Abstract
Background
Manganese Dioxide (MnO) has long been used in industry, and its application has recently been increasing in the form of nanoparticle. Objective: The present study was an attempt to assess the effects of MnO nanoparticles on spermatogenesis in male rats.
Materials and Methods
Micro- and nanoparticles of MnO were injected (100 mg/kg) subcutaneously to male Wistar rats (150 20 gr) once a week for a period of 4 weeks, and the vehicle group received only normal saline (each group included 8 rats). The effect of these particles on the bodyweight, number of sperms, spermatogonia, spermatocytes, diameter of seminiferous tubes, testosterone, estrogen, follicle stimulating factor, and the motility of sperms were evaluated and then compared among the control and vehicle groups as the criteria for spermatogenesis.
Results
The results showed that a chronic injection of MnO nanoparticles caused a significant decrease in the number of sperms, spermatogonia, spermatocytes, diameter of seminiferous tubes (p 0.001) and in the motility of sperms. However, no significant difference was observed in the weight of prostate, epididymis, left testicle, estradiol (p = 0.8) and testosterone hormone (p = 0.2).
Conclusion
It seems that the high oxidative power of both particles was the main reason for the disturbances in the function of the testis. It is also concluded that these particles may have a potential reproductive toxicity in adult male rats. Further studies are thus needed to determine its mechanism of action upon spermatogenesis.
Keywords: MnO2, Nanoparticle, Toxicity, Reproductive, Stereological study
1. Introduction
Human-made materials, especially those made with nanoparticles, have increased the public concern regarding their effects on the body health. Nanoparticles have very particular chemical, physical, and biological characteristics in their size, shape and a high proportion of surface to volume (1). Nano- and micro-metal oxides are recently used as MnO in different industries like Magnetic Resonance Imaging (MRI), drug delivery system, steel casting, catalysis, and consumer products such as batteries, which has undoubtedly led to the human exposure to these particles (2, 3).
Previous reports have shown that magnetic nanoparticles are biosafe and biocompatible and can be used in biomedical materials. Toxicological studies have also shown that these magnetic nanoparticles have adverse effects on the health of human beings as well as other living species. The biological safety of MnO nanoparticles is a controversial issue (4).
Previous studies indicated that MnO could increase the environmental risks and expose human beings to such disorders as Parkinsonism and occupational inhalation. The first report of chronic manganese poisoning was from a manganese ore-crushing plant in France (5). Moreover, invitro and invivo studies on MnO nano- and micro-particles indicated that they cause serious pathological risks to the central nervous system, respiratory system, cardiovascular and liver, as well as reproductive and developmental systems (6, 7).
Recent studies indicated that most nanoparticles insert harmful or toxic effects on spermatogenesis. It seems that such factors as the animal species, drug usage, nanoparticle's dose, and its characteristics (e.g., size, shape, chemical composition, surface, and surface charge) play an important role in determining the nanoparticles' effect on spermatogenesis (6). However, the penetration of nanoparticles to the blood-testis barrier is vital for explaining their toxicity in the process of spermatogenesis (7). Takeda observed sporadic seeds in the injured tubes of testis tissue and showed that Titanium Dioxide (TiO) nanoparticles have a negative effect on spermatogenesis and epididymal sperm motility and induce histopathological changes appeared in the rat's testis (8). Studying the effect of gold nanoparticles on sperm and their penetration into the sperm cells revealed that these nanoparticles can result in the fragmentation of sperm's DNA. It was also shown that the presence of gold nanoparticles affected the sperms' motility; 25% of the sperms exposed to gold nanoparticles did not move (9).
Due to their critical role in the development and future well-being of wildlife, human exposure after maturity and also its health risks, reproductive and developmental toxicity have been used more in toxicology studies. Consequently, MnO nanoparticles are able to cross the blood-testis barrier and accumulate in the testis of rats which, in return, might have certain specific side effects.
Therefore, the aim of this study is to investigate the potential and possible toxic effects of MnO nano- and micro-particles on the reproductive system in male rats.
2. Materials and Methods
Chemicals and dosage
All the chemicals and MnO-MPs with size 5 mm and purity 99% (CAS No. 1313-13-9) were purchased from Merck Company, Hohenbrunn, Germany. MnO nanoparticles, however, were synthetized by Dr. Safi Rahmani. The particles were scrutinized by an electron microscope to ensure that they were 25 to 85 nanometers in size. Both particles were dissolved in normal saline solution. The prepared solutions were sonicated for 30 min before injection. Nano and micro particle doses were selected according to previous investigations with pathological consequences on liver, kidneys, and testes of rats (10, 11).
