐year‐old female, asymptomatic | Nonischemic cardiomyopathy, 38 days, ICD, active fixation | Device interrogation CXR, fluoroscopy, and chest CT | Sternotomy and surgical removal of the right ventricular ICD lead |
| Satpathy et al15 | 72‐year‐old female on steroids, recurrent shocks | Nonischemic cardiomyopathy, 10 months, ICD, active fixation | ECG, Device interrogation, echocardiogram, CXR (angulation and displacement of the ventricular lead tip), chest CT | Surgical removal in the operating room under TEE guidance |
| Park et al16 | 72‐year‐old male, chest discomfort | Ischemic cardiomyopathy and ventricular tachycardia, 6 weeks, ICD, active fixation | Device interrogation CXR, TTE (small pericardial effusion), chest CT (large left pleural effusion) | Emergent transvenous explantation of the device and lead |
| Sakai et al17 | 74‐year‐old male, sharp chest pain | VT in a patient with isolated noncompaction of the ventricular myocardium, 4 weeks, ICD, active fixation | CXR (malpositioning of the RV lead with its tip outside the cardiac silhouette), echocardiogram | Surgical removal of the ICD lead |
| Suri et al19 | N/A, asymptomatic | N/A, 6 weeks, ICD, active fixation | Device interrogation (undersensing on device interrogation), TTE (small pericardial effusion) | Transvenous lead extraction |
| Lopes et al20 | 76‐year‐old female, recurrent syncope | N/A, 2 months, pacemaker, passive fixation | Device interrogation, fluoroscopy | Transvenous lead removal |
| Toal et al21 | 64‐year‐old male, fatigue | Hypertrophic cardiomyopathy, 6 weeks, ICD, active fixation | Device interrogation (sinus rhythm EGM between lead tip to adjacent ring electrode showed an injury current), TTE (trivial pericardial effusion), volume‐rendered cardiac CT | Ventricular lead was transvenously repositioned and injury potential disappeared |
| Laborderie et al22 | 81‐year‐old female, swelling and discoloration of the left breast | N/A, 3 months, pacemaker, active fixation | Device interrogation CXR, TTE, chest CT | Transvenous lead extraction |
| Polin et al9 | 38‐year‐old male, ICD; 55‐year‐old male, ICD; 79‐year‐old female, pacemaker; 85‐year‐old male, pacemaker; 88‐year‐old male, pacemaker; cardiac tamponade in all 5 patients; 2 patients were on Coumadin with therapeutic INR | N/A; 1054, 43, 116, 137, and 433 days, respectively; active fixation leads | Device interrogation, CXR, TTE | Ventricular lead in first 4, atrial lead in last; surgical extraction in 1 hemodynamically unstable patient; transvenous lead replacement in 1 other patient with altered lead parameters; other leads left in place; 3 patients had pericardiocentesis and 1had a pericardial window |
| cardiac tamponade in all 5 patients; 2 patients were on Coumadin with therapeutic INR | ||||
| Khan et al5 | 26‐year‐old female, pleuritic chest pain; 71‐year‐old male, pleuritic chest pain; 84‐year‐old male, asymptomatic | Dual chamber pacemaker for vasoepressor syncope, single chamber ICD for ischemic cardiomyopathy and VT, and dual chamber pacemaker for sinus node dysfunction; 10, 8, and 6 months after implant, respectively; all active fixation leads. | Device interrogation, CXR | Right atrial lead in 2 patients and right ventricular lead in 1, transvenous lead extraction under TEE guidance |
| Sanoussi et al7 | 79‐year‐old female, chest pain | Sick sinus syndrome, 1 month, pacemaker, passive fixation | ECG, CXR, TTE, chest CT; lead found to have migrated to subcutaneous fat under the left breast | Ventricular lead, staged extraction: cutting the tined lead's tip first through a small incision in the left submammary fold followed by transvenous extraction of the lead |
| Akyol et al4 | 24‐year‐old male, decompensated heart failure | Bradycardia due to infrahisian block in the setting of myotonic muscular dystrophy and dilated cardiomyopathy, 6 months, pacemaker, active fixation | ECG, Device interrogation (no ventricular capture and no intracardiac electrogram in the ventricular channel); CXR, TTE, CT abdomen, US abdomen (ventricular lead in the abdomen) | Ventricular lead, transvenous lead extraction, patient died 10 days after lead extraction |
