Abstract
Background:
Surgical resection provides the only potentially curative treatment for pancreatic cancer. Neoadjuvant chemotherapy and/or radiation (NAT) is used to downstage patients with borderline resectable tumors. The objective of this study was to examine the postoperative morbidity and mortality of NAT after pancreaticoduodenectomy (PD) for pancreatic ductal adenocarcinoma (PDA).
Methods:
Using the ACS-NSQIP Targeted Pancreatectomy data, we identified patients who underwent a PD for PDA from 2014–2015. Patients were grouped by receipt of NAT 90-days prior to PD. Bivariable and multivariable analyses was used to compare postoperative outcomes.
Results:
A total of 3,748 patients with PDA underwent PD; 926 (24.7%) received NAT. Those in the NAT group had more major vein resections, and longer operating times (all p<0.001). On pathologic staging, those in the NAT group had smaller tumors (T1 10.9% vs 5.1%, p<0.001) and fewer nodes positive (N0 49% vs 28%, p<0.001). There were no differences in 30-day postoperative mortality or overall complications. On multivariable analysis, patients who received NAT had a lower likelihood of pancreatic fistula (OR 0.67, p<0.001).
Conclusion:
NAT does not increase the overall postoperative morbidity or mortality of PD for PDA. There is a decreased likelihood of pancreatic fistulas in patients that receive neoadjuvant therapy.
Keywords: Neoadjuvant therapy, pancreatic cancer, pancreaticoduodenectomy outcomes, Whipple postoperative complications, postoperative pancreatic fistula
Introduction
Pancreatic adenocarcinoma is the third leading cause of cancer death in the United States.[1,2] A pancreaticoduodenectomy is most commonly performed for patients with adenocarcinoma in the head or neck of the pancreas. The goal of surgery for pancreatic cancer is to obtain a complete (R0) resection; those that do not receive a R0 resection have earlier recurrence and shorter survival.[3–5] Unfortunately, at the time of diagnosis, only 15–20% of patients with adenocarcinoma of the pancreas are candidates for potentially curative surgery due to advanced disease.[6]
Some patients with pancreatic cancer present with borderline resectable tumors. The most recent consensus definition of borderline resectable pancreatic cancer includes anatomical considerations (contact with less than 180 degrees of the superior mesenteric artery and/or celiac artery, short segment contact with the common hepatic artery, and contact or occlusion with the superior mesenteric vein-portal vein confluence with adequate vein proximal and distal for reconstruction), high-risk biologic features, and patient performance status.[7,8] All of these factors make upfront surgery risky, and studies have shown that these patients benefit from neoadjuvant chemotherapy and radiation.[8,9] As a result, the current National Comprehensive Cancer Network (NCCN) guidelines recommend neoadjuvant chemotherapy and chemoradiation for patients with borderline resectable disease.[10]
Initially, neoadjuvant therapy was mainly utilized at large academic centers specializing in pancreatic cancer, and most published studies evaluating the perioperative morbidity and mortality following neoadjuvant therapy for pancreatic cancer have come from these centers.[11] The majority of these single center studies have found no difference between neoadjuvant therapy and initial surgery approaches in terms of postoperative pancreatic fistula formation or total complications.[11,12] For example, in 2015 Cooper et al. published their study using the American College of Surgeons-National Surgical Quality Improvement Project (ACS-NSQIP) Pancreatectomy Demonstration Project pilot data to examine national rates of postoperative complications after neoadjuvant therapy. No difference in the overall postoperative complication rates was identified between groups in that study.[13] However, the sample size of that study was small and the rate of neoadjuvant therapy was still quite low. Since that time, the use of neoadjuvant therapy has become much more widespread and the number of hospitals participating in the ACS-NSQIP Targeted Pancreatectomy database has also increased substantially. As a result, this current study examines whether the findings, specifically the impact of neoadjuvant therapy on 30-day postoperative mortality and morbidity, hold true across this larger population of patients and hospitals.
