Abstract
Objectives:
We examined the association between three inflammatory markers (Interleukin (IL)-6, C-reactive protein (CRP), tumor necrosis factor (TNF)-α) and incident lung cancer using baseline, updated, and averaged inflammatory measures in older adults.
Methods:
We fitted multivariable Cox models to assess whether circulating levels of inflammation markers were associated with incident lung cancers in the Health Aging, Body and Composition (HealthABC) prospective cohort of 3,075 older adults aged 70–79 years at baseline. IL-6 and CRP were measured biennially, whereas TNF-α was measured at baseline.
Results:
Baseline levels of IL-6 were significantly associated with incident lung cancer risk in a model that adjusted for age, gender, race, and site (Model 1) (Hazard RatioT3 vs. T1: 3.34, 95% Confidence Interval: 1.91, 5.85) and in a model adjusted for health factors linked to chronic inflammation (Model 2) (HR T3 vs. T1: 2.57, 95% CI: 1.41, 4.65). The associations observed in time-updated IL-6 (HR T3 vs. T1: 2.47, 95% CI: 1.43, 4.28), cumulatively averaged IL-6 (HR T3 vs. T1: 2.47, 95% CI: 1.43, 4.35), and baseline CRP levels (HR T3 vs. T1: 1.85, 95% CI: 1.11, 3.08) with incident lung cancer in Model 1 were not statistically significant in Model 2.
Conclusions:
Baseline CRP and IL-6 levels were associated with increased risk of lung cancer in Model 1 and both models, respectively. Chronic IL-6 inflammation, as quantified by repeated measures was associated with incident lung cancer in Model 1, but not Model 2. Further research is needed to understand the role of CRP and IL-6 in lung carcinogenesis.
Keywords: lung cancer, inflammatory markers, incidence, pulmonary
1. BACKGROUND
The evidence supporting an association between chronic inflammation and risk of cancer has been limited 1,2. Inflammation leads to proliferation and survival of malignant cells and angiogenesis 3. Chronic inflammatory conditions such as chronic obstructive pulmonary disease (COPD) and bronchitis are established risk factors for lung cancer 1,4. The inflammatory response from cigarette smoking may also be partially implicated in lung carcinogenesis 4,5.
Several prospective studies to date have examined the association between C-reactive protein (CRP) and lung cancer risk, with many reporting statistically significant positive associations 5–9 between the two. The epidemiologic evidence on the association of IL-6 and incident lung cancer is less consistent: three studies reported positive associations 6,10,11, and one found a non-significant inverse association 12. One study assessed the association of the circulating inflammatory marker TNF-α levels with lung cancer, reporting a significant positive association 13.
An important limitation of previous studies is that inflammation at a single time point was used as a surrogate for persistent or chronic inflammation 4–11,13–18. Two recent reviews of inflammatory biomarkers in cancer research cited the need for the assessment of repeated measures of these factors in future research 19,20. A recent study measured the association between repeated measures of inflammatory markers and obesity-related cancers, and found that higher chronic CRP levels were associated with colorectal cancer risk 21.
In this study, we examined repeated measures of chronic inflammatory biomarkers and a comprehensive set of potential time-updated confounders to evaluate the association of circulating levels of three nonspecific serologic markers of chronic low-grade inflammation with incident lung cancer in a prospective biracial cohort study of older adults.
2. METHODS
2.1. Study Population
Participants were enrolled in the Health, Aging and Body Composition (Health-ABC) Study, a prospective cohort study of 3,075 white and black participants aged 70–79 years old at baseline 21. Briefly, between April 1997 and June 1998, a random sample of white Medicare beneficiaries and all black Medicare-eligible residents were recruited in areas around Memphis, Tennessee and Pittsburgh, Pennsylvania. Eligible participants had no reported difficulty in physical functioning, including no history of active cancer in the prior 2 years, no plans to leave the study area for 3 years, and no active participation in a lifestyle intervention trial.
Participants were followed up at yearly clinic examinations for six years (additional examinations conducted in years 8 and 10), and received semiannual phone calls to provide updates to their functional and health statuses for up to 16 years. Follow-up of select disease occurrences and deaths lasted for 16 years. All participants were required to provide informed consent. Protocols were approved by the Institutional Review Boards of the two clinic sites and the data coordinating center. We also obtained expedited IRB approval for secondary data analyses via the University of California, San Francisco.
After excluding subjects with (i) any prevalent cancer (n=527), (ii) those diagnosed with lung cancer during the first two years of follow-up (for whom elevated levels of inflammatory markers may be a result rather than a cause of cancer, n=15), and (iii) missing baseline (1997–1998) inflammatory marker measures (n=210), 2,323 subjects were available for this analysis.
2.2. Exposure Assessment
The primary exposures in our study were serum levels of C-reactive protein (CRP), Interleukin-6 (IL-6) and Tumor Necrosis Factor-alpha (TNF-α). Blood samples were collected after an overnight fast via venipuncture, aliquoted into cryovials, frozen at −70ºC, and shipped to the Health-ABC Core Laboratory at the University of Vermont (Year 1) or Wake Forest University (Years 2–8).
Baseline serum levels of CRP were measured in 1997–1998 in duplicate by enzyme-linked immunosorbent assay (ELISA) based on purified protein and polyclonal anti-CRP antibodies (Calbiochem, San Diego, CA). Standardization was done using WHO International Reference Standard, with a sensitivity of 0.08 mg/L, and a lower limit of detection of 0.007 mg/L. Year 2, 4, 6 and 8 CRP concentrations were determined using an automated chemiluminescent immunoassay system (IMMULITE, Diagnostic Products Corporation, Los Angeles), with a detectable limit of 0.1 µg/mL. Inflammatory markers IL-6 and TNF-α were determined by ELISA, measured in duplicate using a high-sensitivity Quantikine colorimetric immunoassay kit from R&D Systems (Minneapolis, MN), with a detectable limit of 0.10 pg/mL and 0.18 pg/mL for IL-6 and TNF-α, respectively. Blind duplicate analyses (n=150) for IL-6, CRP, and TNF-α showed inter-assay coefficients of variation of 10.3%, 8.0%, and 15.8%, respectively.
