Abstract
The recent decline in global malaria burden has stimulated efforts toward Plasmodium falciparum elimination. Understanding the biology of malaria transmission stages may provide opportunities to reduce or prevent onward transmission to mosquitoes. Immature P. falciparum transmission stages, termed stages I to IV gametocytes, sequester in human bone marrow before release into the circulation as mature stage V gametocytes. This process likely involves interactions between host receptors and potentially immunogenic adhesins on the infected red blood cell (iRBC) surface. Here, we developed a flow cytometry assay to examine immune recognition of live gametocytes of different developmental stages by naturally exposed Malawians. We identified strong antibody recognition of the earliest immature gametocyte-iRBCs (giRBCs) but not mature stage V giRBCs. Candidate surface antigens (n = 30), most of them shared between asexual- and gametocyte-iRBCs, were identified by mass spectrometry and mouse immunizations, as well as correlations between responses by protein microarray and flow cytometry. Naturally acquired responses to a subset of candidate antigens were associated with reduced asexual and gametocyte density, and plasma samples from malaria-infected individuals were able to induce immune clearance of giRBCs in vitro. Infected RBC surface expression of select candidate antigens was validated using specific antibodies, and genetic analysis revealed a subset with minimal variation across strains. Our data demonstrate that humoral immune responses to immature giRBCs and shared iRBC antigens are naturally acquired after malaria exposure. These humoral immune responses may have consequences for malaria transmission potential by clearing developing gametocytes, which could be leveraged for malaria intervention.
INTRODUCTION
Plasmodium falciparum malaria morbidity and mortality have decreased substantially in the past decade (1). These recent gains are threatened by the spread of artemisinin-resistant parasites (2) and insecticide-resistant mosquitoes (3). The recent achievements in malaria control and necessity to contain artemisinin resistance have stimulated malaria elimination initiatives that require a thorough understanding of the biology and epidemiology of malaria transmission and alternative transmission-reducing interventions (4).
P. falciparum transmission to mosquitoes is initiated when a small subset of asexually replicating blood stage parasites produce sexual progeny or gametocytes. Gametocytes develop in human red blood cells (RBCs) along five morphological transitions (stages I to V); stage I to IV development takes place predominantly in the extra-vascular niche of the bone marrow and spleen (5–7). Mature stage V gametocytes are released into the peripheral blood circulation where they may be ingested by a blood-feeding mosquito upon which they egress from RBCs as activated gametes and fuse and form motile zygotes. Further sporogonic development renders the mosquito infectious to humans. Several sexual stage proteins that have no function in gametocyte development but are essential for gamete fertilization (e.g., Pfs48/45 and Pfs230) or post-fertilization development in the mosquito (e.g., Pfs25 and Pfs28) (8) have been identified.
There is currently incomplete evidence for immune responses that affect gametocyte formation, maturation, or circulation time (9). Several field studies suggested mature gametocyte clearance after repeated malaria exposure (10–13), and antibody responses against uncharacterized targets on mature gametocyte-infected RBCs (giRBCs) have been associated with lower gametocyte densities (12, 14). Another field study identified antibodies that bound the surface of stage II to V giRBCs and distorted early gametocyte morphology and maturation (15). Depending on which stage(s) they target, antigametocyte immune responses could be involved in blocking extravascular adhesion of immature giRBCs and/or clearance of circulating mature giRBCs in a manner similar to antibodies against the asexual antigen Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1). PfEMP1 is an immunodominant antigen on the surface of RBCs infected with asexual parasites (aiRBCs); anti-PfEMP1 antibodies have an established role in immune clearance by inhibiting vascular adhesion and by opsonizing aiRBCs for phagocytic clearance (16, 17). aiRBC surface antigens other than PfEMP1 exist (18) and are associated with phagocytosis and cytotoxicity (19). The ligands involved in giRBC adherence may be different from those involved in endothelial binding of aiRBCs; giRBCs are localized to an extravascular compartment (5, 7), show limited binding to human endothelial cell lines, and harbor minimal PfEMP1 on their surface (20). Although no specific giRBC ligand has been identified, 1/10 of the early gametocyte proteome consists of putatively exported antigens called P. falciparum gametocyte-exported proteins (PfGEXPs) (21).
Hypothesizing that developing gametocytes could be targets of antibody responses in the human host, we performed a systematic characterization of gametocyte stage–specific immune recognition and clearance. We demonstrate naturally acquired human immune responses targeting immature (stages I to III) but not more mature stage V giRBCs. Experiments using whole cells and surface-intact and surface-depleted membrane fractions of diverse parasite strains provide evidence for giRBC surface antigens, most of them shared with aiRBCs. We further demonstrate that natural immunity to shared iRBCs correlates with reduced asexual and gametocyte burden and that a subset of the target antigens shows minimal sequence diversity.
RESULTS
Human immune responses recognize secreted gametocyte proteins
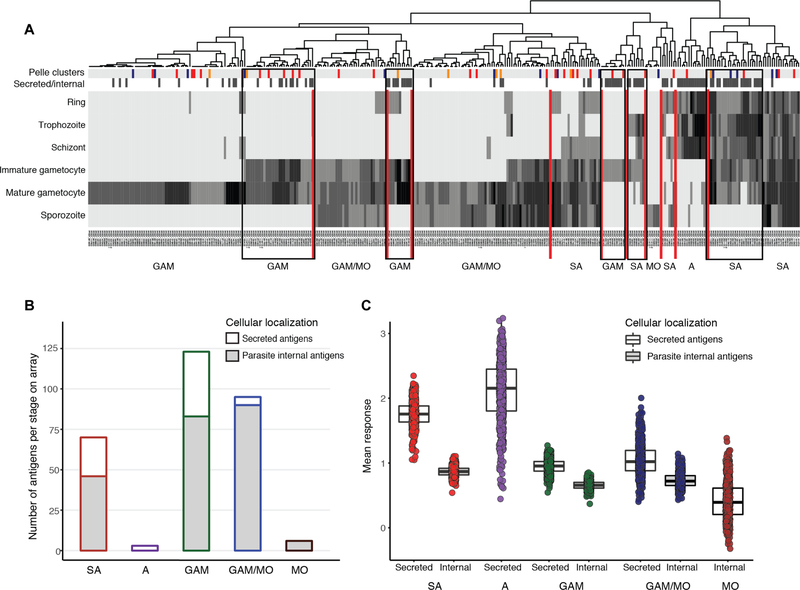
We first probed a P. falciparum peptide array enriched for proteins expressed in the gametocyte and gamete stages (22) with human plasma samples from 579 asymptomatically infected individuals from Cameroon, Burkina Faso, and the Gambia (table S1) (22) to examine natural immunity. Proteins were clustered on the basis of their stage-specific abundance in the blood and mosquito stages in proteomics studies (21, 23, 24), as well as by cellular localization; localization was divided into those proteins that are parasite internal (internal/unknown localization) or secreted onto the merozoite or gamete surface or into the host cell in intra-erythrocytic stages (secreted) (Fig. 1, A and B; see also table S2). Five stage-specific clusters (gametocyte specific or shared with asexual stages) were enriched in secreted antigens (Fig. 1A), and secreted antigens showed significantly higher antibody responses compared to internal antigens for shared, gametocyte-specific and gametocyte/mosquito stage proteins (P≪0.001) (Fig. 1C). Responses to shared secreted proteins increased with age, whereas responses to secreted gametocyte or mosquito stage proteins or to parasite internal proteins did not. Correlations were highly significant for a total of 121 individual peptides (adjusted P < 0.05; table S3). Although responses to numerous protein fragments showed progressive increases with age (fig. S1A), responses to other antigens, including PTP6 (25) and GEXP08, reached a plateau in the 12- to 30-year-old group (fig. S1B). These results indicate that humoral responses to secreted parasite antigens (shared and gametocyte specific) are correlated with cumulative exposure to malaria.
Fig. 1. Human plasma samples recognize secreted asexual (aiRBC) and gametocyte (giRBC) surface antigens.
(A) Heatmap of 344 P. falciparum antigens from 3D7 genome (PlasmoDB release 31) clustering proteins on the array by timing of protein expression (log read counts of number of peptides sequences). Additional annotations are indicated by color bars at the top of the heatmap: The first row indicates cluster stage annotation from (52) (orange, gametocyte rings; red, immature gametocytes; blue, mature gametocytes; gray, others), and the second row indicates cellular localization (black, secreted; white, internal/unknown). Vertical red lines separate stage-specific clusters. Black boxes highlight five clusters of shared or gametocyte-specific secreted antigens. (B) Distribution of 528 P. falciparum protein fragments on the peptide array [developed in (22)] by stage and location. The proteins were selected on the basis of expression during gametocyte stages and predicted export (details in table S2). (C) Mean responses across three malaria-exposed populations are quantified by peptide array (after normalization to controls and quantile normalization), stage of protein expression, and whether they are secreted or not (see table S1). GAM, gametocyte; GAM/MO, gametocyte/mosquito stages; MO, mosquito stages; A, asexual stages; SA, shared antigens.