Material and Methods
Data and Population
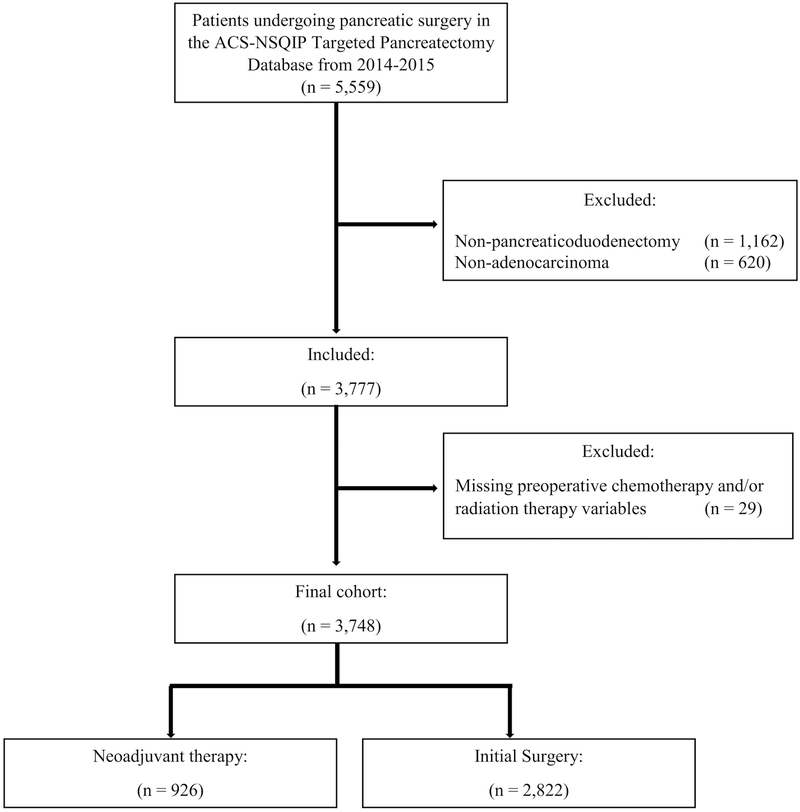
One-hundred and twenty de-identified hospitals in the United States contribute data to the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) and the Targeted Pancreatectomy data program. This program collects 36 additional pancreas specific variables in addition to those captured by the standard ACS-NSQIP program. Patients undergoing a pancreaticoduodenectomy for pancreatic adenocarcinoma between January 1, 2014 to December 31, 2015 were identified in the ACS-NSQIP Targeted Pancreatectomy Participant Use Data Files (PUF) (N= 5,559). This cohort of patients was identified by Current Procedural Terminology (CPT) codes for pancreaticoduodenectomy (48150, 48152, 48153, and 48154) and with a histology diagnosis of pancreatic adenocarcinoma (N= 3,777). Patients were excluded if they had missing data for preoperative chemotherapy or radiation therapy (N= 29). The ACS-NSQIP and the hospitals participating in the ACS-NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors. The University of North Carolina Institutional Review Board deemed this study exempt from further review.
Variables and Outcomes
Patients were divided into two groups: those that received neoadjuvant therapy, and those that had initial surgery. We defined neoadjuvant therapy as any chemotherapy and/or radiation therapy occurring in the 90-days prior to the index operation. The ACS-NSQIP database does not specify the chemotherapy drug regimen, radiation dose, or duration of treatment. Variables extracted from the ACS-NSQIP database included demographics, preoperative risk factors, intraoperative factors, and 30-day postoperative outcomes classified according to the ACS-NSQIP PUF definitions.[14] The primary outcome of interest was 30-day postoperative mortality after pancreaticoduodenectomy for pancreatic adenocarcinoma. The cohort sample size allowed for a 90% power (alpha=0.05) to detect an absolute difference of 2% based on the cohort size (924 in the neoadjuvant group and 2,822 in the initial surgery group), assuming a 30-day mortality of 2%. The secondary outcome was 30-day postoperative complications. Pancreas specific complications included pancreatic fistula, and delayed gastric emptying. Pancreatic fistula is defined by ACS-NSQIP as persistent drainage of amylase rich fluid requiring continued operative drain placement for greater than 7 days, percutaneous drainage, or reoperation. Pancreatic fistula complications were categorized to the International Study Group for Pancreatic Fistula (ISGPF) grades A, B, or C based on available information.[15] Other postoperative complications included superficial and deep surgical site infections, organ space surgical site infection, wound dehiscence, pneumonia, pulmonary embolism/deep vein thromboembolism, unplanned intubation, renal insufficiency/renal failure, urinary tract infection, stroke, cardiac arrest, myocardial infarction, Clostridium difficile, transfusion, sepsis/septic shock, take back to the operating room, and 30-day readmission. Complications were transformed into categorical variables based on their Clavien-Dindo classification (grades 1–5) (Appendix A).[16] Complications were considered minor if they were Clavien-Dindo grades 1–2, and severe if they were grades 3–5.
Statistical Analysis
Chi-square and Student’s t-tests for categorical and continuous variables were used to compare patient demographics, preoperative risk factors, and intraoperative characteristics between the neoadjuvant therapy and initial surgery groups. Thirty-day postoperative outcomes were initially analyzed using unadjusted, bivariate analyses with chi-square and Student’s t-test. Intraoperative and postoperative outcomes that were statistically significant on bivariate analysis were assessed using multivariable logistic regression. Each model was adjusted for age, sex, body mass index (BMI), preoperative steroid use, wound classification, operative time, preoperative biliary stenting, preoperative jaundice, preoperative albumin, preoperative anemia, pancreatic duct size, gland texture, presence of pancreatic fistula, and operative reconstruction (i.e. pylorus-sparing pancreaticoduodenectomy, and pancreaticojejunal vs pancreaticogastrostomy). Presence of pancreatic fistula was removed from the pancreatic fistula model. Each model was tested for effect-measure modification using likelihood ratio tests by creating interaction terms between receipt of neoadjuvant therapy and clinically significant covariates. Clostridium difficile postoperative complication was dropped from the analysis due to 45% missing values. Listwise deletion method was used for analysis of variables if less than 5% of the data was missing. Variables with greater than 5% missing data were reported in the tables and identified as “missing/unknown”. Statistical significance was set at p<0.05 and all tests were 2-sided. All analysis was conducted using STATA 14.1 (StataCorp, Inc., College Station, TX).
