Abstract
Background
The frequency, risk factors for, and effect on long‐term survival of increased troponin I (cTnI) following elective, uncomplicated percutaneous coronary intervention (PCI) remains uncertain.
Methods
We studied 907 patients undergoing elective PCI without recognized PCI complications and with at least 1 measurement of cTnI 12 or more h following the procedure. Patients with pre‐PCI cTnI above 0.1 ng/ml or with myocardial infarction within the previous 48 h were excluded.
Results
Maximal cTnI (TrMX) following PCI averaged 0.8 ng/ml, exceeded the upper normal of 0.1 ng/ml in 65.2% of patients and was 1.5 ng/ml or above in 13.7%. Of several demographic and procedural variables examined, the only significant predictor of TrMX was the number of stents deployed. (p < 0.0023 95% confidence interval [CI]: 0.10–0.46). Significant univariate predictors of survival (Kaplan‐Meier) were older age (p < 0.0001), diabetes (p = 0.02), peripheral vascular disease (p < 0.0001), obstructive lung disease (p < 0.0001), congestive failure (p < 0.0001), renal impairment (p < 0.0001), and TrMX of 3.62 ng/ml or above (p = 0.0451). Independent predictors (Cox) were older age (p < 0.0001), obstructive lung disease (p < 0.0001), congestive failure (p < 0.0001), and TrMX (p = 0.0272).
Conclusions
Elevation of cTnI occurs in most patients undergoing elective, uncomplicated PCI. Deployment of multiple stents is associated with higher values of cTnI. Long‐term survival is primarily influenced by age and pre‐PCI comorbidities, however patients with the highest values of cTnI after PCI are also at increased risk of reduced survival. Significant independent predictors of reduced survival were older age, obstructive pulmonary disease, congestive failure (p < 0.0001 for each), and maximal post‐PCI cTnI (p = 0.0272). Copyright © 2010 Wiley Periodicals, Inc.
Introduction
The ability to perform percutaneous coronary intervention (PCI) safely has improved greatly since its onset, largely as the result of better equipment (imaging, catheters, balloons, wires, stents, distal protection devices, etc.) and improved pharmacologic adjuvant therapy, along with increased operator experience. In spite of these improvements (PCI), with or without stent deployment may still on occasion be associated with myocardial ischemia or necrosis due to transient target vessel obstruction, occlusion of side branches(s), distal embolization or coronary occlusion from dissection, or thrombosis. Although major complications during PCI are usually recognized by angiography or clinical features, less severe complications may not be so easily identified. Accordingly it has become common practice in many laboratories to monitor markers of myocardial cell damage in all patients undergoing PCI. Until recently the marker most commonly measured following PCI was the MB fraction of creatine kinase (CK‐MB), which was felt to be reasonably specific for myocardial cell necrosis. Although the importance of minor increases in CK‐MB after PCI is uncertain1 a rise of 3 to 8 times the upper normal value of CK‐MB appears to be predictive of an adverse outcome.2, 3, 4
Over the past few years the measurement of troponin has largely replaced the measurement of CK‐MB for identification of an acute coronary syndrome in symptomatic patients presenting to a hospital emergency room. Compared to patients with a normal troponin I (cTnI) patients with an elevated cTnI are considered to be at increased risk5, 6, 7 and usually recommended for aggressive antiplatelet therapy coupled with early angiography and revascularization when appropriate.
When PCI is associated with ischemic complications, increases in cTnI can be expected and may indicate an adverse outcome. However in the absence of either angiographic or clinical PCI complications the significance of periprocedural increases of cTnI remains uncertain. We undertook the present study to determine:
-
1.
How often and to what extent does cTnI increase after uncomplicated PCI?
-
2.
What if any clinical or procedural factors are predictive of increased cTnI following PCI?
-
3.
Does increased cTnI following uncomplicated PCI have an impact on long term survival?
Methods
From January 1, 1998 through December 31, 2006, 1,538 patients underwent an initial PCI at our institution. The current study consisted of 907 patients who did not have any of the following exclusions:
-
1.
A clinical diagnosis of myocardial infarction (MI) occurring within 48 h before the procedure or a pre‐PCI cTnI above 0.1 ng/ml (n = 407).
-
2.
Failure to obtain at least 1 measurement of cTnI more than 12 h after the procedure (n = 195).
-
3.
