Abstract
Background
Patients with greater adiposity prior to lung transplantation are at an increased risk for worse post-transplant outcomes. Few studies have examined whether pre-transplant weight loss mitigates this risk. We examined the association between pre-transplant weight loss and post-transplant clinical outcomes.
Methods
We conducted a retrospective cohort study of patients who received a lung transplant at Duke University Hospital from May 1, 2005 to April 30, 2015. The sample included adult transplant recipients with restrictive, obstructive, and vascular diseases. Cox proportional hazards models were used to examine mortality and chronic lung allograft dysfunction (CLAD)-free survival and negative binomial regression analyses were used to examine length of stay (LOS). Weight loss was assessed from change in body mass index (BMI).
Results
The cohort consisted of 810 individuals. Initially 403 (50%) were overweight and 109 (13%) were obese by BMI criteria. Greater pre-transplant weight loss was associated with dose-response improvements in survival (HR = 0.83 [0.72, 0.97], P = .018), with modest (0–3%: HR = 0.91), moderate (7–10%: HR = 0.83), and large (>15%: HR = 0.71) levels of weight loss conferring longer survival, independent of initial weight (P = 0.533 for interaction). Weight loss was also associated with improved CLAD-free survival (HR = 0.84 [0.71, 0.99], P=.034) and shorter LOS (b = −0.17, P <.001).
Conclusions
Weight loss before transplantation was associated with improved short- and long-term clinical outcomes, independent of initial weight. Survival improved proportionally to percentage weight lost. Mechanisms by which weight loss improves clinical outcomes warrant further exploration.
Introduction
Lung transplantation remains an increasingly more common treatment option to extend survival among many individuals with advanced pulmonary disease (1, 2). Despite advances in lung transplantation, median survival remains approximately 6 years, far lower than other solid organ transplant groups (1, 3). Research over the past decade has emphasized the role of potentially modifiable risk factors including body composition (9–11) for improvement of post-transplant clinical outcomes (4–8). Specific to lung transplant, studies have demonstrated an association between recipient obesity at the time of transplant and poorer clinical outcomes, leading many transplant centers to consider a BMI >30 as a relative and a BMI>35 an absolute contraindication to lung transplantation (12–16), consistent with current ISHLT guidelines (17).
Many lung transplant recipients meet overweight or obese criteria, particularly those with pulmonary fibrosis (PF) and other non-cystic fibrosis native diseases (9, 18, 19). United Network for Organ Sharing (UNOS) data suggest that more than 12% of recipients were obese and 32% overweight at the time of transplant (12). In prior studies, obese patients consistently demonstrated poorer clinical outcomes, including greater postoperative graft dysfunction (19), reduced functional status (20), and increased mortality (13). Work has been done to explore the underlying mechanisms for the effects of obesity on lung transplant survival and cytokines produced by adipose cells have drawn interest as potential mediators (9, 21). Adipocytokines influence levels of pro-inflammatory and anti-inflammatory cytokines involved in ischemia-reperfusion injury (22, 23) including PGD (24).
Despite recent data demonstrating the importance of pre-transplant BMI for clinical risk, few studies have examined whether pre-transplant weight loss can modify subsequent clinical outcomes in a large cohort of transplant recipients (11, 25). We therefore sought to examine the effect of pre-transplant weight loss among lung transplant candidates on clinical outcomes including perioperative length of stay (LOS), development of chronic lung allograft dysfunction (CLAD), and mortality.
Methods
Patient Identification
The study included adults who underwent lung transplantation at Duke University Hospital from May 1, 2005 to April 30, 2015. The initiation of our study was set to correspond with introduction of the lung allocation score (LAS) in organ distribution. Our center’s current practice recommends a BMI goal ≤ 27 and ≥ 18.5, though this policy is not absolute and those with a BMI outside this range may still undergo transplantation. Our center strongly recommends any candidate with BMI ≥30 kg/m2 to lose weight before proceeding with listing. Analyses excluded those who underwent multi-organ transplantation, retransplantation, candidates with cystic fibrosis (CF), and those with either no BMI data within three months of transplant or who were underweight BMI ≤ 18.5. The rationale for excluding those with CF is there is a tendency to be underweight in this population and weight loss is generally discouraged. This study was approved by the Institutional Review Board at Duke University Medical Center.