Immune responses target the immature but not the mature giRBC surface
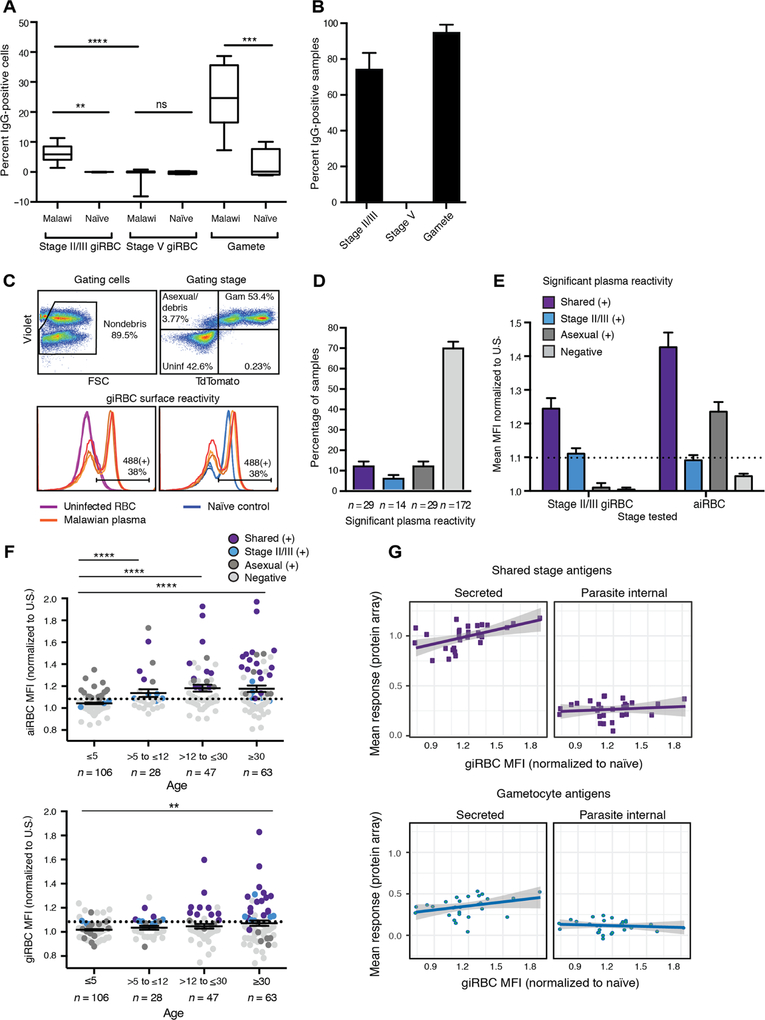
Detection of immune responses against secreted gametocyte proteins prompted us to directly examine immune recognition of giRBC surface antigens among an independent population. In a cross-sectional study, we collected plasma samples from 244 individuals with suspected malaria from southern Malawi (see Materials and Methods and Table 1). A subset of rapid diagnostic test–postive (RDT+) samples and an RDT− control (representative of the entire Malawian study population in terms of age and sex distribution) was incubated with P. falciparum NF54 stage II/III giRBC, stage V giRBCs, or activated gametes. Surface reactivity was measured by comparing the percentage of immunoglobulin G (IgG)–positive cells between incubations with Malawian and naïve control sera (see figs. S2 and S3). To differentiate nonactivated gametocytes (i.e., intact giRBCs) from activated ones (i.e., free gametes), stage V incubations were costained with antibodies recognizing the gametocyte/gamete surface antigen Pfs48/45 (which becomes accessible upon RBC rupture and giRBC activation) and the RBC surface antigen glycophorin C. Highest surface reactivity was found for gametes (mean, 25.80% recognized cells), with substantial reactivity also observed for stage II/III (mean, 6.22%) but not for stage V giRBCs (Fig. 2A). The relatively low percentage of giRBCs recognized suggests low abundance, accessibility, and/or immunogenicity of putative antigen targets. Of the Malawian plasma samples tested, 75.00% (n = 18 of 24) and 95.83% (n = 23 of 24) recognized stage II/III giRBCs or gametes, respectively, whereas no samples were positive for stage V recognition (Fig. 2B).
Table 1. Characteristics of Malawian study population.
Chikhwawa has year-round malaria transmission, whereas Ndirande and Thyolo have more seasonal transmission peaking during the rainy season each year
| Age (years) | Gender | Location | RDT status | ||||
|---|---|---|---|---|---|---|---|
| ≤5 | 106 (43.44%) | Male | 102 (41.8%) | Chikhwawa | 171 (70.1%) | + | 169 (69.3%) |
| >5 to ≤12 | 28 (11.48%) | Female | 142 (58.2%) | Ndirande | 35 (14.3%) | − | 75 (30.7%) |
| >12 to <30 | 47 (19.26%) | Thyolo | 38 (15.6%) | ||||
| ≥30 | 63 (25.82%) | ||||||
| Total | 244 | ||||||
Fig. 2. Immune responses target the immature but not the mature giRBC surface.
(A and B) Results from a pilot flow cytometry study testing reactivity of 24 Malawian plasma samples (22 from Chikhwawa, a high-transmission region, and 2 from Ndirande, a low-transmission region) and 5 naïve controls against stage II/III and stage V gametocytes and gametes. Positive surface reactivity (>3 SDs above mean of naïve controls) is shown both as percentage of significantly positive samples of all those tested (A) and percentage of positive cells among those incubated with an individual plasma sample (B). (C) Schematic for gating strategy of giRBC surface detection in 244 Malawian plasma samples by flow cytometry. IgG positivity is determined using the Pf2004_164/TdTom line that allows selection of the parasite population (positive for DNA dye) and TdTomato (positive for gametocytes). Top: Cells are first gated for live cells and single cells by forward and side scatter (FSC) (left). After debris is gated out, quadrant gates separate gametocytes (Violet+/TdTomato+), asexual/lysed cells/debris (Violet+/TdTomato−), and uninfected cells (right). Bottom: Alexa Fluor 488 surface fluorescence (human IgG secondary antibody conjugates) is compared between uninfected cells and gametocytes (left) and between infected cells incubated with naïve controls and Malawian plasma samples (right). Technical replicates are shown as individual lines. (D and E) Positive recognition of aiRBCs and stage II/III giRBCs (determined by t tests comparing Malawi samples to naïve U.S. controls using the Holm-Sidak method with α = 0.05) by 244 Malawian plasma samples is shown as prevalence (D) and as significant fold change in Alexa Fluor 488 median fluorescence compared to naïve controls (E). The threshold for specific positive reactivity was set to 1.1 based on the highest level of nonspecific reactivity (i.e., reactivity to aiRBCs of human plasma significantly positive for stage II/III giRBC but negative for aiRBC). (F) Correlation of human plasma recognizing aiRBCs (top) and giRBCs (bottom) by flow cytometry with age. MFI, median fluorescence intensity. (G) Correlation of antigen responses by peptide array versus surface recognition by flow cytometry. For the same set of Malawi plasma samples, normalized peptide array signal intensities were averaged across all shared stage antigens (top) or gametocyte-specific antigens (bottom). These mean responses were correlated with giRBC recognition by flow cytometry as measured by median fluorescence (Alexa Fluor 488) fold change compared to naïve controls. Overall, mean responses of shared secreted antigens are significantly correlated with giRBC recognition (P = 0.004), whereas the other correlations are nonsignificant. **P < 0.01, ***P < 0.001, and ****P < 0.0001.
We further investigated antibody specificity to immature giRBC surface antigens as compared to aiRBCs using a transgenic version of the Ghanaian P. falciparum parasite Pf2004 (26, 27), Pf2004/164TdTomato (Pf2004_164/TdTom). This parasite expresses the TdTomato reporter under the control of the PF10_0164 promoter (28) that allows detection by fluorescence microscopy and flow cytometry of gametocytes of all stages except the first 30 hours of development (Fig. 2C). Among 244 Malawian plasma samples, the strongest responses to aiRBCs correlated with the strongest responses to giRBCs, whereas 14 samples were uniquely positive for giRBCs (Fig. 2, D and E). No differences between RDT+ and RDT− individuals in antibody responses for any antigen class were observed (fig. S4A). When we repeated our surface recognition experiments with the 3D7 reference strain (a clone of NF54 used in Fig. 2A, potentially expressing different surface proteins than Pf2004), we observed lower surface antigen expression and lower nonspecific IgG labeling from naïve serum compared to Pf2004 (fig. S4, B and C). These strain disparities are consistent with previous work observing differential reactivity of Kenyan plasma samples to parasite strains of different genetic origins (18). Surface protein removal with trypsin and chymotrypsin treatment revealed that both specific and nonspecific binding of IgG involved antigens on the surface of aiRBCs and giRBCs (fig. S4, D and E). Further experiments using the same patient sera and naïve controls revealed no IgM binding above background and therefore excluded IgM binding as an explanation for the observed nonspecific surface recognition (fig. S5). These data provide strong evidence for IgG-targeted antigens that are shared between asexual and gametocyte stages.
The prevalence (number of samples with substantial aiRBC and/or giRBC recognition) and magnitude (median fluorescence intensity, MFI) of iRBC reactivity was significantly higher for adults compared to children (Fig. 2F). The increased aiRBC reactivity with age (top) corroborates the well-characterized pattern of increasing breadth of antibody response to asexual parasites with cumulative exposure (29–31). The slower age-dependent increase for giRBC responses (bottom) may reflect the lower abundance of immature gametocytes and suggests that giRBC responses differ from those against gametocyte/gamete antigens Pfs48/45 and Pfs230 that appear short-lived (22, 32, 33). We then probed a subset of the Malawian plasma samples (representing a range of reactivity by flow cytometry) on the peptide array to identify recognized targets. Recognition of the giRBC surface by flow cytometry was correlated with mean array responses for shared asexual-gametocyte and gametocyte-specific secreted antigens (Fig. 2G) but not internal proteins. Individuals recognizing giRBCs by flow cytometry had significantly higher reactivity (P < 0.05) to a subset of 22 protein fragments (including 4 shared and 13 gametocyte specific) compared to individuals with minimal reactivity to giRBCs (table S4 and fig. S1C). Together, these data demonstrate that plasma samples recognizing both aiRBCs and giRBCs show the highest magnitude in reactivity, and this signal is driven by antibody responses against secreted antigens across all age groups.
Antigens on the giRBC surface are predominantly shared with aiRBCs
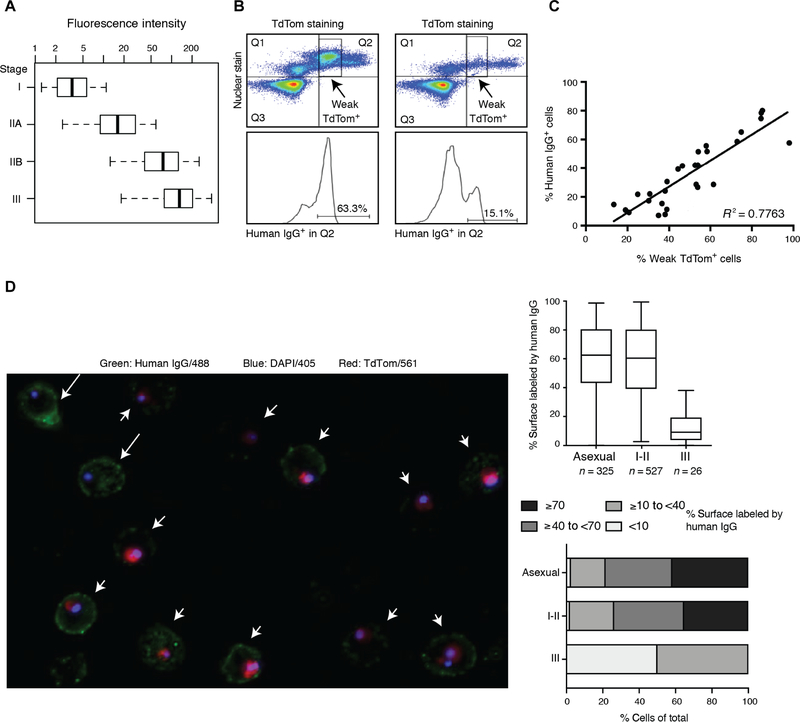
TdTomato fluorescence increases with later stage gametocytes (Fig. 3A), and microscopy and flow experiments indicated that weak TdTomato fluorescence corresponded to stage I/II gametocytes and strong TdTomato fluorescence to stage II/III gametocytes. Three lines of evidence suggest that giRBC surface reactivity is specific for stage I/II gametocytes: (i) A higher percentage of stage I/II weak TdTomato signal consistently corresponded to a higher percentage of cells staining positive for the surface (Fig. 3B), (ii) the intensity of IgG staining correlated with the percentage of weak TdTomato+ cells (Fig. 3C), and (iii) microscopy confirmed significantly higher percentages of surface labeling of aiRBCs and stage I/II giRBCs compared to stage II/III giRBCs (Fig. 3D). These results demonstrate that giRBC reactivity is highest in early stage gametocytes (stage I/II) and decreases during gametocyte development.