Results
A total of 3,748 patients underwent a pancreaticoduodenectomy (Figure 1). Of these patients, 926 (24.7%) received neoadjuvant therapy, with 506 (13.5%) receiving only chemotherapy, 28 (0.8%) receiving only radiation, and 392 (10.5%) receiving chemotherapy and radiation. Patients who received neoadjuvant therapy were more likely to be younger, non-Hispanic white, have normal BMI, have used preoperative steroids, have a higher preoperative albumin, have a biliary stent at the time of surgery, have less preoperative jaundice and have less preoperative hypertension compared to the initial surgery group (all p<0.015, Table 2). Although the majority of patients in both groups underwent an open pancreaticoduodenectomy, there was a larger proportion of patients in the neoadjuvant therapy group who underwent a robotic procedure (4.8% vs 3.3%, p=0.035) compared to the initial surgery group. The neoadjuvant group also required more major vein resections (35.8% vs 17.6%, p<0.001). Thus not surprisingly, the mean operative time was longer by 51 minutes in the neoadjuvant group (413 vs 364 minutes, p<0.001). The neoadjuvant group had more patients with a hard pancreas (66.5% vs 53.2%, p<0.001). Post-surgical pathology revealed smaller tumor size, and negative lymph nodes in the neoadjuvant therapy group (p<0.001, Table 3). Length of stay was shorter for the neoadjuvant therapy group than the initial surgery group (mean 9.7 vs 10.9, p<0.001).
Figure 1:
CONSORT Diagram
Table 2.
Operative Characteristics of Patients with Adenocarcinoma of the Pancreas Undergoing a Pancreaticoduodenectomy
| Neoadjuvant Therapy N=926 | Initial Surgery N=2,828 | p-value | |
|---|---|---|---|
| T Stag, n(%) | |||
| T0 | 22 (2.4%) | 2 (0.1%) | <0.001 |
| Tis | 2 (0.2%) | 3 (0.1%) | 0.384 |
| T1 | 99 (11%) | 142 (5.1%) | <0.001 |
| T2 | 71 (7.9%) | 292 (10.5%) | 0.094 |
| T3 | 682 (75.7%) | 2,222 (79.6%) | ref. |
| T4 | 25 (2.8%) | 129 (4.6%) | 0.038 |
| N Stage, n(%) | <0.001 | ||
| N0 | 442 (49%) | 777 (28%) | |
| N1 | 460 (51%) | 2,002 (72%) | |
| M Stage, n(%) | 0.855 | ||
| M0 | 839 (98.2%) | 2,496 (98.2%) | |
| M1 | 15 (1.8%) | 47 (1.8%) | |
| Total operating time (min.), median (IQR) | 399.5 (322–484) | 354 (274–437) | <0.001 |
| Operative approach, n(%) | |||
| Open | 846 (91.4%) | 2,620 (92.9%) | ref. |
| Laparoscopic | 36 (3.9%) | 110 (3.9%) | 0.945 |
| Robotic | 44 (4.8%) | 92 (3.3%) | 0.035 |
| Wound Class | |||
| Clean | 13 (1.4%) | 44 (1.6%) | 0.851 |
| Clean-contaminated | 732 (79.1%) | 2,334 (82.7%) | ref. |
| Contaminated | 154 (16.7%) | 332 (11.8%) | <0.001 |
| Dirty | 27 (2.9%) | 112 (4.0%) | 0.227 |
| Resection, n(%) | |||
| Artery | 59 (6.4%) | 148 (5.3%) | 0.211 |
| Vein | 329 (35.8%) | 487 (17.6%) | <0.001 |
| Pylorus-preserving surgery, n (%) | 322 (34.77%) | 1,172 (41.5%) | <0.001 |
| Reconstruction, n(%) | |||
| Pancreaticojejunal duct-to-mucosal | 772 (89.4%) | 2,340 (87.7%) | ref. |
| Pancreaticojejunal invagination | 81 (9.4%) | 262 (9.8%) | 0.627 |
| Pancreaticogastrostomy | 11 (1.3%) | 66 (2.5%) | 0.034 |
| Gland texture, n(%) | |||
| Hard | 469 (66.5%) | 1,071 (53.2%) | ref |
| Intermediate | 78 (11.1%) | 226 (11.2%) | 0.094 |
| Soft | 158 (22.4%) | 716 (35.6%) | <0.001 |
| Missing/Unknown | 221 | 809 | |
| Pancreatic duct size, n(%) | |||
| <3 mm | 190 (26.1%) | 507 (23.8%) | 0.354 |
| 3–6 mm | 401 (55.2%) | 1,177 (55.3%) | ref |
| >6 mm | 136 (18.7%) | 445 (20.9%) | 0.339 |
| Missing/Unknown | 199 | 693 |
Table 3.