Data Collection
We abstracted data from the lung transplantation database at Duke Hospital and Duke data submitted to United Network for Organ Sharing (UNOS) including: demographic data, native lung disease (pulmonary fibrosis (PF), chronic obstructive pulmonary disease (COPD), CF, and other lung disease), transplant date, LAS, highest pre-transplant BMI (defined as highest weight within 1 year prior to transplant surgery or BMI at initial transplant evaluation), BMI on day of transplant surgery, LOS immediately post-transplant, albumin levels, and pulmonary function tests (PFTs). BMI categories were defined by World Health Organization (WHO) classification with underweight <18.5, normal weight 18.5–25, overweight 25–30, and obesity >30 (26). PGD was defined as acute lung injury at 72 hours with severity grading according to ISHLT guidelines (27). Analysis was limited to PGD grade 3. In order to qualify for CLAD assessment, an individual had to survive a minimum of 90 days after transplant and have five separate PFTs performed at least 3 weeks apart. CLAD was defined as irreversible 20% decline in the FEV1 relative to the highest post-transplant baseline, found on two separate PFTs at least 3 weeks apart without any other attributable cause(28). Expert review then validated CLAD by medical record review and determined CLAD phenotype between bronchiolitis obliterans syndrome (BOS) or restrictive allograft syndrome (RAS). Missing data points were not imputed.
Statistical Analysis
Statistical analyses used SAS 9.4 and R 3.4.1. All analyses controlled for the following variables: initial weight which was defined as participants maximum weight within the year preceding transplant, percent weight loss ([Initial weight – weight at transplant] / Baseline weight), albumin at time of transplant, native disease, gender, age, PGD, and ethnicity. We also controlled for type of transplantation (bilateral vs. unilateral) in our length of stay (LOS) and survival analyses. LOS analyses used generalized estimating equations with a negative binomial error distribution specification with those that died while in the hospital being assigned the longest LOS in the cohort. Time to death was analyzed using the Cox proportional hazard model. Univariate analyses for predictors of mortality and LOS were performed for age, albumin level, bilateral versus single lung transplant, PF versus COPD, sarcoid versus COPD, other diagnosis versus COPD, gender, race, PGD, pre-transplant BMI, and percent weight lost. Because weight loss was potentially confounded with pre-transplant initial weight (e.g. heavier patients may have lost more weight) we also conducted analyses in which pre-transplant BMI was modeled as an individual predictor. The association between weight loss and PGD (grade 3 at 72 hours) was examined using general linear models, with the same covariates as above. Analysis focused on PGD grade 3 consistent with prior studies (21). Assumptions regarding linearity, independence, and proportional hazards were evaluated and found to be satisfactory prior to analysis. We also tested for potential non-linear associations using the restricted cubic spline function available in Harrell’s RCS package in R.
Results
Pre-Transplant Weight and Weight Loss
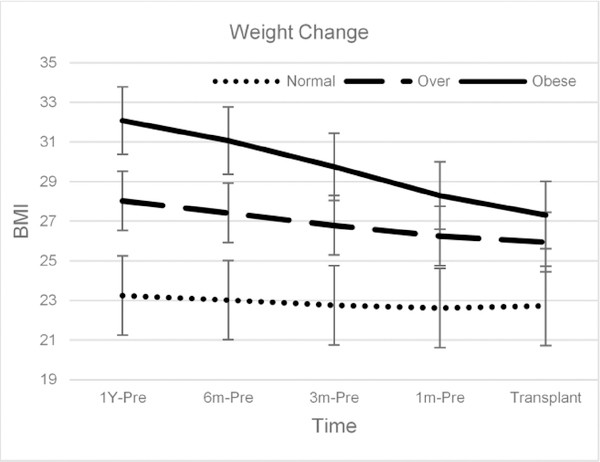
Demographic and clinical characteristics are presented in Table 1 for the total cohort of 810 individuals. The sample included more males (534, 66%), tended to be older (mean age = 60.5 [9.8] years), and white (n=710, 88%). The most common native lung disease was PF (n = 454 [56%]), followed by COPD (n = 231 [29%]), and ‘Other’ disease classifications (e.g. sarcoidosis, PPH, etc.; n = 125 [15%]). LAS scores ranged widely, with a median of 41.9 (mean = 47.7 [SD = 16.5]; range 29.0, 95.1). Participants were slightly above the normal range in BMI on average (mean BMI = 26.3 [SD = 3.6]), with over half of recipients being either overweight (n = 403 [50%]) or obese (n = 109 [13.5%]) at baseline. In this cohort, a very small subset (5%) had a BMI of ≥ 29.0 kg/m2 (range 19.0 to 36.3 kg/m2). Baseline weight was recorded on average 4.8 months pre-transplant [SD = 4]. At baseline, there were substantial differences in BMI levels across native diseases (P<.001), with PF patients weighing the most (mean BMI = 27.3 [27.0, 27.6)]) compared with both COPD (mean BMI = 24.9 [24.5, 25.3]) and ‘other’ native diseases (mean BMI = 25.0 [24.4, 25.5]).