A recognized complication during the PCI likely to be associated with an elevated post‐PCI cTnI (n = 29).
Troponin I values were determined by the clinical laboratory using the heterogeneous immunoassay module Dimension® (Dade Behring Inc. Newark, DE, USA). Values under 0.04 ng/ml were reported by our laboratory as “< 0.04” but are considered as “ = 0.04” ng/ml for the purpose of our study. In our laboratory cTnI values less than 0.1 ng/ml are considered normal, values between 0.10 and 1.49 ng/ml are considered indeterminate, and values equal or more than 1.5 ng/ml are considered to be consistent with myocardial injury.
Because the vast majority of our patients underwent their PCI as a scheduled elective procedure, preprocedural cTnI was usually not measured. Obtaining postprocedural cTnI measurements however was routine and not dependant on clinical or procedural events.
Clinical and procedural related data were prospectively entered into our PCI database upon completion of the procedure. Survival status was determined from the Department of Veterans Affairs computerized medical records. For patients lost to the VA system the Social Security Death Index was searched to identify additional patients who had expired. For patients not known to have died at follow‐up, time was determined from the date of the initial PCI to the date the patient was last seen at a VA healthcare facility.
Statistics
Summary data are expressed as mean ± SD or percent. Predictors of maximal cTnI following the procedure (TrMX) were determined by multiple regression. Cumulative survival curves were constructed according to Kaplan‐Meier with the log‐rank test used to determine the significant univariate predictors of survival after conversion of continuous variables to dichotomous variables with age as equal or below versus above the median value of 65 years, number of treated lesions as 1 versus more than 1, stents as none or 1 versus more than 1, and TrMX as below versus equal or above the 95 percentile which was 3.62 ng/ml. Significant univariate predictors were then entered into the Cox proportional hazards regression model with both age and TrMX entered as continuous variables. A p value of < 0.05 was considered to be significant.
Results
Demographics
The 907 patients averaged 65.5 ± 10.2 years in age (median 65 years) and all but 8 patients were male, reflecting the VA population. Diabetes mellitus (DM) was present in 38.5%, congestive failure (CHF) in 19.5%, peripheral vascular disease (PVD) in 22.4%, obstructive pulmonary disease (COPD) in 13.6%, and abnormal renal function (RF) in 20.7%.
Procedural Data
A single lesion was treated in 623 patients (68.7%) with more than 1 lesion treated in 284 patients (31.3%). Stents were used in 869 patients (95.8%) with at least 1 drug‐eluting stent used in 41.7% of patients receiving stents. In 97 patients (10.7%), at least 1 saphenous vein graft (SVG) was treated. Rotablation atherectomy was used in 33 patients (3.6%) and balloon angioplasty was only used in 26 patients (2.9%). In 865 patients (95.4%), all attempted lesions were improved, in 27 patients (3.0%) some were improved and in 15 patients (1.7%), no lesion was improved.
Determinants of cTnI Increase (Table 1)
Table 1.
Predictors of increased post‐PCI troponin I
| Variable | p value | Lower CI | Upper CI |
|---|---|---|---|
| Age | = 0.0811 | −0.001 | 0.021 |
| Diabetes | = 0.6083 | −0.167 | 0.292 |
| COPD | = 0.6605 | −0.403 | 0.255 |
| PVD | = 0.7654 | −0.229 | 0.312 |
| CHF | = 0.9237 | −0.274 | 0.302 |
| RF | = 0.7461 | −0.239 | 0.333 |
| Number of lesions treated | = 0.0683 | −0.015 | 0.401 |
| Number of stents used | = 0.0023 | 0.100 | 0.462 |
| SVG | = 0.9241 | −0.386 | 0.351 |
| DES | = 0.9274 | −0.244 | 0.222 |
| Rotablation | = 0.2701 | −0.929 | 0.226 |
| Balloon only | = 0.2552 | −0.299 | 1.126 |
Abbreviations: CI = confidence interval; CHF = congestive heart failure; COPD = chronic obstructive lung disease; DES = drug‐eluting stent; PCI = percutaneous coronary angioplasty; PVD = peripheral vascular disease; RF = renal dysfunction; SVG = saphenous vein graft
Following the procedure, TrMX averaged 0.80 ± 1.7 (median 0.2) ng/ml. TrMx exceeded the upper normal value of 0.1 ng/ml in 591 patients (65.2%) and was equal or above 1.5 ng/ml (a value consistent with myocardial injury) in 124 patients (13.7%). In patients having 2 cTnI measurements during the 24 h following the procedure (n = 630) the cTnI at 16.5 h was higher than the cTnI at 6.5 h in two‐thirds.
