Abstract
Background
Cardiac resynchronization therapy (CRT) has been reported to improve cardiac performance. However, CRT in patients with advanced heart failure is not always accompanied by an improvement in survival rates. We investigated the association between hemodynamic studies and long‐term prognosis after CRT.
Methods
A total of 68 consecutive patients receiving CRT devices due to advanced heart failure were assessed by hemodynamic study and long‐term outcome after implantation of the device. Hemodynamic parameters were measured both with the CRT on and off.
Results
Patients demonstrated significant improvement in the maximum first derivative of left ventricular (LV) pressure (LV dP/dtmax) and QRS duration after periods with the CRT on. During the follow‐up period of 34.9 ± 17.6 months, basal LV dP/dtmax and isovolemic LV pressure half‐time (T1/2), but not percent change in LV dP/dtmax, were independent predictors of cardiac mortality or hospitalization due to heart failure after multivariate Cox regression analysis. The Kaplan‐Meier survival analysis revealed that patients in the lowest basal LV dP/dtmax tertile or the longest basal T1/2 tertile exhibited a significantly higher cardiac‐caused mortality or heart failure hospitalization.
Conclusions
Lower LV dP/dtmax or longer T1/2 independently predicts cardiac mortality or heart failure hospitalization in patients receiving CRT. The assessment of the basal LV dP/dtmax and T1/2 could provide useful information in long‐term prognosis after CRT. Copyright © 2010 Wiley Periodicals, Inc.
This work was supported by grant from the Grant‐in‐Aid for Young Scientists, the Nakashima Foundation, and the Kowa Life Science Foundation to RS.
Hirohiko Suzuki, MD and Masayuki Shimano, MD equally contributed to the work.
Introduction
Cardiac resynchronization therapy (CRT) has been established as a therapeutic option in patients with advanced heart failure.1, 2, 3 Previous studies have shown that CRT decreases mortality and hospital admission rates.4, 5 Based on current knowledge of the mechanisms involved, patients with beneficial outcomes from CRT have comprised those suffering impairment of contractility associated with electromechanical ventricular dyssynchrony and reverse remodeling.6, 7, 8 However, it is difficult to actually identify which patients will have the best outcome after CRT from preimplant assessments. We found that approximately one‐third of patients do not respond well to CRT.2, 9 A recent clinical trial10 indicated that the echocardiographic parameters in assessing dyssynchrony did not have enough predictive value to be recommended as selection criteria for CRT beyond current guidelines. Therefore, it is clinically valuable to identify any preimplant characteristics that better predict which patients will have the best outcomes after CRT. In the present study, we investigated what kind of hemodynamic parameters could predict long‐term patient prognosis after CRT.
Methods
Study Population
We retrospectively assessed 72 consecutive patients with heart failure who underwent CRT at Nagoya Daini Red Cross Hospital between June 2001 and September 2006. The patients were selected according to the established CArdiac REsynchronization in Heart Failure Study (CARE‐HF) selection criteria for CRT3: (1) severe heart failure despite optimized medical therapy; (2) left ventricular (LV) systolic dysfunction with a LV ejection fraction < 35%; and (3) QRS duration > 120 ms. In addition, patients with a QRS interval of 120 to 149 ms were required to meet 2 of 3 additional criteria for dyssynchrony as described previously (aortic pre‐ejection delay > 140 ms, interventricular mechanical delay > 40 ms, delayed activation of the posterolateral left ventricular wall).3 We excluded 4 patients with aortic valve replacements for valvular heart disease. All patients enrolled in the study provided written informed consent.
Echocardiography
Both 2‐dimensional and Doppler echocardiography were performed by an experienced sonographer using the Sonos 5500 System (Philips Electronics, Amersterdam, The Netherlands) before the hemodynamic study. The images were recorded on videotape and analyzed off‐line. Left ventricular end‐diastolic diameter (LVEDD) and left atrial dimension (LAD) were measured from standard M‐mode measurements as recommended by the American Society of Echocardiography. Left ventricular ejection fraction (EF) was calculated using a modified Simpson's rule.