Fig. 3. Human IgG selectively recognizes the early (stage I/II) giRBC surface.
(A) Stage-specific expression of the TdTomato (TdTom) reporter in transgenic Pf2004_164/TdTom parasites by flow cytometry. Reporter expression is shown by fluorescence intensity in a time course across stages I to III gametocytes. (B) The Violet+/TdTomato+ gametocyte population detected by flow cytometry can be separated into weak TdTomato+ (stage I/II gametocytes) and strong TdTomato+ (stage II/III) subgroups. (C) Correlation between TdTomato signal and human IgG based on flow cytometry data (Pearson’s correlation, P < 0.0001). (D) Fluorescence microscopy analysis using the same antibody and reporter combination as above. DAPI, 4′,6-diamidino-2-phenylindole. Left: Surface labeling is present on both asexual parasites (arrows) and early gametocytes (arrowheads). Right: High-content image quantification of fluorescence microscopy data based on proportion of the cell surface that is labeled (top) and stratified by intensity (bottom).
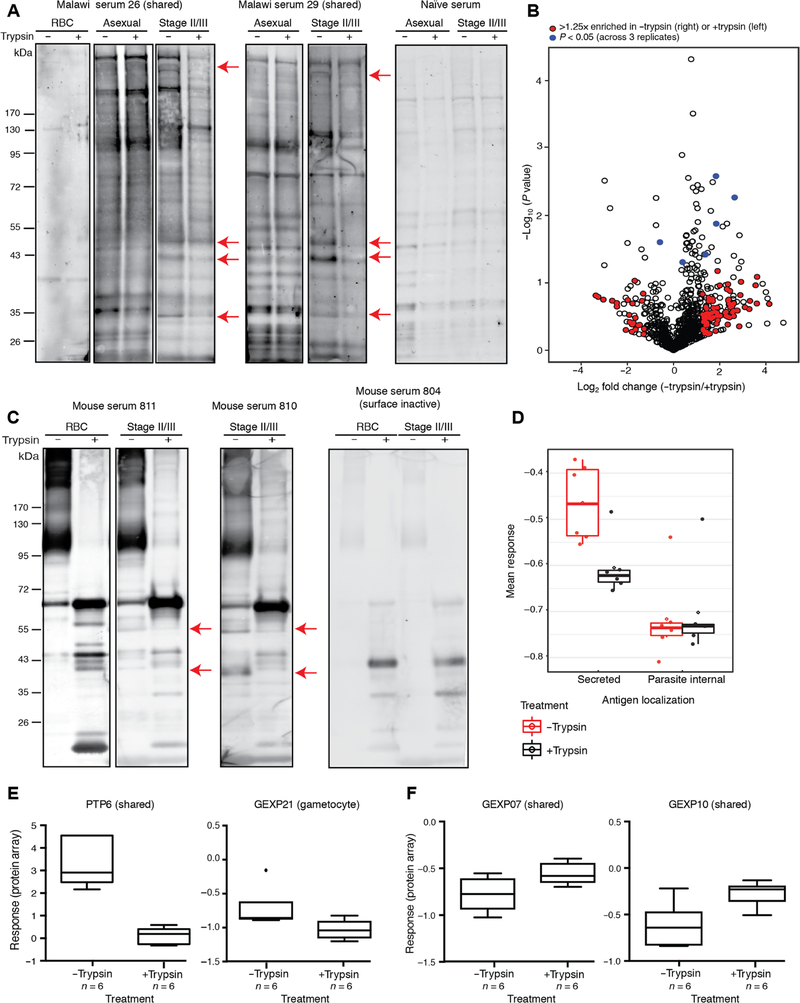
To identify the target giRBC surface antigens, we probed aiRBC and stage I to III giRBC membranes with (+) or without (−) treatment with trypsin and chymotrypsin (hereafter referred to as +trypsin and −trypsin samples) with Malawian plasma samples by Western blot. By comparing differential bands between surface-intact (−trypsin) and surface-depleted (+trypsin) samples, we identified both shared (aiRBC-giRBC) and giRBC-specific trypsin-sensitive protein bands (Fig. 4A), demonstrating the presence of immunogenic antigens on the giRBC surface. Next, we performed mass spectrometry–based proteomics of stage I to III giRBC membrane samples and assessed reactivity of sera from mice immunized with the same giRBC membrane samples. These results were combined with the proteins recognized by individuals with giRBC reactivity by flow cytometry in experiments described above to form an initial list of potential giRBC surface antigens.
Fig. 4. Immunogenic gametocyte antigens identified by three complementary approaches.
(A) Surface-depleted versus surface-intact uninfected and infected RBC membranes were probed with Malawian plasma samples and naïve U.S. sera by Western blot. Each lane represents protein extract from 2.5 × 106 uninfected RBCs (uRBCs) or iRBCs. Differential band patterns between the +trypsin and −trypsin samples are marked with red arrows. (B) Volcano plot showing human and Plasmodium proteins identified by comparing surface-intact versus surface-depleted giRBC membranes. The x axis represents log2 fold change of −trypsin/+trypsin, and the y axis shows the t test P value (P < 0.05 corresponds to P = 0.0004 after Benjamin-Hochberg correction) of −trypsin/+trypsin biological replicates (n = 3). Plasmodium proteins with a log2 fold change of >1.25 are marked in red, and significant Plasmodium proteins across three replicates are marked in blue. (C) Surface-depleted (+trypsin/chymotrypsin) versus surface intact (−trypsin/chymotrypsin) uRBC and giRBC membranes were probed with sera from mice (six per group) immunized with surface-intact or surface-depleted giRBCs by Western blot. Each lane represents protein extract from 2.5 × 106 uRBCs or iRBCs. Differential band patterns between the trypsin(+) and trypsin(−) giRBC samples are shown in red. (D) The array described in Fig. 1 was probed with sera from mice immunized with either surface-depleted or surface-intact giRBC membranes. Responses were normalized to controls and then quantile normalized. (E) PTP6 and GEXP21 differential responses between sera from mice immunized with surface-intact (−trypsin) giRBC membranes and surface-depleted (+trypsin) giRBC membranes. (F) GEXP07 and GEXP10 differential responses from sera from mice immunized with intact and surface-depleted membranes. See table S7 for complete dataset.
In the first approach, we performed whole-lane in-gel digestion with three biological replicates of +trypsin versus −trypsin giRBC membranes and identified differentially enriched protein bands between the two conditions by mass spectrometry (Fig. 4B and table S5). Overall, 72.20% of proteins identified in −trypsin samples were shared between all three replicates, and 92.21% of proteins were identified unequivocally in at least two of the three replicates. Of all 235 proteins that were >1.25× enriched in the −trypsin sample (table S5), a subset of 30 (12.77%) secreted proteins were considered putative surface antigen candidates. Secreted proteins were defined by the presence of at least one transmembrane domain (TM; including the N-terminal signal sequence) and either known localization to membrane/surface or host cell or unknown localization. Within this set of 30 candidates, 28 (93.33%; 11.91% of total candidate list) showed evidence for export into the host cell based on predicted PEXEL motif (21 proteins) or PEXEL/HT-negative exported protein (PNEP) annotation (7 proteins) and 23 were expressed in both asexual and gametocyte stages. This candidate list includes several previously identified secreted antigens such as multiple Plasmodium helical interspersed subtelomeric family proteins such as GEXP02, as well as PIESP2 (21, 34–38).
In a complementary antigen-discovery approach, we immunized mice with the same surface-intact (−trypsin) or surface-depleted (+trypsin) giRBC membranes used for proteomics and probed sera on our gametocyte-enriched protein array. Several bands on Western blot were present only in experiments using sera from mice immunized with surface-intact giRBC membranes and were reduced in intensity when surface-depleted membranes were probed with these sera compared to surface-intact membranes (Fig. 4C). Sera from all mice showed similar responses to parasite-internal peptides on the array, but sera from mice immunized with −trypsin preparations showed significantly higher responses to secreted proteins compared to mice immunized with +trypsin preparations (P = 0.04315; Fig. 4D). Because of lower background using mouse sera compared to human sera, many normalized mean response values were negative; however, the significant differential responses were consistent with observed reduced band intensity after trypsin treatment by Western blot (Fig. 4C) and with the same array probed with human plasma samples described earlier. Consistent with our previous results using the peptide array, 16 individual protein fragments elicited significantly higher differential responses with sera from mice immunized with surface-intact membranes (Fig. 4E and table S6). GEXP07 and GEXP10, two proteins on the iRBC surface that can bind to the chemokine CX3CL1 (37), were recognized both by sera from mice immunized with intact and surface-depleted membranes (Fig. 4F), suggesting that their ectodomain is trypsin insensitive.
In total, we identified an overlapping set of 68 initial candidate giRBC surface antigens: 22 proteins with significantly correlated array versus flow cytometry responses (table S4), 30 proteins from mass spectrometry–based proteomics (table S5), and 16 proteins eliciting significantly higher responses from sera from mice immunized with surface-intact (compared to surface-depleted) giRBC membranes (table S6). This list was then filtered based on detection by gametocyte surface proteomics and presence of at least one TM; subsequently, any proteins with confirmed localization within the parasite or parasitophorous vacuole or Maurer’s clefts were removed. The remaining 30 proteins were therefore deemed potential giRBC surface antigens (table S7): 26 were identified by surface proteomics, 3 were identified by the parallel mouse immune profiling experiment, and 1 hit was identified only by correlating protein array responses and surface reactivity of patient plasma samples. Of the 30 candidate antigens, 26 (86.7%) showed evidence of export into the host cell based on the presence of a PEXEL (23 proteins) or PNEP (3 proteins) motif, and most of the identified proteins (23 proteins; 76.7%) were expressed both in asexual and gametocyte stages (i.e., shared expression profile). There is independent evidence for localization at the iRBC periphery and/or surface for 12 of these 30 candidates from previous studies (table S7), further supporting our data.