| Ellenbogen et al6 | 72‐year‐old female; atypical chest pain, hemoptysis and dyspnea | Polymorphic VT and cardiomyopathy, 1 month, ICD active fixation | Device interrogation (RV lead exit block), CXR, RV ICD lead migrated into the lung chest CT (hemothorax), TTE (pericardial effusion) | RV lead repositioned in the RV outflow tract |
| Kautzner et al23 | 36‐year‐old female, pleuritic chest pain | Syncope and polymorphic ventricular tachycardia, 23 months, ICD, active fixation | Device interrogation, fluoroscopy, right ventriculography | Surgical removal of the lead, suture of the perforation, epicardial patch electrode placement |
| Trigano et al24 | N/A, atrial perforation with penetration of the thoracic wall | N/A, 2 years, pacemaker, Accufix atrial J lead | Device interrogation (normal sensing and pacing parameters), digital fluoroscopy (fracture with extrusion of a short segment of the retention wire) | Surgical atrial lead removal |
| Ramirez et al3 | 68‐year‐old female, intermittent twitching pain at the left subcostal area | Sick sinus syndrome, 1 month, pacemaker implant, type of lead N/A | Device interrogation (loss of sensing and capture of the ventricular lead), fluoroscopy, echocardiography (lead perforated beyond RV apex to left hemidiaphragm) | Thoracotomy, surgical lead extraction, and repair of the perforation |
| Amara et al29 | 82‐year‐old female, left thoracic pain, lifted heavy object 2 weeks after implant | Atrioventricular block, 1 month, Pacemaker, active fixation | Device interrogation (loss of pacing of the ventricular lead and small electrograms), fluoroscopy, chest CT (lead perforated), TTE | Transvenous lead extraction and repositioning to interventricular septum, patient had cardiac tamponade after procedure, had pericardiocentesis |
| Haghjoo et al30 | 37‐year‐old male, chest pain radiating to his back | Idiopathic dilated cardiomyopathy, syncope and monomorphic ventricular tachycardia, 6 weeks, single‐chamber ICD, active fixation | Device interrogation (loss of capture even with maximum output), fluoroscopy, echocardiography (extracardiac intrapericardial location of the lead) | Transvenous lead repositioning to RV high septal region |
| Ferrero‐de‐Loma‐Osorio et al31 | 27‐year‐old female with congenital LQT syndrome, sudden loss of consciousness | Ventricular tachycardia, 4 weeks, ICD, active fixation | Fluoroscopy, echocardiography (ICD lead perforating RV apex) | Median sternotomy, RV apex sutured, ICD lead repositioned to middle septum |
| Tziakas et al32 | 84‐year‐old female; fatigue, dyspnea, and twitching pain of left hemidiaphragm | Complete AV block and syncope, 5 weeks, dual‐chamber pacemaker, type of lead N/A | ECG, device interrogation (loss of ventricular sense and capture), fluoroscopy, echocardiography, chest CT (RV lead perforated myocardium, pericardial cavity, diaphragm, and reached abdominal wall near the stomach) | Median sternotomy and surgical lead explantation, DDD epicardial pacemaker implantation |
| Danik et al33 | 53‐year‐old male, dyspnea | Nonischemic cardiomyopathy, 4 weeks, ICD, type of lead N/A | Device interrogation (high pacing threshold), echocardiography (moderate to large pericardial effusion) | Pericardiocentesis performed, transvenous lead repositioning |
| Celik et al25 | 73‐year‐old male; incessant hiccups | Bradycardia and near‐syncope, 2 years, single chamber pacemaker, passive fixation tined tip | Fluoroscopy, chest CT (tip of RV lead dislodged to epicardial fat layer), echocardiography | Median sternotomy and surgical lead extraction |
Abbreviations: AV, atrioventricular; CT, computerized tomography; CXR, chest x‐ray; ECG, electrocardiogram, EGM, electrogram; ICD, implantable cardioverter‐defibrillator; INR, international normalized ratio; LQT, Long QT; N/A, not available; RT, right ventricle; TEE, transesophageal echocardiogram; TTE, transthoracic echocardiography; US, ultrasound; VT, ventricular tachycardia.