The MnO nanoparticles preparation
In this study, 20 ml of KMnO (0.2 mM/lit) were mixed with 16 ml MnO4 (0.125 mM/lit) for 5 min. The mixture was taken into a steel autoclave with Teflon covering and kept for 16 hr in 160°C. The brown product washed 3 times by distilled water and ethanol, and then dried (12 hr for 80°C). The size of synthetized particles were estimated 25 to 85 nanometers by an electron microscope (Figure 1). MnO nanoparticles were prepared using the hydrothermal procedure introduced by Zhang and his colleagues. (12)
Figure 1.

A) A scanning electron micrograph of MnO microparticles B) A scanning electron micrograph of MnO nanoparticles.
Animals and treatments
For the experimental study, 32 mature male albino Wistar rats weighing 180 5 gr and age 8–10 wk were purchased from Shahid Beheshti University, Tehran, Iran. They were kept in an air-conditioned colony room with a light/dark cycle (temperature of 21–23°C and humidity of 30–40%) and were supplied with standard diet and enough water ad libitum. The rats were divided into four groups (n = 8) including control, vehicle (only received normal saline), nanoparticles (100 mg kg per day), and microparticles (100 mg kg per day). The treatment was carried out once a week for a period of 4 wk which was shown in previous studies to induce greater compassion in the testis.At the end of the treatment period, the rats were weighed and then anesthetized by ether. The reproductive organs including the left testicle, epididymis, seminal vesicle, and the left prostate were dissected and weighted after removing the lipid remnants. Then the blood samples were centrifuged (1500 rpm, 20 min) to measure estradiol, testosterone, follicle-stimulating hormone (FSH), and luteinizing hormone (LH) by immunoassay technique. (Orion Diagnostica; Finland and DRG Instruments GmbH; Germany).
Assessment of the number of sperms
The sperm samples were obtained from the distal region (1.0 cm) of the left vas deferens and placed on a plate containing 2cc of Hank's Balanced Salt Solution and then were gently agitated at 37°C for 3 min. Specimens were spread on a hemocytometer, and the heads were counted manually under a light microscope. Data were expressed as the total number of sperm/ml:
Sperm count = No. of spermatozoa Dilution factor Depth factor No. of areas counted (13, 14).
Sperm morphology
The sperm smears were stained by Eosin Y and evaluated in 10 microscopic fields, which the number of sperms 200-300 per animal. Spermatozoa were categorized as normal and abnormal. The abnormality was considered by a variety of head and tail abnormalities, containing blunt hook, banana-head, amorphous, pin-head, two-head, two-tail, small head, and bent tail. Finally, normal morphology sperm fraction parameter was defined as the mean number of normal sperm 100/total number of sperm.
Stereological study
The left testis was immersed in 10% formalin. Samples were divided into 5 micrometers and stained by hematoxylin and eosin method. The number of primary and secondary spermatocytes, spermatogonia, and diameter of seminiferous tubes were calculated per sample in 12 seminiferous tubes using a light microscope. The orientator method was used to obtain the Isotropic Uniform Random sections. Therefore, the testis was randomly placed on the clock which is divided into nine similar parts. A right cut was made along a randomly selected number (1-9). The first piece was then placed on the clock along with its previous cut surface on the 0-0 axis. The aforementioned procedure was repeated and the second piece was vertically placed on the clock so that its cut surface overlapped the 0-0 axis. A parallel cut was also made on this piece along with a accidentally selected number. Sections with 5 and 20 m thickness were cut by the microtome after tissue processing and stained using Heidenhain's Azan method (15, 16).
Ethical consideration
All experiments were performed in accordance with the guidelines for the care and use of laboratory animals (National Institutes of Health Publication No. 80-23, revised 1996) and were approved by the Research and Ethics Committee of the Pharmaceutical Sciences.
Statistical analysis
The data were analyzed by ANOVA, Tukey Test, and Friedman Test with the statistical significance of p 0.05 using the Stata software, version 13 (Stata Corp, College Station, TX, USA).
