Abstract
Background
The beneficial effects of cardiac resynchronization therapy (CRT) on left ventricular (LV) remodeling have been extensively described. Few data are available about the effects of CRT on right ventricular (RV) function and remodeling.
Hypothesis
We hypothesized that CRT could also induce reverse remodeling in the right ventricle and that RV baseline functional status expressed as tricuspidal annular plane systolic excursion (TAPSE) could affect CRT response.
Methods
Echocardiographic investigation was performed before and 6 months after CRT. In 192 patients, TAPSE, LV, and RV dimensions with functional parameters and LV dyssynchrony index were evaluated.
Results
At 6 months' follow‐up, 86 patients (45%) were responders to CRT according to at least 15% LV end‐systolic volume reduction. Among baseline echocardiographic parameters, responders had significantly lower TAPSE, larger LV volumes, and higher LV dyssynchrony index. In responders, LV volume reduction, ejection fraction increase, and mitral regurgitation improvement were associated with RV dimensions reduction, increased TAPSE, and improved LV dyssynchrony. Receiver operating characteristic curve analysis showed that TAPSE, at 17 mm optimal cutoff, yielded 64% sensitivity and 60% specificity in predicting CRT response; similarly, LV dyssynchrony index, at 41.25 ms optimal cutoff, predicted CRT response with 60% sensitivity and 62% specificity. A subgroup analysis demonstrated that the coexistence of high TAPSE and high dyssynchrony index values increased probability of CRT response.
Conclusions
Our results show that CRT induces RV and LV reverse remodeling and that CRT patient selection can be improved by simply measuring TAPSE value. Copyright © 2010 Wiley Periodicals, Inc.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
Congestive heart failure (CHF) is a major disorder associated with poor quality of life and high mortality.1 At present, cardiac resynchronization therapy (CRT) is a new, well‐established therapeutic strategy for patients with drug‐refractory symptoms, advanced left ventricular (LV) systolic dysfunction, and wide QRS.2, 3, 4, 5, 6, 7 However, because it was primarily developed to improve function and synchronism of the LV, the effects of CRT on right ventricular (RV) function have not been fully examined.
The importance of the right ventricle has often been underestimated; it was long considered mainly as a conduit and the importance of its contractile performance was undervalued. Nowadays, few data are available about the effects of CRT on RV function and remodeling.8, 9, 10 Moreover, data about the relationship between baseline RV functional status and long‐term LV reverse remodeling response to CRT are lacking. The aim of our study was to evaluate the effects of CRT on RV function and to determine if the presence of baseline RV dysfunction could affect LV reverse remodeling using traditional, tissue Doppler imaging (TDI), and strain echocardiography.
Methods
Study Population
A total of 197 CRT patients (CHF with New York Heart Associations [NYHA] class III–IV despite optimal medical treatment and advanced LV systolic dysfunction LV ejection fraction [LVEF] < 35%, QRS > 120 ms) who underwent biventricular device implantation in our institution between January 2003 and Jannuary 2009 were studied. After CRT device implantation all patients underwent an atrioventricular delay optimization according to maximum diastolic filling time.11 The study was approved by the institutional ethics committee. All patients gave written informed consent.
Patients with previous pacemaker implantation or atrial fibrillation were excluded. For each patient, the NYHA functional class and clinical status were assessed at baseline and 6 months after biventricular pacing device implantation. Standard and TDI echocardiography were performed, recorded, and stored for an offline analysis at baseline and 6‐month follow‐up.
Biventricular Pacemaker Implantation
Three transvenous pacing leads were inserted. The right atrial lead (for patients in sinus rhythm) and ventricular leads were positioned conventionally. The LV lead was inserted through the coronary sinus into either the lateral (93 patients, 47%) or the posterolateral cardiac vein (104 patients, 53%). A CRT‐defibrillation (CRT‐D) device was implanted in 106 patients (106/192, 56%) and a CRT device alone was present in 86 patients (86/192, 44%).