CRP and IL-6 concentration measurements taken in the years 2, 4, 6 and 8 were analyzed at a different laboratory (Wake Forest University) than the baseline measurements (Core Laboratory at the University of Vermont). To account for possible laboratory differences, calibration was performed for both IL-6 and CRP serum concentration levels based on a set of 150 blind duplicate measurements obtained from both labs: one set of values was regressed on the other set to create predicted values. Calibrated values were used in our analyses and were confirmed to be significantly correlated with values from the samples at the second laboratory. Measurements of IL-6 were obtained from a cell pack for year 8 and from serum for baseline and years 2, 4 and 6. To account for this difference in sample source, a second calibration was performed on IL-6 for year 8, based on a set of 137 samples for year 6, derived from both serum and cell packs.
Participants were categorized in tertiles of inflammatory markers. The tertiles were updated at each time point to accurately represent the distribution of inflammatory marker levels. We also used a continuous log-transformed version of our inflammatory markers.
2.3. Endpoint Ascertainment
The primary outcome was incident lung cancer. Incident cancers and the date of diagnosis were determined directly from hospital records, or from the underlying cause of death from death certificates. Adjudication of incident cancer events, excluding non-melanoma skin cancer, by the Health ABC Study Diagnosis and Disease Ascertainment Committee was completed through August 31, 2012.
2.4. Covariates
Age, gender, race, geographic site, education, body mass index (BMI), smoking status (including pack-years smoked), alcohol consumption, non-steroidal anti-inflammatory drug (NSAID) use, cardiovascular disease history, and pulmonary disease history were evaluated as potential confounders. BMI, smoking status, and NSAID use were analyzed as time-updated covariates. History of pulmonary conditions was ascertained at baseline, based on previous diagnosis of chronic bronchitis, COPD, or emphysema. History of cardiovascular conditions was measured at baseline, and defined as any previous diagnosis of congestive heart failure, myocardial infarction, high blood pressure or other diagnosed cardiovascular disease. BMI (kg/m2) was derived annually from measured height and weight through year 4, and biennially thereafter until year 822. Smoking history was obtained from baseline interviews for former and never smokers, and updated in the years 2, 5, 8, 9, 12, and 15. Pack-years were reported at baseline along with smoking history; smoking was categorized as never, former smoking of less than 20 pack-years, former smoking of more than 20 pack-years, current smoking of less than 20 pack-years, and current smoking of more than 20 pack-years. NSAID use was measured in each group at baseline and in years 2, 3, 5, 6, 10, 12 and 13.
2.5. Statistical Analysis
To evaluate the long-term effects of low-grade chronic systemic inflammation on lung cancer risk, and reduce random within-person variation, we used updated exposure measures (e.g. most recent), as well as average cumulative exposure, as has been commonly done in analyses measuring the effects of repeated dietary data 23. We also evaluated the association between baseline levels of inflammatory markers and risk of lung cancer 5, 9–12, 14,16,17 to compare results from using one versus repeated measures. In each model, we assessed the tertiles of inflammatory markers as well as a log-transformed continuous exposure. To minimize the risk of reverse causation, baseline exposure measurements were only considered in persons who did not develop lung cancer within the first two years of the Health-ABC study. In the average cumulative exposure analyses, the average levels from baseline and year 2 were used to predict hazard of lung cancer from years 2–4, the average score from baseline and years 2–4 was used to predict lung cancer hazard from years 4–8, and so forth for the remainder of the follow-up.
Cox proportional hazards models were used to estimate the hazard ratios (HR) and 95% confidence intervals (CIs) for the association between each exposure and incident lung cancer. Using Schoenfeld residuals, the proportional hazards assumption was assessed. Fine and Gray competing risks regression models were also fit to calculate subdistribution hazard ratios and corresponding 95% confidence intervals (CIs), treating non-lung cancer related mortality as a competing risk. The date of enrollment marked the beginning of the observation period for each participant, and person-years were accumulated until a lung cancer diagnosis, loss to follow-up, death from other causes, or until the analysis endpoint, whichever occurred first. Two models were used for each analysis: Model 1 adjusted for age, gender, race, and geographic site, similar to a prior study conducted by Il’yasova et al6, and Model 2 additionally adjusted for BMI, smoking status, alcohol consumption, NSAID use, cardiovascular disease history, and pulmonary disease history. The additional factors in Model 2 adjusted for health factors that could be associated with chronic inflammation. All analyses were conducted using SAS software, version 9.2 (SAS Institute, Inc., Cary, North Carolina).
3. RESULTS
Our analytic cohort consisted of 2,323 participants with no lung cancer at baseline, and no missing baseline measure of inflammatory markers, with a mean baseline age of 73.5 years (standard deviation 2.9 years). There were 89 incident cases of lung cancer identified over a median follow-up of 11.9 years. The distributions of demographic and clinical characteristics in the study population by tertiles of baseline inflammatory markers (1997–1998) are presented in Table 1.
Table 1.
