Abstract
Objective:
Health behavior is affected by competing sources of influence like media messages and peers. In the context of alcohol consumption, college students are targeted by anti-drinking media messages, but tend to have pro-alcohol conversations with peers. How do humans integrate competing sources of influence on daily behavior? We observed individuals under exposure to anti-alcohol media messages and pro-alcohol conversations and tested a “common neural value” account of how contradictory influences are integrated to affect behavior.
Methods:
Participants were instructed to cognitively regulate responses to anti-drinking media messages while undergoing fMRI at baseline. Individual differences in success in message-consistent or -derogating regulation were indexed by changes in activity within the neural valuation system (ventral striatum/VS, ventromedial prefrontal cortex/VMPFC), providing a proxy for success in finding value in message-consistent/-derogating engagement. To measure peer influence, we tracked daily drinking-related conversations and drinking behavior for 30 days using mobile electronic diaries.
Results:
Peer conversations, on average, were positive towards drinking. More positive conversations led to more future drinking, particularly for participants who showed greater neural value signals when derogating anti-drinking media. Susceptibility to risky peer influence decreased with increasing success in up-regulating message-consistent neural valuation responses to anti-drinking media. Neural effects were driven by VS-activity.
Conclusions:
Results are consistent with a dynamic value integration process where contradictory influences inform a common neural value signal. Reductions in the value of a behavior (through anti-drinking campaigns) may buffer against future value increases after exposure to competing influences (pro-alcohol peers) with important real-world consequences.
Keywords: Media Campaigns, Social Influence, fMRI, Brain, Mobile Electronic Diaries, Ecological Momentary Assessment (EMA), Cognitive Regulation, Valuation, Alcohol
Behaviors are shaped by multiple, and often competing, sources of influence. For example, media exposure and peer influence (e.g. through conversations) have synergistic as well as antagonistic effects on our actions (David, Cappella, & Fishbein, 2006; Hendriks, de Bruijn, & van den Putte, 2012; Jeong & Bae, 2017). Such interaction effects have been theorized and shown at a large scale (Jeong & Bae, 2017; Katz & Lazarsfeld, 1955), but the psychological mechanisms that underlie the integration of competing sources of influence such as media effects in the context of opposing peer influence (or vice versa) are unknown. To address this gap, we connect three key observations: First, stimuli such as persuasive messages and social influence can change the perceived value of behaviors in individuals and these changes are observable in brain regions associated with valuation (for review, see: Falk & Scholz, 2018). Second, perceived value and subsequent effects of persuasive messages partially depend on people’s conscious appraisal of the stimuli (Doré, Cooper, Scholz, O’Donnell, & Falk, in press). Third,the brain’s value system facilitates the integration of different, inherently incomparable inputs (e.g. taste and monetary value of food) into a final signal of the perceived value of a choice which predicts behavior (Bartra, McGuire, & Kable, 2013; Clithero & Rangel, 2013; Levy & Glimcher, 2012). Bringing these ideas together, we propose a “common value” account where competing sources of influence on the same behavior dynamically heighten and lower the subjective value of that behavior by providing separate inputs to a common, subjective value signal encoded in the brain’s value system. We tested whether individuals who are more successful at cognitively regulating neural valuation of thoughts that are consistent with (or derogate) one source of influence show reduced (or greater) susceptibility to a competing source.
Specifically, we studied the context of alcohol consumption among college students, a significant public health problem which, among others, causes thousands of injuries and deaths yearly (Gore et al., 2011; Hingson, Zha, & Weitzman, 2009). Regularly, anti-drinking media campaigns (DeJong, 2002) compete with the influence of college students’ interpersonal conversations about drinking which, on average, favor alcohol consumption (Hendriks & de Bruijn, 2015; Hendriks et al., 2012). We used functional magnetic resonance imaging (fMRI) to assess an individual’s success in regulating their subjective valuation of message-consistent and message-derogating thoughts about anti-drinking media messages at baseline and linked these data to each individual’s susceptibility to the influence of pro-drinking peer conversations over 30 days following the scan which was assessed through daily mobile electronic diaries (a form of Ecological Momentary Assessment, Wray, Merrill, & Monti, 2014).