Authors reported the underlined tests that were instrumental to the diagnosis
Patient Characteristics and Symptoms
Out of the 51 reports that were reviewed in this article, demographic information and patient characteristics were available on 30 patients with documented late lead perforation. Twenty patients had an age > 65 years (67% of the patients). Of those 20 patients, 12 were women (60%) and 8 were men (40%). Although these numbers confirm the suspicion that elderly women are vulnerable, they also point out that others are not immune. As can be seen in the Table, although a dilated, thin myocardium could be seen as a vulnerable substrate, normal myocardial thickness, or indeed hypertrophy, were not absolutely protective. The same could be concluded regarding concomitant medication; only 1 patient was on steroids and 2 were on Coumadin. Thus, it is erroneous to exclude the possibility of perforation based on time from implant or on patient characteristics alone.
Patients with late lead perforation might have a variety of presenting symptoms.8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33 Symptoms might include any of extracardiac muscle stimulation, chest pain, or shortness of breath reflecting any of pneumothorax, hemothorax, hemopneumothorax, pneumomediastinum, and/or pericardial tamponade.9,10,14 Other reported presenting symptoms included: hiccups, swelling over the pocket, and repetitive shocks as well as chest discomfort related to delayed pericarditis or mammary hematoma next to the device pocket. However, it is important to note that in many reported cases patients were asymptomatic or had vague nonspecific symptoms such as dizziness or fatigue.13,18
Lead Characteristics and Implantation Technique
As can be seen in the Table, most published case reports of late lead perforation involved active fixation leads. Although the majority of cases in the Table are for pacemaker leads, retrospective case series indicate that implantable cardioverter‐defibrillator (ICD) leads might have higher rate of late lead perforation than pacemaker leads. In fact, late lead perforation is reported in 0.1% to 0.8% of pacemakers and 0.6% to 5.2% of ICD implantations.4,5 Some lead models might have a higher perforation incidence rate. A retrospective study of 130 St. Jude Riata series leads (models 1580, 1581, 1590, 1591) at Massachusetts General Hospital showed a high rate of perforations (3.8%) over a 1‐year period, implicating among other factors the small lead size.8 However, a recent study from the University of California San Francisco Medical Center demonstrated a lower rate of perforation (0.65%) of the St. Jude Riata 1581 lead of the same size (7 Fr).26 The lower rate of perforation of the St. Jude Riata was also replicated in another study.34 Lead stiffness as well as lead caliber size affects perforation rate.26
Review of the circumstances surrounding a particular lead that was associated with unacceptably high rates of late lead perforation is of historical and educational value. The atrial lead, Accufix J wire (Telectronics, Englewood, CO) design incorporated a J wire that was found vulnerable to protrusion through the insulation with subsequent injury and/or death. The lead was recalled in November 1994 after 2 deaths and 2 nonfatal injuries. Because the wire perforation occurred only in a fraction of those implanted but in an unusual location with serious consequences, the struggle was to decide who to subject to lead extraction. This was particularly exacerbated by fatalities associated with the extraction procedures.35, 36, 37, 38, 39 Detection of the various stages of the retaining wire protrusion by digital fluoroscopy was demonstrated to be a valuable means of selecting patients at highest risk of imminent perforation. Most recently, the Heart Rhythm Society expert consensus statement recommends extraction of these leads with varying levels of recommendation dependent on the protrusion seen on fluoroscopy.40
Aside from lead characteristics, operator technique might have a role, with over‐torquing of the active fixation mechanism or excessive coiling and increased tension in an implanted lead regardless of fixation mechanism being incriminated as potential contributors to late perforation.8,19 In general, the experience of the operator strongly influences procedure outcomes with a higher incidence of adverse events when the implantation is not performed by a cardiac electrophysiology specialist.41
Physical Exam and Bedside Maneuvers
Evidence of a mammary hematoma, pericardial or pleural effusion, or chest wall bruising are all telltale signs of lead perforation. Device interrogation and stimulation at maximal output to capture chest wall, right or left hemidiaphragm stimulation by an offending atrial or ventricular lead, respectively, have all been instrumental in the diagnosis of lead perforation in the majority of the reviewed reports. Change in impedance or pacing parameters, loss of capture or elevated capture threshold, right bundle branch morphology in lead V1 while pacing the right ventricle, undersensing or noise on the recorded electrogram should all be seen as suggestive of lead perforation and should prompt further investigation including imaging studies. Even though an elevated pacing threshold might indicate a perforation, normal impedance and pacing parameters did not exclude it. In fact, a small perforation might result in the cathode being proximal to the epicardium and the anode proximal to or within the endocardium, resulting in the pacemaker function in the normal range.