Table 1.
Background and clinical characteristics.
| Variable | |
|---|---|
| Age, years, m (SD) | 60.5 (9.8) |
| Native Disease, n (% total cohort) | |
| Pulmonary Fibrosis | 454 (56) |
| Chronic Obstructive Pulmonary Disease | 231 (29) |
| Other | 125 (15) |
| Gender, Male, n (% total cohort) | 534 (66) |
| Bilateral Transplant, n (% total cohort) | 612 (76) |
| Race, Caucasian, n (% total cohort) | 710 (88) |
| LAS, m (SD) | 47.7 (16.5) |
| PGD Grade 3 at 72 hours, n (% total cohort) | 60 (7) |
| Albumin, g/dL, m (SD) | 3.8 (0.4) |
| Baseline BMI, m (SD) | 26.3 (3.6) |
| Pre-Transplant BMI, m (SD) | 25.1 (2.5) |
| Weight Loss, %, m (SD) | 3.9 (6.6) |
BMI=body mass index, PGD=primary graft dysfunction, LAS=lung allocation score, m=mean, SD=standard deviation, n=number participants
Examination of pre-transplant weight changes revealed substantial reductions prior to transplant surgery (median BMI reduction 2.6% [IQR = 0, 7.4%], P<.001). Out of the cohort, 22% gained weight, 5 % had no change in weight, and 73% lost weight. For those that lost weight, the average weight loss was 3.9% (SD=6.6). Examination of predictors of weight loss demonstrated that baseline weight (b = 1.25, P<.001) and female gender (−1.89, P<.001) were the strongest predictors of weight reduction. Changes in weight are shown in Figure 1.
Figure 1.
Weight trends across BMI categories before lung transplant.
Change in BMI and Survival
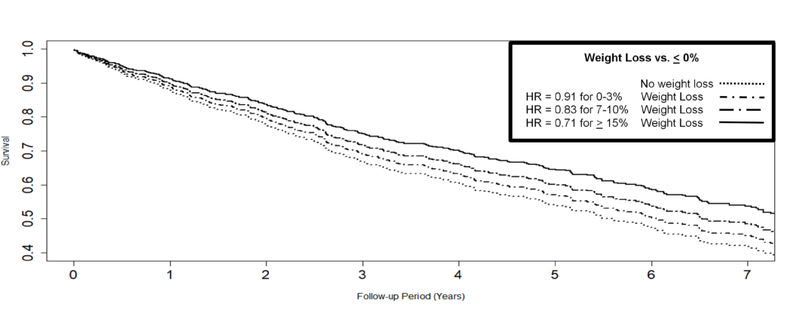
Over a median 6.1 year follow-up (range 0–9.5), 384 participants died (47%). Results demonstrated that weight loss was associated with improved survival (HR = 0.84 [0.72, 0.97] per 10% decrease initial BMI, P=.021). Greater percent weight loss was associated with dose-response improvements in survival: modest (0–3% decrease initial BMI: HR = 0.91), moderate (7–10% decrease baseline BMI: HR = 0.83), and large (>15% decrease initial BMI: HR = 0.71). Weight loss was protective regardless of initial BMI (P = 0.533 for interaction). For example, individuals in the lower IQR for weight loss (HR = 0.69), median (HR = 0.68), and upper IQR for weight loss (HR = 0.67) of initial weight did not experience differential survival. In addition, older age (HR = 1.39 [1.18, 1.65], P<.01), lower albumin (HR = 0.82 [0.73, 0.92], P = .002), and PGD (HR = 2.09 [1.42, 3.07], P <.001) also were predictive of poorer survival shown in Table 2.