Peer Influence and Alcohol in College Students
College drinking is highly social and driven by social motivations (e.g. Kuntsche, Knibbe, Gmel, & Engels, 2005; LaBrie, Hummer, & Pedersen, 2007), social norms (e.g. Borsari & Carey, 2003), and by the presence and behavior of others (Collins & Marlatt, 1981). Unsurprisingly, drinking features often in interpersonal conversations (Dorsey, Scherer, & Real, 1999; Hendriks & de Bruijn, 2015), which impact drinking intentions and behavior (e.g. Boyle, LaBrie, Froidevaux, & Witkovic, 2016; Dorsey et al., 1999; Real & Rimal, 2007). In the context of this type of peer influence, both whether and how drinking is discussed matters. Pro-drinking conversations are associated with more drinking (Dorsey et al., 1999; Real & Rimal, 2007) and reductions in drinking are related to anti-drinking conversations (Hendriks et al., 2012; Hendriks, van den Putte, de Bruijn, & de Vreese, 2014). On average, students’ conversations favor drinking (Hendriks & de Bruijn, 2015; Hendriks et al., 2012), highlighting the riskiness of peer influence.
Existing evidence for effects of conversational valence on drinking is mainly cross-sectional, often based on self-reports that aggregate conversations over long periods of time (e.g. Dorsey et al., 1999; Hendriks & de Bruijn, 2015) or on experimentally engineered conversations in the laboratory (Hendriks, van den Putte, & de Bruijn, 2015). To achieve a closer approximation of dynamic peer influence on behavior we studied time-sensitive, bi-directional relationships between daily conversational valence and drinking over 30 days.
Neural Valuation of Media Messages as a Buffer Against Risky Peer Influence
Anti-drinking media campaigns frequently target alcohol consumption on college campuses (DeJong, 2002) where they compete with pro-drinking peer influence. We propose that cognitive regulation of the perceived value of thoughts that are consistent with or derogating anti-drinking media messages moderates risky peer influence on drinking.
The neural value system consists of clusters within ventromedial prefrontal cortex (VMPFC) and ventral striatum (VS). Here, activity scales with the subjective value of ideas and stimuli across domains (Bartra et al., 2013). Specifically, these structures encode and integrate the perceived value of diverse choice options to arrive at a weighted sum representing the value of a choice based on all available inputs (Bartra et al., 2013; Clithero & Rangel, 2013; Levy & Glimcher, 2012). This value signal underlies persuasion by both mediated and social sources of influence (for a review, see Falk & Scholz, 2018). Media messages which are more successful in engaging regions of the neural value system are more effective in motivating message-consistent behaviors like calls to smoking quit-lines (Falk, Berkman, & Lieberman, 2012), micro-lending (Genevsky, Yoon, & Knutson, 2017), and music sales (Berns & Moore, 2012). Similarly, individuals who show more activity in these regions across multiple persuasive messages are more likely to show message-consistent behaviors (Chua et al., 2011; Cooper, Tompson, O’Donnell, & Falk, 2015). Further, social influence modulates neural value-related responses to diverse stimuli and this mechanism determines an individual’s susceptibility to social influence (Klucharev, Munneke, Smidts, & Fernandez, 2011; Zaki, Schirmer, & Mitchell, 2011).
Extending this work, we argue that competing sources of influence on the same behavior heighten and lower the subjective value of that behavior, respectively. Consequently, cognitively regulating neural valuation of thoughts that are consistent with (/derogate) one source of influence may change the computation of the weighted sum of overall behavioral value and, thereby, lower (/heighten) the impact of competing influences on behavior. We collected fMRI data from the neural value system indicating an individual’s success when cognitively up-regulating message-consistent or message-derogating responses to anti-drinking messages. We linked neural valuation responses to repeated measures of the valence of peer conversations about drinking and drinking behavior collected over the following month. In this context, we argue that being successful when cognitively up-regulating neural valuation of thoughts that are consistent with (/derogating of) anti-drinking media messages should reduce (/increase) an individual’s susceptibility to the competing influence of pro-drinking peer conversations. Insight into these relationships will enhance our mechanistic understanding of how competing sources of influence on behavior are integrated neurally and psychologically.
Finally, changes in neural value-related activity can be construed as an outcome of the cognitive regulation process which, itself, is thought to be encoded in the neural executive function network (e.g. dorsolateral prefrontal cortex; Buhle et al., 2014). In orthogonal analyses of the neural data discussed here, we report that cognitive regulation during the fMRI task was indeed associated with activity within this network and cognitive regulation activity ultimately impacted neural valuation of the stimuli within VMPFC (Doré et al., in press). Here, we examined whether susceptibility to peer influence was impacted, not only by the successfulness/outcomes of cognitive regulation (i.e. neural value-related activity), but also by the effort participants put into this process (i.e. neural cognitive regulation activity).