Comparison of Baseline Characteristics for 2,323 Participants in the Health, Aging and Body Composition (Health-ABC) Study
| Tertiles of Inflammatory Markers |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | C-reactive Protein (ug/ml) | Interleukin-6 (pg/ml) | Tumor Necrosis Factor-α (pg/ml) | |||||||
| T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | ||
| Total Participants (Cases) | 2323 (89, 4%) | 718 (26, 4%) | 715 (21, 3%) | 716 (39, 5%) | 734 (17, 2%) | 735 (23, 3%) | 734 (46, 6%) | 721 (20, 3%) | 720 (32, 4%) | 720 (28, 4%) |
| Exposure, Median(SD) | 5.9 (9.8) | 0.7 (0.6) | 3.1 (0.9) | 19.9 (52.7) | 1.1 (0.3) | 2.0 (0.3) | 5.2 (3.2) | 2.1 (0.4) | 3.1 (0.3) | 5.0 (1.9) |
| Age at baseline, mean (SD) | 73.5 (2.9) | 73.5 (2.8) | 73.6 (2.9) | 73.2 (2.8) | 73.2 (2.7) | 73.6 (2.9) | 73.5 (2.9) | 73.3 (2.9) | 73.3 (2.8) | 73.9 (2.9) |
| Smoking, Pack-years, mean(SD) | 17.6 (26.7) | 15.1 (24.6) | 19 (27.9) | 19.0 (26.5) | 12.2 (20.5) | 18.6 (29) | 21.9 (28.7) | 15.0 (23.7) | 16.6 (24.2) | 20.8 (30.4) |
| Body mass index (kg/m2), mean (SD) | 27.5 (4.9) | 26.1 (4.0) | 27.2 (4.5) | 29.1 (5.4) | 26.1 (4.0) | 27.9 (4.5) | 28.5 (5.5) | 27.1 (5.0) | 27.5 (4.8) | 27.9 (4.8) |
| Gender, % Male | 46 | 54 | 49 | 36 | 41 | 49 | 49 | 42 | 46 | 50 |
| Race, % Caucasian | 55 | 64 | 61 | 45 | 61 | 57 | 50 | 48 | 59 | 62 |
| Site, % Memphis | 50 | 46 | 50 | 50 | 46 | 50 | 53 | 53 | 46 | 49 |
| Education % | ||||||||||
| Less than High School | 26 | 22 | 24 | 30 | 23 | 26 | 29 | 28 | 23 | 27 |
| High School | 33 | 32 | 35 | 34 | 33 | 32 | 34 | 30 | 33 | 37 |
| Post-secondary | 41 | 45 | 41 | 36 | 44 | 42 | 37 | 41 | 44 | 36 |
| Smoking Status, % | ||||||||||
| Never | 45 | 50 | 44 | 42 | 52 | 45 | 39 | 48 | 45 | 43 |
| Former | 45 | 41 | 48 | 45 | 42 | 45 | 47 | 40 | 45 | 48 |
| Current | 10 | 9 | 8 | 13 | 7 | 10 | 14 | 12 | 10 | 9 |
| Alcohol Consumption, % | ||||||||||
| None in Last Year | 51 | 49 | 48 | 54 | 50 | 49 | 55 | 51 | 48 | 53 |
| Less than Once per Week | 21 | 22 | 21 | 20 | 22 | 22 | 19 | 21 | 21 | 22 |
| 1–7 Times per Week | 20 | 22 | 23 | 19 | 23 | 20 | 18 | 20 | 24 | 17 |
| More than 1 per Day | 7 | 7 | 8 | 7 | 5 | 8 | 8 | 7 | 7 | 7 |
| Medication Use, % | ||||||||||
| NSAIDs | 22 | 22 | 20 | 22 | 25 | 22 | 20 | 20 | 24 | 24 |
| Corticosteroids | 4 | 3 | 4 | 5 | 2 | 4 | 5 | 4 | 4 | 4 |
| Statins | 12 | 14 | 13 | 13 | 12 | 12 | 13 | 11 | 12 | 15 |
| Multivitamins | 18 | 18 | 19 | 17 | 25 | 14 | 15 | 19 | 19 | 17 |
| Comorbid Conditions, % | ||||||||||
| Cardiovascular Disease | 54 | 49 | 52 | 62 | 46 | 54 | 60 | 49 | 51 | 60 |
| Pulmonary Disease (COPD, Chronic Bronchitis, Emphysema) | 13 | 12 | 13 | 14 | 10 | 12 | 16 | 13 | 12 | 13 |
Participants in the highest tertile of each baseline inflammatory marker value were more likely to be former or current smokers, to have a history of cardiovascular disease, and a higher BMI than participants in the lowest tertiles. Participants with higher IL-6 concentrations were more likely to have a previous diagnosis of pulmonary disease, and to have a smoking history of nearly ten pack-years more than participants in the lowest tertile. For TNF-α, participants in the highest tertile were more likely to be male and Caucasian.
3.1. The association of inflammatory markers with lung cancer risk
Cumulative incidence functions for each inflammatory marker stratified by tertiles and adjusted based on each analytic model are included in Fig. 1 (1a, 1b, 1c, 1d, 1e, 1f). We computed the HRs and corresponding 95% CIs for incident lung cancer using baseline, cumulative average, updated and log-transformed levels of inflammatory markers CRP, IL-6 and TNF-α (Table 2). The highest tertile of baseline CRP was associated with increased hazard of incident lung cancer in Model 1 (HR for highest tertile vs. lowest tertile = 1.85, 95% CI: 1.11, 3.08). After further adjustment for additional covariates in Model 2, the HR was still elevated but the association was no longer statistically significant (HRT3 vs. T1: 1.53, 95% CI: 0.88, 2.65). CRP was not significantly associated with hazard of incident lung cancer in cumulative average exposure, updated exposure, or log-transformed exposure models.
Figure 1a.
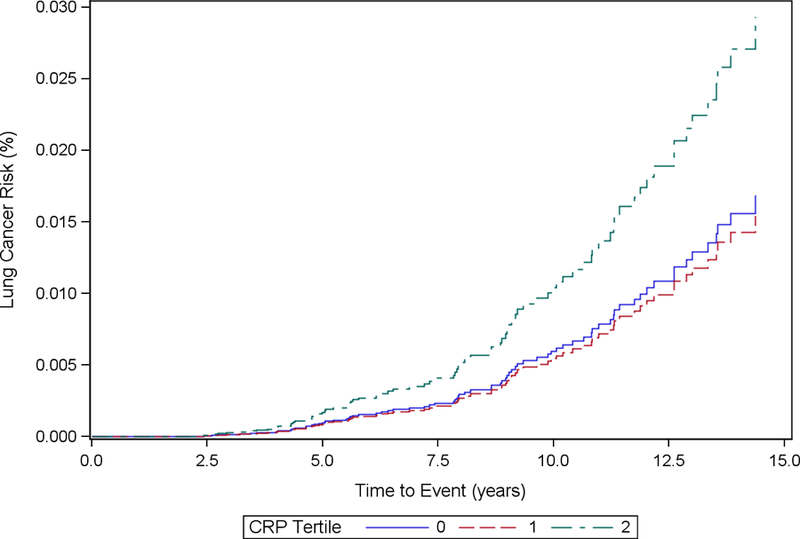
Cumulative Incidence Function Plot by CRP Tertiles: Model 1
Figure 1b.