3. Results
Effects of micro- and nanoparticles of MnO on clinical signs and body weight changes
All the animals survived the experiments. Figure 2 illustrates the body weight of rats in each group compared to their average body weight in the 1st wk of the experiment. The results of the Friedman Test indicated a significant difference between the experimental groups compared to the vehicle and control groups. The body weight gain of the animals treated with nanoparticles significantly decreased during the 2 wk and increased in the 4 and 5 wk after injection, compared to the untreated control group.
The body weight gain of the animals treated with microparticles was continuous during the whole treatment and significantly increased compared to the untreated control group in the 4 and 5 wk after injection. The body weight gain of the rats receiving nanoparticles in most weeks was significantly lower than that of rats receiving the same dose of microparticles.
The effect of micro- and nanoparticles of MnO on all genital organs
The effect of micro- and nanoparticles of MnO on all genital organs compared to the body weight after 28 days of treatment in rats is shown in Table I. No significant difference was found in testis (p = 0.7), epididymis (p = 0.8), prostate (p = 0.6), and large and small diameter of left testis (p = 0.9) parameters among all groups.
Effect of micro- and nanoparticles of MnO on epididymal sperm count and motility
The mean number of epididymal sperm showed a highly significant reduction in the nano- and micro groups compared to other groups (p 0.001) (Table II).
The results of sperm motion analysis are shown in Table II. The percentage of immotile sperm significantly (p 0.000) increased in the nano- and micro groups by 100% after 4 wk treatment compared to the vehicle and control groups.
Table 1.
The effect of the MnO on the weights of prostate, seminal vesicle, left and right testicle, and diameter of left testis
|
| ||||||
| Groups | Left Testis (g) | Right Testis (g) | Left Epididymis (g) | Prostate (g) | Large diameter of Left Testis (mm) | Small diameter of Left Testis (mm) |
| Control | 0.69 0.03 | 0.67 0.03 | 0.024 0.01 | 0.24 0.01 | 19.9 0.17 | 10.7 0.05 |
| Vehicle | 0.61 0.01 | 0.61 0.003 | 0.23 0.009 | 0.19 0.03 | 20.14 0.35 | 10.9 0.08 |
| Micro-MnO | 0.67 0.09 | 0.57 0.04 | 0.22 0.02 | 0.22 0.02 | 20.18 0.39 | 11.3 0.33 |
| Nano-MnO | 0.60 0.01 | 0.58 0.01 | 0.22 0.01 | 0.24 0.03 | 20.12 0.27 | 11.5 0.17 |
| Note: Values are means STD for 8 rats; MnO: Manganese Dioxide | ||||||
Table 2.
The effect of micro- and nanoparticles of MnO on sperm motility and epidydimal sperm count of rats
|
| |||||
| Groups | Sperm Count (ml) | Sperm Motility (%) | |||
| Rapid | Slow | None | Immotile | ||
| Control | 316333 27284 | 71 | 10 | 15 | 5 |
| Vehicle | 320666 7218 | 61 | 18 | 11 | 10 |
| Micro-MnO | 20666 3051### | – | – | – | 100 |
| Nano-MnO | 311266 6241### | – | – | – | 100 |
| Note: Values are means STD for 8 rats; ****p 0.0001 compared to vehicle and control groups; ###p 0.001 compared to the vehicle and control groups (one-way ANOVA followed by Tukey post-hoc test) | |||||
Figure 2.

Comparison of changes in body weight per week relative to the weight of the first week, values expressed as mean for 8 rats.
The effect of micro- and nanoparticles of MnO on the number of spermatogonia, spermatocyte, spermatid and seminiferous tubules
The mean number of spermatogonia showed a highly significant reduction in the micro- and nanoparticles of MnO groups compared to other groups (p 0.001). Moreover, the reduction in the mean number of spermatocyte in the nano- and micro groups was highly significant when compared to other groups (p 0.001). The mean of spermatid and seminiferous tubules diameter showed a highly significant (p 0.001) reduction in the micro- and nanoparticles of MnO groups compared to other groups. A significant decrease (p 0.05) was also observed in the mean spermatid and seminiferous tubules' diameter of nanoparticles of MnO group compared to the micro-group (Table III).
The effect of micro- and nanoparticles of MnO on serum hormones level
Serum concentrations of FSH (p = 0.37), Estradiol (p = 0.8), and Testosterone (p = 0.2) in all four groups showed no significant difference (Table IV).