Investigation
In addition to symptoms and/or device interrogation, late lead perforation is often identified by simple anteroposterior or posteroanterior/lateral chest radiography showing forward movement of the lead tip compared to a radiograph performed soon after implant as a reference point. Chest x‐ray showed displacement of the right ventricular lead in many of the reports (Table).
Transthoracic echocardiography is also useful by demonstrating pericardial effusion on many patients with late lead perforation.8,16,19,21 However, diagnostic assessment by chest x‐ray and echocardiography might fail in detecting lead perforation in some patients. In such cases, fluoroscopy can be of diagnostic value by demonstrating abnormal motion or a hinge point of a lead trapped in the myocardium.11
Chest CT images can be a valuable tool in the diagnosis of lead perforation when other modalities are nondiagnostic.16,18 In the absence of symptoms or signs, however, the higher sensitivity of this modality might jeopardize the specificity of findings. In one series of asymptomatic patients with pacemakers or ICDs, late lead perforation was found in 15 patients out of 100 patients with chest CTs performed for other reasons.18 The study was limited, however, by the difficulty in precisely locating the lead tip due to star artifact.
Management
Simple repositioning of the lead was utilized in many of the cases. However, the length of time elapsed since implant increases the challenges of repositioning a previously implanted lead due to fibrotic adhesions. Out of the 25 reviewed cases that were managed by lead extraction, 14 cases were managed by open chest lead extraction, whereas 11 cases had a percutaneous transvenous lead extraction. One patient died 10 days after percutaneous lead extraction,4 whereas another had cardiac tamponade after percutaneous lead extraction and was successfully managed by pericardiocentesis.29 Transvenous percutaneous extraction was pursued in some cases with utilization of transesophageal echocardiographic guidance as an added safety measure.7,15
Discussion
Lead perforation rates range from 0.1% to 0.8% for pacemaker leads and 0.6% to 5.2% for ICD leads.27 Late lead perforation is relatively rare, although the diagnosis is being made more frequently thanks to a higher index of suspicion and advanced imaging techniques. However, reported rates of perforation underestimate the public health burden due to dependence on voluntary reporting, hence the inherent publication bias and the frequent loss of follow‐up to the implanting center.26 In recent years there has been recognition of the need for proactive surveillance of this often overlooked problem.27
A low threshold to suspect lead perforation is needed. The two cases presented in this article illustrate the potential for late lead perforation after an uneventful implantation of a tined passive fixation lead. The patient can be asymptomatic as demonstrated in case 2. The presentation in case 1 with elevated ventricular capture threshold, chest wall capture, and an unexplained left pleural effusion was sufficient to make a tentative diagnosis. A higher clinical suspicion should be maintained in thin elderly women who appear to be more vulnerable, and in patients who are taking steroids or possibly anticoagulants.28
Chest CT was helpful in confirming the diagnosis of the patient described in case 1, whereas on plain film x‐ray the lateral border of the heart and apex were obscured by the left pleural effusion, and the extent of the tip of the lead could not be discerned. It should be noted that in many instances near perforation might be detected on CT where the lead tip is seen close to the epicardium, and if there are no symptoms, no pericardial effusion, or no abnormality in the lead measurements the implications of such a finding are less clear cut. Such CT findings alone are not an indication for lead removal; however, increased vigilance in follow‐up of these patients is indicated especially if the lead has been recently implanted. On the other hand, many cases could be missed on CT scan due to a reverberation artifact and acoustic shadowing from the metallic lead.