Validation of giRBC antigen surface localization
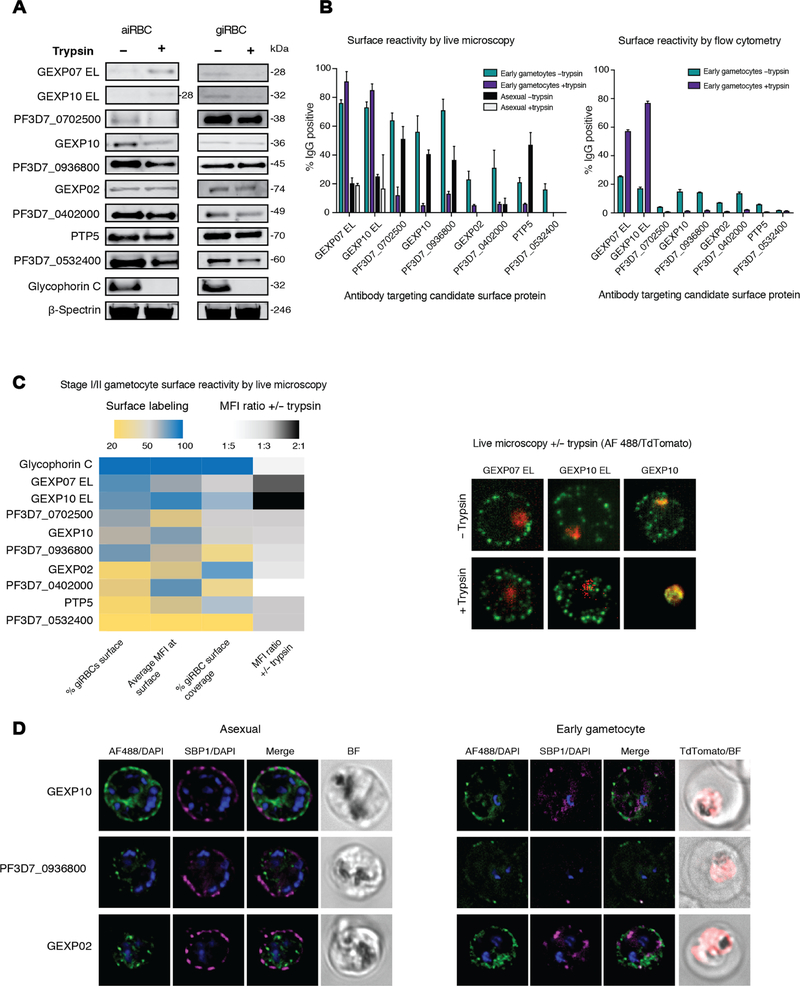
From the 30 proteins, we selected 9 for experimental validation of surface expression using antibodies against peptides [PF3D7_0402000, PF3D7_0702500, PF3D7_0936800, PTP5, PTP6, GEXP02, GEXP07, and GEXP10] or a recombinant protein [PF3D7_0532400 (39)] (table S8) in Western blots (Fig. 5A), flow cytometry (Fig. 5B), and live immunofluorescence assays (IFAs; Fig. 5, B and C). In addition, we performed IFAs using fixed, permeabilized cells to determine the cellular distribution of the candidate proteins (Fig. 5D). We obtained a band of the expected size by Western blot, and candidate antigens showed variable degrees of trypsin sensitivity (Fig. 5A and fig. S6). All antibodies except PTP6, which did not detect giRBCs, were then tested by flow cytometry (Fig. 5B) and immunofluorescence microscopy (Fig. 5, B and C) using live Pf2004_164/TdTom parasites. By flow cytometry, all antibodies, except those against GEXP10 and GEXP07, showed significantly reduced recognition of surface-depleted asexual stages and early gametocytes, although cell binding was low for some antibodies (Fig. 5B, right). The overall percentage of cells labeled and the magnitude of decreased labeling after trypsin treatment were higher in live IFAs [Fig. 5, B (left) and C] compared to flow cytometry. Again, GEXP10 and GEXP07 appeared insensitive to trypsin treatment in these assays. Apart from trypsin sensitivity, we quantified the proportion of surface-labeled aiRBCs and giRBCs, the fluorescence intensity of surface labeling, and the average percentage of surface coverage among labeled cells by live microscopy (Fig. 5C, left). Whereas GEXP10 and GEXP07 showed high levels for all three measurements, other antibodies had high values for one or two parameters (Fig. 5C). The two candidates with major expression in asexual stages and minimal expression in gametocytes based on our proteomic clustering (PF3D7_0402000 and PF3D7_0532400) showed the lowest levels of giRBC surface staining by live microscopy. Last, immunofluorescence microscopy using fixed and permeabilized cells confirmed significant labeling at the iRBC periphery, as well as colabeling with the Maurer’s Cleft marker SBP1, for three of these candidates across asexual and immature gametocyte stages (Fig. 5D). Antibodies against all three candidates showed markedly weaker labeling in gametocytes compared to asexual parasites. Together, analysis of a subset of candidates using peptide antibodies validated our analysis pipeline and confirmed giRBC surface recognition of six antigens.
Fig. 5. Six candidate antigens expressed during gametocyte stages are validated on the giRBC surface.
Previously published GEXP07 and GEXP10 antibodies (37) target the putative extracellular loops of these proteins and will be referred to as “GEXP07 EL” and “GEXP10 EL” to distinguish from our newly produced peptide antibodies targeting the same proteins. (A) Magnetic-activated cell sorter (MACS)–purified aiRBC or giRBC membranes (+/− pretreatment with trypsin and chymotrypsin, hereafter referred to as +/− trypsin) are probed with polyclonal antibodies targeting candidate antigens by Western blot (see full blots in fig S6). Antibodies against glycophorin C (trypsin-sensitive, surface expressed) and β-spectrin (trypsin resistant, internally localized) are included as controls. Each lane represents protein extract from 2.5 × 106 iRBCs. (B) Reactivity of candidate antibodies to surface of MACS-purified Pf2004_164/TdTom iRBCs (+/− trypsin/chymotrypsin) was detected by live microscopy (left) and flow cytometry (right) using the same sample preparations in parallel. For live microscopy, the percentage of surface-labeled aiRBCs or stage I giRBCs (weak TdTomato+) is shown for all antibodies tested. No asexual samples were tested for GEXP02 and PF3D7_0402000. For flow cytometry, cells were gated for live cells, single cells, and then uRBCs, and giRBCs were gated on the basis of Vybrant violet and TdTomato fluorescence and surface reactivity measured using Alexa Fluor 488–conjugated secondary antibody. The TdTomato+ population was further split into “weak TdTomato+” (corresponding to earlier gametocytes) and “strong TdTomato+” (corresponding to later gametocytes) populations. (C) Antibodies were clustered (automatic independent clustering) on the basis of the imaging parameters shown in the heatmap: percentage of labeled giRBCs, average MFI at the giRBC cell surface, % giRBC surface covered, and ratio of MFI at the surface of −trypsin samples compared to +trypsin. Glycophorin C is included as a control. Live representative images of early giRBCs +/− trypsin treatment are shown for GEXP07 and GEXP10. (D) Immunofluorescence analysis of the localization of GEXP02, GEXP10, and PF3D7_0936800 (detected with antipeptide antibodies) in fixed, permeabilized aiRBCs and giRBCs (days 2 and 4 of the induction, corresponding to stages I to IIA and IIA to IIB, respectively). Candidate protein is shown in green, SBP1 in magenta, TdTomato in red, and nuclear staining in blue. BF, brightfield.
A subset of secreted parasite antigens shows minimal sequence diversity and elicits responses that are correlated with reduced gametocyte burden
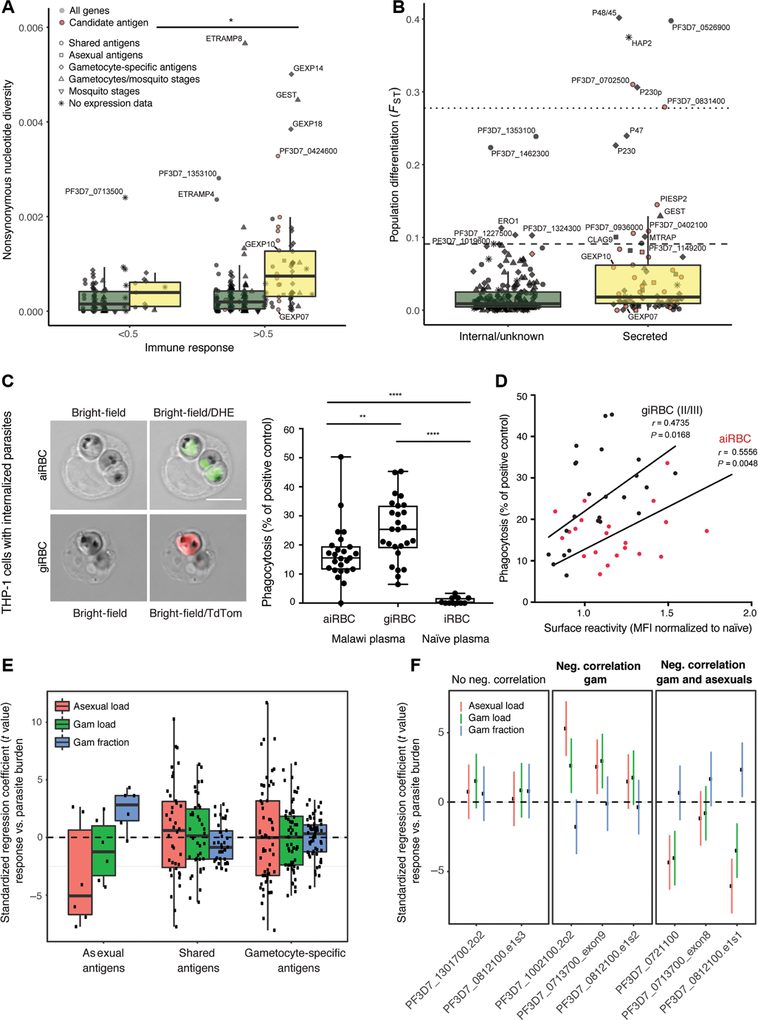
To determine the extent of sequence polymorphisms among the antigens analyzed in this study, we measured signatures of selection in the encoding genes from clinical isolates collected from two patient populations in Senegal and Malawi (table S9). Analysis of nonsynonymous pairwise nucleotide diversity (πNS) demonstrated significantly elevated levels of genetic diversity in genes encoding secreted antigens compared to internal antigens across all stages [Mann-Whitney U test, P = 8 × 10−13 (Senegal) and P = 3.7 × 10−11 (Malawi); Fig. 6A and fig. S7A]. Genes with Tajima’s D values above the genome-wide 95th percentile (D > −0.343), indicating balancing selection, were also enriched in secreted relative to internal antigens [Fisher’s exact test, P = 0.0153 (Senegal) and P = 0.00660 (Malawi); fig. S7B]. These data support the hypothesis that acquired immunity drives genetic diversity in genes encoding secreted P. falciparum blood stage antigens (both shared and gametocyte specific). We measured a positive correlation between immune responses against secreted antigens and the levels of πNS of the encoding genes [Pearson’s correlation, r = 0.221, P = 0.000141 (Senegal); r = 0.199, P = 0.000623 (Malawi)]. Levels of πNS were significantly increased at mean responses greater than 0.5 across secreted antigens, suggesting a threshold effect inducing positive selection through antibody-mediated immunity [Mann-Whitney U test, P = 0.0265 (Senegal) and P = 0.0441 (Malawi); Fig. 6A]. We also quantified genetic differentiation between the two geographically separated parasite populations in Malawi and Senegal using the fixation index (FST). This analysis demonstrated that genes encoding secreted antigens show significantly higher FST indices (Mann-Whitney U test, P = 2.3 × 10−5) and that most of the genes had high corresponding indices (FST > 0.1) [Mann-Whitney U test, P = 2.3 × 10−5; Fig. 6B]. Among our 30 candidate antigens, 9 showed minimal levels of nucleotide diversity across parasite populations in Malawi and Senegal (fig. S7C and table S9). Seven antigens, including GEXP07 and PTP5, show both minimal levels of nucleotide diversity across parasite populations and low levels of population divergence between populations (fig. S7C and table S9). Together, genetic analysis demonstrates that genes encoding secreted antigens show significantly higher signatures of selection compared to internal antigens, whereas a subset of eight candidate antigens show minimal levels of genetic diversity and may thus elicit strain-transcending immunity (Table 2).