Histopathological findings
Histopathological micrographs of the testis show the structure and cellular arrangement of the seminiferous tubules. Germinal epithelium is normal in control and vehicle groups containing different germline cells, spermatogonia, spermatocyte, spermatid and spermatozoa. In nano- and micro-MnO groups, the germline cells were also seen but the interstitial space hyalinized because of fluid accumulation (Figure 3).
Table 3.
Comparing the number of spermatogonia, spermatocyte, spermatid and seminiferous tubules in different groups of rats after 28 days of treatment
|
| |||
| Groups | Spermatogonia 10 | Spermatocyte 10 | seminiferous tubules 10 |
| Control | 64.9 0.26 | 203.9 1.1 | 65.2 0.86 |
| Vehicle | 64.96 0.48 | 203.2 0.28 | 64.7 0.98 |
| Micro-MnO | 50.6 0.52 | 162.1 0.62 | 58.7 0.31 |
| Nano-MnO | 56.4 0.31 | 171.9 0.50 | 56.1 0.6 |
| Note: Values are mean STD; ***p 0.001 compared to the vehicle and control groups; p 0.001 compared to the Nano-group; MnO: Manganese Dioxide | |||
Table 4.
The effect of micro- and nanoparticles of MnO on serum concentrations of FSH, estradiol, and testosterone
|
| |||
| Groups | FSH (µIU/L) | Estradiol (Pg/mL) | Testosterone(ng/mL) |
| Control | 1.75 0.43 | 46 2.8 | 0.85 0.05 |
| vehicle | 3.6 1.05 | 40 5.7 | 4.01 2.4 |
| Micro-MnO | 4.9 1.55 | 39.6 6.01 22 | 2.91 0.73 |
| Nano-MnO | 3.4 0.4 | 39.4 4.04 | 4.37 0.88 |
| Note: Values are mean STD; MnO: Manganese Dioxide; FSH: Follicle-stimulating Hormone | |||
Figure 3.
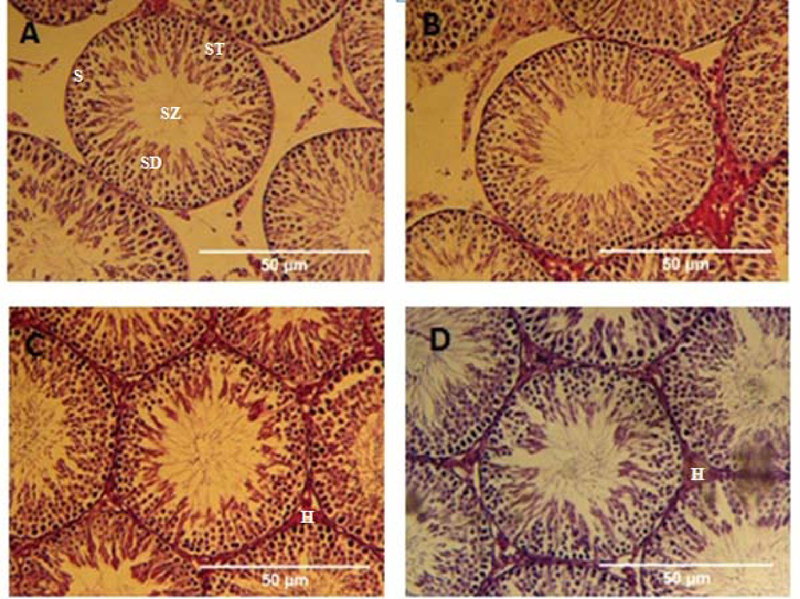
Histopathological micrographs of the testis's Control group (a), Vehicle group (b), Nanoparticles of MnO group (c), and Microparticles of MnO group (d). Germinal epithelium is normal in control and vehicle groups containing different germline cells. Spermatogonia(S), Spermatocyte (ST), Spermatid (SD), and Spermatozoa (SZ) in nano- and micro-MnO groups, the germline cells were also seen but the interstitial space hyalinized(H) because of fluid accumulation. All samples were stained with H&E (A, B, C, and D: 10).