Table 2.
Univariate Predictors of mortality following transplantation.
| Predictor (IQR) | Hazard Ratio | 95% CI | P-value |
|---|---|---|---|
| Age (12 years) | 1.39 | 1.18, 1.65 | <0.001 |
| Albumin (0.5 g/dL) | 0.82 | 0.73, 0.92 | <0.01 |
| Transplant Type | 1.07 | 0.80, 1.43 | 0.662 |
| PF vs. COPD | 1.24 | 0.93, 1.65 | 0.141 |
| Sarcoid vs. COPD | 1.13 | 0.64, 2.00 | 0.661 |
| ‘Other vs. COPD’ | 1.39 | 0.92, 2.10 | 0.114 |
| Gender | 1.07 | 0.85, 1.33 | 0.572 |
| Race | 1.08 | 0.74, 1.57 | 0.696 |
| PGD | 2.09 | 1.42, 3.07 | <0.001 |
| Pre-transplant BMI (3.3) | 1.00 | 0.87, 1.16 | 0.965 |
| Weight loss, (%10) | 0.84 | 0.72, 0.97 | 0.021 |
PF, pulmonary fibrosis; COPD, chronic obstructive pulmonary disease; PGD, primary graft dysfunction; BMI, body mass index
Predictors of mortality following transplantation. Continuous predictors were scaled using the interquartile range (IQR), with the exception of weight loss, which was scaled in 10% units.
Change in BMI and CLAD-free Survival
Over the same follow-up time period, 279 participants either developed CLAD (n = 238) or died before 90 days post-transplant (15%). Among the confirmed 238 CLAD cases, 153 (64%) were BOS and 85 (36%) were RAS. Results demonstrated that greater weight loss was associated with improved CLAD-free survival (HR = 0.84 [0.71, 0.99] per 10%, P=.034). Weight loss was not associated with whether a recipient developed BOS versus CLAD (mean BMI decrease BOS 3.7 % (6.3), mean BMI decrease RAS 5.1 % (7.0), p=.118). When individuals who died before 90 days were excluded, this effect was attenuated (HR = 0.87 [0.72, 1.03], P = .109), suggesting that this association was partly driven by individuals with early mortality.
BMI, LOS, and PGD
Following transplantation, participants were hospitalized for a median of 16 days (IQR = 11, 28), while 34 participants died during hospitalization. Sixty participants (7.4%) developed grade 3 PGD following transplantation. Having a greater BMI at time of transplant surgery was not significantly associated with longer LOS (p=0.851), although greater BMI at the time of transplant tended to be associated with a greater likelihood of PGD grade 3(P = .115). We found that greater weight loss was associated with a lower incidence of PGD following transplant (P = .046). Specifically, individuals with PGD at 72 hours had lesser weight loss compared with participants without PGD (0.9 kg / m2 or 2.6% [1.1, 4.2] vs. 1.3 kg / m2 or 4.2% [3.7, 4.6]). Within our model, lower age (b = 0.11, P<.017), greater albumin (b = −0.2, P <.001), single-lung transplant (0.70, P<.001), male gender (−0.21, P= .010), COPD native disease (P<.01 compared with PF and ‘Other’), PGD grade 3 at 72 hours (b = 0.39, P <.001) and greater weight loss (b = −0.17, P <.001) were associated with shorter LOS (Supplemental Table 3).
Sensitivity Analyses Incorporating Functional Capacity
A subset of 238 patients had 6MWD available for analysis (mean 6MWD = 1375 ft. [IQR = 1175, 1557]). Sensitivity analyses were conducted to determine the influence of pre-transplant functional capacity on the observed associations between pre-transplant weight loss and clinical outcomes. Controlling for 6MWD as an additional predictor did not alter the observed pattern of findings, and weight loss remained predictive of post-transplant mortality (HR = 0.63 [0.40, 1.00], P = .048), CLAD-free survival (HR = 0.53 [0.32, 0.91], P = .020), and LOS (b = −0.36, P <.001).
Discussion
Our study suggests that in candidates who are not underweight at the time of initial transplant evaluation, greater weight loss in all other BMI categories is associated with improved short- and long-term clinical outcomes among lung transplant recipients. We found that over half of our recipients were overweight or obese before transplant, and that the majority of recipients lost substantial weight prior to transplant, likely in the context of intensive pulmonary rehabilitation that is mandated during this time period. Most notably, the beneficial effects of weight loss were dose-dependent and persisted after controlling for weight at the time of transplantation or functional status, suggesting a unique contribution of weight loss in improving clinical outcomes.