Echocardiographic Protocol
Echocardiography data were collected using commercially available equipment and transducer (Vivid 7; GE Medical Equipment, Horten, Norway). All two‐dimensional grey‐scale echocardiographic images were acquired using the second harmonic mode. Data were obtained while the subjects were at rest in a lateral decubitus position. Three cardiac cycles were stored in a cine‐loop format for an offline analysis. M‐mode measurement of left atrium diameter, right ventricular transverse diameters (RVTD), and right ventricular longitudinal diameters (RVLD) were obtained according to the recommendation of the European Society of Echocardiography.12
The following parameters were also evaluated: LV end diastolic volume (LVEDV), LV end systolic volume (LVESV), LVEF assessed by Simpson's equation,13 and left atrium area (LAA) and right atrium area evaluated from the apical 4‐chamber view at the end of systole. RV myocardial performance index (RV MPI) was calculated, as described by Tei14 as the sum of isovolumic contraction and isovolumic relaxation time divided by the ejection time. Tricuspid annular plane systolic excursion (TAPSE) was estimated by two‐dimensional echo‐guided M‐mode recordings from the apical 4‐chamber view with the cursor placed at the free wall side of the tricuspid annulus. Pulse wave (PW) Doppler pulmonary acceleration time was also evaluated as an index of pulmonary hypertension. The interventricular delay (IVD) was calculated as the difference between the aortic pre‐ejection time and the pulmonary pre‐ejection time at PW Doppler. Pulsed TDI‐derived peak early diastolic lateral mitral annular velocity (E′) and the mitral E‐wave to E′ ratio were evaluated respectively as an index of LV ventricular relaxation and filling pressure.
Two‐dimension echocardiography with TDI was performed with a 2.5 or 3.5 MHz phase array transducer for the long axis motion of the ventricles. From the apical 4‐chamber view, the 2‐chamber view, and the long axis view, a 6 basal and 6 mid‐segmental model was obtained in the LV. The time to systolic peak velocity (TS) was measured in every segment. For the TS, the beginning of the QRS complex was used as the reference point. Standard deviation of the TS (TS‐SD) was assumed as the LV dyssynchrony index.15,16
Myocardial strain curves were reconstituted offline from the TDI color images. An elliptic region of interest (ROI), 12 × 6 mm, was used with careful alignment of the cursor along the direction of the wall in any given region measured. A semiautomatic tracking algorithm was applied to maintain the sample volume in the ROI throughout the cardiac cycle. RV free wall, basal, and mid segment were evaluated (RV Bas Str, RV mid Str) from the apical 4‐chamber view. Three beats were measured and averaged for each measurement. At the 6‐month follow‐up, patients were divided into responders and nonresponders on the basis of at least 15% LVESV reduction.
Statistical Analysis
SPSS 13.0 for Windows (SPSS Inc., Chicago, IL) was used for all analyses. All values were expressed as frequencies (percentages) and mean ± standard deviation. For comparisons, χ2 and paired sample t test analysis were used. Linear regression analysis was performed to assess the relationship between RV dimension and function parameters (ie, RVTD, RVLD, RV MPI, TAPSE, RV Bas Str, and RV mid Str) at baseline and changes in end systolic volume at the 6‐month follow‐up. Receiver operating curve (ROC) analysis was performed to determine sensitivity and specificity of TAPSE and TS‐SD in predicting CRT response. A probability value of P < 0.05 was considered statistically significant.
Results
Out of a total of 197 patients initially assessed, 5 had poor echocardiographic images of the right ventricle and were excluded. Therefore, a total of 192 patients (152 males, age 69.6 ± 10.8 yrs) with QRS duration of 146 ± 33 ms were included in the study. Underling heart failure etiology was ischemic in 98 patients (98/192, 51%), and nonischemic in 94 patients (94/192, 49%). No differences were observed in baseline and follow‐up RV function between CRT and CRT‐Dpatients.