4. Discussion
This study was an effort to investigate the effects of sub-chronic injection of MnO nanoparticles on spermatogenesis of male rats compared to the injection of MnO microparticles. The effects of MnO nano- and micro-particles on the body weight of rats during 4 wk of injection illustrated that the vehicle and control groups had a normal weight gain, but the experimental groups had a significant weight loss during the 2nd wk. They also had a significant weight gain after 2 wk compared to the vehicle and control groups. The previous studies demonstrated that the application of MnO using this method can cause hyperglycemia and hyperlipidemia; therefore, the significant weight gain of the experimental groups compared to the vehicle and control groups can be attributed to this phenomenon (3). Generally, the body weight of rats is measured to show their general health after nanoparticle injection. The increase of oxidative stress and metabolic disturbance as a result of free radicals have been mentioned as the main reasons for the decrease of weight gain after nanoparticle injection (17).
In the present study, the toxicity of MnO nanoparticles was investigated through subcutaneous injection. It was shown that these nanoparticles might cause the appearance of certain clinical symptoms such as weight loss. In this regard, Wang studied the high toxicity of nano- and microparticles of zinc powder in rats and reported severe symptoms of nausea, vomiting, and diarrhea in the rats treated with zinc powder nanoparticles in the first days of treatment. These findings were in agreement with the previous reports regarding the excessive oral prescription of zinc salts. Since the nanoscale materials are easily accumulated in different environments, it is believed that nano-scale zinc powder can also be easily accumulated in the body of animals (18).
Therefore, since the power of injected nanoparticles was stronger in inducing the oxidative stress, the effect of nanoparticles on the body weight was more significant. Another reason for the existing difference in the results can be attributed to different methods used in each study, including the rate, procedure, and period of injection (10). The findings of the present study showed that nano- and microparticles of MnO decrease the fertility factors such as the number of sperms, sperm motility, spermatogonia, spermatocytes, and diameter of seminiferous tubes. Since there have been no studies on the effects of MnO nanoparticles on the spermatogenesis, the effects of these nanoparticles have been compared to the effects of other forms of nanoparticles. Statistical analysis review of the effect of MnO nanoparticles on the serum level of sex hormones indicated that these particles significantly increased the hormones 14 days after treatment compared to the control group. This increase can be attributed to the effect of MnO nanoparticles on Leydig cells. MnO nanoparticles can also have a lower mitochondrial activity, leading to severe tissue damage and an increase in their secretion. On one hand, MnO nanoparticles increased the number of reactive oxygen molecules such as super oxidase as well as the oxidation of such molecules as proteins that accordingly leads to apoptosis (19). Several studies showed that nanoparticles can increase the transcription of mRNA as the steroidogenic acute regulatory protein of testis which influences the survival of Leydig cells and the production of steroid hormones (20). Moreover, the harmful effects of nanoparticles with their anti-androgenic features have been recognized as the cause of disturbances in the performance of the male reproductive system (testis tissue damage and sperm reduction). The steroidogenic acute regulatory protein plays an important role in regulating the cholesterol transfer into the inner membrane of mitochondria and increasing the steroid hormones (21, 22). On the other hand, it is assumed that silver nanoparticles can affect spermatogenesis and the number of spermatogenic cells and also the acrosome reaction in sperm cells; Miresmaeili. determined that nanoparticles have a negative effect on the spermatogenesis process and can influence reproductive potential in animal models (23). In the present study, the data indicated that FSH hormone level in those groups treated with nanoparticles significantly increased compared to the control group. The increase of FSH hormone level cannot be related to Gonadotropin-releasing hormone (GnRH). However, it can be attributed to the release of Inhibin hormone from Sertoil cells. Given the results of FSH hormone level, it is assumed that the feedback mechanisms are not applied just by the testis' steroids. But they play a role in regulating the FSH level by affecting the production of GnRH. The histopathologic study of testicular tissue sections with hematoxylin-eosin coloring in mice treated with zinc oxide nanoparticles with different doses of 1 and 2.5 ppm indicated various changes including, vacuolation of seminiferous tubes, reduction of germ cell lines in different stages of spermatogonia, irregularity in the order of germ cells, destruction of some seminiferous tubes involved in spermatogonia process, and the existence of colloidal liquid in lumen of seminiferous tubes. Lan and Ema showed that titanium dioxide nanoparticles could pass testicular blood barrier and accumulate in the form of granules in Sertoli cells (6, 24). This can accordingly lead to the reduction of Sertoli cells and thus damage and disorder of seminiferous tubes. Morphological changes of testis in animals treated with zinc oxide nanoparticles were observed after their exposure to nanoparticles. Spermatogonia process is disturbed due to the destruction of seminiferous tubes which is proved by the reduction of understudy parameters in this study (25).