Cardiac Hemodynamic Analysis
Invasive hemodynamic study using a micro‐manometer‐tipped catheter (Millar Instruments, Houston, Texas) was performed immediately after CRT device implantation with a standard pacing protocol described previously.11, 12 Hemodynamic and electrical signals were digitized at a sampling rate of 12 kHz and stored on a PC. Left ventricular end‐diastolic pressure (LVEDP), the maximum first derivative of LV pressure (LV dP/dtmax) as an index of contractility, and the pressure half‐time (T1/2) to evaluate LV isovolumic relaxation according to Mirsky's method were measured both with CRT on (CRT basal) and off.13 Hemodynamic data were analyzed by 2 independent observers who were unaware of the clinical and echocardiographic data.
Follow‐Up and Assessment of Cardiac Events
All patients underwent regular follow‐ups (typically every 2 mo) by means of outpatient clinical visits or telephone interviews. Hospitalizations for worsening of heart failure were adjudicated by Nagoya Daini Red Cross Hospital staff cardiologists. Causes of death were ascertained by reviewing the clinical records.
Statistical Analysis
All data are expressed as mean ± standard deviation (SD). Univariate and multivariate Cox regression analysis were performed to control for potentially confounding hemodynamic, demographic, and clinical variables. Freedom from all causes of death, cardiac death, or hospitalization for heart failure was determined by Kaplan‐Meier analysis with the log‐rank test. A level of P < 0.05 indicated statistical significance. All analyses were performed using SPSS 16.0 (SAS Institute Inc., Cary, NC).
Results
Baseline Characteristics
Baseline characteristics are shown in Table 1. A total of 68 patients who received CRT were 66.2 ± 9.2 years of age, 58.8% male, and predominantly classified as New York Heart Association (NYHA) functional class III and IV with QRS prolongation (169.4 ± 29.5 ms). Patients had severe LV dysfunction (mean LVEF 27.1% ± 9.5%), with extensive dilatation (LVEDD 67.6 ± 9.0 mm). The etiology of heart failure in 10.3% of patients was ischemic heart disease. Hemodynamic data immediately after CRT device implantation demonstrated significant improvement in LV dP/dtmax (24.4%), LVEDP (−17.9%), and T1/2 (−6.8%) as compared with basal conditions. CRT also made QRS width shortened (26.0%) as compared with basal conditions.
Table 1.
Patients Characteristics (n=68)
| Age (yrs) | 66.2 ± 9.2 |
| Male (%) | 40 (58.8%) |
| NYHA class III/IV (%) | 54(79.4%)/14 (20.6%) |
| QRS width (ms) | 169.4 ± 29.5 |
| Echocardiography | |
| Left ventricular end diastolic diameter (mm) | 67.6 ± 9.0 |
| Left ventricular ejection fraction (%) | 27.1 ± 9.5 |
| Mitral regurgitation (grade) | 1.6 ± 0.7 |
| Left atrial dimension (mm) | 45.2 ± 7.9 |
| Ischemic heart disease (%) | 7 (10.3%) |
| History of atrial fibrillation (%) | 13 (19.1%) |
| Upgrading of existing device | 23 (33.8%) |
| Hemodynamic data | |
| Positive LV dP/dtmax (mm Hg/sec) | 748.1 ± 246.4 |
| Peak LV pressure (mm Hg) | 102.2 ± 22.2 |
| Negative LV dP/dtmin (mm Hg/sec) | 758.2 ± 222.4 |
| LVEDP (mm Hg) | 14.2 ± 8.1 |
| T1/2 (ms) | 47.7 ± 7.6 |
| eGFR (mL/min) | 51.2 ± 16.3 |
| BNP (pg/mL) | 576.6 ± 414.3 |
| Preferable LV lead position | 63 (92.6%) |
| Follow‐up (mo) | 34.9 ± 17.6 |
Abbreviations: BNP, brain natriuretic peptide; eGFR, estimated glomerular filtration rate; LV, left ventricle; LVEDP, left ventricular end‐diastolic pressure; NYHA, New York Heart Association; T1/2, isovolemic pressure half‐time.