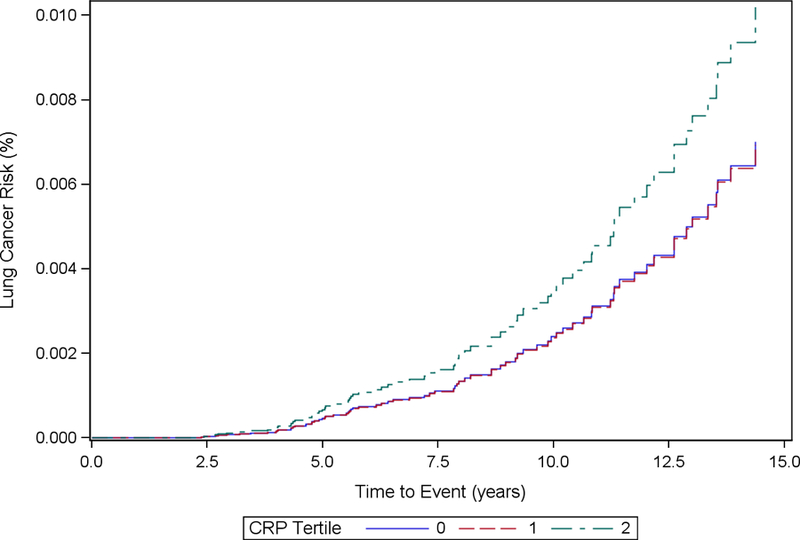
Cumulative Incidence Function Plot by CRP Tertiles: Model 2
Figure 1c.
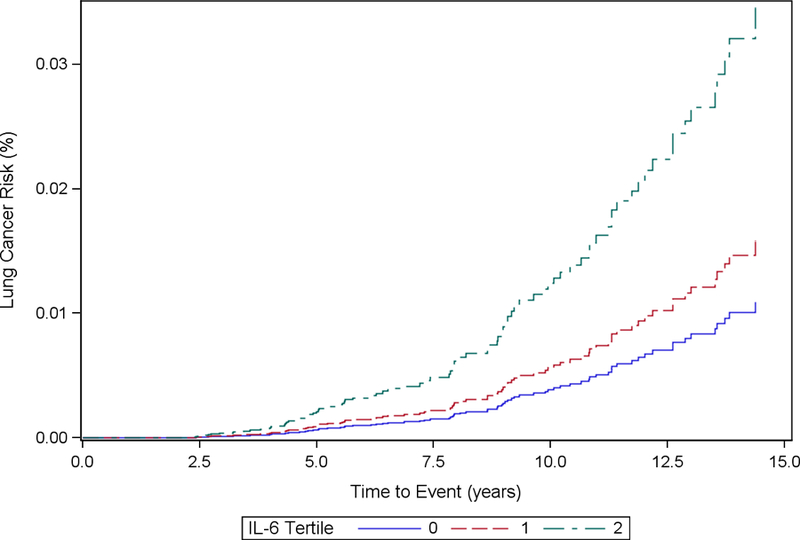
Cumulative Incidence Function Plot by IL-6 Tertiles: Model 1
Figure 1d.
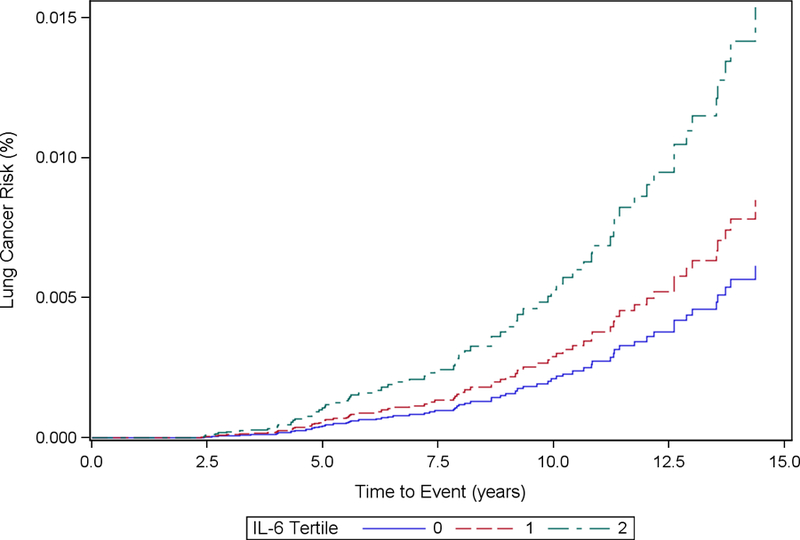
Cumulative Incidence Function Plot by IL-6 Tertiles: Model 2
Figure 1e.
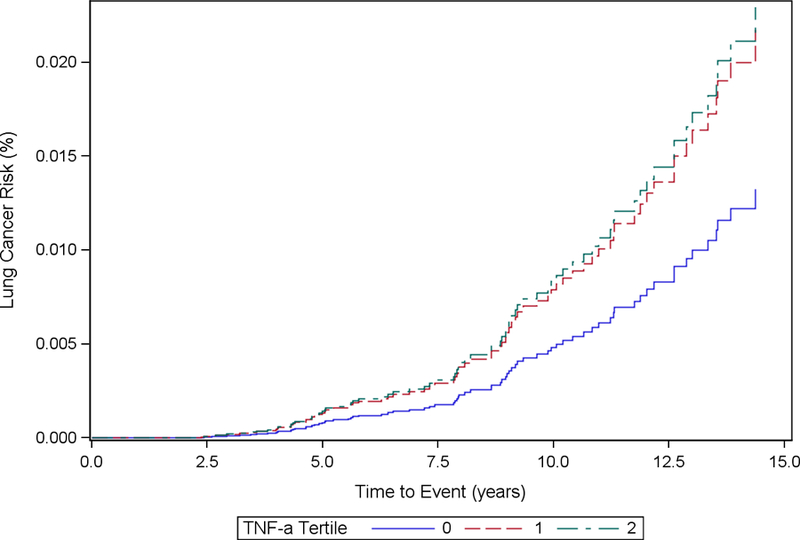
Cumulative Incidence Function Plot by TNF-α Tertiles: Model 1
Figure 1f.
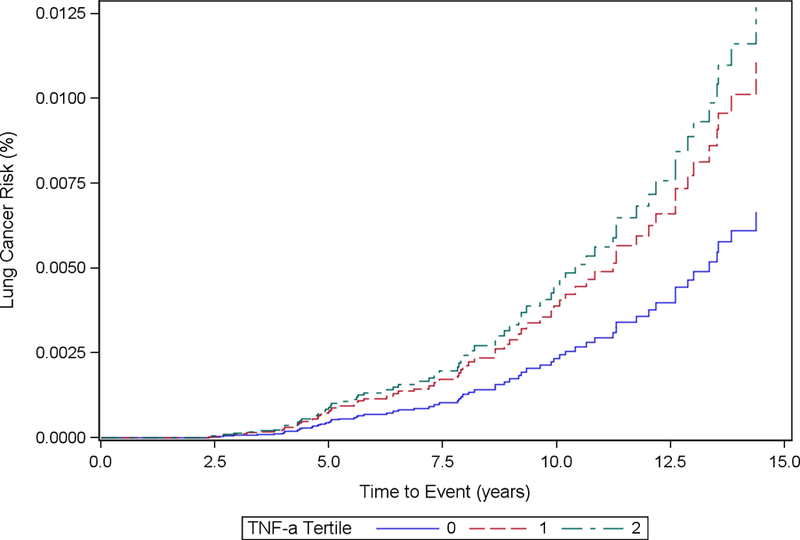
Cumulative Incidence Function Plot by TNF-α Tertiles: Model 2
Table 2.