Ema showed that TiO nanoparticles decrease the daily production and motility of sperms by causing disturbance in Sertoli cells (24). A study conducted in 2012 demonstrated that the treatment of mice with gold nanoparticles for 4 days had no adverse effects on testis epithelium and spermatogenesis (26). However, cytogenetic evaluations illustrated that gold nanoparticles could have affected the chromosomes of primary spermatocytes. Nevertheless, these nanoparticles caused no disturbance in chromosomes of spermatogonia cells. Another study, conducted in 2009, showed that gold nanoparticles had high toxicity power in spermatogenesis. These nanoparticles inhibit the motility of sperms. They also prevent the separation of chromatins and the formation of gametes (9, 27). In another study, the researchers demonstrated that the FeO nanoparticles destroy the seminiferous tubes and, in consequence, decrease the number of spermatogonia, primary spermatocytes, spermatids, and sperms (28). In 2013, a study on the evaluation of the toxicity of ZnO nanoparticles in spermatogenesis of mice showed that the spermatogenesis factors such as the number and motility of sperms changed significantly in the experimental group compared to the control group. ZnO nanoparticles decrease the diameter and height of the seminiferous tubes significantly and stop the maturity of sperm cell lines (7). Therefore, researchers concluded that ZnO nanoparticles can cause disturbance to spermatogenesis as a toxic compound. Kobayashi indicated a steady decrease in the number of alive and active sperms in relation to the increase of the amount of ROS (29). Therefore, the presence of ROS decreases the quality of sperms. This phenomenon occurs by the activation of oxidative stress by nanoparticles. According to this study and a other study, it can be mentioned that the chemical materials that are disruptive to endocrine system can cause oxidative damages to biomolecules such as DNA and protein through the production of free radicals (ROS) (26, 30). It is probable that free radicals cause mutation in testis, especially in spermatogonia, primary spermatocytes, spermatids, and spermatosoides, and can also seriously damage and destroy these cells (30).
5. Conclusion
The results of the present study demonstrated that nano- and microparticles of MnO decrease the number and motility of sperms significantly and reduce spermatogonia, spermatocytes, and the diameter of seminiferous tubes. Therefore, it seems that nano- and microparticles of MnO cause disturbance to spermatogenesis as toxic materials.
The present study showed that the prescription of MnO nanoparticles can cause significant changes in spermatogonia, sex hormones, and histology of male's testis that can also affect the performance of the male reproductive system and its fertility. Therefore, given the role of MnO nanoparticles in health product and medical equipment, they are considered as one of the harmful factors for reproductive systems that affect the fertility and, thus, should be evaluated and controlled with respect to their toxicity level in daily use.
Conflict of Interest
None of the authors have any potential conflict of interest of a funding source for this study.
Acknowledgments
This study was supported by the Pharmaceutical Sciences Branch, Islamic Azad University, Tehran, Iran. The authors have no support or funding to report.
