Abstract
Background
Disturbances in autonomic nervous system function have been reported to occur in patients suffering from mitochondrial cytopathies. However, there is paucity of literature on the occurrence of orthostatic intolerance (OI) in these patients. We report on a series of patients diagnosed with mitochondrial cytopathy who developed features of autonomic dysfunction in the form of OI.
Methods
This was a single‐center report on a series of 6 patients who were followed in our clinic for orthostatic intolerance. All of these patients had a diagnosis of mitochondrial cytopathy on the basis of muscle biopsy and were being followed at a center specializing in the treatment of mitochondrial disorders. This study was approved by our local institutional review board. Each of the patients had suffered from symptoms of fatigue, palpitations, near syncope, and syncope. The diagnosis of OI was confirmed by head‐up tilt test. Collected data included demographic information, presenting symptoms, laboratory data, tilt‐table response, and treatment outcomes.
Results
Six patients (3 females) were identified for inclusion in this report. The mean age of the group was 48 ± 8 years (range, 40–60 years). All of these patients underwent head‐up tilt table testing and all had a positive response that reproduced their clinical symptoms. Among those having an abnormal tilt‐table pattern, 1 had a neurocardiogenic response, 1 had a dysautonomic response, and 4 had a postural orthostatic tachycardia response. All but 1 patient reported marked symptom relief with pharmacotherapy. The patient who failed pharmacotherapy received a dual‐chamber closed‐loop pacemaker and subsequently reported marked improvement in her symptoms with elimination of her syncope.
Conclusions
Orthostatic intolerance might be a significant feature of autonomic nervous system dysfunction in patients suffering from mitochondrial cytopathy. Copyright © 2010 Wiley Periodicals, Inc.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
Autonomic nervous system dysfunction has been reported to occur in patients suffering from mitochondrial cytopathy. The most common manifestations of autonomic dysfunction in these patients include gastrointestinal dysmotility, central apnea, bladder dysfunction, and dysregulation of both heart rate and blood pressure during stress.1 Herein we report a series of 6 patients suffering from a mitochondrial cytopathy who presented with autonomic dysfunction in the form of orthostatic intolerance (OI).
Methods
This is a single‐center report on a series of 6 patients who were referred to our clinic due to persistent symptomatic orthostatic intolerance. All of these patients carried a diagnosis of mitochondrial cytopathy that was made on the basis of muscle biopsy results and were being followed at a center that specialized in the treatment of mitochondrial disorders. The Figure 1 demonstrates modified Gormori stain of the muscle biopsy in a patient with mitochondrial cytopathy. This study was approved by our local institutional review board. OI refers to a heterogeneous group of disorders of hemodynamic regulation characterized by excessive pooling of blood in the dependent areas of the body during upright posture resulting in insufficient cerebral perfusion causing symptoms during upright posture relieved by recumbency. Symptoms included syncope, near syncope fatigue, palpitations, exercise intolerance, lightheadedness, diminished concentration, and headache.2 Collected data included demographic information, presenting symptoms, laboratory data, tilt‐table response, and treatment outcomes. The protocol used for tilt‐table testing has been described in detail elsewhere, but basically consisted of a 70‐degree baseline upright tilt for a period of 30 minutes, during which time heart rate and blood pressure were monitored continually.3 If symptomatic hypotension and bradycardia occurred reproducing the patient's symptoms, the test was ended. If no symptoms occurred, the patient was lowered to the supine position and an intravenous infusion of isoproterenol was started with a dose sufficient to raise the heart rate 20% to 25% above the resting value. Upright tilt was then repeated for a period of 15 minutes. These data were categorized into 3 groups based on the positive tilt‐table pattern: neurocardiogenic, dysautonomic, and postural orthostatic tachycardia syndrome (POTS). Details of these patterns are given elsewhere.2, 3, 4
Figure 1.