Values are mean ± standard deviation
Clinical Outcome of Patients During Follow‐Up
The mean duration of follow‐up was 34.9 ± 17.6 months. There were 13 (19.1%) deaths. Causes of death included sudden cardiac death in 5 patients, heart failure in 3 patients, and noncardiac‐related deaths in 5 patients (2 deaths from cancer, 1 of infection, 1 traumatic accident, and 1 renal failure). A total of 6 patients were hospitalized due to exacerbation of heart failure after CRT device implantation.
Prediction of Prognosis After CRT
We first performed univariate Cox regression analysis to examine the relationship between long‐term prognosis (cardiac mortality and heart failure hospitalization) after CRT and cardiac parameters including conventional risk factors. We found the statistical significance in NYHA functional class (P < 0.01), plasma brain natriuretic peptide (BNP) levels (P < 0.05), estimated glomerular filtration rate (eGFR; P < 0.05), LV dP/dtmax during CRT (P < 0.001) and T1/2 during CRT (P < 0.01; Table 2). There were no significant differences in age, LVEDD, LVEF, QRS duration, percent change in QRS duration, percent change in LV dP/dtmax, LVEDP, preexisting atrial fibrillation (AF), etiology of cardiomyopathy, and preferable LV lead position (posterolateral or lateral tributary of coronary sinus).
Basal LV dP/dtmax (P < 0.05) and T1/2 (P < 0.05) during CRT are independent predictors of cardiac death or heart failure hospitalization in multivariate Cox regression models, after controlling for NYHA functional class, plasma BNP levels, eGFR, basal LV dP/dtmax, and basal T1/2 (Table 2).
Table 2.
Predictors of Cardiac Mortality and Heart Failure Hospitalization
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | CI (OR) | P value | OR | CI (OR) | P value | |
| Age | 0.956 | 0.909– 1.006 | 0.081 | |||
| NYHA | 4.097 | 1.497– 11.213 | 0.008 | 2.217 | 0.772– 6.366 | 0.139 |
| Atrial fibrillation | 1.201 | 0.335– 4.307 | 0.779 | |||
| Ischemic etiology | 3.453 | 0.956– 12.469 | 0.059 | |||
| LVEDD | 1.054 | 0.993– 1.119 | 0.085 | |||
| LVEF | 0.986 | 0.931– 1.044 | 0.619 | |||
| BNP | 1.001 | 1.000– 1.002 | 0.026 | 1.001 | 0.999– 1.002 | 0.213 |
| eGFR | 0.965 | 0.933– 0.997 | 0.033 | 0.971 | 0.939– 1.005 | 0.095 |
| LV dP/dtmax | 0.994 | 0.990– 0.998 | 0.001 | 0.996 | 0.992– 0.995 | 0.018 |
| % change in LV dP/dtmax | 0.993 | 0.956– 1.030 | 0.693 | |||
| LVEDP | 1.018 | 0.955– 1.085 | 0.587 | |||
| T1/2 | 1.092 | 1.028– 1.159 | 0.004 | 1.076 | 1.011– 1.145 | 0.021 |
| QRS width | 1.007 | 0.990– 1.025 | 0.417 | |||
| % change in QRS width | 0.983 | 0.960– 1.006 | 0.135 | |||
| LV lead position | 2.323 | 0.516– 10.451 | 0.272 | |||
Abbreviations: BNP, brain natriuretic peptide; CI, 95% confidence interval; eGFR, estimated glomerular filtration rate; LV, left ventricle; LVEDD, left ventricular end‐diastolic diameter; LVEDP, left ventricular end‐diastolic pressure; NYHA, New York Heart Association; OR, odds ratio; T1/2, isovolemic pressure half‐time
The Kaplan‐Meier survival analysis demonstrated that patients in the lowest tertile of basal LV dP/dtmax during CRT (<750 mm Hg/s) had a significantly higher cardiac‐caused mortality and hospitalization for heart failure compared with those in the upper tertile (>750 mm Hg/s; Figure 1A,B). In addition, patients in the lowest tertile of basal LV dP/dtmax during CRT (<750 mm Hg/s) had exhibited higher all‐cause death (Figure 1C). Kaplan‐Meier survival analysis also presented that patients in the longest tertile of basal T1/2 during CRT (>50 ms) had significantly higher cardiac‐based mortality and hospitalization for heart failure (Figure 1D,E).