At the 6‐month follow‐up, patients were divided into responders (86/192, 45%) to LV reverse remodeling with reduction of at least 15% LVESV, and nonresponders (106/192, 55%) in whom the reduction in LVESV was < 15%. No differences were observed in cardiomyopathy etiology between responder and nonresponder patients. Comparing baseline echocardiographic parameters in both responders and nonresponders, LV volumes were significantly larger, and both interventricular and intraventricular dyssynchrony indexes were significantly higher in the former. Moreover, TAPSE was significantly lower in the latter, whereas no differences were observed for either RV dimension or other RV functional parameters or mean pulmonary artery pressure (Supporting Information). LV systolic and diastolic function were similar in the two groups as well. No difference was observed between the two groups regarding ischemic or nonischemic disease.
At the 6‐month follow‐up, LV volumes and LAA were significantly decreased in responders when compared with baseline, whereas in nonresponders LVESV and LVEDV were significantly further dilated.
In responder patients, LV reverse remodeling was associated with a significant reverse remodeling of the RV with an improvement of all RV function indexes. Moreover, a significant reduction in pulmonary acceleration time was observed, suggesting a reduction in pulmonary artery pressure. A significant reduction in TS‐SD and IVD, indicating an improvement of interventricular and LV intraventricular dyssynchrony, were observed only in the responder group. In nonresponders, all these parameters remained unchanged, with the exception of RV MPI, which improved significantly.
A significant reduction in NYHA class and mitral regurgitation degree was observed in both groups (Supporting Information).
RV Baseline Function and CRT Response
In the overall population, linear regression analysis showed a significant correlation between changes in LVESV and baseline TAPSE (R = 0.20, P = 0.03), whereas no correlation was observed between other RV functional parameters or RV dimension and CRT reverse remodeling. As previously reported in the literature, a significant correlation between TS‐SD and changes in LVESV were observed (R = 0.21, P = 0.005).
ROC curve analysis was performed to define the appropriate cutoff value for baseline TAPSE. An optimal TAPSE cutoff value of 17 mm yielded sensitivity of 64% and specificity of 60% (area under the curve 0.63; 95% confidence interval [CI], 0.53–0.71; P = 0.009). Similar predictive values were achieved by TS‐SD. In fact, in our population, a baseline TS‐SD with an optimal cutoff of 41.25 ms yielded a sensitivity of 60% and a specificity of 62% (area under the curve 0.65; 95% CI, 0.56–0.73; P = 0.001). On the contrary, at baseline a QRS duration cutoff value of 145 ms yielded sensitivity of 50% and specificity of 58% (area under the curve 0.52; 95% CI, 0.43–0.60; P = 0.51) (Figure 1).
Figure 1.
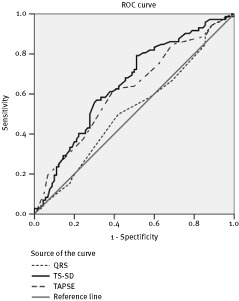
The receiver operating characteristics (ROC) for identification of left ventricular reverse remodeling in heart failure patients, for tricuspidal annular plane systolic excursion (TAPSE) (area under the curve 0.63; 95% confidence interval [CI], 0.53–0.71; P = 0.009), time to systolic peak velocity‐standard deviation (TS‐SD) (area under the curve 0.63; 95% CI, 0.55–0.70; P = 0.002), and QRS duration (area under the curve 0.52; 95% CI, 0.43–0.60; P = 0.51)
At baseline, out of 192 subjects, 96 showed a TAPSE value < 17 mm. Among them, only 30 subjects (30/96, 32%) resulted as responders at 6 months' follow‐up, whereas among the patients with a baseline TAPSE ≥ 17 mm, 56 subjects resulted as CRT responders at 6 months' follow‐up (56/96, 58%; P < 0.001) (Figure 2A).
Figure 2.