Hazard ratios (HR) and 95% confidence intervals (CI) of inflammatory markers for lung cancer (Cases n=89)
| Log-Transformed | T1 | T2 | T3 | |
|---|---|---|---|---|
| CRP (ug/mL), N Cases | 718 (26) | 715 (21) | 716 (39) | |
| Baseline | ||||
| Model 1 | 1.09 (0.97, 1.22) | 1.00 | 0.86 (0.49, 1.54) | 1.85 (1.11, 3.08) |
| Model 2 | 1.03 (0.92, 1.15) | 1.00 | 0.94 (0.52, 1.69) | 1.53 (0.88, 2.65) |
| Cumulative Averaged | ||||
| Model 1 | 1.12 (0.95, 1.33) | 1.00 | 0.83 (0.48, 1.45) | 1.55 (0.96, 2.51) |
| Model 2 | 1.04 (0.88, 1.24) | 1.00 | 0.82 (0.47, 1.45) | 1.24 (0.74, 2.10) |
| Time-Updated | ||||
| Model 1 | 1.12 (0.95, 1.33) | 1.00 | 0.89 (0.52, 1.54) | 1.46 (0.89, 2.40) |
| Model 2 | 1.04 (0.88, 1.24) | 1.00 | 0.84 (0.48, 1.49) | 1.21 (0.70, 2.06) |
| IL-6 (pg/mL), Cases | 734 (17) | 735 (23) | 734 (46) | |
| Baseline | ||||
| Model 1 | 1.29 (1.04, 1.60) | 1.00 | 1.41 (0.75, 2.64) | 3.34 (1.91, 5.85) |
| Model 2 | 1.18 (0.98, 1.41) | 1.00 | 1.34 (0.70, 2.55) | 2.57 (1.41, 4.65) |
| Cumulative Averaged | ||||
| Model 1 | 1.43 (1.09, 1.89) | 1.00 | 1.15 (0.60, 2.19) | 2.47 (1.41, 4.35) |
| Model 2 | 1.18 (0.88, 1.58) | 1.00 | 0.81 (0.41, 1.61) | 1.78 (0.97, 3.24) |
| Time-Updated | ||||
| Model 1 | 1.43 (1.09, 1.89) | 1.00 | 1.28 (0.69, 2.36) | 2.47 (1.43, 4.28) |
| Model 2 | 1.18 (0.88, 1.58) | 1.00 | 0.97 (0.52, 1.83) | 1.71 (0.96, 3.05) |
| TNF-α (pg/mL), Cases | 721 (20) | 720 (32) | 720 (28) | |
| Baseline | ||||
| Model 1 | 0.96 (0.88, 1.05) | 1.00 | 1.64 (0.94, 2.89) | 1.59 (0.89, 2.85) |
| Model 2 | 0.97 (0.88, 1.06) | 1.00 | 1.76 (0.96, 3.24) | 1.82 (0.96, 3.42) |
Model 1: Adjusted for age in years, race, gender, site
Model 2: Adjusted for age in years, race, gender, site, smoking, history of pulmonary conditions, NSAID use,
body mass index, alcohol consumption, history of cardiovascular disease
Participants with the highest tertile levels of baseline IL-6 had significantly increased hazards of incident lung cancer in Model 1 (HRT3 vs. T1: 3.34, 95% CI: 1.91, 5.85) and Model 2 (HRT3 vs. T1: 2.57, 95% CI: 1.41, 4.65) relative to the reference group. Cumulative average concentrations of IL-6 were significantly associated with increased hazard of incident lung cancer in Model 1 (HRT3 vs. T1: 2.47, 95% CI: 1.41, 4.35), though the effect was attenuated in Model 2 (HRT3 vs. T1: 1.78, 95% CI: 0.97, 3.24). Similarly, updated exposure concentrations of IL-6 were significantly associated with increased hazard of incident lung cancer in Model 1 (HRT3 vs. T1: 2.47, 95% CI: 1.43, 4.28), but the effects were attenuated in Model 2 (HRT3 vs. T1: 1.71, 95% CI: 0.96, 3.05). Log-transformed exposure concentrations of IL-6 were significantly associated with increased hazard of incident lung cancer in Model 1 (HR: 1.43, 95% CI: 1.09, 1.89), but not in Model 2 (HR: 1.18, 95% CI: 0.88, 1.58).
Baseline TNF-α concentrations were not significantly associated with increased hazard of incident lung cancer in Model 1 (HRT3 vs. T1: 1.59, 95% CI: 0.89, 2.85) or Model 2 (HRT3 vs. T1 = 1.82, 95% CI: 0.96, 3.42). Log-transformed exposure concentrations of TNF- α were not significantly associated with increased hazard of incident lung cancer.
Fine and Gray competing risk regression model results are shown in Table 3. Unlike in the Cox model, baseline (SHR: 1.27, 95% CI: 0.90, 1.79), cumulatively averaged and time-updated (SHR: 1.39, 95% CI: 0.93, 2.08) log-transformed IL-6 concentrations were not significantly associated with hazard of incident lung cancer in Model 1. Among other analyses, there was small attenuation of effect sizes due to adjustment for competing risk, but no change in statistical significance.
Table 3.