Methods
Recruitment materials advertised an “fMRI Drinking Research Study” for those “who sometimes drink alcohol” to University of Pennsylvania (UPenn) students, on flyers and Facebook advertisements distributed in the Philadelphia area. Recruited individuals provided initial consent to complete an online survey assessing screening criteria and, for those eligible, individual-difference measures. Based on their availability for fMRI appointments, 60 respondents were invited for an in-person appointment where they provided full informed consent for a study (“about emotions, attitudes, social relationships, and alcohol consumption”) based on general descriptions of all study tasks. Afterwards, they completed a baseline appointment, including a 60-min fMRI scan evaluating their responses to anti-drinking media messages. Finally, participants completed a 30-day field period, providing daily reports on drinking-related conversations and alcohol consumption (Figure 1). Participants received up to $105 ($10 online survey, $45 in-person appointment, $50 Amazon gift card for answering at least 70% of text messages). UPenn’s Institutional Review Board approved all study procedures.
Figure 1.
Study Timeline; fMRI = functional magnetic resonance imaging, SMS = short message service
Participants
Sixty participants completed all study tasks, fulfilled standard fMRI screening criteria (no metal in their body, self-report to not currently take mood-altering or psychoactive medication and to have no history of major physical (e.g. heart disease, diabetes, cancer, untreated high blood pressure) or neurological illness (e.g. stroke, epilepsy), not claustrophobic, not pregnant/breast feeding, right-handed, native English speaker) and reported to own a mobile phone. Based on an NIAAA ‘Recommended Alcohol Question’ (NIAAA, 2003), eligible participants further self-reported to have consumed any alcohol-containing drink (standard drink of beer, wine, liquor) on 2–3 occasions per month or more (10-item scale from daily to never), on average, over the last 12 months. Participants were told that a standard alcoholic drink refers to half an ounce of absolute alcohol (e.g. a 12 ounce can of beer, a 5 ounce glass of wine, or a drink containing 1 shot of liquor). A minimum drinking requirement ensured a sufficient number of drinking occasions during the field period. Sample size was determined in advance of data collection based on resource availability and corresponds to about double the median size of published neuroimaging samples (Yeung, 2018).
Survey data for one participant was lost, disqualifying them from all analyses. Thus, 59 participants (54% female, Age: M = 22.52, SD = 2.63, range=19–33) were included in the analyses. One participant was excluded from neuroimaging analyses (N=58 with at least partial neuroimaging data) due to severe signal drop-out. For a second participant two runs of neural data were lost and a total of four fMRI task runs across three participants were excluded due to excessive head motion (> 3 mm translation). An additional 6–7 participants were excluded from statistical models (final N = 51–52) due to the need for co-occurrences of drinking occasions and peer conversations, given our variable definitions (see details below).
Procedures
Baseline questionnaire.
At baseline, participants reported the typical number of drinks consumed during a typical drinking occasion over the last year: “During the last 12 months, how many alcoholic drinks did you have on a typical day when you drank alcohol?” (11-point Likert-type scale: “I didn’t drink alcohol in the last year.”, “1 drink”, “2 drinks”, “3 to 4 drinks”, “5 to 6 drinks”, “7 to 8 drinks”, “9 to 11 drinks”, “12 to 15 drinks”, “16 to 18 drinks”, “19 to 24 drinks”, and “25 or more drinks”) and binge drinking attitudes using the question: “If you were to drink more than 3 or 4 drinks within a 2 hour period in the next month, that would be:” (harmful, beneficial, enjoyable/fun, unenjoyable/dull, healthy, unhealthy, sad/negative, happy/positive; 1 = not at all, 5 = extremely). Both variables were used in statistical models to control for a participant’s average affinity for alcohol. Finally, we collected demographics including gender. Given that drinking patterns (Johnston, 2010), motivations, and consequences (LaBrie et al., 2007) vary across genders, all models controlled for gender.
fMRI task.
During the fMRI scan, participants saw 90 English-language media messages from 26 anti-drinking campaigns published in the USA, Australia, the UK, Finland, New Zealand, and Canada. Ads were obtained through an online search in December 2016. All messages discourage harmful alcohol consumption, for instance by highlighting negative outcomes of (excessive) drinking and consist of still images and some text. Messages were presented in five conditions which asked participants to either look naturally (LOOK condition, 30 trials), or to cognitively regulate their responses to each message in four conditions (15 trials each). For each trial type, participants were given specific instructions and examples of thought processes required by the task before the scan session. In the LOOK condition, they were told to look naturally at the message and have whatever thoughts or feelings they would normally have. In regulation trials, participants were asked to (a) up-regulate message consistent thoughts by considering why the message was persuasive, for instance why it was relevant to them or why the arguments were strong (WHY PERSUASIVE condition), (b) up-regulate message-derogating thoughts by considering why the message was not persuasive, for instance why it did not apply to them or included weak argumentation (WHY NOT PERSUASIVE condition), (c) up-regulate message-consistent emotions, namely feeling more negative upon learning about negative outcomes of drinking, for instance by thinking about the consequences of the depicted situation happening to them personally (INCREASE NEGATIVE condition) or (d) regulate their emotions in a message-derogating manner by decreasing negative emotions, for instance by thinking about how the depicted situation is staged or not personally relevant to them (DECREASE NEGATIVE condition). As such, this task adopts well-validated paradigms from the emotion-regulation literature to the context of alcohol campaigns (Gross, 2015).