Postoperative Complications for Patients with Adenocarcinoma of the Pancreas after Pancreaticoduodenectomy within 30-days of the procedure
| Neoadjuvant Therapy | Initial Surgery | p-value | |
|---|---|---|---|
| N= 926 | N= 2,822 | ||
| Postoperative death, n (%) | 16 (1.7%) | 56 (2.0%) | 0.622 |
| Overall complication, n (%) | 517 (55.8%) | 1,554 (55.1%) | 0.685 |
| 30-day Readmission, n (%) | 154 (16.6%) | 424 (15.0%) | 0.24 |
| Superficial SSI, n (%) | 89 (9.6%) | 253 (9.0%) | 0.554 |
| Deep incisional SSI, n (%) | 20 (2.2%) | 59 (2.1%) | 0.899 |
| Organ space SSI, n (%) | 76 (8.2%) | 321 (11.4%) | 0.007 |
| UTI, n (%) | 40 (4.3%) | 92 (3.3%) | 0.129 |
| Pneumonia, n (%) | 21 (2.3%) | 108 (3.8%) | 0.024 |
| Pulmonary embolism, n (%) | 6 (0.7%) | 35 (1.2%) | 0.133 |
| DVT, n (%) | 27 (2.9%) | 79 (2.8%) | 0.853 |
| Sepsis, n (%) | 74 (8.0%) | 232 (8.2%) | 0.825 |
| Septic Shock, n (%) | 26 (2.8%) | 84 (3.0%) | 0.792 |
| Acute renal insufficiency, n (%) | 2 (0.2%) | 19 (0.7%) | 0.106 |
| Acute renal failure, n (%) | 5 (0.5%) | 26 (0.9%) | 0.266 |
| Stroke, n (%) | 1 (0.1%) | 9 (0.3%) | 0.28 |
| Myocardial infarction, n (%) | 6 (0.7%) | 27 (1.0%) | 0.383 |
| Cardiac Arrest, n (%) | 8 (0.9%) | 35 (1.2%) | 0.351 |
| Wound dehiscence, n (%) | 9 (1.0%) | 34 (1.2%) | 0.564 |
| Reoperation, n (%) | 49 (5.3%) | 157 (5.5%) | 0.784 |
| Pancreatic fistula, n (%) | 85 (9.2%) | 414 (14.8%) | <0.001 |
| Grade A (ISGPF) | 55 (6.0%) | 278 (10.0%) | < 0.001 |
| Grade B (ISGPF) | 28 (3.0%) | 104 (3.7%) | 0.331 |
| Grade C (ISGPF) | 2 (0.2%) | 32 (1.2%) | 0.01 |
| Delayed gastric emptying, n (%) | 125 (13.8%) | 481 (17.6%) | 0.008 |
| Percutaneous drainage, n (%) | 91 (10.0%) | 297 (10.8%) | 0.473 |
| Perioperative transfusion, n (%) | 255 (27.5%) | 596 (21.1%) | <0.001 |
| Clavien-Dino Complication, n (%) | |||
| Grade 1 | 2 (0.2%) | 19 (0.7%) | 0.106 |
| Grade 2 | 430 (46.4%) | 1,247 (44.2%) | 0.233 |
| Grade 3 | 117 (12.6%) | 419 (14.9%) | 0.095 |
| Grade 4 | 54 (5.8%) | 192 (6.8%) | 0.3 |
| Grade 5 (death) | 16 (1.7%) | 56 (2.0%) | 0.622 |
| Complication, n (%) | |||
| Minor (Clavien-Dino 1–2) | 426 (46%) | 1,232 (43.7%) | 0.212 |
| Severe (Clavien-Dino 3–5) | 155 (16.7%) | 516 (18.3%) | 0.287 |
SSI surgical site infection; UTI urinary tract infection; DVT deep venous thrombus; ISGPF International Study Group for Pancreatic Fistula
The 30-day mortality was similar between the neoadjuvant therapy group and the initial surgery group (1.7% vs 2%, p=0.622). There was no difference in 30-day overall complications or readmission rates between the two groups (Table 3). After stratifying complications based on Clavien-Dindo Grade, there was no statistically significant difference in complications between the groups. On bivariate analyses of individual complications, there were statistically significant differences in postoperative complications for organ space surgical site infection, pneumonia, postoperative blood transfusion, pancreatic fistula and delayed gastric emptying between the neoadjuvant therapy and initial surgery groups (Table 3). There were significantly fewer organs space surgical site infections, pneumonias, pancreatic fistulas, and delayed gastric emptying in the neoadjuvant group. The neoadjuvant group had both fewer grade A (6% vs 10%, p<0.001) and grade C (0.2% vs 1.2%) pancreatic fistulas complications. The neoadjuvant group did have higher rates of blood transfusion within 72 hours of the pancreaticoduodenectomy.
On multivariable logistic regression analysis, individuals who had neoadjuvant therapy were less likely to have a pancreatic fistula complication (OR, 0.67; 95% CI, 0.49–0.92; p=0.015) after controlling for clinically and statistically significant preoperative and operative characteristics (Table 3). Independent predictors for the development of a pancreatic fistula in addition to initial surgery included, having a preoperative biliary stent, having a small pancreatic duct (<3mm), having soft pancreatic tissue intraoperatively compared to hard, and having a longer operation (Table 4). After controlling for clinically and statistically significant perioperative characteristics, there was no statistically significant association between receipt of neoadjuvant therapy and organ space surgical site infection, pneumonia, delayed gastric emptying, or need for blood.
Table 4.