Figure 1.
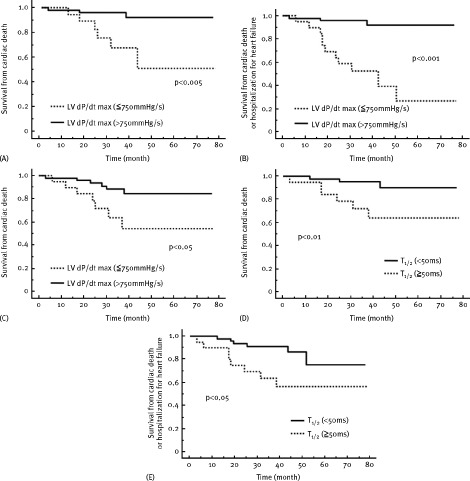
Kaplan‐Meier estimates of the time to various clinical end points; (A,D) cardiac death, (B,E) cardiac death or hospitalization for heart failure, (C) all‐cause death. Patients were stratified according to (A,B,C) peak positive LV dP/dt and (D,E) isovolemic LV pressure half‐time (T1/2)
Discussion
This study has demonstrated for the first time that LV dP/dtmax and T1/2 during CRT just after implantation estimated by invasive hemodynamic study independently predicts cardiac mortality and morbidity in patients receiving CRT.
The reason that LV dP/dtmax is such a sensitive index14, 15, 16, 17 for response to CRT is thought to involve the manner in which mechanical dyssynchrony rises during early isovolumic contraction and peaks at end systole or early relaxation.18 Myocardial dyssynchrony as assessed by magnetic resonance imaging was an independent predictor of mortality after CRT.19 Doppler‐derived LV dP/dtmax also predicts survival in patients with heart failure in the settings without CRT.20 In addition, it has been noted that CRT provides long‐term beneficial effects to patients in early stages of heart failure,21 thus indicating those who do not have low basal LV dP/dtmax. Left ventricular dyssynchrony caused the prolongation of isovolemic relaxation through asynchronous relaxation time.22 Consistent with these findings, the present study demonstrates that lower peak positive LV dP/dt and longer T1/2 at baseline independently predicts cardiac death or hospitalization for heart failure in patients receiving CRT. These data suggest that measurements of hemodynamics before CRT implantation could provide useful clinical information regarding cardiac mortality and morbidity after CRT.
It was recently reported that echocardiographic assessments of the acute hemodynamic response to CRT such as percent change in LV dP/dtmax predict long‐term clinical outcomes.23, 24, 25, 26 However, the association between the acute hemodynamic response to CRT and long‐term outcome is controversial. In contrast, conclusions from the Predictors of Response to CRT (PROSPECT) trial10 and contemporary review27 suggested that single echocardiographic measure of dyssynchrony might not be recommended to improve patient selection for CRT. The differences between the results of the various studies may be due to underlying differences in the severity of heart failure or frequency of ischemic cardiomyopathy. Our findings also showed that percent change in LV dP/dtmax by invasive hemodynamic study did not predict cardiac mortality in patients receiving CRT. Therefore, it appears that the acute hemodynamic response to CRT is not an independent predictor of long‐term outcome after CRT.
The present study has several limitations. First, the sample size is relatively small. Second, because we excluded patients with aortic valve replacements, there were no heart failure patients with valvular heart diseases in this study. Finally, the frequency of ischemic etiology was relatively low in this population. This probably reflects the lower cardiac mortality compared with the various CRT‐related studies. Accordingly, inconsistent with previous studies,28, 29, 30 ischemic etiology was not a major predictor of cardiac mortality.