By multiple regression the only significant predictor of TrMX was the number of stents deployed (p < 0.0023, 95% CI: 0.10–0.46).
Determinants of Survival (Table 2, Figure 1)
Table 2.
Predictors of survival
| Variable | p value | |
|---|---|---|
| Log rank | Cox | |
| Age | <0.0001 | <0.0001 |
| Diabetes | =0.0201 | =0.0661 |
| COPD | <0.0001 | <0.0001 |
| PVD | <0.0001 | =0.1006 |
| CHF | <0.0001 | <0.0001 |
| RF | <0.0001 | =0.0557 |
| Number of treated lesions | =0.4931 | — |
| Number of stents used | =0.7999 | — |
| SVG | =0.0525 | — |
| DES | =0.5852 | — |
| Rotablation | =0.3562 | — |
| Balloon only | =0.3968 | — |
| TrMX | =0.0451 | =0. 0272 |
Abbreviations: CHF = congestive heart failure; COPD = chronic obstructive lung disease; DES = drug‐eluting stent; PVD = peripheral vascular disease; RF = renal dysfunction; SVG = saphenous vein graft
Figure 1.
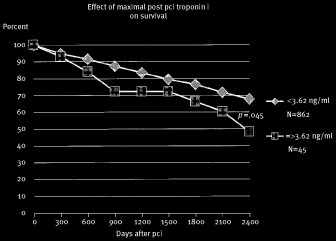
Kaplan‐Meier cumulative survival after PCI comparing patients with a maximum post‐PCI cTnI equal or above the 95 percentile of 3.62 ng/ml (n = 45) to those with a maximum cTnI of less than 3.62 ng/ml (n = 862). The curves are significantly different from the log‐rank test (p = 0.045).
Significant univariate predictors of survival (log‐rank test) were older age, DM, CHF, RF, COPD, PVD, and TrMX equal or above 3.62 ng/ml (95 percentile for TrMX). When entered into the Cox proportional hazards model however, only age, COPD, CHF, and TrMX were significant independent predictors of survival.
Discussion
Our data show that small increases in cTnI occur in the majority of patients within 24 h following uncomplicated PCI and in a significant number of patients (13.7%) TrMX reached a value suggestive of myocardial injury. Multiple investigators have reported increased cTnI following PCI with an incidence ranging from 16% to 73%. This rather wide range can be explained by the fact that some studies included patients who were unstable prior to PCI or had recognized complications during the procedure. In addition, the criteria used to define what constitutes an abnormal cTnI following PCI has yet to be standardized.
Mandadi et al.8 reported increased cTnI in 27% of 405 post‐PCI patients. In their study an elevated cTnI was defined as either > 2.0 ng/ml or a > 20% rise from elevated pre‐PCI values. Natarajan et al.9 reported cTnI above the upper normal value in 17% of 1,128 post‐PCI patients after exclusion of patients with abnormal pre‐PCI cTnI values or elevated pre‐PCI or post‐PCI creatine kinase values. Kini and colleagues3 reported elevated cTnI (defined as equal to or greater than 2 ng/ml) in 39% of 2,873 post‐PCI patients after excluding patients with cardiogenic shock or pre‐PCI increases of CK‐MB. Cantor et al.10 found a positive cTnI (defined as cTnI > 1.5 ng/ml) in 26% of 151 patients undergoing PCI in whom pre‐PCI cTnI had been negative. Although these studies excluded patients with an elevated pre‐PCI cTnI they did not exclude patients with recognized periprocedural complications likely to cause cTnI release.
In our study, we exluded patients with an abnormal pre‐PCI cTnI as well as patients undergoing PCI for an acute coronary syndrome even if the initial cTnI was not elevated. We also excluded patients with a recognized PCI complication likely to be associated with a procedural rise in cTnI. We believe therefore that our results in a large number of patients reflect what can be realistically expected with regard to cTnI elevation following uncomplicated, elective PCI.