Odds Ratios for 30-day Postoperative Complications for Patients with Adenocarcinoma of the Pancreas after Pancreaticoduodenectomy
| Crude | Adjusted* | |||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Organ space SSI | ||||
| Neoadjuvant Therapy | 0.70 (0.54, 0.91) | 0.007 | 0.86 (0.63–1.17) | 0.353 |
| Initial Surgery | 1.0, Ref | Ref. | ||
| Pneumonia | ||||
| Neoadjuvant Therapy | 0.58 (0.36, 0.94) | 0.026 | 1.15 (0.59–2.27) | 0.681 |
| Initial Surgery | Ref. | Ref. | ||
| Pancreatic fistula | ||||
| Neoadjuvant Therapy | 0.66 (0.50–0.88) | <0.001 | 0.67 (0.49–0.92) | 0.015 |
| Initial Surgery | Ref. | Ref. | ||
| Delayed gastric emptying | ||||
| Neoadjuvant Therapy | 0.75 (0.61–0.93) | 0.008 | 0.80 (0.59–1.06) | 0.124 |
| Initial Surgery | Ref. | Ref. | ||
| Perioperative transfusion | ||||
| Neoadjuvant Therapy | 1.42 (1.20–1.68) | <0.001 | 1.12 (0.88–1.43) | 0.344 |
| Initial Surgery | Ref. | Ref. | ||
SSI surgical site infection; CI confidence interval;
Odds Ratio was adjusted for: age, sex, body mass index, preoperative steroid use, wound classification, operative time (hours), preoperative biliary stenting, preoperative jaundice, preoperative albumin, preoperative anemia, pancreatic duct size, gland texture, presence of pancreatic fistula, and operative reconstruction (i.e. pylorus-sparing pancreaticoduodenectomy, and pancreaticojejunal vs pancreaticogastrostomy
Discussion
Neoadjuvant therapy is increasingly being utilized in borderline resectable and locally advanced pancreatic cancer to improve margin negative resection rates or increase resectability, particularly in high-volume and academic centers.[17–19] One meta-analysis found 43% of patients with borderline resectable and locally advanced disease were able to be resected after preoperative FOLFIRINOX with or without radiation, with a complete resection (R0) rate of 85%.[17] Similarly, another systematic review of locally advanced pancreatic cancer patients found that 28% underwent resection after FOLFIRINOX with or without radiation, with a 77% R0 resection rate.[18] Consistent with these prior studies, this study demonstrated that patients treated with neoadjuvant therapy had smaller tumors and fewer nodes positive on pathologic staging compared to patients treated with surgery first (see Table 2), supporting the idea that neoadjuvant therapy results in downstaging of the tumor. Still, there are no published randomized control trials comparing neoadjuvant therapy to initial surgery in borderline resectable patients, so the best approach remains disputed.
Neoadjuvant therapy may have benefits beyond making an R0 resection possible. At the time of diagnosis, pancreatic cancer is a systemic disease and requires not only local control but also systemic treatment with chemotherapy to improve survival.[20] However, only 57.7% of patients receive adjuvant chemotherapy after curative-intent pancreatic resection.[21] Among patients that have a serious postoperative complication, only 43.6% receive adjuvant therapy.[21] Neoadjuvant therapy ensures that systemic therapy is not delayed or omitted due to prolonged postoperative recovery. Neoadjuvant therapy may also identify patients that would not benefit from surgical resection due to rapidly progressive metastatic disease.[22] Approximately 25% of patients who undergo preoperative chemotherapy or chemoradiation for pancreatic cancer do not undergo resection due to either preoperative disease progression, decline in performance status, or extrapancreatic disease found at the time of surgery.[22–24]
Despite these potential advantages, concerns remain regarding higher rates of perioperative complications in patients undergoing neoadjuvant therapy.[11,25,26] However, existing data do not support this concern. Two separate single high-volume center studies found no difference in 90-day postoperative morbidity or mortality.[27,28] Prior ACS-NSQIP studies examined this on a national level but these studies had limitations. The first study using the ACS-NSQIP Targeted Pancreatectomy was limited in its conclusions due to the combination of both pancreaticoduodenectomy and distal pancreatectomy patients and the small proportion of patients that received neoadjuvant therapy (12.7%).[13] Other studies using the ACS-NSQIP data found no difference in overall morbidity and mortality, but were not able to capture pancreas specific complications (i.e. pancreatic fistula, and delayed gastric emptying).[29,30] This current study is unique in that we examined pancreaticoduodenectomy in the setting of neoadjuvant therapy across 120 hospitals in the modern era of pancreatic surgery. We found no statistically significant differences in mortality, overall complications, and major or minor complications between the initial surgery and neoadjuvant therapy groups.
Overall there was a pancreatic fistula rate of 13.4%, which is concordant with the previously published studies.[31] Postoperative pancreatic fistula was associated with preoperative biliary stenting, having a soft pancreas, small pancreatic duct (<3mm), longer operative time, and initial surgery (compared to neoadjuvant therapy). Patients who received neoadjuvant therapy (compared to initial surgery) had a decreased the likelihood of developing a pancreatic fistula, even after controlling for the other common risk factors.[32,33] Decreased pancreatic fistula rates in patients who receive neoadjuvant therapy is in alignment with previous single-center reports.[28,34] The proposed mechanism is impairment of pancreatic function and induction of pancreatic fibrosis, making the pancreas more favorable for pancreatic ductal anastomosis.[35] Additionally, intraoperative characteristic of having a soft pancreas and pancreatic duct size <3mm were even more strongly associated with developing a pancreatic fistula than exposure to neoadjuvant therapy alone. This is consistent with previous single-center studies that have shown that a fatty pancreas and lack of pancreatic fibrosis were significant risk factors for pancreatic fistula development.[36,37]
The rate of neoadjuvant therapy documented in this study (approximately 25% of patients undergoing a pancreaticoduodenectomy for pancreatic adenocarcinoma) was much higher than what had been previously reported.[29,30,38] The increased use may reflect increased adoption of neoadjuvant therapy for resectable pancreatic cancer patients in addition to borderline resectable and locally advanced disease. Alternatively, there could be selection bias as the high-volume pancreas centers that tend to participate in the ACS NSQIP Pancreas Group may also be more likely to be both early adopters of innovation such as neoadjuvant therapy.