In conclusion, our findings suggest that basal LV dP/dtmax and T1/2 during CRT—but not percent change in LV dP/dtmax—predict cardiac mortality in patients receiving CRT. Measurements of hemodynamics just after CRT implantation could provide useful information in long‐term prognosis after CRT. More aggressive utilization of CRT should be considered in early stages of heart failure characterized by relatively intermediate LV dP/dtmax values.
Acknowledgements
The authors gratefully acknowledge the assistance of Yutaka Kose and Toru Kaneko.
REFERENCES
- 1. Cazeau S, Leclercq C, Lavergne T, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med 2001; 344: 873–880. [DOI] [PubMed] [Google Scholar]
- 2. Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002; 346: 1845–1853. [DOI] [PubMed] [Google Scholar]
- 3. Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005; 352: 1539–1549. [DOI] [PubMed] [Google Scholar]
- 4. Rivero‐Ayerza M, Theuns DA, Garcia‐Garcia HM, et al. Effects of cardiac resynchronization therapy on overall mortality and mode of death: a meta‐analysis of randomized controlled trials. Eur Heart J 2006; 27: 2682–2688. [DOI] [PubMed] [Google Scholar]
- 5. McAlister FA, Ezekowitz J, Hooton N, et al. Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction: a systematic review. JAMA 2007; 297: 2502–2514. [DOI] [PubMed] [Google Scholar]
- 6. Bax JJ, Bleeker GB, Marwick TH, et al. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol 2004; 44: 1834–1840. [DOI] [PubMed] [Google Scholar]
- 7. Yu CM, Bleeker GB, Fung JW, et al. Left ventricular reverse remodeling but not clinical improvement predicts long‐term survival after cardiac resynchronization therapy. Circulation 2005; 112: 1580–1586. [DOI] [PubMed] [Google Scholar]
- 8. Di Biase L, Auricchio A, Sorgente A, et al. The magnitude of reverse remodelling irrespective of aetiology predicts outcome of heart failure patients treated with cardiac resynchronization therapy. Eur Heart J 2008; 29(20): 2497–2505. [DOI] [PubMed] [Google Scholar]
- 9. Hawkins NM, Petrie MC, MacDonald MR, et al. Selecting patients for cardiac resynchronization therapy: electrical or mechanical dyssynchrony? Eur Heart J 2006; 27: 1270–1281. [DOI] [PubMed] [Google Scholar]
- 10. Chung ES, Leon AR, Tavazzi L, et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation 2008; 117: 2608–2616. [DOI] [PubMed] [Google Scholar]
- 11. Shimano M, Inden Y, Yoshida Y, et al. Does RV lead positioning provide additional benefit to cardiac resynchronization therapy in patients with advanced heart failure? Pacing Clin Electrophysiol 2006; 29: 1069–1074. [DOI] [PubMed] [Google Scholar]
- 12. Shimano M, Tsuji Y, Yoshida Y, et al. Acute and chronic effects of cardiac resynchronization in patients developing heart failure with long‐term pacemaker therapy for acquired complete atrioventricular block. Europace 2007; 9: 869–874. [DOI] [PubMed] [Google Scholar]
- 13. Parker JD, Landzberg JS, Bittl JA, et al. Effects of beta‐adrenergic stimulation with dobutamine on isovolumic relaxation in the normal and failing human left ventricle. Circulation 1991; 84: 1040–1048. [DOI] [PubMed] [Google Scholar]
- 14. Kass DA, Chen CH, Curry C, et al. Improved left ventricular mechanics from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation 1999; 99: 1567–1573. [DOI] [PubMed] [Google Scholar]
- 15. Auricchio A, Stellbrink C, Block M, et al. Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. The Pacing Therapies for Congestive Heart Failure Study Group. The Guidant Congestive Heart Failure Research Group. Circulation 1999; 99: 2993–3001. [DOI] [PubMed] [Google Scholar]