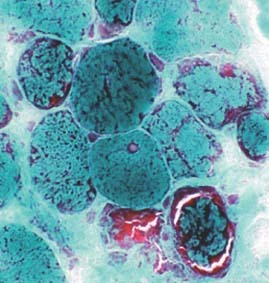
A modified Gormori trichrome stain of the muscle fibers in various stages of degeneration. The peripheral red staining material is dead and clumped mitochondrial structures. Courtesy of Dr. Bruce Cohen, Cleveland Clinic Foundation, Cleveland, Ohio
The treatment protocols employed were based on our previous experiences with orthostatic disorders and are described in detail elsewhere.2, 3, 4
Briefly, a sequence of therapies was employed that included aerobic reconditioning resistance training and physical counter maneuvers as well as increased dietary fluids and sodium. If these were ineffective, pharmacotherapy was initiated in a sequence generally consisting of fludrocortisone, midodrine, methylphenidate, selective serotonin reuptake inhibitors, pyridostigmine, and erythropoietin (either alone or in combination). The rationale for this sequence and the doses employed are described in detail elsewhere.2, 3, 4, 5, 6 A treatment was considered successful if it provided symptomatic relief.
Results
Six patients (3 females) were identified for inclusion in this report. The mean age of the group was 48 ± 8 years (range, 40–60 years). Table 1 summarizes the main clinical features of the study group. Two patients had Leigh's syndrome, 2 had carnitine deficiency, and another 2 had an undifferentiated mixed type of mitochondrial cytopathy. Three patients reported painful neuropathy of distal extremities.
Table 1.
Clinical Characteristics in Patients of Orthostatic Intolerance With Mitochondrial Cytopathy
| Symptoms | |||||||
|---|---|---|---|---|---|---|---|
| Patient No. | Age (years) | Sex | Tilt | Fatigue | Palpitations | Near Syncope | Syncope |
| 1 | 43 | F | POTS | + | + | − | − |
| 2 | 53 | F | POTS | + | + | + | − |
| 3 | 60 | F | NCS | + | − | + | + |
| 4 | 53 | M | Dysautonomia | + | + | + | + |
| 5 | 43 | M | POTS | + | + | + | + |
| 6 | 40 | M | POTS | + | + | + | − |
Abbreviations: F, female; M, male; NCS, neurocardiogenic syncope; POTS, postural orthostatic tachycardia
Every patient underwent extensive laboratory testing that included a complete blood count, including serum electrolytes, calcium, phosphorus, blood urea nitrogen, creatinine, glucose, cortisol, liver function studies, lipid profile, thyroid function; and blood levels of folic acid, iron, and vitamins D and B12.
All values were within normal limits in all patients. All 6 patients had undergone echocardiographic and Doppler examinations, 4 of which were normal and 2 of which showed mild left‐ventricular hypertrophy. One of the patients had atypical chest pain and underwent coronary angiography, which was reported as normal. One patient had undergone standard dual‐chamber pacemaker implantations prior to referral to our center with little or no change in her symptoms. All of these patients had been previously evaluated at a center that specialized in the diagnosis and treatment of mitochondrial disorders and had been referred due to significant OI.
All patients had head‐up tilt table testing and all had a positive tilt table study that reproduced their clinical symptoms. Among those having an abnormal tilt table pattern, 1 had a neurocardiogenic response, 1 had a dysautonomic response, and 4 had postural orthostatic tachycardic responses.
All of these patients reported having suffered from limitations of their daily activity due to symptoms of OI. Three had become homebound, and all 6 patients had lost their employment. All but 1 patient responded to a combination of pharmacotherapies (midodrine, methylphenidate, pyridostigmine, and modafinil) directed at correcting their OI. The nonresponsive patient had demonstrated a neurocardiogenic response during tilt table testing. She had also received a standard dual‐chamber pacemaker for her symptoms prior to her referral to our center. The pacemaker pulse generator was subsequently changed to one with closed‐loop pacing capabilities (Cylos Biotronik Inc., Berlin, Germany). After an implantation of the closed‐loop pacemaker she reported improvement in her symptoms and frequency of syncope. These patients were also being treated with coenzyme Q, carnitine, vitamin D, and riboflavin.
Two patients also reported features of bowel dysmotility in addition to OI.