There are some other limitations of these data, largely due to the limitations of registry data. The ACS-NSQIP Targeted Pancreatectomy data do not capture details such as chemotherapy drug regimen, radiation dosing, or duration of treatment, so we cannot determine the granular details of the planned therapy, or if patients completed a full course of treatment. In addition, as ACS-NSQIP data only capture surgical outcomes up to 30 days from the index operation; however, a prior single center study that followed patients out to 90-days after surgery found no difference in morbidity or mortality after neoadjuvant chemoradiation.[27] Finally, we were unable to adjust for surgeon, center, or center volume leaving the possibility that differences in outcomes between the neoadjuvant group and the surgery first group may have been in part due to differential use of neoadjuvant therapy amongst high and low volume providers.
Despite these limitations, our study has many strengths. The cohort is comprised of over 900 patients who underwent neoadjuvant therapy after pancreaticoduodenectomy at 120 hospitals, allowing for a broader examination of outcomes compared to previous single center studies. Additionally, we were able to evaluate pancreas specific complications (i.e. pancreatic fistula, and delayed gastric emptying) that are often not captured with large database studies.
Conclusion
Neoadjuvant therapy does not appear to increase the 30-day overall postoperative morbidity or mortality of a pancreaticoduodenectomy for adenocarcinoma of the pancreas, and in fact, patients receiving neoadjuvant therapy have a decreased likelihood of pancreatic fistula. Despite the increased utilization of neoadjuvant therapy, prospective randomized trials are needed to establish the best approach for sequencing of therapy for the different subsets of patients with resectable, borderline resectable, and locally advanced pancreatic cancer.
Supplementary Material
Table 1.
Demographics and Preoperative Characteristics of Patients with Adenocarcinoma of the Pancreas Undergoing a Pancreaticoduodenectomy from 2014–2015 in the ACS-NSQIP Targeted Pancreatectomy Database
| Neoadjuvant Therapy | Initial Surgery | p-value | |
|---|---|---|---|
| N=926 | N=2,822 | ||
| Age, mean (IQR) | 64 (57–71) | 67 (60–74) | <0.001 |
| Male, n(%) | 456 (49.2%) | 1,536 (54.4%) | 0.006 |
| Race/Ethnicity, n(%) | |||
| NHW | 717 (79.1%) | 2,001 (73.2%) | ref. |
| NHB | 138 (15.3%) | 497 (18.2%) | 0.013 |
| Hispanic | 28 (3.1%) | 116 (4.2%) | 0.062 |
| Asian | 20 (2.2%) | 110 (4%) | 0.005 |
| AI/NA | 3 (0.3%) | 11 (0.4%) | 0.671 |
| BMI, n(%) | |||
| Normal | 350 (37.8%) | 892 (31.8%) | ref. |
| Underweight | 16 (1.7%) | 46 (1.6%) | 0.685 |
| Overweight | 323 (34.9%) | 1,047 (37.3%) | 0.007 |
| Obese | 237 (25.6%) | 824 (29.3%) | 0.001 |
| Smokers, n(%) | 168 (18.1%) | 503 (17.8%) | 0.826 |
| Diabetes, n(%) | 267 (28.8%) | 767 (27.2%) | 0.328 |
| Hypertension, n(%) | 455 (49.1%) | 1,560 (55.3%) | 0.001 |
| CHF, n(%) | 1 (0.1%) | 12 (0.4%) | 0.154 |
| COPD, n(%) | 39 (4.2%) | 133 (4.6%) | 0.585 |
| Functional Status, n (%) | |||
| Independent | 919 (99.4%) | 2,783 (99%) | ref. |
| Partially Dependent | 5 (0.5%) | 27 (1%) | 0.23 |
| Totally Dependent | 1 (0.1%) | 0 | 0.082 |
| Preoperative steroid use, n (%) | 31 (3.4%) | 53 (1.9%) | 0.009 |
| >10% weight lossŧ, n (%) | 186 (20.1%) | 609 (21.6%) | 0.335 |
| Albumin, median (IQR) | 3.8 (3.4–4.1) | 3.7 (3.3–4.1) | <0.001 |
| Albumin <3.5 g/dL | 235 (26.6%) | 932 (35.4%) | <0.001 |
| Albumin >3.5 g/dL | 649 (73.4%) | 1,703 (64.6%) | |
| Bilirubin, median (IQR) | 0.4 (0.3–0.6) | 1.2 (0.6–3.5) | <0.001 |
| Anemia, n(%) | 438 (48.0%) | 1,162 (41.8%) | 0.001 |
| Jaundice, n(%) | 332 (36.2%) | 1,920 (68.5%) | <0.001 |
| Biliary stent, n(%) | 612 (66.1%) | 1,744 (61.8%) | 0.019 |
| ASA, n(%) | |||
| 1 | 1 (0.1%) | 3 (0.1%) | 0.996 |
| 2 | 166 (17.9%) | 550 (19.5%) | 0.287 |
| 3 | 694 (75.0%) | 2,070 (73.4%) | ref. |
| 4 | 65 (7%) | 197 (7%) | 0.915 |
IQR Interquartile Range; BMI body mass index, Normal (18.5–24.9), Underweight (<18.5), Overweight (25–29.9), Obese (>30); NHW non-Hispanic white; NHB non-Hispanic black; AI/NA American Indian/Native Alaskan; ASA American Society of Anesthesia physical status classification; CHF congestive heart failure; COPD chronic obstructive pulmonary disease;
weight loss 6 months prior to operation
Table 5:
Independent Predictors of Pancreatic Fistula
| Risk Factor (Ref) | OR | 95% CI | p-value |
|---|---|---|---|
| Neoadjuvant (Initial surgery) | 0.67 | 0.49–0.92 | 0.015 |
| Pre-op biliary stent (none) | 1.3 | 1.01–1.69 | 0.043 |
| <3mm pancreatic duct (3–6mm duct) | 1.45 | 1.08–1.96 | 0.014 |
| Soft pancreatic texture (Hard) | 2.97 | 2.33–3.78 | <0.001 |
| Time (hours) | 1.06 | 1.00–1.12 | 0.035 |
In addition to variables listed, model controls for: age, sex, body mass index, preoperative steroid use, wound classification, preoperative jaundice, preoperative albumin, preoperative anemia, and operative reconstruction.