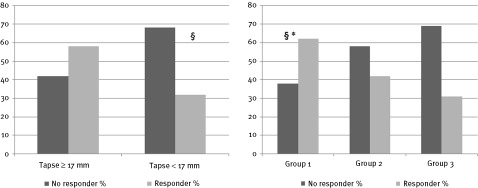
(A) Response to cardiac resynchronization therapy. Distribution of 6 months' follow‐up of left ventricular (LV) reverse remodeling cardiac resynchronization therapy (CRT) responders and nonresponders among patients with baseline tricuspidal annular plane systolic excursion (TAPSE) ≥ 17 mm and TAPSE < 17 mm. §P < 0.001. (B) Distribution of 6 months' follow‐up of LV reverse remodeling CRT responders and nonresponders among group 1 (TAPSE ≥ 17 mm, time to systolic peak velocity‐standard deviation [TS‐SD] ≥ 41 ms), group 2 (TAPSE < 17 mm, TS‐SD ≥ 41 ms, or TAPSE ≥ 17 mm and TS‐SD < 41 ms), group 3 (TAPSE < 17 mm, TS‐SD < 41 ms). P < 0.001 group 1 vs group 3. *P < 0.05 group 1 vs group 2
Moreover, the best 6‐month follow‐up response rate (32/52, 62%) was achieved in patients in whom a TS‐SD ≥ 41.25 ms was combined with a TAPSE ≥ 17 mm. Patients with a low asynchrony index and reduced RV function (TS‐SD < 41.25 ms and TAPSE < 17 mm) achieved the worst response rate (13/42, 31%). Patients with a high LV dyssynchrony and reduced RV function (TS‐SD ≥ 41.25 ms and TAPSE < 17 mm) or patients with low LV dyssynchrony index and preserved RV function (TS‐SD < 41.25 ms and TAPSE ≥ 17 mm) achieved an intermediate response rate (41/98, 42%) (Figure 2B).
Discussion
The main findings of our study were that CRT induces reverse remodeling of both the left and the right ventricles and that this double effect can be predicted by LV dyssynchrony index and by TAPSE value. Moreover, the maximum rate of CRT response is achieved in those patients in whom high values of LV dyssynchrony are combined with high values of TAPSE.
These results emphasize the presence of a close relationship between LV dyssynchrony, LV function, and RV function.
There is evidence of a ventricular interaction by which LV contraction significantly affects RV systolic function. Experimental studies have shown that about 20% to 40% of the RV systolic pressure and volume outflow result from LV contraction.17
Oboler et al in 1973,18 by recording high‐fidelity pressure from the left and right ventricles of anesthetized dogs, demonstrated that the derivative of the RV pressure is composed of the intrinsically generated pressure plus the LV component.
This LV contribution to the RV systolic function occurs during the isovolumetric contraction phase when the rapid increase of pressure, developed by the left ventricle, is transmitted through the septum to the right ventricle. Systolic coupling between the ventricles, and thus LV assistance, strictly depends on the synchronism of the left and RV contraction, which is depressed when the presence of conduction disturbances produces a delay of the LV contraction, as in patients treated with CRT. In our patients, RV and LV reverse remodeling with systolic functional parameters improvement were closely associated and were achieved only in those patients in whom CRT had induced recovery of both interventricular and intraventricular synchrony.
It has been demonstrated that RV systolic function is not the sole determinant of TAPSE because it depends not only on RV systolic function but also on LV systolic function.19 Moreover, in a group of patients admitted to the intensive care unit for respiratory or circulatory failure, changes in TAPSE following dynamic interventions have been shown to be linearly related to changes in LVEF rather than to changes in RV functional parameters.20 Interestingly, Gupta et al,21 in a cohort of heart failure patients, showed that TAPSE was inversely correlated with invasive and echocardiographic measures of dyssynchrony. The same authors observed that TAPSE had the highest predictive value of LV dyssynchrony when taking into consideration other parameters of RV systolic function, such as RV myocardial performance index or RV ejection fraction. Considering this inverse relation between TAPSE and LV dyssynchrony, it might be surprising that low rather than high values of TAPSE are associated with a poor response of CRT, because LV dyssynchrony should be the crucial premise for CRT action.