The finding that weight loss prior to transplant may be associated with improved outcomes in some lung transplant patients has been shown previously (11). Chandrashekaran and colleagues previously found that weight loss prior to lung transplantation was associated with improved survival among patients with a BMI over 25(11). Our study extends these findings by 1) replicating this association in a much larger and more heterogeneous sample, 2) extending the breadth of our clinical outcomes (e.g. incorporating LOS and CLAD-free survival), and 3) patients were required to attend intensive pulmonary rehabilitation and have formal nutritional consultation prior to transplantation. Moreover, by controlling for additional confounders, such as pre-transplant albumin and 6MWD, we were able to better isolate the independent effects of weight loss, whereas weight loss in the previously reported study may have been influenced by unintentional weight loss or confounded by functional status gains (11).
Our finding that weight loss was associated with improved clinical outcomes regardless of initial weight is novel and warrants further investigation. Previous studies have demonstrated that patients who are either obese or underweight at the time of transplantation are at elevated risk of poor post-transplant clinical outcomes (9, 19). Recent studies have extended these findings to demonstrate that components of metabolic syndrome likely explain some of association observed between obesity and poor clinical outcomes (5). It is plausible that through dietary modification and exercise in the context of pulmonary rehabilitation, patients who lost weight in our cohort exhibited improved clinical outcomes through improved metabolic function (29).
Our study must be viewed with several limitations in mind. First, the present results were based on a single-center, retrospective cohort. Second, we used BMI to quantify weight loss, which does not allow for the delineation of regional body composition or the characterization of informative clinical phenotypes, such as sarcopenia. In addition, many of our participants likely experienced variations in weight due to concomitant muscle gain, which could paradoxically confound any analysis of weight loss and clinical outcomes (30). Future studies would therefore benefit from a more refined assessment of body composition characteristics. There are numerous potential surgical and intraoperative confounders that may have influenced the present pattern of results, but were beyond the scope of this study. Future studies may therefore benefit from a systematic examination of additional mechanisms that may have influenced the present pattern of findings. Lastly, future studies would benefit from examining the impact of pre-transplant weight loss on additional, clinically-relevant outcomes, including quality of life and post-transplant functional status.
In conclusion, our study suggests that pre-transplant weight loss improves clinical outcomes following lung transplantation. Future studies would benefit from more refined examination of metabolic mechanisms explaining the observed associations, such as leptin and adiponectin levels both before and after lung transplant. If replicated, these findings could have important implications for the management of pre-transplant candidates.
Supplementary Material
Figure 2.
Greater pre-transplant weight loss was associated with dose-response improvements in survival.
Acknowledgments
The project described was supported by Grant Number 5T32HL007538-32 from NIH awarded to Emily Siu Clausen. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. There are no other authors’ conflict of interests or financial disclosures to acknowledge.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Valapour M, Skeans MA, Smith JM, Edwards LB, Cherikh WS, Uccellini K, Israni AK, Snyder JJ, Kasiske BL. OPTN/SRTR 2015 Annual Data Report: Lung. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2017; 17 Suppl 1: 357–424. [DOI] [PubMed] [Google Scholar]