Synopsis for Table of Contents:
Neoadjuvant chemotherapy and radiation are increasingly utilized in pancreatic cancer. This article examines the postoperative morbidity and mortality of neoadjuvant therapy following pancreaticoduodenectomy for pancreatic adenocarcinoma.
Grant Support:
This work is supported by the Cancer Care Quality Research Training Program from a National Institutes of Health grant R25CA116339.
Disclosures and Funding Sources: The authors have no disclosures relevant to the research covered in this submitted manuscript.
References:
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA: a cancer journal for clinicians. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61(2):69–90. [DOI] [PubMed] [Google Scholar]
- 3.Neoptolemos JP, Stocken DD, Dunn JA, et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg. 2001;234(6):758–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richter A, Niedergethmann M, Sturm JW, Lorenz D, Post S, Trede M. Long-term results of partial pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head: 25-year experience. World J Surg. 2003;27(3):324–329. [DOI] [PubMed] [Google Scholar]
- 5.Kuhlmann KF, de Castro SM, Wesseling JG, et al. Surgical treatment of pancreatic adenocarcinoma; actual survival and prognostic factors in 343 patients. European journal of cancer (Oxford, England : 1990). 2004;40(4):549–558. [DOI] [PubMed] [Google Scholar]
- 6.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371(11):1039–1049. [DOI] [PubMed] [Google Scholar]
- 7.Isaji S, Mizuno S, Windsor JA, et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology. 2018;18(1):2–11. [DOI] [PubMed] [Google Scholar]
- 8.Katz MHG, Pisters PW, Evans DB, et al. BORDERLINE RESECTABLE PANCREATIC CANCER: THE IMPORTANCE OF THIS EMERGING STAGE OF DISEASE. Journal of the American College of Surgeons. 2008;206(5):833–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillen S, Schuster T, zum Buschenfelde CM, Friess H, Kleeff J. Preoperative/Neoadjuvant Therapy in Pancreatic Cancer: A Systematic Review and Meta-analysis of Response and Resection Percentages. PLos Med. 2010;7(4):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Network NCC. Pancreatic Adenocarcinoma (Version 1.2018). https://www.nccn.org/professionals/physician_gls/PDF/pancreatic.pdf. Accessed May 4, 2018.
- 11.Laurence JM, Tran PD, Morarji K, Eslick GD, Lam VW, Sandroussi C. A systematic review and meta-analysis of survival and surgical outcomes following neoadjuvant chemoradiotherapy for pancreatic cancer. J Gastrointest Surg. 2011;15(11):2059–2069. [DOI] [PubMed] [Google Scholar]
- 12.Verma V, Li J, Lin C. Neoadjuvant Therapy for Pancreatic Cancer: Systematic Review of Postoperative Morbidity, Mortality, and Complications. American journal of clinical oncology. 2016;39(3):302–313. [DOI] [PubMed] [Google Scholar]
- 13.Cooper AB, Parmar AD, Riall TS, et al. Does the Use of Neoadjuvant Therapy for Pancreatic Adenocarcinoma Increase Postoperative Morbidity and Mortality Rates? J Gastrointest Surg. 2015;19(1):80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ACS NSQIP User Guide for the 2015 Participant Use Data File. 2015.
