Abstract
Background
Sudden cardiac death (SCD) from cardiac arrest, one of the most common types of cardiac‐related death, is most often triggered by ventricular arrhythmia (VA). It has been reported that aldosterone antagonists (AAs) have the benefit of reducing SCD in patients with heart failure (HF). It also has been indicated in animal experiments and clinical trials that AAs may have an antiarrhythmic effect.
Hypothesis
AAs have an effect on VA in patients with HF or coronary artery disease.
Methods
We searched the Cochrane Central Register of Controlled Trials, PubMed, Current Controlled Trials, and the National Research Register, and identified randomized controlled trials on the effect of AAs on VA.
Results
All together, 7 trials with a total of 8635 patients were identified and extracted. AAs reduced the risk of SCD in patients with HF by 21% (relative risk [RR]: 0.79, 95% confidence interval [CI]: 0.67–0.93). AAs significantly reduced the episodes of ventricular premature complexes (mean difference 705 ± 646 episodes per 24 hours). Risk of ventricular tachycardia was reduced by 72% (RR: 0.28, 95% CI: 0.10–0.77).
Conclusions
The additional administration of AAs in patients with HF or coronary artery disease shows a benefit in reducing the risk of SCD and may also be effective for reducing episodes of ventricular premature complexes and ventricular tachycardia. Copyright © 2010 Wiley Periodicals, Inc.
The work was supported by grants from the Chinese National Nature Science Foundation (grant numbers 30600607 and 30770877, Beijing, China), and the National High‐tech Research and Development Program of China (2006AA02A406, Beijing, China).
Introduction
Sudden cardiac death (SCD), defined as sudden natural death from cardiac causes, generally occurs within 1 hour of the onset of prodromal symptoms and accounts for more than 50% of cardiac‐related deaths. Coronary artery disease (CAD) causes the majority of SCD, and cardiomyopathies are responsible for most of the remaining cases. Although trigger factors can vary, ventricular arrhythmia (VA) progression from ventricular tachycardia (VT) to ventricular fibrillation and then to asystole constitutes the terminal pathophysiological process of SCD. In a pooled analysis, arrhythmic mortality was higher than nonarrhythmic mortality in high‐risk post–myocardial infarction (MI) patients.1 Another registry research showed that ventricu‐ lar premature complexes (VPCs) plus nonsustained ventricular tachycardia (NSVT) was associated with high long‐term mortality after acute MI.2
As a component of the renin‐angiotensin‐aldosterone system (RAAS), aldosterone plays an important role in the underlying pathophysiology of atrial and ventricular arrhythmias, although the exact proarrhythmic effects have not been clearly documented.3, 4, 5, 6 Patients with primary aldosteronism are reported to have a higher risk of sustained arrhythmia and atrial fibrillation.7,8 Therefore, aldosterone antagonists (AAs), with their effect on RAAS, appear to have an antiarrhythmic effect to some extent, which has been indicated in several recent experiments.9, 10, 11, 12 Stambler et alfound that eplerenone attenuated ventricular electrical remodeling and tachyarrhythmia vulnerability,12 whereas Shroff et alfound a similar effect for suppressing atrial tachyarrhythmias in a ventricular tachypacing–induced heart failure (HF) model.13 Another animal experiment showed that canrenone‐reduced left ventricular (LV) remodeling and increased ventricular fibrillation threshold.14
The effect of AAs as an important component of RAAS has not been analyzed systematically on VA, which has been evaluated in several recent clinical trials. The objective of this review was to evaluate the effect of AAs on VA.
Methods
Criteria for Study Consideration
Types of Studies
We included randomized controlled trials; however, because the number of such trials could be low, prospective cohort studies were also included.
Types of Participants
The included studies enrolled patients at high risk of VA, such as those with ischemic heart disease or HF induced by cardiomyopathy, hypertension, or MI.
Types of Interventions
We reviewed studies assessing the preventive and therapeutic effects of AAs including spironolactone and eplerenone, in addition to others ongoing treatment for arrhythmia. Baseline conditions were included.
Types of Outcome Measures
The outcome measures were: 1) new‐onset VA, 2) SCD, 3) hospitalization for VA, 4) frequency of VPCs, 5) episodes of NSVT, and 6) episodes of sustained ventricular tachycardia (SVT).