Competing Risks Subdistribution hazard ratios (SHR) and 95% confidence intervals (CI) of inflammatory markers for lung cancer (Cases n=89)
| Log-Transformed | T1 | T2 | T3 | |
|---|---|---|---|---|
| CRP (ug/mL), N Cases | 718 (26) | 715 (21) | 716 (39) | |
| Baseline | ||||
| Model 1 | 1.08 (0.92, 1.27) | 1.00 | 0.86 (0.48, 1.52) | 1.83 (1.11, 3.01) |
| Model 2 | 1.02 (0.89, 1.18) | 1.00 | 0.93 (0.52, 1.65) | 1.51 (0.87, 2.60) |
| Cumulative Averaged | ||||
| Model 1 | 1.12 (0.88, 1.41) | 1.00 | 0.83 (0.48, 1.45) | 1.52 (0.94, 2.47) |
| Model 2 | 1.03 (0.80, 1.34) | 1.00 | 0.82 (0.46, 1.44) | 1.21 (0.72, 2.04) |
| Time-Updated | ||||
| Model 1 | 1.12 (0.88, 1.41) | 1.00 | 0.89 (0.51, 1.54) | 1.43 (0.88, 2.34) |
| Model 2 | 1.03 (0.80, 1.34) | 1.00 | 0.84 (0.48, 1.48) | 1.17 (0.69, 2.00) |
| IL-6 (pg/mL), Cases | 734 (17) | 735 (23) | 734 (46) | |
| Baseline | ||||
| Model 1 | 1.27 (0.90, 1.79) | 1.00 | 1.39 (0.75, 2.58) | 3.23 (1.87, 5.56) |
| Model 2 | 1.17 (0.92, 1.48) | 1.00 | 1.32 (0.71, 2.44) | 2.48 (1.42, 4.33) |
| Cumulative Averaged | ||||
| Model 1 | 1.39 (0.93, 2.08) | 1.00 | 1.14 (0.59, 2.18) | 2.37 (1.35, 4.15) |
| Model 2 | 1.15 (0.75, 1.75) | 1.00 | 0.81 (0.41, 1.60) | 1.70 (0.93, 3.11) |
| Time-Updated | ||||
| Model 1 | 1.39 (0.93, 2.08) | 1.00 | 1.27 (0.69, 2.34) | 2.37 (1.38, 4.07) |
| Model 2 | 1.15 (0.75, 1.75) | 1.00 | 0.97 (0.51, 1.84) | 1.62 (0.89, 2.97) |
| TNF-α (pg/mL), Cases | 721 (20) | 720 (32) | 720 (28) | |
| Baseline | ||||
| Model 1 | 0.96 (0.88, 1.05) | 1.00 | 1.63 (0.93, 2.84) | 1.54 (0.88, 2.70) |
| Model 2 | 0.97 (0.88, 1.06) | 1.00 | 1.75 (0.97, 3.18) | 1.76 (0.93, 3.31) |
Model 1: Adjusted for age in years, race, gender, site
Model 2: Adjusted for age in years, race, gender, site, smoking, history of pulmonary conditions, NSAID use,
body mass index, alcohol consumption, history of cardiovascular disease
4. DISCUSSION
Our findings indicated a positive association between baseline CRP and IL-6 exposure concentrations, and incident lung cancer risk. Baseline CRP concentration was significantly associated with incident lung cancer risk in Model 1 that adjusted for age, gender, race, and site. Cumulative-average and time-updated IL-6 concentrations were also significantly associated with incident lung cancer risk in Model 1. Further adjustment led to a positive trend toward increased risk of lung cancer in baseline CRP and cumulative-average and time-updated IL-6 models that did not reach statistical significance, likely due to a relatively small number of lung cancer cases in this cohort (n=89). We found no significant associations between cumulative-average and time-updated CRP concentrations, or baseline TNF-α and incident lung cancer risk in multivariable models.
Our findings for IL-6 aligned with findings from five previous studies 6, 10,11,13. Findings from two nested case control studies, the National Cancer Institute-Maryland (NCI-MD) study and the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial—both measured by Pine et al.—reported that IL-6 levels were increased in those who were diagnosed with lung cancer (NCI-MD), and those who developed lung cancer within 2 years (PLCO) 10. In addition, IL-6 has been found in higher quantities in the lungs of smokers experiencing chronic inflammation 24. While the findings are similar to our study, concerns about temporal instability, or reverse causality serve as an important limitation of these studies 10. To address this issue, studies have excluded cases within two years of blood sampling 9, excluded the first year of follow-up 7, or excluded cases within three months of measurement 25. Given that diagnosis of lung cancer may have occurred between 2 and 16 years from the initial baseline measurement of inflammatory markers, temporal instability or reverse causality are less likely in our study, compared to the previous work 10.
Our study reported log-CRP concentration findings, which did not reach statistical significance in Model 2. These results are consistent with the five studies that produced null findings 11,12, 15,25,26, but contradict five other studies that showed statistically significant associations of CRP with lung cancer risk 5–9. Of the five that reported positive findings, two studies measured effect sizes based on one unit increases in log-CRP concentration 6,8, versus one standard deviation increases in CRP in another study 7, versus categorical groupings of CRP indicative of low, medium, and high concentrations in two other studies 5,9.
The five studies stratified results by smoking status to test for interaction with CRP levels 5, 8, 10, 16,27. While two studies conducted by Pine et al., found higher odds of incident lung cancer among current and former versus never smokers 10, none of the studies reported a significant interaction between CRP levels and smoking status 5, 8, 10, 16,27. Our study lacked adequate power to assess this potential interaction, since current smokers made up less than 15% of the study population. Given that lung inflammation can be exacerbated by cigarette smoke 14, populations with a variety of smoking histories would provide an ideal setting to further evaluate this interaction.
Although findings for chronic levels of IL-6 when adjusting for health factors of chronic inflammation in Model 2 were not significant, there is an indication that the significant Model 1 findings represent local inflammation, influencing the development of malignant cells. It is possible that higher levels of IL-6 serve as signs of senescence, the body’s evolved stress response to the development of cancer 28. However, IL-6 is also one of the most prominent inflammatory markers associated with senescence-associated secretory phenotype (SASP), which can turn senescent cells into proinflammatory cells that promote tumor progression 29,30. IL-6 is also known to become elevated as a result of a variety of conditions independent of tumorigenesis 4, 9, 19,30. In particular, IL-6 is known to be involved in COPD and emphysema-like inflammation 4, 9,31. Our findings are primarily generalizable to older individuals, particularly those aged 70 and older, and could shed further light on how to protect older adults from developing cancer by focusing on the body’s inflammatory response. However, more research must be conducted into understanding the mechanisms by which inflammation promotes tumorigenesis, and whether this effect persists in more diverse populations.