Fig. 6. Candidate gametocyte surface antigens elicit responses correlated with reduced gametocyte burden and a subset show minimal genetic diversity.
(A) Nonsynonymous nucleotide diversity for all antigens present on the protein array, stratified by stage, localization, and level of immune response (Mann-Whitney U test, P < 0.05). Genome data are from a set of parasite samples in Senegal. (B) Population differentiation between Senegal and Malawi parasite samples for secreted and internal proteins (FST at nonsynonymous sites; Mann-Whitney U test, P = 2.0 × 10−5). The dotted and dashed lines mark the 99th and 95th percentile of genome-wide nonsynonymous FST values. (C) Left: Internalized aiRBCs and giRBCs upon phagocytosis by THP-1 cells. aiRBCs are stained with the nuclear dye dihydroethidium (DHE) and giRBCs show TdTomato (TdTom) reporter fluorescence. Right: Phagocytosis index of Malawi plasma samples relative to positive control (rabbit antihuman RBC) and naïve U.S. serum. (D) iRBC phagocytosis versus surface recognition. (E) Associations were estimated between gametocyte fraction (gametocytes/total parasites), gametocyte and asexual parasite load, and secreted antigen fragment responses by peptide array [after normalization to in vitro transcription-translation (IVTT) controls and quantile normalization as in Fig. 1] across three malaria-exposed populations. Median standardized regression coefficient, −0.86; Wilcoxon test, P = 0.087. (F) Regression coefficients (and 95% confidence intervals) between individual protein fragments of the prioritized candidate antigens and parasite parameters (gametocyte fraction, gametocyte load, or asexual parasite load). Fragments are stratified by their correlation with parasite parameters. *P < 0.05, **P < 0.01, and ****P < 0.0001.
Table 2. Prioritized candidate gametocyte antigens.
Eight candidate gametocyte antigens were identified by (i) predicted or known host secretion, (ii) proteomics of +trypsin and −trypsin giRBC membranes, (iii) correlations between plasma reactivity by protein array and flow cytometry, (iv) array reactivity of serum from mice immunized with trypsin-treated and trypsin-untreated giRBC membranes, (v) exposure-dependent increase of IgG in malaria-positive individuals, (vi), negative correlation with asexual and gametocyte load, and (vii) low genetic diversity and divergence. Three candidates (marked in bold) fulfill at least 6 out of the 7 criteria listed above. Previously described stage annotation and localization was retrieved from plasmodb.org (21, 23, 24, 53). Further details on candidates are provided in table S7 and for three top candidates in fig. S8. Pf, P. falciparum; Pv, P. vivax; Pb, P. berghei.
| Accession ID | Export motif | Protein description | Stage annotation | Previously described localizations | Detection method | Host phenotypes | Conservation | Validated surface expression |
|---|---|---|---|---|---|---|---|---|
| PF3D7_0601900 | PNEP | Conserved Plasmodium protein, unknown function | Shared | Maurer’s clefts | Proteomics | Pf | ||
| PF3D7_0713700 | NO | Conserved Plasmodium protein, unknown function | Gam/Mo | Unknown | Proteomics | Neg. correlation with asex./gam. load | Pf, Pv, Pb | |
| PF3D7_0721100 | PEXEL | Conserved Plasmodium protein, unknown function | Shared | RBC surface | Proteomics array/flow correlation | Pos. correlation with age/neg. correlation with asex./gam. load | Pf, Pv, Pb | |
| PF3D7_0812100 | NO | Conserved Plasmodium protein, unknown function | Shared | Unknown | Proteomics | Pos. correlation with age/neg. correlation with asex./gam. load | Pf, Pv, Pb | |
| PF3D7_0831400 | PEXEL | Plasmodium exported protein, unknown function (Hyp12) | Shared | Unknown | Proteomics | Pf | ||
| PF3D7_1002100 | PEXEL | EMP1-trafficking protein (PTP5) | Shared | Maurer’s clefts | Proteomics | Pf | X | |
| PF3D7_1038000 | NO | Antigen UB05 | Shared | Unknown | Proteomics | Pf, Pv, Pb | ||
| PF3D7J301700 | PEXEL | Plasmodium-exported protein (hyp8), unknown function (GEXP07) | Shared | RBC surface | Proteomics/mouse sera array (+trypsin enriched) | Pf | X |
It is currently unknown whether antibodies recognizing shared or gametocyte-specific surface antigens may inhibit giRBC binding/sequestration and/or increase phagocytosis efficiency by opsonization, as implicated in responses to PfEMP1 (18, 40, 41) and merozoite antigens (42, 43). To directly test this hypothesis, we opsonized iRBCs with the same Malawian plasma samples used for iRBC surface labeling and determined the level of iRBC phagocytosis by THP-1 cells (18). Significant levels of iRBC phagocytosis were detected (Fig. 6C), and the magnitude of surface reactivity was significantly correlated with induction of phagocytosis both for aiRBCs and giRBCs (Fig. 6D). Together, these data demonstrate the existence of functional antibodies targeting both aiRBCs and giRBCs and provide evidence for antibody-mediated clearance of giRBCs. In support of these functional assays, the intensity of recognition of shared secreted antigens by plasma samples from individuals in Cameroon, Burkina Faso, or the Gambia was overall negatively associated with the gametocyte fraction in these individuals (quantified by coefficients of regressing antigen response on logit-transformed gametocyte fraction). In contrast, normalized recognition of asexual antigens was overall negatively associated with asexual stage and gametocyte load (also quantified by regression coefficients and antigen response on log-transformed asexual/gametocyte load), whereas normalized recognition of gametocyte-specific antigens did not show any negative association (Fig. 6E). Furthermore, the proportion of total parasites that were gametocytes was negatively associated with breadth of response to the 76 fragments representing the 31 candidate surface antigens on the peptide array [coefficient, −0.002 (95% confidence interval, −0.004/−0.0004), P = 0.019]. Responses to a total of 12 candidate surface antigens, including three of our final candidates (Table 2), showed significant (P < 0.05) negative correlation between immune response and both peripheral gametocyte and asexual stage load (Fig. 6F, fig. S8, and table S10). These data support the phagocytosis data and suggest that iRBC immunity may be able to simultaneously reduce total parasite burden and gametocyte burden.
DISCUSSION
In this study, we systematically addressed immune recognition of antigens on the surface of giRBCs and provide evidence for the identity of these proteins. Our combination of a flow cytometry assay using distinct gametocyte stages, immune profiling by protein microarray, three parallel methods of antigen discovery, and a functional assay to quantify antibody-mediated iRBC phagocytosis provides evidence for naturally acquired antibodies recognizing shared asexual/gametocyte and gametocyte-specific antigens on the surface of immature giRBCs.
Two previous studies reported immune recognition of mature giRBCs (12, 14) but did not specifically control for gametocyte activation. We regularly observed glycophorin-negative gametocyte populations where the giRBC membrane was lost because of activation or permeabilization. It is conceivable that earlier studies have similarly experienced a loss in RBC integrity and may thus have detected antibodies against gamete proteins, which are common in endemic populations (22), instead of mature giRBC responses. Less stringent methods of giRBC purification also could have hindered the detection of responses targeting the most immature stages. When we carefully prevented activation by using a compound that prevents gamete egress (44) and confirmed the intact RBC membrane by counterstains (the gamete surface antigen Pfs48/45 and the RBC surface antigen glycophorin C), we did not detect substantial recognition of stage V giRBCs. In addition, we observed strong reactivity to stage I/II gametocytes but negligible reactivity to stage V gametocytes in our highly synchronous TdTomato transgenic parasite line (45). Our data demonstrate that plasma from naturally exposed individuals strongly recognizes early stage I/II giRBCs and aiRBCs; most of the immunogenic giRBC antigens in our study are also expressed in asexual stage parasites. These observations have potential implications for our understanding of parasite biology. Asexual and early gametocyte stages of P. falciparum, Plasmodium vivax, and Plasmodium berghei are abundantly present in the bone marrow parenchyma (5, 7, 46, 47), suggesting environmental characteristics supporting both gametocyte development and a genuine asexual replication cycle. An independent study recently confirmed that both bone marrow and spleen represent major reservoirs for parasite development in rodent malaria (48). We hypothesize that shared antigens present on aiRBC and giRBC surfaces are involved in cellular interactions in the bone marrow parenchyma and are critical for the maturation of both asexual and gametocyte stages. In such a model, the aiRBC surface serves the dual purpose of vascular adherence and extravascular binding, whereas the giRBC surface is optimized for extravascular binding only. Recent work demonstrated trypsin-sensitive binding of aiRBCs and immature, but not mature, giRBCs to human bone marrow mesenchymal stromal cells (49). Two antigens we identified on the giRBC and aiRBC surface, GEXP07 and GEXP10, were recently described as aiRBC surface proteins that bind the chemokine CX3CL1 (37). Because expression of this chemokine on bone marrow stromal cells is involved in homing and retention of monocytes (50), it is tempting to speculate that GEXP07 and GEXP10 are involved in such interactions between iRBCs and other cell types. It remains to be determined why human IgG levels recognizing giRBC antigens are generally lower compared to aiRBCs and why recognition is restricted to young gametocyte stages despite their continued presence in the extravascular niche until maturity. Although we only examined stages I/II, III, and V gametocytes and not the intermediate stage IV, our data suggest reduced antigen expression on the giRBC surface over the course of gametocyte development, the mechanism of which could include a combination of membrane remodeling, protease activity, or release via extracellular vesicles. As the molecular mechanisms of the bone marrow sequestration process become further elucidated, the ability and function of natural antibodies to access this compartment in meaningful concentrations and effectively target parasites in this niche are likely to also be revealed.