Search Methods for Identification of Studies
We conducted online searches of the Cochrane Central Register of Controlled Trials (CENTRAL) and PubMed through September 20, 2009. We also searched 2 databases of ongoing trials: Current Controlled Trials (www.controlled‐trials.com) and the National Research Register (https://portal.nihr.ac.uk/Pages/Portfolio.aspx). We attempted to identify additional studies by searching the reference lists of relevant studies, reviews, and conference proceedings. In particular, with respect to journals, we searched those not indexed in the electronic databases. The following search‐term strategy was used for all databases: 1) spironolactone, 2) eplerenone, 3) canrenoate, 4) aldactone, 5) arrhythmia, and 6) 1 or 2 or 3 or 4 with 5.
Data Collection and Analysis
Selection of Studies
Titles and abstracts were reviewed to identify trials which met criteria of inclusion. Full texts were identified, as any trial appeared eligible. The selection process was conducted by 2 authors independently. Disagreements were resolved through discussion among authors of our group to achieve consensus.
Data Extraction and Management
The characteristics of trials were extracted into a table including design, blinding, sample size, participants, interventions, outcome measures, and results.
Data Analysis
Data analysis was performed with Review Manager version 5.0.21 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). Data of sufficient quality and sufficient similarity were included in a meta‐analysis. Results from dichotomous data were presented as relative risks. Results from continuous data were presented as weighted mean differences. Overall results were calculated based on the random‐effects model as heterogeneity was observed. A fixed‐effects model was used if no heterogeneity existed. Heterogeneity was tested using the z score and the χ2 test, with statistical significance considered as P < 0.1. Possible sources of heterogeneity were assessed by sensitivity and subgroup analyses as needed.
Results
Description of Studies
Seven trials with a total of 8635 patients were included and the relevant data extracted. The characteristics of included trials are described in the Table 1. Six trials in English were retrieved with full text of journal articles, and 1 trial in Russian was retrieved with abstract only. Five trials were randomized placebo‐controlled trials, and the other 2 randomized controlled trials were blank‐controlled. One of these studies was of crossover design and enrolled patients with CAD but without HF, while the others enrolled patients with HF secondary to CAD, dilated cardiomyopathy, or decompensated hypertension. Patients with HF secondary to primary valvular heart disease were excluded in the above trials. Spironolactone, a nonselective AA, was assessed in 6 trials, and eplerenone, a selective AA, in 1 trial. No trials evaluated the prophylactic effect of AAs for new‐onset arrhythmia. Two large trials compared AAs with placebo on SCD. Five trials evaluated the effect on VPCs during 24‐hour electrocardiographic (ECG) monitoring.
Table 1.
Characteristics of Included Studies
| Study ID | Double‐Blind? | Patient Criteria | Total (Male) | Interventions | Follow‐Up | Results |
|---|---|---|---|---|---|---|
| Barr et al, 199518 | Yes | HF (CAD); NYHA class II– III | 42 (32) | Spironolactone 50– 100 mg/d; placebo | 2 mo | VPCs/24 h: Exp (2974 ± 823) vs Con (4026 ± 1222), P = 0.002 |
| Gao et al, 200717 | Yes | HF, NYHA class II or IV; LVEF <45% | 116 (75) | Spironolactone 20 mg/d; placebo | 6 mo | VPCs/24 h: Exp (375 ± 296) vs Con (650 ± 592), P = 0.002; NSVT: Exp (0/58) vs Con (3/58), P = 0.19 |
| Pitt et al, 199915 | Yes | NYHA class III or IV; LVEF ≤35% | 1663 (1217) | Spironolactone 25 mg/d; placebo | 24 mo | SCD: Exp (82/822) vs Con (110/841), P = 0.02; hospitalization for VA: Exp (23/822) vs Con (24/841), P = 0.95 |
| Pitt et al, 200316 | Yes | HF (post‐AMI); LVEF ≤40% | 6632 (4714) | Eplerenone 50 mg/d; placebo | 16 mo | SCD: Exp (162/3319) vs Con (201/3313), P = 0.03; hospitalization for VA: Exp (52/3319) vs Con (54/3313), P = 0.79 |
| Ramires et al, 200019 | No | HF, NYHA class III; mean LVEF 33% | 35 (32) | Spironolactone 25 mg/d; blank | 4 mo | VPCs/24 h: Exp (408 ± 1569) vs Con (1536 ± 1440), P = 0.04; NSVT/24 h: Exp (0.6 ± 1.3) vs Con (2 ± 2), P = 0.02 |
| Shah et al, 200720, a | Yes | CAD (without HF), LVEF ≥50%) | 98 (65) | Spironolactone 12.5– 50 mg/d; placebo | 3 mo | VPCs/24 h: Exp (48, 19.2– 288) vs Con (192, 48– 744), P = 0.003; QTc: Exp (425 ± 24.9) vs Con (440 ± 27.5), P < 0.001 |
| Skvortsov 200821 | No | HF, NYHA class II or IV, LVEF ≤35% | 49 (44) | Spironolactone 25– 75 mg/d; blank | 12 mo | VPCs/24 h: Exp (12, 0– 15) vs Con (75, 39– 477), P = 0.043; VT: Exp (3/19) vs Con (15/30), P = 0.02 |
Abbreviations: AMI, acute myocardial infarction; CAD, coronary artery disease; Con, control group; DCM, dilated cardiomyopathy; Exp, experimental group; HF, heart failure; HTN, hypertension; LVEF, left ventricular ejection fraction; NSVT, nonsustained ventricular tachycardia; QTc, corrected QT interval; SCD, sudden cardiac death; VA, ventricular arrhythmia; VPCs, ventricular premature complexes; VT, ventricular tachycardia.