Limitations of this study included a small number of incident lung cancer cases, and reduced statistical power, which meant we were unable to conduct interaction analyses, particularly by smoking status. Furthermore, our study population of adults ages 70 and older tended to be healthier than the general population of older adults, who may be afflicted with more age-related diseases (i.e. cardiovascular disease), which may affect external generalizability of our findings. The use of two different lab assays and measurement protocols was also a limitation, requiring calibration of blood sample measurements at first visit to the protocols conducted at subsequent visits. Although the long duration of follow-up and use of repeated measures may raise a concern of survival bias, the lack of appreciably different results between baseline and updated exposure models, indicates that the risk is minimal.
Our study had several strengths. The Health-ABC cohort is a large, racially and geographically diverse population of older men and women with detailed medical history and medication use information from all participants. Our study is also the first to evaluate repeated measures of inflammatory markers and lung cancer incidence, enabling us to categorize a participant’s longer term inflammatory status. Studies using only one measurement can be affected by regression dilution 7,9, which can attenuate the potential association. Our study also used tertiles to address concerns about the potential influence of acute inflammation at the time of measurement. While previous studies conducted sensitivity analyses excluding individuals with higher levels of CRP 9, the use of tertiles mitigated the effect of potential outliers, which only accounted for a small number of individuals (n=20) in our study (CRP concentration greater than 50 ug/mL).
5. CONCLUSION
In conclusion, we found that elevated IL-6 levels at baseline were significantly associated with risk of lung cancer; in analyses using repeated measures of IL-6 over time, findings were significant in the previously applied Model 1, but not in Model 2, which accounted for additional covariates. Baseline CRP was associated with increased hazard of incidence lung cancer in Model 1, but not in the model adjusting for additional covariates. TNF-α levels were not statistically significantly associated with increased hazard of incident lung cancer in either model. Future prospective studies that plot trajectories of repeated measures of TNF-α, IL-6, and CRP, and leverage larger sample sizes, will provide further insight into the association of chronic inflammation with the risk of lung cancer.
Acknowledgements:
We would like to thank the Health, Aging and Body Composition Study Research Team and the Statistical Coordinating Center at the University of California, San Francisco for their support throughout the project. We would also like to thank all of the participants in the HealthABC study for giving their time.
Funding Statement: Preparation of this manuscript was supported by grant 121891-MRSG-12-007-01-CPHPS from the American Cancer Society. The Health ABC study was supported by the National Institute on Aging (NIA) Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grants R01-AG028050, and P30-AG15272, and NINR grant R01-NR012459. We would also like to thank all the men and women who participated in this study.
Abbreviations and Acronyms:
- Health ABC Study
Health, Aging, and Body Composition Study
- Vs.
versus
- CRP
C-reactive protein
- IL-6
Interleukin-6
- (TNF-α)
Tumor necrosis factor-α
- BMI
body mass index
- HR
hazard ratio
- SHR
Subdistribution Hazard Ratio
- 95% CI
95% confidence interval
- kg
kilograms
- L
liters
- mg
milligrams
- mL
milliliters
- µg
micrograms
- pg
picograms
- cm
centimeters
- ICD
International Classification of Diseases
- IQ
interquartile range
Footnotes
DECLARATIONS
Ethics Approval and Consent to Participate: All participants in the Dynamics of Health, Aging and Body Composition study gave informed written consent to participate in the study. The protocol for the study was approved by the institutional review boards of the clinical sites in Pittsburgh, PA and Memphis, TN, as well as at the Data Coordinating Center of the University of California, San Francisco. Researchers also obtained expedited IRB approval for secondary data analyses via the University of California, San Francisco.
Consent to Publish: Not applicable. In no part of this manuscript do we include details, images, or videos relating to an individual person.
Availability of Data and Materials: The datasets generated and/or analyzed during the current study are available on request with the completion of a data access proposal from the Dynamics of Health, Aging, and Body Composition research team. (https://healthabc.nia.nih.gov/) To request data, submit analysis plan proposals to the Health ABC Publications Committee Coordinator at the University of California, San Francisco Coordinating Center for approval.
Conflict of Interest Statement: We have read and understood the Elsevier policy on declaration of conflicts of interests and declare we have no conflicts of interest.
References:
- 1.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res 2006;4(4):221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 3.Gomes M, Teixeira AL, Coelho A, Araújo A, Medeiros R. The role of inflammation in lung cancer. Adv Exp Med Biol 2014;816:1–23. doi: 10.1007/978-3-0348-0837-8_1. [DOI] [PubMed] [Google Scholar]
- 4.Agassandian M, Shurin GV, Ma Y, Shurin MR. C-reactive protein and lung diseases. Int J Biochem Cell Biol 2014;53:77–88. doi: 10.1016/j.biocel.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi AK, Caporaso NE, Katki HA, Wong H-L, Chatterjee N, Pine SR, Chanock SJ, Goedert JJ, Engels EA. C-reactive protein and risk of lung cancer. J Clin Oncol 2010;28(16):2719–2726. doi: 10.1200/JCO.2009.27.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Il’yasova D, Colbert LH, Harris TB, Newman AB, Bauer DC, Satterfield S, Kritchevsky SB. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev 2005;14(10):2413–2418. doi: 10.1158/1055-9965.EPI-05-0316. [DOI] [PubMed] [Google Scholar]
- 7.Trichopoulos D, Psaltopoulou T, Orfanos P, Trichopoulou A, Boffetta P. Plasma C-reactive protein and risk of cancer: a prospective study from Greece. Cancer Epidemiol Biomarkers Prev 2006;15(2):381–384. doi: 10.1158/1055-9965.EPI-05-0626. [DOI] [PubMed] [Google Scholar]