Our data reveal a positive correlation between antibody-mediated immunity and genetic diversity in secreted parasite antigens. Nevertheless, we identified a small set of immunogenic candidate antigens with minimal genetic diversity within and between populations, suggesting that they may induce strain-transcendent immunity. Our plasma samples were from cross-sectional surveys in asymptomatic populations. Although this makes it unlikely that inflammation or acute disease have influenced the results, our sampling approach means that we were lacking details on gametocyte commitment and maturation and were thus unable to test causality between antibody responses and parasite and gametocyte dynamics. We observed that the proportion of the total parasite biomass that is gametocyte [indicating what fraction of parasites successfully develops into circulating mature gametocytes (51)], was reduced in infections of individuals who responded to peptides mapping to shared asexual/gametocyte antigens. The negative associations between responses to asexual secreted antigens and asexual parasite load suggest a specific role for these proteins in reducing asexual parasite burden, in addition to the established contribution of anti-PfEMP1 antibodies (18, 19). A total of 12 candidate antigens, including 3 of our 8 top candidates with low sequence diversity, showed negative correlations between antibody titer and both asexual and gametocyte load, suggesting an association with reduced parasite growth and gametocyte maturation or clearance. This possible phenotype of the detected antibody responses is supported by our finding that plasma samples with increased aiRBC and giRBC surface recognition demonstrate increased phagocytosis of aiRBCs and giRBCs by THP-1 cells. This phenotype and the identification of a small set of target immunogenic antigens present on the giRBC surface with low sequence diversity provide a rationale for a novel transmission blocking vaccine strategy that may interfere with gametocyte maturation. Such a vaccine approach would reduce the number of gametocytes in the circulation and hence transmission potential.
Together, we provide compelling evidence for natural immune responses targeting young gametocytes and their antibody-mediated immune clearance. We identify a small set of eight candidate antigens that are (i) expressed in gametocytes (seven of them are also expressed in asexual stages), (ii) elicit natural antibody responses, and (iii) display low sequence diversity.
MATERIALS AND METHODS
Study design
For the Malawi study, samples were collected over 4 weeks from July to August 2013. Two weeks were spent in Chikhwawa because this region had higher malaria transmission during this time of year, and 1 week each was spent in Ndirande and Thyolo. All individuals receiving an RDT at the clinic were referred to our study, and samples were taken from all of those individuals who consented to the study. The end of data collection was not determined by any factor other than the end of the defined sample collection period. Samples from two individuals who withdrew their consent after participation were discarded; all other samples were shipped to the United States for further experiments. We aimed to detect natural antibody responses among the study participants that recognize giRBCs and then to determine the targets of these antibody responses. To examine antibody binding to the giRBC surface, we used a surface reactivity flow cytometry assay, immunofluorescence microscopy, and a protein array enriched for proteins expressed during gametocyte stages. In these experiments, samples were identified only by number and patient age, and corresponding clinical data were unblinded only after experiments finished. Three technical replicates were used for all samples, and two biological replicates were performed for a subset of samples. In cases where the result from one technical replicate was of a different magnitude than the other two replicates, this value was removed. To determine the identity of antigens targeted by the identified antibodies, we used mass spectrometry and immunization of mice with giRBC membranes, each using three biological replicates for preparation of giRBC membranes. Surface expression of candidate antigens was validated by Western blot, flow cytometry, and immunofluorescence microscopy. Functional activity was assessed using a THP-1 cell phagocytosis assay. Sequence diversity was assessed using standard methods (nonsynonymous pairwise nucleotide diversity, balancing selection measured by Tajima’s D, and genetic differentiation measured by the fixation index).
Statistical analysis
The appropriate statistical test for each experiment was determined on the basis of the type of data being compared. False discovery rate corrections were performed for all analyses involving multiple comparisons, and P values of <0.05 were considered significant. Simple univariate linear regressions were performed for examining the correlation between levels of IgG responses against individual fragments on the protein array and covariates including (ordinally categorized) age, burden, and iRBC recognition by flow cytometry. P values across fragments were corrected with the Bonferroni method. Pairwise, two-sided Student’s t tests were used to test for difference in mean IgG response against proteins across stages. Linear regressions were used to test for associations between IgG response against fragments and parasite load, gametocyte load, and gametocyte fraction, with adjustment for age by including age groups as covariates. The regression t statistics (estimated coefficients/SE) of internal and secreted protein fragments are compared by two-sided Mann-Whitney U test. The association of gametocyte fraction and breadth of response (number of proteins seropositive) was conducted on gametocyte positive individuals for whom asexual and gametocyte stages had been quantified. Analysis on breadth and fraction on continuous scales was performed with linear regression, adjusting for gametocyte density. Analysis with breadth as a binary variable was performed with logistic regression, adjusting for gametocyte density. Throughout the manuscript, significant P values are reported either as is or with the corresponding α level (all, P < 0.05).
Supplementary Material
Acknowledgments:
Antibodies to full-length PF3D7_0532400 and to N-terminal SBP1 were provided by H.-P. Beck (Swiss Tropical and Public Health Institute, Basel) and T. Spielmann (Bernhard-Nocht-Institut für Tropenmedizin, Hamburg), respectively. R. Tweedell (Johns Hopkins) helped with protein sample in-gel digestion for mass spectrometry. We would like to acknowledge the following people for help in collecting plasma samples in Blantyre: N. Chimbiya, C. Mlangali, A. Mwafulirwa, A. Kapito-Tembo, P. Pensulo, A. Bauleni, A. Saidi, E. Gondwe, and the entire facility-based surveillance team (Malawi ICEMR). We also thank all of the individuals who participated in the study, as well as their families.
Funding: This work was supported by the U.S. NIH (R01A1077558 to M.M., AI095916 and U19 AI089686 to P.F, U19AI089683 to T.T., Intramural Research Program of the NIH, National Center for Advancing Translational Sciences to D.T., and Intramural Research Program of the NIH, National Institute on Aging to C.U.-M.), a career development award from the Burroughs Wellcome Fund (to M.M.), a European Research Council award BoneMalar ERC-2015-CoG 682360 (to M.M.). and a Sanofi Innovation Award and a Wellcome Trust Center award (104111). D.T., C.U.-M, and R.R.D. were supported by the Bloomberg Family Foundation through the Johns Hopkins Malaria Research Institute. S.R., W.J.R.S., and T.B. are supported by the Netherlands Organization for Scientific Research through a VIDI fellowship grant to T.B. (no. 016.158.306) and a fellowship from the European Research Council (ERC-2014-StG 639776) to T.B. K.W.D. was supported by a Herchel Smith Graduate Fellowship. M.D.N. was supported by a postdoctoral fellowship from the Swiss National Science Foundation (P2BEP3_165396), and S.K.N.B. was supported by a postdoctoral fellowship from the American Heart Association. P.H. and P.D. were supported by “Fondation pour la Recherche Médicale” (Equipes FRM 2016) and by “Agence Nationale de la Recherche” (grant no. CE-15-0019-01, CMOS).
Footnotes
Competing interests: P.F. is inventor on patent application no. US20180016299A1 submitted by University of California that covers protein microarray construction.
Data and materials availability: All data associated with this study are present in the paper or Supplementary Materials. Sample meta data and protein microarray data are available at https://datadryad.org/resource/doi:10.5061/dryad.8bp05. Whole-genome sequence data for the Senegal and Malawi samples are publicly available on the MalariaGen Pf3k website (www.malariagen.net/projects/pf3k). Scripts and example input files used for calculating population genetics statistics are available at https://github.com/amearly/Dantzler_et_al_Diversity_Calcs.
SUPPLEMENTARY MATERIALS
stm.sciencemag.org/cgi/content/full/11/495/eaav3963/DC1
Materials and Methods
Fig. S1. Correlations between age and reactivity by peptide array or between reactivity by peptide array and reactivity by flow cytometry.
Fig. S2. Schematic of gating strategy for measuring giRBC surface reactivity by flow cytometry.
Fig. S3. Activation of stage V gametocytes and the impact of protein kinase G inhibitors on activation.
Fig. S4. Stage-specific reactivity of human plasma with iRBCs by flow cytometry.
Fig. S5. Human IgM binding to iRBCs.
Fig. S6. Specificity of polyclonal antibodies against candidate antigens by Western blot.
Fig. S7. Genetic diversity and divergence of candidate antigens.
Fig. S8. Antibody correlations and protein details from three top candidates (Table 2).
Table S1. Protein array details and mean responses of patient plasma samples tested on protein array.
Table S2. Annotation of proteins on the array.
Table S3. Correlations between mean responses and age.
Table S4. Correlations between mean responses and giRBC surface reactivity by flow cytometry.
Table S5. Proteomics hits identified by liquid chromatography–tandem mass spectrometry.
Table S6. IgG responses from mice immunized with gametocyte ghosts.
Table S7. Candidate gametocyte antigens identified by three complementary methods (expanded from Table 2).
Table S8. Amino acid sequences for peptide antibodies generated in this study.
Table S9. Genetic diversity data for all genes analyzed in this study.
Table S10. Correlations between mean responses by array with parasite load.