This study had a crossover design
Effects of Interventions
Two trials evaluated the risk of SCD and the risk of readmission for VA.15,16 AAs reduced the risk of SCD in patients with heart failure by 21% (relative risk [RR]: 0.79, 95% confidence interval [CI]: 0.67–0.93). However, there was no significant difference in the risk of readmission for VA (RR: 0.97, 95% CI: 0.71–1.32).
Episodes of VPC in 24‐hour ECG monitoring were analyzed in 4 trials.17, 18, 19, 20 Data from 3 of these trials were available for meta‐analysis. Heterogeneity was observed when analyzing these trials. Overall, AAs reduced episodes of VPCs (mean difference 705 episodes/24 h, 95% CI: 59–1350; Figure 1). Data from the fourth trial showed that spironolactone reduced episodes of VPCs in patients with ischemic heart disease but without HF (P = 0.003).
Figure 1.
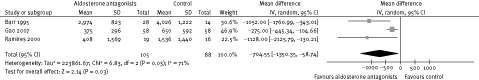
The episodes of ventricular premature complexes in 24‐hour ECG monitoring.
Episodes of NSVT during a 24‐hour period were analyzed in 1 trial, which indicated that NSVT was significantly reduced in the spironolactone group (mean difference 1.4 episodes/24 h, 95% CI: 0.26–2.54).19
Risk of NSVT was evaluated in 1 trial, and no significant difference was observed between patients with and without spironolactone.17 The risk of VT was also assessed in another trial, in which the benefit of spironolactone was observed; however, the types of VT were not reported.21 When the 2 above studies were pooled for analysis, the risk of VT was reduced in the spironolactone group by 72% (RR: 0.28, 95% CI: 0.10–0.77; Figure 2).17,21
Figure 2.
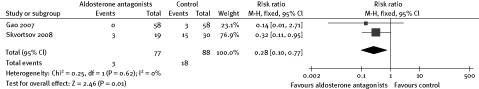
Risk of ventricular tachycardia.
Discussion
This meta‐analysis based on 2 large randomized controlled trials, Eplerenone Post–Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) and Randomized Aldactone Evaluation Study (RALES), indicated that AAs reduced the risk of SCD. This benefit of AAs has also been reported in another meta‐analysis.22 Yet the mechanism by which AAs reduce SCD in patients with HF was not completely clear. Based on the limited data included in the analysis, we could not draw further conclusions on the relation between benefits of AAs on reduction of SCD and benefits of AAs on reduction of VA.
Readmission for VA was not decreased by use of AAs; however, this outcome measure might not be related to the effect on VA. VA in patients with advanced HF was common and could be asymptomatic. The readmission for VA could be unrelated to the prevalence of VA. Unfortunately, trials evaluating new‐onset VA were not found and analysis of the effect of AAs for primary prevention could not be conducted.
Overall, ventricular extrasystoles were reduced by use of AAs in patients with HF. In addition, the risk of VT was also reduced in 1 trial, but without detailed evidence on NSVT and SVT, respectively. In patients with ischemic heart disease but without HF, spironolactone was also observed to have the effect of reducing risk of VA in 1 trial.