Messages were pseudo-randomly assigned to condition for each participant in a counter-balanced manner and the task was presented in four runs (22–23 trials each). Each run exclusively included either trials in which participants regulated cognitive or affective responses together with LOOK trials. Run order was randomized across participants.
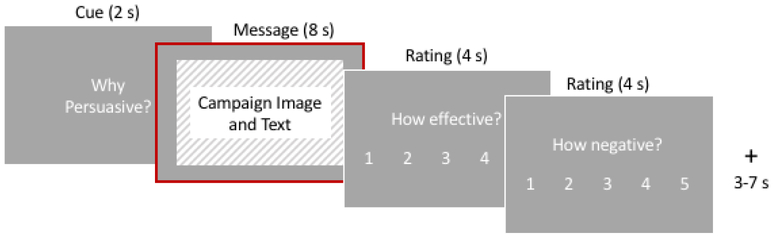
Each trial presented a 2 s cue screen showing the current condition. Then, the anti-drinking message was shown (8 s), while participants applied condition-specific thought processes. Finally, they rated their current negative affect and the perceived effectiveness of each anti-alcohol ad (4 s each). Trials were separated by jittered fixation periods (3–7 s; Figure 2).
Figure 2.
fMRI Task. Example trial (‘Why Persuasive?’ condition). Analyses focus on neural activity extracted from the message screen periods (outlined in red).
Regions of interest (ROIs).
We relied on a meta-analysis of over 200 neuroimaging studies to identify voxels for which increased activity can be used to probabilistically infer subjective valuation (Figure 9 in Bartra et al., 2013). We also examined activity within ventral striatal (VS) and ventromedial prefrontal cortex (VMPFC) portions of this subjective value map separately. In addition, we defined a cognitive regulation ROI based on the omnibus effect of trial type (message-inconsistent, look naturally, message-consistent) in a whole-brain ANOVA (FWE p < .05). In line with prior work on cognitive regulation (Buhle et al., 2014), this ROI consists of clusters in bilateral ventrolateral prefrontal cortex, dorsolateral prefrontal cortex, dorsomedial prefrontal cortex, and posterior parietal cortex. In addition, clusters within ventromedial prefrontal cortex and posterior cingulate were identified. Full results of this and other orthogonal analyses of this dataset are reported elsewhere (Doré et al., in press).
Mobile Electronic Diaries.
Every day during the field period, participants answered three short surveys through text messaging (two questions each). The four questions of relevance here are: (1 & 2, sent together) “In the past 24 hours, how many standard alcoholic drinks have you had? Were you around other people drinking alcohol? Reply # and Y/N”, and (3 & 4, sent together) “Have you talked to someone else about drinking or alcohol in the last 24 hours? How negatively or positively? Reply Y/N and 1(very negative) - 7(very positive).”
Drinking behavior and peer presence in the past 24 hours were measured at 11 AM and, on average, participants answered within 10 min (SD = 13.26 min). The occurrence and valence of conversations in the last 24 hours was measured at 3 PM. Participants answered, on average, within 9.17 min (SD = 12.76 min). Protocol compliance was high with an average completion rate of 81.18% (SD = 22.00%) for drinking behavior and peer presence, and 74.32% (SD = 20.86%) for conversational valence. We assessed drinking behavior and peer presence in close temporal proximity to night-time drinking occasions, but not at night to minimize participant burden and given large variability of what constitutes the end of a night. Conversational valence was assessed mid-day when participants are likely awake, not intoxicated, and socially active. Additional daily diary questions which are not the main focus here measured current mood.
Variables
Conversational valence.
We analyzed same-day and one-day lagged versions of the valence of daily conversations towards drinking and alcohol (1: very negative – 7: very positive) during the field period to estimate bi-directional, longitudinal relationships between drinking and conversational valence. This measure showed acceptable split-half reliability (comparing averages of odd- to even-numbered reports) (r = 0.74, p < .001) and test-retest reliability (comparing averages of the first and second half of ratings; r = 0.72, p < .001). An aggregate measure of conversational valence (mean valence of all conversations per participant) was used to isolate time-sensitive effects by controlling for general dispositions towards alcohol.