Discussion
OI is a form of autonomic nervous system disturbance that often occurs due to the body's inability to maintain adequate amounts of peripheral vasoconstriction in the presence of orthostatic stress, thereby resulting in an abnormal degree of pooling of blood into the lower half of the body while upright. This shift is then compensated for by a reflex‐mediated increase in the rate and myocardial contractility, which although adequate enough to prevent syncope is not completely sufficient to meet the body's circulatory requirements. OI can be divided into different subgroups, such as neurocardiogenic syncope, dysautonomic syncope, and POTS. Details on the diagnosis and management of POTS and OI can be found elsewhere.2, 3, 4, 5
The mitochondrial cytopathies are a group of multisystem disorders characterized by structural or biochemical defects in the mitochondria that impair normal oxidative phosphorylation.7 Autonomic nervous system dysfunction can be severe enough to overshadow the myopathic features of the disorder and might delay the definitive diagnosis of mitochondrial cytopathy.8 Autonomic features that include gastrointestinal dysmotility, impaired respiratory control, and cardiac arrhythmias have been observed with both Leigh syndrome and Kerns‐Sayre syndrome and in myoneurogastrointestinal disorders with encephalopathy.9, 10, 11, 12 In addition, cardiac conduction defects or hypothermia and feeding problems might occasionally occur in other mitochondrial diseases, including Leber hereditary optic neuropathy and X‐linked recessive kinky hair disease.12, 13 Other features of autonomic dysfunction reported include decreased lacrimation, vasomotor disturbances (characterized by blotchy erythema and skin mottling), altered sweating, and postural hypotension. Autonomic dysfunction might result from structural abnormalities of the mitochondria within either the central or peripheral nervous system.7
OI (and its subtypes) have not been reported to occur in patients suffering from mitochondrial cytopathy. Patients suffering from OI experience postural palpitations, severe fatigue, dyspnea on exertion, lightheadedness, and significant exercise intolerance. Severe forms of the disorder can be very disabling, resulting in a state of functional impairment not unlike that seen in congestive heart failure and chronic obstructive lung disease.14, 15, 16, 17, 18 Initial therapy consists of an increase in salt and fluid intake as well as aerobic reconditioning with resistance training to increase lower extremity strength. Physical counter measures are also employed. Pharmacotherapy can be used alone or in combination in the following order: fludrocortisone, 0.1 mg, orally, twice a day; midodrine, 5 to 10 mg, orally, three times a day; propranolol, 10 mg, orally, three times a day; pyridostigmine, 60 mg, orally, twice a day; serotonin reuptake inhibitor and modafinil, 100 mg, orally, every morning.
Following recognition and diagnosis of OI, each patient received pharmacotherapy and all but 1 patient reported marked improvement in their symptoms. This patient, who failed pharmacotherapy, received a dual‐chamber closed‐loop system pacemaker and subsequently reported marked improvement in her symptoms with elimination of syncope. Details regarding the use of this pacing system have been reported elsewhere.19 The exact mechanism for the development of POTS in patients suffering mitochondrial cytopathy remains unknown. A long‐term follow‐up of patients suffering from mitochondrial cytopathies might be helpful in estimating the incidence of OI in these patients.
Limitations
This was a retrospective report on a relatively small number of patients. Because of the retrospective nature and small number we could not estimate any incidence of OI in patients suffering from mitochondrial cytopathies. Also, some questions, such as why only some mitochondrial cytopathy patients develop OI, remain unanswered from this report.
Despite these limitations we still feel that OI intolerance can occur in patients suffering from mitochondrial cytopathies. A high index of suspicion for OI might allow for its early recognition and treatment in patients of mitochondrial cytopathy. Further studies are warranted in the future to answer the questions that this report could not address.
Conclusion
Orthostatic intolerance might be a significant feature of autonomic nervous system dysfunction in patients suffering from mitochondrial cytopathy.