- 15.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138(1):8–13. [DOI] [PubMed] [Google Scholar]
- 16.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrelli F, Coinu A, Borgonovo K, et al. FOLFIRINOX-based neoadjuvant therapy in borderline resectable or unresectable pancreatic cancer: a meta-analytical review of published studies. Pancreas. 2015;44(4):515–521. [DOI] [PubMed] [Google Scholar]
- 18.Rombouts SJ, Walma MS, Vogel JA, et al. Systematic Review of Resection Rates and Clinical Outcomes After FOLFIRINOX-Based Treatment in Patients with Locally Advanced Pancreatic Cancer. Ann Surg Oncol. 2016;23(13):4352–4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz MH, Wang H, Balachandran A, et al. Effect of neoadjuvant chemoradiation and surgical technique on recurrence of localized pancreatic cancer. J Gastrointest Surg. 2012;16(1):68–78; discussion 78–69. [DOI] [PubMed] [Google Scholar]
- 20.Bakkevold KE, Arnesjo B, Dahl O, Kambestad B. Adjuvant combination chemotherapy (AMF) following radical resection of carcinoma of the pancreas and papilla of Vater--results of a controlled, prospective, randomised multicentre study. European journal of cancer (Oxford, England : 1990). 1993;29a(5):698–703. [DOI] [PubMed] [Google Scholar]
- 21.Merkow RP, Bilimoria KY, Tomlinson JS, et al. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg. 2014;260(2):372–377. [DOI] [PubMed] [Google Scholar]
- 22.Crane CH, Varadhachary G, Wolff RA, Pisters PW, Evans DB. The argument for pre-operative chemoradiation for localized, radiographically resectable pancreatic cancer. Best practice & research Clinical gastroenterology. 2006;20(2):365–382. [DOI] [PubMed] [Google Scholar]
- 23.Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26(21):3496–3502. [DOI] [PubMed] [Google Scholar]
- 24.Marsh RD, Talamonti MS, Baker MS, et al. Primary systemic therapy in resectable pancreatic ductal adenocarcinoma using mFOLFIRINOX: A pilot study. Journal of surgical oncology. 2018;117(3):354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vento P, Mustonen H, Joensuu T, Karkkainen P, Kivilaakso E, Kiviluoto T. Impact of preoperative chemoradiotherapy on survival in patients with resectable pancreatic cancer. World J Gastroenterol. 2007;13(21):2945–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allendorf JD, Lauerman M, Bill A, et al. Neoadjuvant chemotherapy and radiation for patients with locally unresectable pancreatic adenocarcinoma: feasibility, efficacy, and survival. J Gastrointest Surg. 2008;12(1):91–100. [DOI] [PubMed] [Google Scholar]
- 27.Denbo JW, Bruno ML, Cloyd JM, et al. Preoperative Chemoradiation for Pancreatic Adenocarcinoma Does Not Increase 90-Day Postoperative Morbidity or Mortality. J Gastrointest Surg. 2016;20(12):1975–1985. [DOI] [PubMed] [Google Scholar]
- 28.Marchegiani G, Andrianello S, Nessi C, et al. Neoadjuvant Therapy Versus Upfront Resection for Pancreatic Cancer: The Actual Spectrum and Clinical Burden of Postoperative Complications. Ann Surg Oncol. 2018;25(3):626–637. [DOI] [PubMed] [Google Scholar]
- 29.Teng A, Lee DY, Yang CK, Rose KM, Attiyeh F. The effects of neoadjuvant chemoradiation on pancreaticoduodenectomy-the American College of Surgeon’s National Surgical Quality Improvement Program analysis. The Journal of surgical research. 2015;196(1):67–73. [DOI] [PubMed] [Google Scholar]
- 30.Cho SW, Tzeng CWD, Johnston WC, et al. Neoadjuvant radiation therapy and its impact on complications after pancreaticoduodenectomy for pancreatic cancer: analysis of the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP). HPB (Oxford). 2014;16(4):350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexakis N, Sutton R, Neoptolemos JP. Surgical treatment of pancreatic fistula. Digestive surgery. 2004;21(4):262–274. [DOI] [PubMed] [Google Scholar]
- 32.Muscari F, Suc B, Kirzin S, et al. Risk factors for mortality and intra-abdominal complications after pancreatoduodenectomy: multivariate analysis in 300 patients. Surgery. 2006;139(5):591–598. [DOI] [PubMed] [Google Scholar]
- 33.Ban D, Shimada K, Konishi M, Saiura A, Hashimoto M, Uesaka K. Stapler and nonstapler closure of the pancreatic remnant after distal pancreatectomy: multicenter retrospective analysis of 388 patients. World J Surg. 2012;36(8):1866–1873. [DOI] [PubMed] [Google Scholar]
- 34.Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg. 2015;261(1):12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishikawa O, Ohigashi H, Imaoka S, et al. Concomitant benefit of preoperative irradiation in preventing pancreas fistula formation after pancreatoduodenectomy. Archives of surgery (Chicago, Ill : 1960). 1991;126(7):885–889. [DOI] [PubMed] [Google Scholar]
- 36.Gaujoux S, Cortes A, Couvelard A, et al. Fatty pancreas and increased body mass index are risk factors of pancreatic fistula after pancreaticoduodenectomy. Surgery. 2010;148(1):15–23. [DOI] [PubMed] [Google Scholar]
- 37.El Nakeeb A, Salah T, Sultan A, et al. Pancreatic anastomotic leakage after pancreaticoduodenectomy. Risk factors, clinical predictors, and management (single center experience). World J Surg. 2013;37(6):1405–1418. [DOI] [PubMed] [Google Scholar]
- 38.Youngwirth LM, Nussbaum DP, Thomas S, et al. Nationwide trends and outcomes associated with neoadjuvant therapy in pancreatic cancer: An analysis of 18 243 patients. Journal of surgical oncology. 2017;116(2):127–132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.