In some instances, definitive diagnosis has been established by fluoroscopy employing different projections, demonstrating that the lead tip does not follow the heart wall motion and revealing the extracardiac localization of the lead tip.11
The management question of late lead perforation is whether to extract the lead or not, and if so, whether to extract percutaneously or surgically. It has been postulated that if the lead's tip is inside the mediastinum and there is no bleeding complication, an additional lead could be inserted without performing lead extraction.7 However, the implications of retaining a nonfunctional lead, and the potential for further migration need to be considered.40 In the presence of tamponade secondary to a presumed perforation, drainage of the effusion and a conservative strategy has borne out well in a single‐center experience.8 Whenever an uncontrolled bleeding occurs or when a lead migrates outside the pericardium with a potential risk of vascular or pulmonary damage, extraction must be performed. If the lead has an active fixation tip, some electrophysiologists would favor transvenous extraction under TEE monitoring and general anesthesia as the treatment of choice because of the low risk of complications. Lead extraction can be done in the electrophysiology laboratory or operating room system with availability of emergency back‐up thoracotomy. Higher success rates have been claimed with TEE monitoring and utilization of excimer laser sheath.14 On the other hand, tined electrodes have bulky tips that might damage tissues during withdrawal. In such cases, two‐stage surgery is generally considered. Cutting the lead's tip first decreases the risk of bleeding or damaging tissues during extraction.7 In all late perforations, particularly those that have traversed through the pericardium, it is important to alert the cardiothoracic surgeon whether transvenous removal or repositioning is planned.
Limitations
One limitation to this study of late lead perforation including both presented cases is that the perforation occurred sometimes between the day after implant and the checkup. Few cases are misclassified as the lead perforation goes unnoticed when the perforation itself actually occur within a month from the procedure but was not recognized clinically due to the lack of clinical clues as to when the perforation might have occurred.
REFERENCES
- 1. Haq SA, Heitner JF, Lee L, et al. Late presentation of a lead perforation as a complication of permanent pacemaker insertion: a case report. Angiology 2008;59:619–621. [DOI] [PubMed] [Google Scholar]
- 2. Selcuk H, Selcuk MT, Maden O, et al. Uncomplicated heart and lung perforation by a displaced ventricular pacemaker lead: a case report. Pacing Clin Electrophysiol 2006;29:429–430. [DOI] [PubMed] [Google Scholar]
- 3. Ramirez MF, Ching CK, Ho KL, et al. The attack of the 52 cm lead: an unusual case of late cardiac perforation by a passive‐fixation permanent pacemaker lead. Int J Cardiol 2007;115:e5–e7. [DOI] [PubMed] [Google Scholar]
- 4. Akyol A, Aydin A, Erdinler I, et al. Late perforation of the heart, pericardium, and diaphragm by an active‐fixation ventricular lead. Pacing Clin Electrophysiol 2005;28:350–351. [DOI] [PubMed] [Google Scholar]
- 5. Khan MN, Joseph G, Khaykin Y, et al. Delayed lead perforation: a disturbing trend. Pacing Clin Electrophysiol 2005;28:251–253. [DOI] [PubMed] [Google Scholar]
- 6. Ellenbogen KA, Wood MA, Shepard RK. Delayed complications following pacemaker implantation. Pacing Clin Electrophysiol 2002;25:1155–1158. [DOI] [PubMed] [Google Scholar]