References
- 1. Nissenkorn A, Zeharia A, Lev D, et al. Neurological presentation of mitochondrial disorders. J Child Neurol 2000; 15: 44–48. [DOI] [PubMed] [Google Scholar]
- 2. Grubb BP. Dysautonomic (orthostatic) syncope In: Grubb BP, Olshansky B, eds. Syncope: Mechanisms and Management. Malden, MA: Blackwell Publishing; 2005; 72–91. [Google Scholar]
- 3. Grubb BP. Neurocardiogenic syncope In: Grubb BP, Olshansky B, eds. Syncope: Mechanisms and Management. Malden, MA: Blackwell Publishing; 2005; 47–71. [Google Scholar]
- 4. Grubb BP. Neurocardiogenic syncope and related disorders of orthostatic intolerance. Circulation 2005; 111: 2997–3006. [DOI] [PubMed] [Google Scholar]
- 5. Grubb BP. Postural orthostatic tachycardia. Circulation 2008;117: 2814–2817. [DOI] [PubMed] [Google Scholar]
- 6. Grubb BP, Kanjwal Y, Kosinski DJ. The postural tachycardia syndrome: a concise guide to diagnosis and management. J Cardiovasc Electrophysiol 2006; 17: 108–112. [DOI] [PubMed] [Google Scholar]
- 7. DiMauro S. Mitochondrial myopathies In: Rosenberg RN, Pruisner SB, DiMauro S, et al, eds. The Molecular and Genetic Basis of Neurological Diseases. Boston, MA: Butterworth‐Henemann; 1993; 665–694. [Google Scholar]
- 8. Zelnik N, Axelrod FB, Leshinsky E, et al. Mitochondrial encephalopathies presenting with features of autonomic and visceral dysfunction. Pediatr Neurol 1996; 14: 251–254. [DOI] [PubMed] [Google Scholar]
- 9. Berenberg RA, Pellock JM, DiMauro S, et al. Lumping or splitting? “Ophthalmoplegia plus” or Kearns‐Sayre syndrome? Ann Neurol 1977; 1: 37–43. [DOI] [PubMed] [Google Scholar]
- 10. Pincus JH. Subacute necrotizing encephalomyopathy (Leigh's disease): a consideration of clinical features and etiology. Dev Med Neurol 1972; 14: 87–101. [DOI] [PubMed] [Google Scholar]
- 11. Bardosi A, Creutzfeld W, DiMauro S, et al. Myo‐neurogastrointestinal encephalopathy (MNGIE syndrome) due to partial deficiency of cytochrome c‐oxidase: a new mitochondrial multisystem disorder. Acta Neuropathol (Berl) 1987; 74: 248–258. [DOI] [PubMed] [Google Scholar]
- 12. Newman NJ, Wallace DC. Mitochondria and Leber's hereditary optic neuropathy. Am J Ophthalmol 1990; 109: 726–730. [DOI] [PubMed] [Google Scholar]
- 13. Kodama H. Recent developments in Menkes disease. J Inherit Metab Dis 1993; 16: 791–799. [DOI] [PubMed] [Google Scholar]
- 14. Benrud‐Larson LM, Dewar MS, Sandroni P, et al. Quality of life in patients with postural tachycardia syndrome. Mayo Clin Proc 2002; 77: 531–537. [DOI] [PubMed] [Google Scholar]
- 15. van Dijk N, Sprangers MA, Boer KR, et al. Quality of life within one year following presentation after transient loss of consciousness. Am J Cardiol 2007; 100: 672–676. [DOI] [PubMed] [Google Scholar]
- 16. van Dijk N, Sprangers MA, Colman N, et al. Clinical factors associated with quality of life in patients with transient loss of consciousness. Cardiovasc Electrophysiol 2006; 17: 998–1003. [DOI] [PubMed] [Google Scholar]
- 17. Giada F, Silvestri I, Rossillo A, et al. Psychiatric profile, quality of life and risk of syncopal recurrence in patients with tilt‐induced vasovagal syncope. Europace 2005; 7: 465–471. [DOI] [PubMed] [Google Scholar]
- 18. Linzer M, Varia I, Pontinen M, et al. Medically unexplained syncope: relationship to psychiatric illness [review]. Am J Med 1992; 92: 18S–25S. [DOI] [PubMed] [Google Scholar]
- 19. Kanjwal K, Karabin B, Kanjwal Y, et al. Preliminary observations on the use of closed‐loop cardiac pacing in patients with refractory neurocardiogenic syncope. J Interv Card Electrophysiol 2010; 27: 69–73. [DOI] [PubMed] [Google Scholar]