A low TAPSE value is likely to be a marker of a severe stage of heart failure, where this index could be principally affected by the structural RV myocardial involvement. This therapy has been demonstrated less efficacious in very advanced stages of heart failure.22 Concordantly, our data show that in patients with high TAPSE values, CRT is more effective and produces beneficial effects in both the left and the right ventricles.
Few studies have already described the effects of CRT on RV functional parameters. Bleeker et alshowed improvement in RV dimensions and function in patients with heart failure after 6 months of CRT.9 Moreover, Donal et al8 demonstrated that a significant increase in TDI‐derived RV peak systolic velocity was induced by biventricular pacing compared to acute atrial, RV, and lone LV pacing. Rajagopalan et al10 confirmed this observation by demonstrating an increased TDI‐derived RV peak systolic velocity at midterm follow‐up after CRT. Recently, Scuteri et al23 demonstrated that a baseline reduced TAPSE value in patients treated with CRT was associated with a poor response and adverse prognosis.
Conclusion
Our study confirms and expands all of these observations demonstrating that CRT induces a significant RV reverse remodeling only in those patients in whom an LV reverse remodelling was achieved, and that by combining TAPSE and LV dyssynchrony index evaluation, improvement in the selection of patients could be obtained. Further studies with longer follow‐up might confirm our findings. Thus, TAPSE and LV dyssynchrony evaluation should be taken into account when considering resynchronization as they may be determining factors of the patient's overall improvement.
REFERENCES
- 1. Dickstein K, Cohen‐Solal A, Filippatos G, et al ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Eur Heart J 2008; 29: 2388–2442. [DOI] [PubMed] [Google Scholar]
- 2. Strickberger SA, Conti J, Daoud EG, et al; Council on Clinical Cardiology Subcommittee on Electrocardiography and Arrhythmias and the Quality of Care and Outcomes Research Interdisciplinary Working Group; Heart Rhythm Society. Patient selection for cardiac resynchronization therapy: from the Council on Clinical Cardiology Subcommittee on Electrocardiography and Arrhythmias and the Quality of Care and Outcomes Research Interdisciplinary Working Group, in collaboration with the Heart Rhythm Society. Circulation 2005; 111: 2146–2150. [DOI] [PubMed] [Google Scholar]
- 3. Leclercq C, Kass DA. Retiming the failing heart: principles and current clinical status of cardiac resynchronization. J Am Coll Cardiol 2002; 39: 194–201. [DOI] [PubMed] [Google Scholar]
- 4. Cazeau S, Leclercq C, Lavergne T, et al; Multisite Stimulation in Cardiomyopathies (MUSTIC) Study Investigators. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med 2001; 344: 873–80. [DOI] [PubMed] [Google Scholar]
- 5. Abraham WT, Fisher WG, Smith AL, et al; Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002; 346: 1845–1853. [DOI] [PubMed] [Google Scholar]
- 6. Bradley DJ, Bradley EA, Baughman KL, et al Cardiac resynchronization and death from progressive heart failure a meta‐analysis of randomized controlled trials. JAMA 2003; 289: 730–740. [DOI] [PubMed] [Google Scholar]
- 7. Cleland JGF, Daubert JC, Erdmann E, et al; Cardiac Resynchronization‐Heart Failure (CARE‐HF) Study Investigators. The effect of cardiac resynchronization therapy on morbidity and mortality in heart failure [the Cardiac Resynchronization‐Heart Failure (CARE‐HF) Trial]. N Eng J Med 2005; 352: 1539–1549. [DOI] [PubMed] [Google Scholar]
- 8. Donal E, Vignat N, De Place C, et al Acute effects of biventricular pacing on right ventricular function assessed by tissue Doppler imaging. Europace 2007; 9: 108–112. [DOI] [PubMed] [Google Scholar]