References
- 1.Wani MY., Hashim MA., Nabi F., Malik MA., Nanotoxicity. Advances in Physical Chemistry. 1-15; 2011. dimensional and morphological concerns; p. 15. [Google Scholar]
- 2.Kim T., Momin E., Choi J., Yuan K., Zaidi H., Kim J., Park M., Lee N., McMahon M. T., Quinones-Hinojosa A., Bulte J. W. M., Hyeon T., Gilad A. A. Mesoporous silica-coated hollow manganese oxide nanoparticles as positive T 1 contrast agents for labeling and MRI tracking of adipose-derived mesenchymal stem cells. Journal of the American Chemical Society. 2011;133(9):2955–2961. doi: 10.1021/ja1084095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mousavi Z., Hassanpourezatti M., Najafizadeh P., Rezagholian S., Rhamanifar M. S., Nosrati N. Effects of subcutaneous injection MnO2 micro- and nanoparticles on blood glucose level and lipid profile in rat. Iranian Journal of Medical Sciences. 2016;41(6):518–524. [PMC free article] [PubMed] [Google Scholar]
- 4.Mahmoudi M., Hofmann H., Rothen-Rutishauser B., Petri-Fink A. Assessing the In Vitro and In Vivo Toxicity of Superparamagnetic Iron Oxide Nanoparticles. Chemical Reviews. 2012;112(4):2323–2338. doi: 10.1021/cr2002596. [DOI] [PubMed] [Google Scholar]
- 5.Huang C.-C. Parkinsonism induced by chronic manganese intoxication - An experience in Taiwan. Chang Gung Medical Journal. 2007;30(5):385–395. [PubMed] [Google Scholar]
- 6.Lan Z., Yang W. Nanoparticles and spermatogenesis: how do nanoparticles affect spermatogenesis and penetrate the blood–testis barrier. Nanomedicine. 2012;7(4):579–596. doi: 10.2217/nnm.12.20. [DOI] [PubMed] [Google Scholar]
- 7.Talebi A. R., Khorsandi L., Moridian M. The effect of zinc oxide nanoparticles on mouse spermatogenesis. Journal of Assisted Reproduction and Genetics. 2013;30(9):1203–1209. doi: 10.1007/s10815-013-0078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeda K., Suzuki K.-I., Ishihara A., Kubo-Irie M., Fujimoto R., Tabata M., Oshio S., Nihei Y., Ihara T., Sugamata M. Nanoparticles transferred from pregnant mice to their offspring can damage the genital and cranial nerve systems. Journal of Health Science. 2009;55(1):95–102. doi: 10.1248/jhs.55.95. [DOI] [Google Scholar]
- 9.Wiwanitkit V., Sereemaspun A., Rojanathanes R. Effect of gold nanoparticles on spermatozoa: the first world report. Fertility and Sterility. 2009;91(1):e7–e8. doi: 10.1016/j.fertnstert.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 10.Nosrati N., Hassanpour-Ezzati M., Mousavi SZ., Rezagholiyan MS. Rezagholiyan Sh. Comparison of MnO2 nanoparticles and microparticles distribution in CNS and muscle and effect on acute pain threshold in rats. Nanomedicine Journal. 2014;1:180–190. [Google Scholar]
- 11.Rezagolian S., Hassanpourezatti M., Mousavi SZ., Rhamanifar M., Nosrati N. Comparison of chronic administration of manganese oxide micro and nanoparticles on liver function parameters in male rats. Daneshvar Med. 35-46: 20 (Suppl; 2013. [Google Scholar]
- 12.Zhang Y., Yang Y., Zhang T., Ye M. Heterogeneous oxidation of naproxen in the presence of α-MnO2 nanostructures with different morphologies. Applied Catalysis B: Environmental. 2012;127:182–189. doi: 10.1016/j.apcatb.2012.08.014. [DOI] [Google Scholar]
- 13.Najafizadeh P., Dehghani F., Shahin M. P., Taj S. H. The effect of a hydro-alcoholic extract of olive fruit on reproductive argons in male sprague-dawley rat. Iranian Journal of Reproductive Medicine. 2013;11(4):293–300. [PMC free article] [PubMed] [Google Scholar]
- 14.Seed J., Chapin R. E., Clegg E. D., Dostal L. A., Foote R. H., Hurtt M. E., Klinefelter G. R., Makris S. L., Perreault S. D., Schrader S., Seyler D., Sprando R., Treinen K. A., Veeramachaneni D. N. R., Wise L. D. Methods for assessing sperm motility, morphology, and counts in the rat, rabbit, and dog: a consensus report. Reproductive Toxicology. 1996;10(3):237–244. doi: 10.1016/0890-6238(96)00028-7. [DOI] [PubMed] [Google Scholar]
- 15.Noorafshan A., Karbalay-Doust S. A simple method for unbiased estimating of ejaculated sperm tail length in subjects with normal and abnormal sperm motility. Micron. 2010;41(1):96–99. doi: 10.1016/j.micron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Karbalay-Doust S., Noorafshan A., Dehghani F., Panjehshahin M. R., Monabati A. Effects of hydroalcoholic extract of matricaria chamomilla on serum testosterone and estradiol levels, spermatozoon quality, and tail length in rat. Iranian Journal of Medical Sciences. 2010;35(2):122–128. [Google Scholar]