- 2.Scarborough JE, Bennett KM, Davis RD, Lin SS, Tracy ET, Kuo PC, Pappas TN. Temporal trends in lung transplant center volume and outcomes in the United States. Transplantation 2010; 89: 639–643. [DOI] [PubMed] [Google Scholar]
- 3.Vock DM, Durheim MT, Tsuang WM, Finlen Copeland CA, Tsiatis AA, Davidian M, Neely ML, Lederer DJ, Palmer SM. Survival Benefit of Lung Transplantation in the Modern Era of Lung Allocation. Ann Am Thorac Soc 2017; 14: 172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diamond JM, Lee JC, Kawut SM, Shah RJ, Localio AR, Bellamy S, Lederer DJ, Cantu E, Kohl BA, Lama VN, Bhorade S, Crespo M, Demissie E, Sonnett J, Wille K, Orens J, Shah AS, Weinacker A, Arcasoy S, Shah PD, Wilkes DS, Ware LB, Palmer SM, Christie JD. Clinical Risk Factors for Primary Graft Dysfunction after Lung Transplantation. Am J Resp Crit Care 2013. [Google Scholar]
- 5.Singer JP, Diamond JM, Gries CJ, McDonnough J, Blanc PD, Shah R, Dean MY, Hersh B, Wolters PJ, Tokman S, Arcasoy SM, Ramphal K, Greenland JR, Smith N, Heffernan P, Shah L, Shrestha P, Golden JA, Blumenthal NP, Huang D, Sonett J, Hays S, Oyster M, Katz PP, Robbins H, Brown M, Leard LE, Kukreja J, Bacchetta M, Bush E, D’Ovidio F, Rushefski M, Raza K, Christie JD, Lederer DJ. Frailty Phenotypes, Disability, and Outcomes in Adult Candidates for Lung Transplantation. Am J Respir Crit Care Med 2015; 192: 1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castleberry AW, Englum BR, Snyder LD, Worni M, Osho AA, Gulack BC, Palmer SM, Davis RD, Hartwig MG. The utility of preoperative six-minute-walk distance in lung transplantation. Am J Resp Crit Care 2015; 192: 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castleberry AW, Englum BR, Snyder LD, Worni M, Osho AA, Pietrobon RS, Palmer SM, Davis RD, Hartwig MG. Utility of Six-Minute Walk Distance in Predicting Outcomes after Lung Transplant: A Nationwide Survival Analysis. J Heart Lung Transpl 2013; 32: S147-S147. [Google Scholar]
- 8.Martinu T, Babyak MA, O’Connell CF, Carney RM, Trulock EP, Davis RD, Blumenthal JA, Palmer SM, Investigators I. Baseline 6-min walk distance predicts survival in lung transplant candidates. Am J Transplant 2008; 8: 1498–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singer JP, Peterson ER, Snyder ME, Katz PP, Golden JA, D’Ovidio F, Bacchetta M, Sonett JR, Kukreja J, Shah L, Robbins H, Van Horn K, Shah RJ, Diamond JM, Wickersham N, Sun L, Hays S, Arcasoy SM, Palmer SM, Ware LB, Christie JD, Lederer DJ. Body composition and mortality after adult lung transplantation in the United States. Am J Respir Crit Care Med 2014; 190: 1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Didsbury M, McGee RG, Tong A, Craig JC, Chapman JR, Chadban S, Wong G. Exercise training in solid organ transplant recipients: a systematic review and meta-analysis. Transplantation 2013; 95: 679–687. [DOI] [PubMed] [Google Scholar]
- 11.Chandrashekaran S, Keller CA, Kremers WK, Peters SG, Hathcock MA, Kennedy CC. Weight loss prior to lung transplantation is associated with improved survival. J Heart Lung Transplant 2015; 34: 651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen JG, Arnaoutakis GJ, Weiss ES, Merlo CA, Conte JV, Shah AS. The impact of recipient body mass index on survival after lung transplantation. J Heart Lung Transplant 2010; 29: 1026–1033. [DOI] [PubMed] [Google Scholar]
- 13.Lederer DJ, Wilt JS, D’Ovidio F, Bacchetta MD, Shah L, Ravichandran S, Lenoir J, Klein B, Sonett JR, Arcasoy SM. Obesity and underweight are associated with an increased risk of death after lung transplantation. Am J Respir Crit Care Med 2009; 180: 887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Culver DA, Mazzone PJ, Khandwala F, Blazey HC, Decamp MM, Chapman JT, Group CCFLT. Discordant utility of ideal body weight and body mass index as predictors of mortality in lung transplant recipients. J Heart Lung Transplant 2005; 24: 137–144. [DOI] [PubMed] [Google Scholar]
- 15.Kanasky WF Jr., Anton SD, Rodrigue JR, Perri MG, Szwed T, Baz MA. Impact of body weight on long-term survival after lung transplantation. Chest 2002; 121: 401–406. [DOI] [PubMed] [Google Scholar]