- 9. Bleeker GB, Schalij MJ, Nihoyannopoulos P, et al Left ventricular dyssynchrony predicts right ventricular remodeling after cardiac resynchronization therapy. J Am Coll Cardiol 2005; 46: 2264–2269. [DOI] [PubMed] [Google Scholar]
- 10. Rajagopalan N, Suffoletto MS, Tanabe M, et al A right ventricular function following cardiac resynchronization therapy. Am J Cardiol 2007; 100: 1434–1436. [DOI] [PubMed] [Google Scholar]
- 11. Kindermann M, Frohlig G, Doerr T. Optimizing the AV delay in DDD pacemaker patients with high degree AV block: mitral valve Doppler vs. impedance cardiography. Pacing Clin Electrophysiol 1997; 20: 2453–2462. [DOI] [PubMed] [Google Scholar]
- 12. Lang RM, Bierig M, Devereux RB, et al; American Society of Echocardiography's Nomenclature and Standards Committee; Task Force on Chamber Quantification; American College of Cardiology Echocardiography Committee; American Heart Association; European Association of Echocardiography, European Society of Cardiology. Recommendations for chamber quantification. Eur J Echocardiogr 2006; 7: 79–108. [DOI] [PubMed] [Google Scholar]
- 13. Schiller NB. Two‐dimensional echocardiographic determination of left ventricular volume, systolic function and mass. Summary and discussion of the 1989 recommendations of the American Society of Echocardiography. Circulation 1991; 84(suppl 1): 280–287. [PubMed] [Google Scholar]
- 14. Tei C. New non‐invasive index for combined systolic and diastolic function. J Cardiol 1995; 26: 135–136. [PubMed] [Google Scholar]
- 15. Yu CM, Fung WH, Lin H, et al Predictors of left ventricular reverse remodeling after cardiac resynchronization therapy for heart failure secondary to idiopathic dilated or ischemic cardiomyopathy. Am J Cardiol 2003; 91: 684–688. [DOI] [PubMed] [Google Scholar]
- 16. Yu CM, Fung JW, Zhang Q, et al Tissue Doppler imaging is superior to strain rate imaging and postsystolic shortening on the prediction of reverse remodeling in both ischemic and nonischemic heart failure after cardiac resynchronization therapy. Circulation 2004; 110: 66–73. [DOI] [PubMed] [Google Scholar]
- 17. Santamore WP, Dell'Italia LJ. Ventricular interdependence: significant left ventricular contributions to right ventricular systolic function. Prog Cardiovasc Dis 1998; 40: 289–308. [DOI] [PubMed] [Google Scholar]
- 18. Oboler AA, Keefe JF, Gaasch WH, et al Influence of left ventricular isovolumic pressure upon right ventricular pressure transients. Cardiology 1973; 58: 32–44. [DOI] [PubMed] [Google Scholar]
- 19. Lopez‐Candales A, Rajagopalan N, Saxena N, et al Right ventricular systolic function is not the sole determinant of tricuspid annular motion. Am J Cardiol 2006; 98: 973–977. [DOI] [PubMed] [Google Scholar]
- 20. Lamia B, Teboul JL, Monnet X, et al Relationship between the tricuspid annular plane systolic excursion and right and left ventricular function in critically ill patients. Intensive Care Med 2007; 33: 2143–2149. [DOI] [PubMed] [Google Scholar]
- 21. Gupta S, Khan F, Shapiro M, et al The associations between tricuspid annular plane systolic excursion (TAPSE), ventricular dyssynchrony, and ventricular interaction in heart failure patients. Eur J Echocardiogr 2008; 9: 766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Bommel RJ, Bax JJ, Abraham WT, et al Characteristics of heart failure patients associated with good and poor response to cardiac resynchronization therapy: a PROSPECT (Predictors of Response to CRT) sub‐analysis. Eur Heart J 2009; 30: 2470–2477. [DOI] [PubMed] [Google Scholar]
- 23. Scuteri L, Rordorf R, Marsan NA, et al Relevance of echocardiographic evaluation of right ventricular function in patients undergoing cardiac resynchronization therapy. Pacing Clin Electrophysiol 2009; 32: 1040–1049. [DOI] [PubMed] [Google Scholar]