Several risk factors, in addition to procedural complications, have been reported to be associated with an elevation of CK‐MB or cTnI after PCI. These include multivessel or multilesion PCI, lesion length and complexity, presence of thrombus, rest angina, PCI of SVGs, use of multiple stents, older age, increased fluoroscopy time, and increased use of contrast media.3, 8, 9, 11 In a study of 2,256 patients undergoing PCI with intravascular ultrasound imaging, Mehran et al.12 found plaque burden to be a significant independent predictor of post‐PCI CK‐MB elevation.
In our study we found an increased number of stents deployed to be the only significant independent variable associated with an increased post‐PCI cTnI while older age (p = 0.081) and the number of treated lesions (p = 0.068) were of borderline significance.
In the absence of a recognized procedural complication, the causes of post‐PCI cTnI elevation remains speculative. Atheromatous material is frequently found on filters used for distal protection during balloon or stent expansion and distal embolization, coronary vasospasm, or small side branch occlusion are factors which could go unrecognized and yet result in small increases of cTnI.
The impact of cardiac enzyme release after PCI on subsequent outcome is an area of controversy.1 Ricciardi and colleagues13 reported an increased incidence of major adverse cardiac events among 286 patients who had an elevated cTnI following elective PCI. Saadeddin et al.14 reported a significant increase in adverse cardiac events in patients whose cTnI was above 2.0 ng/ml within 24 h following PCI. Nageh et al.15 reported adverse events at 18 mo after PCI when associated with elevated cTnI. Drzewiecka‐Gerber and colleagues16 reported a cTnI above 0.1 ng/ml in 77% of their patients following PCI, but found adverse events only when cTnI exceeded 1.0 ng/ml.
Contrary to the previous studies, Kini and colleagues3 followed 2,873 patients without acute myocardial infarction who underwent PCI. Survival was significantly reduced in patients with a post‐PCI CK‐MB of > 5 times the upper normal value as well as in patients with heart failure, peripheral vascular disease, or post‐PCI renal failure; however, an elevated post‐PCI cTnI was not predictive. Natarajan et al.9 found that cTnI elevation even at 5 times the upper normal value did not predict adverse events following hospital discharge in patients who did not have a concomitant elevation of creatine kinase.
In a recent publication by the ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction17 it was stated that “there is currently no solid scientific basis for defining a biomarker threshold for the diagnosis of peri‐procedural myocardial infarction.” It was suggested that until additional data becomes available elevations more than 3 times the 99th percentile be considered as indicative of PCI‐related myocardial infarction.
In our study, we found age and other comorbid conditions to be strongly predictive of worsened long‐term survival. We also found that survival was significantly worse in patients with a higher post‐PCI cTnI, however this effect appeared to be small when compared to comorbidities and was essentially limited to elevations of cTnI at or above the 95 percentile. It is possible that the relationship between increased cTnI and reduced survival is the result of unrecognized myocardial injury sustained during the procedure. However it is also possible that higher post‐PCI cTnI values identify patients with more complex or advanced coronary disease with a less favorable long‐term prognosis.18
Study Limitations
We excluded from our analysis patients with a pre‐PCI diagnosis of acute myocardial infarction and/or patients with a pre‐PCI cTnI of 0.1 ng/ml or above. The patients included in our study were undergoing elective PCI and although only a few had cTnI measured prior to the procedure we think it quite reasonable to assume that in these patients an abnormal elevation of cTnI observed after the PCI resulted from the procedure rather than from events occurring prior.
We attempted to eliminate from the study any patient who had a PCI‐related complication likely to cause a rise in cTnI. However it is possible that some procedural related complications were unrecognized.
Our study did not include an analysis of lesion morphology or complexity nor did we attempt to analyze the effects of adjuvant pharmacotherapy or the use of distal protection devices all of which have been shown in other studies to have an impact on cardiac enzyme release following PCI.
Conclusions
-
1.
The majority of low risk patients undergoing elective and uncomplicated PCI can be expected to have small increases of cTnI within 24 h following the procedure.
-
2.
Higher values of cTnI occur in patients in whom multiple stents are used.
-
3.
In our study, long‐term survival was primarily effected by the presence of associated comorbid conditions, however it was also adversely effected in those patients with the largest increases in post‐PCI cTnI.