- 16. Leclercq C, Kass DA. Retiming the failing heart: principles and current clinical status of cardiac resynchronization. J Am Coll Cardiol 2002; 39: 194–201. [DOI] [PubMed] [Google Scholar]
- 17. Steendijk P, Tulner SA, Bax JJ, et al. Hemodynamic effects of long‐term cardiac resynchronization therapy: analysis by pressure‐volume loops. Circulation 2006; 113: 1295–1304. [DOI] [PubMed] [Google Scholar]
- 18. Kass DA. An epidemic of dyssynchrony: but what does it mean? J Am Coll Cardiol 2008; 51: 12–17. [DOI] [PubMed] [Google Scholar]
- 19. Chalil S, Stegemann B, Muhyaldeen S, et al. Intraventricular dyssynchrony predicts mortality and morbidity after cardiac resynchronization therapy: a study using cardiovascular magnetic resonance tissue synchronization imaging. J Am Coll Cardiol 2007; 50: 243–252. [DOI] [PubMed] [Google Scholar]
- 20. Kolias TJ, Aaronson KD, Armstrong WF. Doppler‐derived dP/dt and ‐dP/dt predict survival in congestive heart failure. J Am Coll Cardiol 2000; 36: 1594–1599. [DOI] [PubMed] [Google Scholar]
- 21. Landolina M, Lunati M, Gasparini M, et al. Comparison of the effects of cardiac resynchronization therapy in patients with class II versus class III and IV heart failure (from the InSync/InSync ICD Italian Registry). Am J Cardiol 2007; 100: 1007–1012. [DOI] [PubMed] [Google Scholar]
- 22. Mottram PM, Marwick TH. Assessment of diastolic function: what the general cardiologist needs to know. Heart 2005; 91: 681–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oguz E, Dagdeviren B, Bilsel T, et al. Echocardiographic prediction of long‐term response to biventricular pacemaker in severe heart failure. Eur J Heart Fail 2002; 4: 83–90. [DOI] [PubMed] [Google Scholar]
- 24. Sogaard P, Egeblad H, Kim WY, et al. Tissue Doppler imaging predicts improved systolic performance and reversed left ventricular remodeling during long‐term cardiac resynchronization therapy. J Am Coll Cardiol 2002; 40: 723–730. [DOI] [PubMed] [Google Scholar]
- 25. Heist EK, Taub C, Fan D, et al. Usefulness of a novel “response score” to predict hemodynamic and clinical outcome from cardiac resynchronization therapy. Am J Cardiol 2006; 97: 1732–1736. [DOI] [PubMed] [Google Scholar]
- 26. Tournoux FB, Alabiad C, Fan D, et al. Echocardiographic measures of acute haemodynamic response after cardiac resynchronization therapy predict long‐term clinical outcome. Eur Heart J 2007; 28: 1143–1148. [DOI] [PubMed] [Google Scholar]
- 27. Anderson LJ, Miyazaki C, Sutherland GR, et al. Patient selection and echocardiographic assessment of dyssynchrony in cardiac resynchronization therapy. Circulation 2008; 117: 2009–2023. [DOI] [PubMed] [Google Scholar]
- 28. Gasparini M, Mantica M, Galimberti P, et al. Is the outcome of cardiac resynchronization therapy related to the underlying etiology? Pacing Clin Electrophysiol 2003; 26: 175–180. [DOI] [PubMed] [Google Scholar]
- 29. Woo GW, Petersen‐Stejskal S, Johnson JW, et al. Ventricular reverse remodeling and 6‐month outcomes in patients receiving cardiac resynchronization therapy: analysis of the MIRACLE study. J Interv Card Electrophysiol 2005; 12: 107–113. [DOI] [PubMed] [Google Scholar]
- 30. Mangiavacchi M, Gasparini M, Faletra F, et al. Clinical predictors of marked improvement in left ventricular performance after cardiac resynchronization therapy in patients with chronic heart failure. Am Heart J 2006; 151: 477. [DOI] [PubMed] [Google Scholar]