- 7. Sanoussi A, El Nakadi B, Larkinois I, et al. Late right ventricular perforation after permanent pacemaker implantation: how far can the lead go? Pacing Clin Electrophysiol 2005;28:723–725. [DOI] [PubMed] [Google Scholar]
- 8. Danik SB, Mansour M, Singh J, et al. Increased incidence of subacute lead perforation noted with one implantable cardioverter‐defibrillator. Heart Rhythm 2007;4:439–442. [DOI] [PubMed] [Google Scholar]
- 9. Polin GM, Zado E, Nayak H, et al. Proper management of pericardial tamponade as a late complication of implantable cardiac device placement. Am J Cardiol 2006;98:223–225. [DOI] [PubMed] [Google Scholar]
- 10. Greenberg S, Lawton J, Chen J. Images in cardiovascular medicine. Right ventricular lead perforation presenting as left chest wall muscle stimulation. Circulation 2005;111:e451–e452. [DOI] [PubMed] [Google Scholar]
- 11. Mortensen K, Aydin MA, Goldmann B, et al. Fluoroscopy to assess late heart and lung perforation by a permanent ventricular pacemaker lead. A case complicated by isolated hemothorax. Int J Cardiol 2008;128:104–106. [DOI] [PubMed] [Google Scholar]
- 12. Krivan L, Kozak M, Vlasinova J, et al. Right ventricular perforation with an ICD defibrillation lead managed by surgical revision and epicardial leads—case reports. Pacing Clin Electrophysiol 2008;31:3–6. [DOI] [PubMed] [Google Scholar]
- 13. Fisher JD, Fox M, Kim SG, et al. Asymptomatic anterior perforation of an ICD lead into subcutaneous tissues. Pacing Clin Electrophysiol 2008;31:7–9. [DOI] [PubMed] [Google Scholar]
- 14. Lloyd MS, Shaik MN, Riley M, et al. More late perforations with the Riata defibrillator lead from a high‐volume center: an update on the numbers. Pacing Clin Electrophysiol 2008;31:784–785. [DOI] [PubMed] [Google Scholar]
- 15. Satpathy R, Hee T, Esterbrooks D, et al. Delayed defibrillator lead perforation: an increasing phenomenon. Pacing Clin Electrophysiol 2008;31:10–12. [DOI] [PubMed] [Google Scholar]
- 16. Park RE, Melton IC, Crozier IG. Delayed defibrillator lead perforation. Pacing Clin Electrophysiol 2008;31:785–786. [DOI] [PubMed] [Google Scholar]
- 17. Sakai Y, Sato Y, Matsuo S, et al. Perforation of the right ventricular free wall by an ICD lead in a patient with isolated noncompaction of the ventricular myocardium. Int J Cardiol 2007;117:e104–e106. [DOI] [PubMed] [Google Scholar]
- 18. Hirschl DA, Jain VR, Spindola‐Franco H, et al. Prevalence and characterization of asymptomatic pacemaker and ICD lead perforation on CT. Pacing Clin Electrophysiol 2007;30:28–32. [DOI] [PubMed] [Google Scholar]
- 19. Suri R, Keller S. Lead perforation with a small body diameter implantable defibrillator lead. Heart Rhythm 2007;4:1248–1249. [DOI] [PubMed] [Google Scholar]
- 20. Lopes LR, Brandao L, Carrageta M. Single‐step transvenous extraction of a passive fixation lead with delayed perforation of the right ventricle. Europace 2007;9:672–673. [DOI] [PubMed] [Google Scholar]
- 21. Toal SC, Nanthakumar K. Injury potential as a diagnostic tool for implantable cardioverter‐defibrillator lead perforation. Heart Rhythm 2007;4:381. [DOI] [PubMed] [Google Scholar]
- 22. Laborderie J, Bordachar P, Reuter S, et al. Myocardial pacing lead perforation revealed by mammary hematoma next to the device pocket. J Cardiovasc Electrophysiol 2007;18:338. [DOI] [PubMed] [Google Scholar]
- 23. Kautzner J, Bytesnik J. Recurrent pericardial chest pain: a case of late right ventricular perforation after implantation of a transvenous active‐fixation ICD lead. Pacing Clin Electrophysiol 2001;24:116–118. [DOI] [PubMed] [Google Scholar]
- 24. Trigano AJ, Caus T. Lead explantation late after atrial perforation. Pacing Clin Electrophysiol 1996;19:1268–1269. [DOI] [PubMed] [Google Scholar]