REFERENCES AND NOTES
- 1.Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, Battle KE, Moyes CL, Henry A, Eckhoff PA, Wenger EA, Briet O, Penny MA, Smith TA, Bennett A, Yukich J, Eisele TP, Griffin JT, Fergus CA, Lynch M, Lindgren F, Cohen JM, Murray CL, Smith DL, Hay SI, Cibulskis RE, Gething PW, The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526, 207–211 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, Sopha C, Chuor CM, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Thuy-Nhien NT, Thanh NV, Phu NH, Htut Y, Han KT, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, Hossain MA, Samad R, Rahman MR, Hasan MM, Islam A, Miotto O, Amato R, MacInnis B, Stalker J, Kwiatkowski DP, Bozdech Z, Jeeyapant A, Cheah PY, Sakulthaew T, Chalk J, Intharabut B, Silamut K, Lee SJ, Vihokhern B, Kunasol C, Imwong M, Tarning J, Taylor WJ, Yeung S, Woodrow CJ, Flegg JA, Das D, Smith J, Venkatesan M, Plowe CV, Stepniewska K, Guerin PJ, Dondorp AM, Day NP, White NJ; Tracking Resistance to Artemisinin Collaboration (TRAC), Spread of artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med 371, 411–423 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hemingway J, Ranson H, Magill A, Kolaczinski J, Fornadel C, Gimnig J, Coetzee M, Simard F, Roch DK, Hinzoumbe CK, Pickett J, Schellenberg D, Gething P, Hoppe M, Hamon N, Averting a malaria disaster: Will insecticide resistance derail malaria control? Lancet 387, 1785–1788 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alonso PL, Brown G, Arevalo-Herrera M, Binka F, Chitnis C, Collins F, Doumbo OK, Greenwood B, Hall BF, Levine MM, Mendis K, Newman RD, Plowe CV, Rodriguez MH, Sinden R, Slutsker L, Tanner M, A research agenda to underpin malaria eradication. PLOS Med 8, e1000406 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aguilar R, Magallon-Tejada A, Achtman AH, Moraleda C, Joice R, Cistero P, Li Wai Suen CS, Nhabomba A, Macete E, Mueller I, Marti M, Alonso PL, Menendez C, Schofield L, Mayor A, Molecular evidence for the localization of Plasmodium falciparum immature gametocytes in bone marrow. Blood 123, 959–966 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farfour E, Charlotte F, Settegrana C, Miyara M, Buffet P, The extravascular compartment of the bone marrow: A niche for Plasmodium falciparum gametocyte maturation? Malar. J 11, 285 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joice R, Nilsson SK, Montgomery J, Dankwa S, Egan E, Morahan B, Seydel KB, Bertuccini L, Alano P, Williamson KC, Duraisingh MT, Taylor TE, Milner DA, Marti M, Plasmodium falciparum transmission stages accumulate in the human bone marrow. Sci. Transl. Med 6, 244re245 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sauerwein RW, Bousema T, Transmission blocking malaria vaccines: Assays and candidates in clinical development. Vaccine 33, 7476–7482 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Sutherland CJ, Surface antigens of Plasmodium falciparum gametocytes—A new class of transmission-blocking vaccine targets? Mol. Biochem. Parasitol 166, 93–98 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Bousema JT, Gouagna LC, Drakeley CJ, Meutstege AM, Okech BA, Akim IN, Beier JC, Githure JI, Sauerwein RW, Plasmodium falciparum gametocyte carriage in asymptomatic children in western Kenya. Malar. J 3, 18 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunyo S, Milligan P, Edwards T, Sutherland C, Targett G, Pinder M, Gametocytaemia after drug treatment of asymptomatic Plasmodium falciparum. PLOS Clin. Trials 1, e20 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saeed M, Roeffen W, Alexander N, Drakeley CJ, Targett GA, Sutherland CJ, Plasmodium falciparum antigens on the surface of the gametocyte-infected erythrocyte. PLOS ONE 3, e2280 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baird JK, Jones TR, Purnomo, Masbar S, Ratiwayanto S, Leksana B, Evidence for specific suppression of gametocytemia by Plasmodium falciparum in residents of hyperendemic Irian Jaya. Am. J. Trop. Med. Hyg 44, 183–190 (1991). [DOI] [PubMed] [Google Scholar]
- 14.Dinko B, King E, Targett GA, Sutherland CJ, Antibody responses to surface antigens of Plasmodium falciparum gametocyte-infected erythrocytes and their relation to gametocytaemia. Parasite Immunol 38, 352–364 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tonwong N, Sattabongkot J, Tsuboi T, Iriko H, Takeo S, Sirichaisinthop J, Udomsangpetch R, Natural infection of Plasmodium falciparum induces inhibitory antibodies against gametocyte development in human hosts. Jpn. J. Infect. Dis 65, 152–156 (2012). [PubMed] [Google Scholar]
- 16.Chan JA, Fowkes FJ, Beeson JG, Surface antigens of Plasmodium falciparum-infected erythrocytes as immune targets and malaria vaccine candidates. Cell. Mol. Life Sci 71, 3633–3657 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bengtsson A, Joergensen L, Rask TS, Olsen RW, Andersen MA, Turner L, Theander TG, Hviid L, Higgins MK, Craig A, Brown A, Jensen AT, A novel domain cassette identifies Plasmodium falciparum PfEMP1 proteins binding ICAM-1 and is a target of cross-reactive, adhesion-inhibitory antibodies. J. Immunol 190, 240–249 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan JA, Howell KB, Reiling L, Ataide R, Mackintosh CL, Fowkes FJ, Petter M, Chesson JM, Langer C, Warimwe GM, Duffy MF, Rogerson SJ, Bull PC, Cowman AF, Marsh K, Beeson JG, Targets of antibodies against Plasmodium falciparum-infected erythrocytes in malaria immunity. J. Clin. Invest 122, 3227–3238 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arora G, Hart GT, Manzella-Lapeira J, Doritchamou JY, Narum DL, Thomas LM, Brzostowski J, Rajagopalan S, Doumbo OK, Traore B, Miller LH, Pierce SK, Duffy PE, Crompton PD, Desai SA, Long EO, NK cells inhibit Plasmodium falciparum growth in red blood cells via antibody-dependent cellular cytotoxicity. eLife 7, 10.7554/eLife.36806 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tibúrcio M, Silvestrini F, Bertuccini L, Sander AF, Turner L, Lavstsen T, Alano P, Early gametocytes of the malaria parasite Plasmodium falciparum specifically remodel the adhesive properties of infected erythrocyte surface. Cell. Microbiol 15, 647–659 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Silvestrini F, Lasonder E, Olivieri A, Camarda G, van Schaijk B, Sanchez M, Younis Younis S, Sauerwein R, Alano P, Protein export marks the early phase of gametocytogenesis of the human malaria parasite Plasmodium falciparum. Mol. Cell. Proteomics 9, 1437–1448 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stone WJR, Campo JJ, Ouédraogo AL, Meerstein-Kessel L, Morlais I, Da D, Cohuet A, Nsango S, Sutherland CJ, van de Vegte-Bolmer M, Siebelink-Stoter R, van Gemert GJ, Graumans W, Lanke K, Shandling AD, Pablo JV, Teng AA, Jones S, de Jong RM, Fabra-Garcia A, Bradley J, Roeffen W, Lasonder E, Gremo G, Schwarzer E, Janse CJ, Singh SK, Theisen M, Felgner P, Marti M, Drakeley C, Sauerwein R, Bousema T, Jore MM, Unravelling the immune signature of Plasmodium falciparum transmission-reducing immunity. Nat. Commun 9, 558 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lasonder E, Janse CJ, van Gemert GJ, Mair GR, Vermunt AM, Douradinha BG, van Noort V, Huynen MA, Luty AJ, Kroeze H, Khan SM, Sauerwein RW, Waters AP, Mann M, Stunnenberg HG, Proteomic profiling of Plasmodium sporozoite maturation identifies new proteins essential for parasite development and infectivity. PLOS Pathog 4, e1000195 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oehring SC, Woodcroft BJ, Moes S, Wetzel J, Dietz O, Pulfer A, Dekiwadia C, Maeser P, Flueck C, Witmer K, Brancucci NM, Niederwieser I, Jenoe P, Ralph SA, Voss TS, Organellar proteomics reveals hundreds of novel nuclear proteins in the malaria parasite Plasmodium falciparum. Genome Biol 13, R108 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maier AG, Rug M, O’Neill MT, Brown M, Chakravorty S, Szestak T, Chesson J, Wu Y, Hughes K, Coppel RL, Newbold C, Beeson JG, Craig A, Crabb BS, Cowman AF, Exported proteins required for virulence and rigidity of Plasmodium falciparum-infected human erythrocytes. Cell 134, 48–61 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elliott SR, Payne PD, Duffy MF, Byrne TJ, Tham WH, Rogerson SJ, Brown GV, Eisen DP, Antibody recognition of heterologous variant surface antigens after a single Plasmodium falciparum infection in previously naive adults. Am. J. Trop. Med. Hyg 76, 860–864 (2007). [PubMed] [Google Scholar]
- 27.Hommel M, Elliott SR, Soma V, Kelly G, Fowkes FJ, Chesson JM, Duffy MF, Bockhorst J, Avril M, Mueller I, Raiko A, Stanisic DI, Rogerson SJ, Smith JD, Beeson JG, Evaluation of the antigenic diversity of placenta-binding Plasmodium falciparum variants and the antibody repertoire among pregnant women. Infect. Immun 78, 1963–1978 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aingaran M, Zhang R, Law SK, Peng Z, Undisz A, Meyer E, Diez-Silva M, Burke TA, Spielmann T, Lim CT, Suresh S, Dao M, Marti M, Host cell deformability is linked to transmission in the human malaria parasite Plasmodium falciparum. Cell. Microbiol 14, 983–993 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marsh K, Kinyanjui S, Immune effector mechanisms in malaria. Parasite Immunol 28, 51–60 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Schofield L, Mueller I, Clinical immunity to malaria. Curr. Mol. Med 6, 205–221 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Ryg-Cornejo V, Ly A, Hansen DS, Immunological processes underlying the slow acquisition of humoral immunity to malaria. Parasitology 143, 199–207 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Bousema T, Roeffen W, Meijerink H, Mwerinde H, Mwakalinga S, van Gemert GJ, van de Vegte-Bolmer M, Mosha F, Targett G, Riley EM, Sauerwein R, Drakeley C, The dynamics of naturally acquired immune responses to Plasmodium falciparum sexual stage antigens Pfs230 & Pfs48/45 in a low endemic area in Tanzania. PLOS ONE 5, e14114 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drakeley CJ, Bousema JT, Akim NI, Teelen K, Roeffen W, Lensen AH, Bolmer M, Eling W, Sauerwein RW, Transmission-reducing immunity is inversely related to age in Plasmodium falciparum gametocyte carriers. Parasite Immunol 28, 185–190 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Sargeant TJ, Marti M, Caler E, Carlton JM, Simpson K, Speed TP, Cowman AF, Lineage-specific expansion of proteins exported to erythrocytes in malaria parasites. Genome Biol 7, R12 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nilsson Bark SK, Ahmad R, Dantzler K, Lukens AK, De Niz M, Szucs MJ, Jin X, Cotton J, Hoffmann D, Bric-Furlong E, Oomen R, Parrington M, Milner D, Neafsey DE, Carr SA, Wirth DF, Marti M, Quantitative proteomic profiling reveals novel Plasmodium falciparum surface antigens and possible vaccine candidates. Mol. Cell. Proteomics 17, 43–60 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vincensini L, Richert S, Blisnick T, Van Dorsselaer A, Leize-Wagner E, Rabilloud T, Braun Breton C, Proteomic analysis identifies novel proteins of the Maurer’s clefts, a secretory compartment delivering Plasmodium falciparum proteins to the surface of its host cell. Mol. Cell. Proteomics 4, 582–593 (2005). [DOI] [PubMed] [Google Scholar]