There are several possible mechanisms by which AAs might reduce episodes of cardiac VA. RAAS is considered to play an important role in the development of VA. It has been indicated that angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers might have a prophylactic effect on atrial fibrillation in a meta‐analysis based on a number of large randomized controlled trials.23 AAs, as the other main agents blocking RAAS, might have a similar antiarrhythmia effect, which was indicated in this meta‐analysis. It was reported that aldosterone and overexpression of mineralocorticoid receptor could influence gene expression and ion‐channel remodeling, which might be a potential proarrhythmic mechanism.6,24
Another possible explanation for the reduction of arrhythmia could be related to electrolyte regulation promoted by AAs. The use of AAs could maintain stable levels of potassium and magnesium, which is known to be of benefit in controlling arrhythmias.25 In this meta‐analysis, levels of serum potassium and magnesium were higher in patients in the AA group than in the placebo group (Figures 3, 4). Two other trials also indicated that patients not receiving AAs had a higher rate of hypokalemia. In patients with severe HF, digoxin was commonly used, which was a risk factor for arrhythmia, especially in patients with hypokalemia. One trial showed that the QT interval was shortened in patients with ischemic heart disease receiving spironolactone,20 which also indicated the antiarrhythmic effect.
Figure 3.
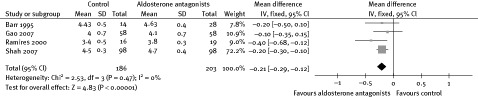
Effect of aldosterone antagonists on serum potassium.
Figure 4.
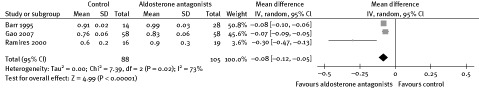
Effect of aldosterone antagonists on serum magnesium.
In this review, the antiarrhythmic effect of spironolactone was evaluated and mainly based on 5 trials of small size. The strength of the evidence could be limited to the small sample size. In addition, 2 trials were open‐label design and placebo was not used in the control group, which could create high risk of performance and detection bias. However, the outcome measures, such as VPCs during ECG monitoring, were objective parameters.
Conclusion
Implications for Practice
The additional administration of AAs showed a benefit for reducing the risk of SCD. AAs also may be effective in reducing episodes of VPCs and VT in patients with HF or CAD; however, it could be difficult to draw profound implications based on the modest evidence.
Implications for Research
More prospective studies of large size are required to determine the antiarrhythmic effect of AAs. It is not clear whether the reduction of SCD could be attributed to the reduction of VA, to improvement of heart function by AAs, or to both. The relation among arrhythmia, risk of SCD, and AAs still needs to be further elucidated. Because the beneficial mechanism of AAs might involve blockade of RAAS, the double inhibition offered through addition of an angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker could exhibit a larger effect on arrhythmia.
References
- 1. Yap YG, Duong T, Bland M, et al. Temporal trends on the risk of arrhythmic vs. non‐arrhythmic deaths in high‐risk patients after myocardial infarction: a combined analysis from multicentre trials. Eur Heart J 2005; 26: 1385–1393. [DOI] [PubMed] [Google Scholar]
- 2. Drögemüller A, Seidl K, Schiele R, et al. Prognostic value of non‐sustained ventricular tachycardias after acute myocardial infarction in the thrombolytic era: importance of combination with frequent ventricular premature beats. Z Kardiol 2003; 92: 164–172. [DOI] [PubMed] [Google Scholar]
- 3. Brilla CG, Matsubara LS, Weber KT. Antifibrotic effects of spironolactone in preventing myocardial fibrosis in systemic arterial hypertension. Am J Cardiol 1993; 7: 12A–16A. [DOI] [PubMed] [Google Scholar]
- 4. Brilla CG, Matsubara LS, Weber KT. Anti‐aldosterone treatment and the prevention of myocardial fibrosis in primary and secondary hyperaldosteronism. J Mol Cell Cardiol 1993; 25: 563–575. [DOI] [PubMed] [Google Scholar]
- 5. Brilla CG, Pick R, Tan LB, et al. Remodeling of the rat right and left ventricles in experimental hypertension. Circ Res 1990; 67: 1355–1364. [DOI] [PubMed] [Google Scholar]