- 25. Celik T, Kose S, Bugan B, et al. Hiccup as a result of late lead perforation: report of two cases and review of the literature. Europace 2009;11:963–965. [DOI] [PubMed] [Google Scholar]
- 26. Turakhia M, Prasad M, Olgin J, et al. Rates and severity of perforation from implantable cardioverter‐defibrillator leads: a 4‐year study. J Interv Card Electrophysiol 2009;24:47–52. [DOI] [PubMed] [Google Scholar]
- 27. Carlson MD, Freedman RA, Levine PA. Lead perforation: incidence in registries. Pacing Clin Electrophysiol 2008;31:13–15. [DOI] [PubMed] [Google Scholar]
- 28. Mahapatra S, Bybee KA, Bunch TJ, et al. Incidence and predictors of cardiac perforation after permanent pacemaker placement. Heart Rhythm 2005;2:907–911. [DOI] [PubMed] [Google Scholar]
- 29. Amara W, Cymbalista M, Sergent J. Delayed right ventricular perforation with a pacemaker lead into subcutaneous tissues. Arch Cardiovasc Dis 2010;103:53–54. [DOI] [PubMed] [Google Scholar]
- 30. Haghjoo M, Alizadeh A, Fazelifar AF, et al. Delayed cardiac perforation by one small body diameter defibrillator lead. J Electrocardiol 2010;43:71–73. [DOI] [PubMed] [Google Scholar]
- 31. Ferrero‐de‐Loma‐Osorio A, Albors‐Martin J, Ruiz‐Granell R, et al. Delayed right ventricular perforation by a transvenous active fixation implantable cardioverter‐defibrillator lead: echocardiographic diagnosis and surgical management. Circulation 2009;119:2112–2113. [DOI] [PubMed] [Google Scholar]
- 32. Tziakas D, Alexoudis A, Konstantinou F, et al. A rare case of late right ventricular perforation by a passive‐fixation permanent pacemaker lead. Europace 2009;11:968–969. [DOI] [PubMed] [Google Scholar]
- 33. Danik SB, Mansour M, Heist EK, et al. Timing of delayed perforation with the St. Jude Riata lead: a single‐center experience and a review of the literature. Heart Rhythm 2008;5:1667–1672. [DOI] [PubMed] [Google Scholar]
- 34. Porterfield JG, Porterfield LM, Kuck KH, et al. Clinical performance of the St. Jude medical Riata defibrillation lead in a large patient population. J Cardiovasc Electrophysiol 2010;21:551–556. [DOI] [PubMed] [Google Scholar]
- 35. Lloyd MA, Hayes DL, Holmes DR, et al. Extraction of the Telectronics Accufix 330‐801 atrial lead: the Mayo Clinic experience. Mayo Clin Proc 1996;71:230–234. [DOI] [PubMed] [Google Scholar]
- 36. Daoud EG, Kou W, Davidson T, et al. Evaluation and extraction of the Accufix atrial J lead. Am Heart J 1996;131:266–269. [DOI] [PubMed] [Google Scholar]
- 37. Kawanishi DT, Brinker JA, Reeves R, et al. Kaplan‐Meier analysis of freedom from extraction or death in patients with an Accufix J retention wire atrial permanent pacemaker lead: a potential management tool. Pacing Clin Electrophysiol 1998;21:2318–2321. [DOI] [PubMed] [Google Scholar]
- 38. Kawanishi DT, Brinker JA, Reeves R, et al. Spontaneous versus extraction related injuries associated with Accufix J‐wire atrial pacemaker lead: tracking changes in patient management. Pacing Clin Electrophysiol 1998;21:2314–2317. [DOI] [PubMed] [Google Scholar]
- 39. Kay GN, Brinker JA, Kawanishi DT, et al. Risks of spontaneous injury and extraction of an active fixation pacemaker lead. Report of the Accufix Multicenter Clinical Study and Worldwide Registry. Circulation 1999;100:2344–2352. [DOI] [PubMed] [Google Scholar]
- 40. Wilkoff BL, Love CJ, Byrd CL, et al. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management. Heart Rhythm 2009;6:1085–1104. [DOI] [PubMed] [Google Scholar]
- 41. Curtis JP, Luebbert JJ, Wang Y, et al. Association of physician certification and outcomes among patients receiving an ICD. JAMA 2009;301:1661–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]