- 8.Siemes C, Visser LE, Coebergh J-WW, Splinter TAW, Witteman JCM, Uitterlinden AG, Hofman A, Pols HAP, Stricker BHC. C-reactive protein levels, variation in the C-reactive protein gene, and cancer risk: the Rotterdam Study. J Clin Oncol 2006;24(33):5216–5222. doi: 10.1200/JCO.2006.07.1381. [DOI] [PubMed] [Google Scholar]
- 9.Allin KH, Bojesen SE, Nordestgaard BG. Baseline C-reactive protein is associated with incident cancer and survival in patients with cancer. J Clin Oncol 2009;27(13):2217–2224. doi: 10.1200/JCO.2008.19.8440. [DOI] [PubMed] [Google Scholar]
- 10.Pine SR, Mechanic LE, Enewold L, Chaturvedi AK, Katki HA, Zheng Y-L, Bowman ED, Engels EA, Caporaso NE, Harris CC. Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. J Natl Cancer Inst 2011;103(14):1112–1122. doi: 10.1093/jnci/djr216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heikkilä K, Harris R, Lowe G, Rumley A, Yarnell J, Gallacher J, Ben-Shlomo Y, Ebrahim S, Lawlor DA. Associations of circulating C-reactive protein and interleukin-6 with cancer risk: findings from two prospective cohorts and a meta-analysis. Cancer Causes Control 2009;20(1):15–26. doi: 10.1007/s10552-008-9212-z. [DOI] [PubMed] [Google Scholar]
- 12.Heikkilä K, Ebrahim S, Lawlor DA. A systematic review of the association between circulating concentrations of C reactive protein and cancer. J Epidemiol Community Health 2007;61(9):824–833. doi: 10.1136/jech.2006.051292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song X-Y, Zhou S-J, Xiao N, Li Y-S, Zhen D-Z, Su C-Y, Liu Z-D. Research on the relationship between serum levels of inflammatory cytokines and non-small cell lung cancer. Asian Pac J Cancer Prev 2013;14(8):4765–4768. http://www.ncbi.nlm.nih.gov/pubmed/24083740. Accessed October 10, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Shiels MS, Pfeiffer RM, Hildesheim A, Engels EA, Kemp TJ, Park J-H, Katki HA, Koshiol J, Shelton G, Caporaso NE, Pinto LA, Chaturvedi AK. Circulating inflammation markers and prospective risk for lung cancer. J Natl Cancer Inst 2013;105Shiels,(24):1871–1880. doi: 10.1093/jnci/djt309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki K, Ito Y, Wakai K, Kawado M, Hashimoto S, Seki N, Ando M, Nishino Y, Kondo T, Watanabe Y, Ozasa K, Inoue T, Tamakoshi A. Serum heat shock protein 70 levels and lung cancer risk: a case-control study nested in a large cohort study. Cancer Epidemiol Biomarkers Prev 2006;15(9):1733–1737. doi: 10.1158/1055-9965.EPI-06-0005. [DOI] [PubMed] [Google Scholar]
- 16.Xu M, Zhu M, Du Y, Yan B, Wang Q, Wang C, Zhao J. Serum C-reactive protein and risk of lung cancer: a case-control study. Med Oncol 2013;30(1):319. doi: 10.1007/s12032-012-0319-4. [DOI] [PubMed] [Google Scholar]
- 17.Zhou B, Liu J, Wang Z-M, Xi T. C-reactive protein, interleukin 6 and lung cancer risk: a meta-analysis. Ljubimov AV, ed. PLoS One 2012;7(8):e43075. doi: 10.1371/journal.pone.0043075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang K-T, Huang C-YF, Tsai C-M, Chiu C-H, Lok Y-Y. Role of IL-6 in neuroendocrine differentiation and chemosensitivity of non-small cell lung cancer. Am J Physiol Lung Cell Mol Physiol 2005;289(3):L438–45. doi: 10.1152/ajplung.00033.2005. [DOI] [PubMed] [Google Scholar]
- 19.Chaturvedi AK, Moore SC, Hildesheim A. Invited commentary: circulating inflammation markers and cancer risk--implications for epidemiologic studies. Am J Epidemiol 2013;177(1):14–19. doi: 10.1093/aje/kws357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brenner DR, Scherer D, Muir K, Schildkraut J, Boffetta P, Spitz MR, Le Marchand L, Chan AT, Goode EL, Ulrich CM, Hung RJ. A review of the application of inflammatory biomarkers in epidemiologic cancer research. Cancer Epidemiol Biomarkers Prev 2014;23(9):1729–1751. doi: 10.1158/1055-9965.EPI-14-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Izano M, Wei EK, Tai C, Swede H, Gregorich S, Harris TB, Klepin H, Satterfield S, Murphy R, Newman AB, Rubin SM, Braithwaite D. Chronic inflammation and risk of colorectal and other obesity-related cancers: The health, aging and body composition study. Int J Cancer 2016;138(5):1118–1128. doi: 10.1002/ijc.29868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tammemagi CM, Pinsky PF, Caporaso NE, Kvale PA, Hocking WG, Church TR, Riley TL, Commins J, Oken MM, Berg CD, Prorok PC. Lung cancer risk prediction: Prostate, Lung, Colorectal And Ovarian Cancer Screening Trial models and validation. J Natl Cancer Inst 2011;103(13):1058–1068. doi: 10.1093/jnci/djr173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 1999;149(6):531–540. http://www.ncbi.nlm.nih.gov/pubmed/10084242. Accessed October 27, 2014. [DOI] [PubMed] [Google Scholar]
- 24.Crotty Alexander LE, Shin S, Hwang JH. Inflammatory Diseases of the Lung Induced by Conventional Cigarette Smoke. Chest 2015;148(5):1307–1322. doi: 10.1378/chest.15-0409. [DOI] [PubMed] [Google Scholar]
- 25.Van Hemelrijck M, Holmberg L, Garmo H, Hammar N, Walldius G, Binda E, Lambe M, Jungner I. Association between levels of C-reactive protein and leukocytes and cancer: three repeated measurements in the Swedish AMORIS study. Cancer Epidemiol Biomarkers Prev 2011;20(3):428–437. doi: 10.1158/1055-9965.EPI-10-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.dos Santos Silva I, De Stavola BL, Pizzi C, Meade TW. Circulating levels of coagulation and inflammation markers and cancer risks: individual participant analysis of data from three long-term cohorts. Int J Epidemiol 2010;39(3):699–709. doi: 10.1093/ije/dyq012. [DOI] [PubMed] [Google Scholar]
- 27.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999;282(22):2131–2135. http://www.ncbi.nlm.nih.gov/pubmed/10591334. Accessed June 2, 2015. [DOI] [PubMed] [Google Scholar]
- 28.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol 2013;75(1):685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coppé J-P, Desprez P-Y, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 2014;69 Suppl 1(Suppl 1):S4–9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 31.Ochoa CE, Mirabolfathinejad SG, Ruiz VA, Evans SE, Gagea M, Evans CM, Dickey BF, Moghaddam SJ. Interleukin 6, but not T helper 2 cytokines, promotes lung carcinogenesis. Cancer Prev Res (Phila) 2011;4(1):51–64. doi: 10.1158/1940-6207.CAPR-10-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]