- 16.Madill J, Gutierrez C, Grossman J, Allard J, Chan C, Hutcheon M, Keshavjee SH, Toronto Lung Transplant P. Nutritional assessment of the lung transplant patient: body mass index as a predictor of 90-day mortality following transplantation. J Heart Lung Transplant 2001; 20: 288–296. [DOI] [PubMed] [Google Scholar]
- 17.Weill D, Benden C, Corris PA, Dark JH, Davis RD, Keshavjee S, Lederer DJ, Mulligan MJ, Patterson GA, Singer LG, Snell GI, Verleden GM, Zamora MR, Glanville AR. A consensus document for the selection of lung transplant candidates: 2014--an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2015; 34: 1–15. [DOI] [PubMed] [Google Scholar]
- 18.Benden C, Ridout DA, Edwards LB, Boehler A, Christie JD, Sweet SC. Body mass index and its effect on outcome in children after lung transplantation. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation 2012. [DOI] [PubMed] [Google Scholar]
- 19.Lederer DJ, Kawut SM, Wickersham N, Winterbottom C, Bhorade S, Palmer SM, Lee J, Diamond JM, Wille KM, Weinacker A, Lama VN, Crespo M, Orens JB, Sonett JR, Arcasoy SM, Ware LB, Christie JD. Obesity and primary graft dysfunction after lung transplantation: the Lung Transplant Outcomes Group Obesity Study. Am J Resp Crit Care 2011; 184: 1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sager JS, Kotloff RM, Ahya VN, Hadjiliadis D, Simcox R, Blumenthal NP, Mendez J, Bilker WB, Pochettino A, Christie JD. Association of clinical risk factors with functional status following lung transplantation. Am J Transplant 2006; 6: 2191–2201. [DOI] [PubMed] [Google Scholar]
- 21.Lederer DJ, Kawut SM, Wickersham N, Winterbottom C, Bhorade S, Palmer SM, Lee J, Diamond JM, Wille KM, Weinacker A, Lama VN, Crespo M, Orens JB, Sonett JR, Arcasoy SM, Ware LB, Christie JD, Lung Transplant Outcomes G. Obesity and primary graft dysfunction after lung transplantation: the Lung Transplant Outcomes Group Obesity Study. Am J Respir Crit Care Med 2011; 184: 1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX2-dependent mechanisms. Nat Med 2005; 11: 1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shibata R, Numaguchi Y, Matsushita K, Sone T, Kubota R, Ohashi T, Ishii M, Kihara S, Walsh K, Ouchi N, Murohara T. Usefulness of adiponectin to predict myocardial salvage following successful reperfusion in patients with acute myocardial infarction. Am J Cardiol 2008; 101: 1712–1715. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman SA, Wang L, Shah CV, Ahya VN, Pochettino A, Olthoff K, Shaked A, Wille K, Lama VN, Milstone A, Ware LB, Orens J, Weinacker A, Demissie E, Bellamy S, Kawut SM, Hancock WW, Christie JD, Lung Transplant Outcomes G. Plasma cytokines and chemokines in primary graft dysfunction post-lung transplantation. Am J Transplant 2009; 9: 389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singer LG, Brazelton TR, Doyle RL, Morris RE, Theodore J, International Lung Transplant Database Study G. Weight gain after lung transplantation. J Heart Lung Transplant 2003; 22: 894–902. [DOI] [PubMed] [Google Scholar]
- 26.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000; 894: i-xii, 1–253. [PubMed] [Google Scholar]
- 27.Snell GI, Yusen RD, Weill D, Strueber M, Garrity E, Reed A, Pelaez A, Whelan TP, Perch M, Bag R, Budev M, Corris PA, Crespo MM, Witt C, Cantu E, Christie JD. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, part I: Definition and grading-A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2017; 36: 1097–1103. [DOI] [PubMed] [Google Scholar]
- 28.Meyer KC, Raghu G, Verleden GM, Corris PA, Aurora P, Wilson KC, Brozek J, Glanville AR, Committee IAEBTF, Committee IAEBTF. An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Eur Respir J 2014; 44: 1479–1503. [DOI] [PubMed] [Google Scholar]
- 29.Calvert LD, Singh SJ, Morgan MD, Steiner MC. Exercise induced skeletal muscle metabolic stress is reduced after pulmonary rehabilitation in COPD. Respir Med 2011; 105: 363–370. [DOI] [PubMed] [Google Scholar]
- 30.Chaikriangkrai K, Jhun HY, Graviss EA, Jyothula S. Overweight-mortality paradox and impact of six-minute walk distance in lung transplantation. Annals of thoracic medicine 2015; 10: 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.