- 6. Ouvrard‐Pascaud A, Sainte‐Marie Y, Benitah JP, et al. Conditional mineralocorticoid receptor expression in the heart leads to life‐threatening arrhythmias. Circulation 2005; 111: 3025–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Catena C, Colussi G, Nadalini E, et al. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Intern Med 2008; 168: 80–85. [DOI] [PubMed] [Google Scholar]
- 8. Milliez P, Girerd X, Plouin PF, et al. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol 2005; 45: 1243–1248. [DOI] [PubMed] [Google Scholar]
- 9. Beck L, Blanc‐Guillemaud V, Cherif OK, et al. Effects of spironolactone and fosinopril on the spontaneous and chronic ventricular arrhythmias in a rat model of myocardial infarction. Cardiology 2001; 96: 85–93. [DOI] [PubMed] [Google Scholar]
- 10. Yang SS, Han W, Zhou HY, et al. Effects of spironolactone on electrical and structural remodeling of atrium in congestive heart failure dogs. Chin Med J (Engl) 2008; 121: 38–42. [PubMed] [Google Scholar]
- 11. De Mello WC. Beneficial effect of eplerenone on cardiac remodelling and electrical properties of the failing heart. J Renin Angiotensin Aldosterone Syst 2006; 7: 40–46. [DOI] [PubMed] [Google Scholar]
- 12. Stambler BS, Laurita KR, Shroff SC, et al. Aldosterone blockade attenuates development of an electrophysiological substrate associated with ventricular tachyarrhythmias in heart failure. Heart Rhythm 2009; 6: 776–783. [DOI] [PubMed] [Google Scholar]
- 13. Shroff SC, Ryu K, Martovitz NL, et al. Selective aldosterone blockade suppresses atrial tachyarrhythmias in heart failure. J Cardiovasc Electrophysiol 2006; 17: 534–541. [DOI] [PubMed] [Google Scholar]
- 14. Cittadini A, Monti MG, Isgaard J, et al. Aldosterone receptor blockade improves left ventricular remodeling and increases ventricular fibrillation threshold in experimental heart failure. Cardiovasc Res 2003; 58: 555–564. [DOI] [PubMed] [Google Scholar]
- 15. Pitt B, Zannad F, Remme WJ, et al; for Randomized Aldactone Evaluation Study Investigators. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 341: 709–717. [DOI] [PubMed] [Google Scholar]
- 16. Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348: 1309–1321. [DOI] [PubMed] [Google Scholar]
- 17. Gao X, Peng L, Adhikari CM, et al. Spironolactone reduced arrhythmia and maintained magnesium homeostasis in patients with congestive heart failure. J Card Fail 2007; 13: 170–177. [DOI] [PubMed] [Google Scholar]
- 18. Barr CS, Lang CC, Hanson J, et al. Effects of adding spironolactone to an angiotensin‐converting enzyme inhibitor in chronic congestive heart failure secondary to coronary artery disease. Am J Cardiol 1995; 76: 1259–1265. [DOI] [PubMed] [Google Scholar]
- 19. Ramires FJ, Mansur A, Coelho O, et al. Effect of spironolactone on ventricular arrhythmias in congestive heart failure secondary to idiopathic dilated or to ischemic cardiomyopathy. Am J Cardiol 2000; 85: 1207–1211. [DOI] [PubMed] [Google Scholar]
- 20. Shah NC, Pringle SD, Donnan PT, et al. Spironolactone has antiarrhythmic activity in ischaemic cardiac patients without cardiac failure. J Hypertens 2007; 25: 2345–2351. [DOI] [PubMed] [Google Scholar]
- 21. Skvortsov AA, Mareev V, Orlova Ia A, et al. Effect of long term therapy with spironolactone on parameters of 24‐hour heart rhythm variability and ventricular arrhythmias in patients with heart failure receiving optimal therapy[in Russian]. Kardiologiia 2008; 48: 52–64. [PubMed] [Google Scholar]
- 22. Anand K, Mooss AN, Mohiuddin SM. Aldosterone inhibition reduces the risk of sudden cardiac death in patients with heart failure. J Renin Angiotensin Aldosterone Syst 2006; 7: 15–19. [DOI] [PubMed] [Google Scholar]
- 23. Healey JS, Baranchuk A, Crystal E, et al. Prevention of atrial fibrillation with angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers: a meta‐analysis. J Am Coll Cardiol 2005; 45: 1832–1839. [DOI] [PubMed] [Google Scholar]
- 24. Muto T, Ueda N, Opthof T, et al. Aldosterone modulates I(f) current through gene expression in cultured neonatal rat ventricular myocytes. Am J Physiol Heart Circ Physiol 2007, 293: H2710–2718. [DOI] [PubMed] [Google Scholar]
- 25. Crippa G, Sverzellati E, Giorgi‐Pierfranceschi M, et al. Magnesium and cardiovascular drugs: interactions and therapeutic role. Ann Ital Med Int 1999; 14: 40–45. [PubMed] [Google Scholar]