References
- 1. Bhatt DL, Topol EJ. Does creatine kinase‐MB elevation after percutaneous coronary intervention predict outcomes in 2005. Circulation 2005; 112: 906–923. [DOI] [PubMed] [Google Scholar]
- 2. Nageh T, Sherwood RA, Harris BM, et al. Cardiac troponin T and I and creatine kinase‐MB as markers of myocardial injury and predictors of outcome following percutaneous coronary intervention. Int J Cardio 2003; 92: 285–293. [DOI] [PubMed] [Google Scholar]
- 3. Kini AS, Lee P, Marmur JD, et al. Correlation of postpercutaneous coronary intervention creatine kinase‐MB and troponin I elevation in predicting mid‐term mortality. Am J Cardiol 2004; 93: 18–23. [DOI] [PubMed] [Google Scholar]
- 4. Stone GW, Mehran R, Dangas G, et al. Differential impact on survival of electrocardiographic Q‐wave versus enzymatic myocardial infarction after percutaneous intervention: A device specific analysis of 7,147 patients. Circulation 2001; 104: 642–647. [DOI] [PubMed] [Google Scholar]
- 5. Hamm CW, Goldmann BU, Heeschen C, et al. Emergency room triage of patients with acute chest pain by means of rapid testing for cardiac troponin T or troponin I. N Engl J Med 1997; 337: 1648–1657. [DOI] [PubMed] [Google Scholar]
- 6. Newby LK, Roe MT, Chen AY, et al. Frequency and clinical implications of discordant creatine kinase‐MB and troponin measurements in acute coronary syndromes. J Am Coll Cardiol 2006; 47: 312–318. [DOI] [PubMed] [Google Scholar]
- 7. Kontos MC, Shah R, Fritz LM, et al. Implication of different cardiac troponin I levels for clinical outcomes and prognosis of acute chest pain patients. J Am Coll Cardiol 2004; 43: 958–965. [DOI] [PubMed] [Google Scholar]
- 8. Mandadi VR, DeVoe MC, Ambrose JA, et al. Predictors of troponin elevation after percutaneous coronary intervention. Am J Cardiol 2004; 93: 747–750. [DOI] [PubMed] [Google Scholar]
- 9. Natarajan MK, Kreatsoulas C, Velianou JL, et al. Incidence, predictors and clinical significance of troponin‐I elevation without creatine kinase elevation following percutaneous coronary interventions. Am J Cardiol 2004; 93: 750–753. [DOI] [PubMed] [Google Scholar]
- 10. Cantor WJ, Newby LK, Christenson RH, et al. Prognostic significance of elevated troponin I after percutaneous coronary intervention. J Am Coll Cardiol 2002; 39: 1738–1744. [DOI] [PubMed] [Google Scholar]
- 11. Sergy A, Goldman LE, Cantor LE, et al. Elevated troponin‐I after percutaneous coronary interventions: Incidence and risk factors. Cardiovasc Radiat Med 2004; 5: 59–63. [DOI] [PubMed] [Google Scholar]
- 12. Mehran R, Dangas G, Mintz GS, et al. Atherosclerotic plaque burden and CK‐MB enzyme elevation after coronary interventions: Intravascular ultrasound study of 2,256 patients. Circulation 2000; 101: 604–610. [DOI] [PubMed] [Google Scholar]
- 13. Ricciardi MJ, Davidson CJ, Gubernikoff G, et al. Troponin I elevation and cardiac events after percutaneous coronary intervention. Am Heart J 2003; 145: 522–528. [DOI] [PubMed] [Google Scholar]
- 14. Saadeddin SM, Habbab MA, Sobki SH, et al. Biochemical detection of minor myocardial injury after elective, uncomplicated, successful percutaneous coronary intervention in patients with stable angina: Clinical outcome. Ann Clin Biochem 2002; 39: 392–397. [DOI] [PubMed] [Google Scholar]
- 15. Nageh T, Sherwood RA, Harris BM, et al. Prognostic role of cardiac troponin I after percutaneous coronary intervention in stable coronary disease. Heart 2005; 91: 1181–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Drzewiecka‐Gerber A, Drzewiecki J, Wita K, et al. Prognostic value of troponin I after elective percutaneous coronary interventions. Kardiol pol 2004; 61: 117–126. [PubMed] [Google Scholar]
- 17. Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol 2007; 50: 2173–2195. [DOI] [PubMed] [Google Scholar]
- 18. Zethelius B, Johnston N, Venge P. Troponin I as a predictor of coronary heart disease and mortality in 70‐year‐old men. A community‐based cohort study. Circulation 2005; 113: 1071–1078. [DOI] [PubMed] [Google Scholar]