- 37.Hermand P, Ciceron L, Pionneau C, Vaquero C, Combadiere C, Deterre P, Plasmodium falciparum proteins involved in cytoadherence of infected erythrocytes to chemokine CX3CL1. Sci. Rep 6, 33786 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tarr SJ, Moon RW, Hardege I, Osborne AR, A conserved domain targets exported PHISTb family proteins to the periphery of Plasmodium infected erythrocytes. Mol. Biochem. Parasitol 196, 29–40 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oberli A, Slater LM, Cutts E, Brand F, Mundwiler-Pachlatko E, Rusch S, Masik MF, Erat MC, Beck HP, Vakonakis I, A Plasmodium falciparum PHIST protein binds the virulence factor PfEMP1 and comigrates to knobs on the host cell surface. FASEB J 28, 4420–4433 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beeson JG, Mann EJ, Elliott SR, Lema VM, Tadesse E, Molyneux ME, Brown GV, Rogerson SJ, Antibodies to variant surface antigens of Plasmodium falciparum-infected erythrocytes and adhesion inhibitory antibodies are associated with placental malaria and have overlapping and distinct targets. J. Infect. Dis 189, 540–551 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghumra A, Khunrae P, Ataide R, Raza A, Rogerson SJ, Higgins MK, Rowe JA, Immunisation with recombinant PfEMP1 domains elicits functional rosette-inhibiting and phagocytosis-inducing antibodies to Plasmodium falciparum. PLOS ONE 6, e16414 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hill DL, Eriksson EM, Li Wai Suen CS, Chiu CY, Ryg-Cornejo V, Robinson LJ, Siba PM, Mueller I, Hansen DS, Schofield L, Opsonising antibodies to P. falciparum merozoites associated with immunity to clinical malaria. PLOS ONE 8, e74627 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osier FH, Feng G, Boyle MJ, Langer C, Zhou J, Richards JS, McCallum FJ, Reiling L, Jaworowski A, Anders RF, Marsh K, Beeson JG, Opsonic phagocytosis of Plasmodium falciparum merozoites: Mechanism in human immunity and a correlate of protection against malaria. BMC Med 12, 108 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donald RG, Zhong T, Wiersma H, Nare B, Yao D, Lee A, Allocco J, Liberator PA, Anticoccidial kinase inhibitors: Identification of protein kinase targets secondary to cGMP-dependent protein kinase. Mol. Biochem. Parasitol 149, 86–98 (2006). [DOI] [PubMed] [Google Scholar]
- 45.Brancucci NM, Goldowitz I, Buchholz K, Werling K, Marti M, An assay to probe Plasmodium falciparum growth, transmission stage formation and early gametocyte development. Nat. Protoc 10, 1131–1142 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Obaldia III N, Meibalan E, Sa JM, Ma S, Clark MA, Mejia P, Moraes Barros RR, Otero W, Ferreira MU, Mitchell JR, Milner DA, Huttenhower C, Wirth DF, Duraisingh MT, Wellems TE, Marti M, Bone marrow is a major parasite reservoir in Plasmodium vivax infection. MBio 9, e00625–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Niz M, Meibalan E, Mejia P, Ma S, Brancucci NMB, Agop-Nersesian C, Mandt R, Ngotho P, Hughes KR, Waters AP, Huttenhower C, Mitchell JR, Martinelli R, Frischknecht F, Seydel KB, Taylor T, Milner D, Heussler VT, Marti M, Plasmodium gametocytes display homing and vascular transmigration in the host bone marrow. Sci. Adv 4, eaat3775 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee RS, Waters AP, Brewer JM, A cryptic cycle in haematopoietic niches promotes initiation of malaria transmission and evasion of chemotherapy. Nat. Commun 9, 1689 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Messina V, Valtieri M, Rubio M, Falchi M, Mancini F, Mayor A, Alano P, Silvestrini F, Gametocytes of the malaria parasite Plasmodium falciparum interact with and stimulate bone marrow mesenchymal cells to secrete angiogenetic factors. Front. Cell. Infect. Microbiol 8, 50 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamon P, Loyher PL, Baudesson de Chanville C, Licata F, Combadiere C, Boissonnas A, CX3CR1-dependent endothelial margination modulates Ly6Chigh monocyte systemic deployment upon inflammation in mice. Blood 129, 1296–1307 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Drakeley C, Sutherland C, Bousema JT, Sauerwein RW, Targett GA, The epidemiology of Plasmodium falciparum gametocytes: Weapons of mass dispersion. Trends Parasitol 22, 424–430 (2006). [DOI] [PubMed] [Google Scholar]
- 52.Pelle KG, Oh K, Buchholz K, Narasimhan V, Joice R, Milner DA, Brancucci NM, Ma S, Voss TS, Ketman K, Seydel KB, Taylor TE, Barteneva NS, Huttenhower C, Marti M, Transcriptional profiling defines dynamics of parasite tissue sequestration during malaria infection. Genome Med 7, 19 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Florens L, Liu X, Wang Y, Yang S, Schwartz O, Peglar M, Carucci DJ, Yates III JR, Wu Y, Proteomics approach reveals novel proteins on the surface of malaria-infected erythrocytes. Mol. Biochem. Parasitol 135, 1–11 (2004). [DOI] [PubMed] [Google Scholar]
- 54.Harris C, Morlais I, Churcher TS, Awono-Ambene P, Gouagna LC, Dabire RK, Fontenille D, Cohuet A, Plasmodium falciparum produce lower infection intensities in local versus foreign Anopheles gambiae populations. PLOS ONE 7, e30849 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morlais I, Nsango SE, Toussile W, Abate L, Annan Z, Tchioffo MT, Cohuet A, Awono-Ambene PH, Fontenille D, Rousset F, Berry A, Plasmodium falciparum mating patterns and mosquito infectivity of natural isolates of gametocytes. PLOS ONE 10, e0123777 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Drakeley CJ, Secka I, Correa S, Greenwood BM, Targett GA, Host haematological factors influencing the transmission of Plasmodium falciparum gametocytes to Anopheles gambiae s.s. mosquitoes. Trop. Med. Int. Health 4, 131–138 (1999). [DOI] [PubMed] [Google Scholar]
- 57.Drakeley CJ, Akim NI, Sauerwein RW, Greenwood BM, Targett GA, Estimates of the infectious reservoir of Plasmodium falciparum malaria in The Gambia and in Tanzania. Trans. R. Soc. Trop. Med. Hyg 94, 472–476 (2000). [DOI] [PubMed] [Google Scholar]
- 58.Targett G, Drakeley C, Jawara M, von Seidlein L, Coleman R, Deen J, Pinder M, Doherty T, Sutherland C, Walraven G, Milligan P, Artesunate reduces but does not prevent posttreatment transmission of Plasmodium falciparum to Anopheles gambiae. J. Infect. Dis 183, 1254–1259 (2001). [DOI] [PubMed] [Google Scholar]
- 59.Dunyo S, Ord R, Hallett R, Jawara M, Walraven G, Mesa E, Coleman R, Sowe M, Alexander N, Targett GA, Pinder M, Sutherland CJ, Randomised trial of chloroquine/sulphadoxine-pyrimethamine in Gambian children with malaria: Impact against multidrug-resistant P. falciparum. PLOS Clin. Trials 1, e14 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hallett RL, Dunyo S, Ord R, Jawara M, Pinder M, Randall A, Alloueche A, Walraven G, Targett GA, Alexander N, Sutherland CJ, Chloroquine/sulphadoxine-pyrimethamine for gambian children with malaria: Transmission to mosquitoes of multidrug-resistant Plasmodium falciparum. PLOS Clin. Trials 1, e15 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sutherland CJ, Ord R, Dunyo S, Jawara M, Drakeley CJ, Alexander N, Coleman R, Pinder M, Walraven G, Targett GA, Reduction of malaria transmission to Anopheles mosquitoes with a six-dose regimen of co-artemether. PLOS Med 2, e92 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ouedraogo AL, Goncalves BP, Gnémé A, Wenger EA, Guelbeogo MW, Ouédraogo A, Gerardin J, Bever CA, Lyons H, Pitroipa X, Verhave JP, Eckhoff PA, Drakeley C, Sauerwein R, Luty AJ, Kouyate B, Bousema T, Dynamics of the human infectious reservoir for malaria determined by mosquito feeding assays and ultrasensitive malaria diagnosis in Burkina Faso. J. Infect. Dis 213, 90–99 (2016). [DOI] [PubMed] [Google Scholar]
- 63.Da DF, Churcher TS, Yerbanga RS, Yameogo B, Sangaré I, Ouedraogo JB, Sinden RE, Blagborough AM, Cohuet A, Experimental study of the relationship between Plasmodium gametocyte density and infection success in mosquitoes; implications for the evaluation of malaria transmission-reducing interventions. Exp. Parasitol 149, 74–83 (2015). [DOI] [PubMed] [Google Scholar]
- 64.Mathanga DP, Walker ED, Wilson ML, Ali D, Taylor TE, Laufer MK, Malaria control in Malawi: Current status and directions for the future. Acta Trop 121, 212–217 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davies DH, Liang X, Hernandez JE, Randall A, Hirst S, Mu Y, Romero KM, Nguyen TT, Kalantari-Dehaghi M, Crotty S, Baldi P, Villarreal LP, Felgner PL, Profiling the humoral immune response to infection by using proteome microarrays: High-throughput vaccine and diagnostic antigen discovery. Proc. Natl. Acad. Sci. U.S.A 102, 547–552 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bolstad BM, Irizarry RA, Astrand M, Speed TP, A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19, 185–193 (2003). [DOI] [PubMed] [Google Scholar]
- 67.Benjamini Y, Hochberg Y, Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57, 289–300 (1995). [Google Scholar]
- 68.Trager W, Jensen JB, Human malaria parasites in continuous culture. Science 193, 673–675 (1976). [DOI] [PubMed] [Google Scholar]
- 69.Buchholz K, Burke TA, Williamson KC, Wiegand RC, Wirth DF, Marti M, A high-throughput screen targeting malaria transmission stages opens new avenues for drug development. J Infect Dis 203, 1445–1453 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fivelman QL, McRobert L, Sharp S, Taylor CJ, Saeed M, Swales CA, Sutherland CJ, Baker DA, Improved synchronous production of Plasmodium falciparum gametocytes in vitro. Mol. Biochem. Parasitol 154, 119–123 (2007). [DOI] [PubMed] [Google Scholar]
- 71.Tao D, Ubaida-Mohien C, Mathias DK, King JG, Pastrana-Mena R, Tripathi A, Goldowitz I, Graham DR, Moss E, Marti M, Dinglasan RR, Sex-partitioning of the Plasmodium falciparum stage V gametocyte proteome provides insight into falciparum-specific cell biology. Mol. Cell. Proteomics 13, 2705–2724 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.R. C. Team, R: A language and environment for statistical computing (R Foundation for Statistical Computing, 2014); www.R-project.org. [Google Scholar]
- 73.Teo A, Hasang W, Boeuf P, Rogerson S, A robust phagocytosis assay to evaluate the opsonic activity of antibodies against Plasmodium falciparum-infected erythrocytes. Methods Mol. Biol 1325, 145–152 (2015). [DOI] [PubMed] [Google Scholar]
- 74.Weir BS, Cockerham CC, Estimating F-statistics for the analysis of population structure. Evolution 38, 1358–1370 (1984). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.