- 17.Merry B. J. Molecular mechanisms linking calorie restriction and longevity. The International Journal of Biochemistry & Cell Biology. 2002;34(11):1340–1354. doi: 10.1016/S1357-2725(02)00038-9. [DOI] [PubMed] [Google Scholar]
- 18.Wang B., Feng W.-Y., Wang T.-C., Jia G., Wang M., Shi J.-W., Zhang F., Zhao Y.-L., Chai Z.-F. Acute toxicity of nano- and micro-scale zinc powder in healthy adult mice. Toxicology Letters. 2006;161(2):115–123. doi: 10.1016/j.toxlet.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Carlson C., Hussein S. M., Schrand A. M., Braydich-Stolle L. K., Hess K. L., Jones R. L., Schlager J. J. Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. The Journal of Physical Chemistry B. 2008;112(43):13608–13619. doi: 10.1021/jp712087m. [DOI] [PubMed] [Google Scholar]
- 20.Komatsu T., Tabata M., Kubo-Irie M., Shimizu T., Suzuki K., Nihei Y., Takeda K. The effects of nanoparticles on mouse testis Leydig cells in vitro. Toxicology in Vitro. 2008;22(8):1825–1831. doi: 10.1016/j.tiv.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Arakane F., King S. R., Du Y., Kallen C. B., Walsh L. P., Watari H., Stocco D. M., Strauss J. F. Phosphorylation of Steroidogenic Acute Regulatory Protein (StAR) Modulates Its Steroidogenic Activity. The Journal of Biological Chemistry. 1997;272(51):32656–32662. doi: 10.1074/jbc.272.51.32656. [DOI] [PubMed] [Google Scholar]
- 22.Ajdary M., Ghahnavieh M., Naghsh N. Sub-chronic toxicity of gold nanoparticles in male mice. Advanced Biomedical Research. 2015;4(1):67. doi: 10.4103/2277-9175.153890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miresmaeili S. M., Halvaei I., Fesahat F., Fallah A., Nikonahad N., Taherinejad M. Evaluating the role of silver nanoparticles on acrosomal reaction and spermatogenic cells in rat. Iranian Journal of Reproductive Medicine. 2013;11(5):423–430. [PMC free article] [PubMed] [Google Scholar]
- 24.Ema M., Kobayashi N., Naya M., Hanai S., Nakanishi J. Reproductive and developmental toxicity studies of manufactured nanomaterials. Reproductive Toxicology. 2010;30(3):343–352. doi: 10.1016/j.reprotox.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Mohammadi Fartkhooni F., Noori A., Momayez M., Sadeghi L., Shirani K. Yousefi Babadi V. The effects of nano titanium dioxide (TiO2) in spermatogenesis in wistar rat. Euro J Exp Bio. 2013;3:145–149. [Google Scholar]
- 26.Zakhidov S. T., Pavlyuchenkova S. M., Marshak T. L., Rudoy V. M., Dement’eva O. V., Zelenina I. A., Skuridin S. G., Makarov A. A., Khokhlov A. N., Evdokimov Y. M. Effect of gold nanoparticles on mouse spermatogenesis. The Biological Bulletin. 2012;39(3):229–236. doi: 10.1134/S1062359012030156. [DOI] [PubMed] [Google Scholar]
- 27.Nazari M., Talebi A. R., Hosseini Sharifabad M., Abbasi A., Khoradmehr A., Danafar A. H. Acute and chronic effects of gold nanoparticles on sperm parameters and chromatin structure in Mice. International Journal of Reproductive BioMedicine. 2016;14(10):637–642. doi: 10.29252/ijrm.14.10.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sundarraj K., Manickam V., Raghunath A., Periyasamy M., Viswanathan M. P., Perumal E. Repeated exposure to iron oxide nanoparticles causes testicular toxicity in mice. Environmental Toxicology. 2017;32(2):594–608. doi: 10.1002/tox.22262. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi H., Larson K., Sharma R. K., Nelson D. R., Evenson D. P., Toma H., Thomas A. J., Agarwal A. DNA damage in patients with untreated cancer as measured by the sperm chromatin structure assay. Fertility and Sterility. 2001;75(3):469–475. doi: 10.1016/S0015-0282(00)01740-4. [DOI] [PubMed] [Google Scholar]
- 30.Choi SM., Yoo SD., Lee BM. Toxicological characteristics of endocrine-disrupting chemicals: developmental toxicity, carcinogenicity, and mutagenicity. J Toxicol Environ Health BCrit Rev. 2004;7:24. doi: 10.1080/10937400490253229. [DOI] [PubMed] [Google Scholar]