Drinking behavior.
Drinking behavior is defined as the daily number of drinks consumed during the field period, provided any alcohol was consumed that day. This behavior is targeted by most drinking campaigns which advocate drinking in moderation rather than abstinence. Further, this measure optimally uses the high temporal resolution of our data, increasing statistical power and approximating dynamic relationships between social interactions and health behavior. We excluded extreme values of ≥ 40 drinks per drinking occasion from analysis. Based on conservative parameters assuming 40 standard alcoholic drinks for a 200 pound male within a 24 hour period, we estimate a blood alcohol concentration of 340 mg/100 ml which approximates a fatal dose (Heatley & Crane, 1990). These values are assumed to be reporting mistakes or exaggerations. This applied to three data points for one participant. In line with prior work (Collins & Muraven, 2007; Shiffman, 2009; Wray et al., 2014) this measure showed acceptable split-half reliability (r = 0.92, p < .001) and test-retest reliability (r = 0.74, p < .001). Field period drinking behavior significantly correlated with baseline typical number of drinks per occasion over the last year (r = .46, p < .001), implying acceptable concurrent validity.
Drinking behavior was strongly right-skewed (skew = 3.04). We log-transformed the count data to be able to report readily interpretable parameter estimates produced by standard regression techniques. Same-day and one-day lagged versions of this variable were analyzed.
Binge Drinking Attitudes.
Attitude items were re-coded so that higher scores correspond to more negative (i.e. healthier) attitudes towards binge drinking. The final attitude score corresponds to the average of the 8 items (α = 0.76, M = 3.01, SD = 0.57).
Peer Presence.
Is a dichotomous daily indicator of whether the participant was in the presence of others who consumed alcohol in the last 24 hours (0: No, 1: Yes).
Neural activity.
Participants’ success and effort in cognitively regulating message-consistent and -derogating responses was indexed by increases in neural activity within value-related and cognitive regulation ROIs, respectively in (1) a message-consistent contrast, comparing neural activity in trials encouraging message-consistent processing of anti-drinking messages (INCREASE NEGATIVE and WHY PERSUASIVE trials) to natural responses (the LOOK condition), and (2) a message-derogation contrast, comparing message derogation (DECREASE NEGATIVE and WHY NOT PERSUASIVE trials) to LOOK trials. Thereby, we focused on individual differences in neural activity measured during the message-exposure period of each trial (Figure 2). In an orthogonal analysis to those presented here, Doré and colleagues (in press) show that, on average (across participants), the modulation of neural value-related activity mediates effects of the cognitive regulation strategy employed during message exposure on perceived message effectiveness, but individuals differed in their ability to find value in the stimuli. In addition, this previous work did not find differences across cognitive and affective regulation strategies. Thus, we do not distinguish between them here.
Typical drinking behavior.
The typical number of drinks consumed at drinking occasions over the last year is used as a control variable to isolate time-sensitive effects of conversations on drinking. The variable was right skewed and, thus, log-transformed.
Daily Mobile Electronic Diary Analysis
Conversational influence model.
To estimate time-sensitive effects of conversational influence on drinking, we built a multi-level model regressing daily drinking behavior during the field period on lagged conversational valence controlling for typical baseline drinking behavior, binge drinking attitudes, aggregate conversational valence during the field period, peer presence, and gender. Varying intercepts across participants and dates were entered to control for unmeasured between-person differences and history effects (e.g. exam periods), and to account for the nested data structure. All variables were grand mean centered. In a null-model using only random effects as predictors of conversational valence 5.38% in the outcome variance lay between dates, 34.34% between participants, and 60.28% within participants, leaving substantial variance to be explained by our within-participant and individual-difference measures.
Given our variable definitions, inclusion in the conversational influence model required, per participant, at least one co-occurrence of a drinking occasion (> 0 drinks in the last 24 hours) and a relevant conversation one day prior. Participants with only one eligible data point are included to improve the estimation of the residual variance and fixed effects (Martin, Nussey, Wilson, & Réale, 2011). The model is based on 52 participants.
Hangover model.
To assess whether results are driven by a general liking of alcohol consumption and pro-drinking conversations, we also regressed conversational valence on lagged drinking behavior, controlling for typical drinking at baseline, binge drinking attitudes, aggregate conversational valence across the field period, peer presence, and gender. Varying intercepts for participants and dates were included. All variables were grand mean centered. A null-model using only random effects as predictors of drinking behavior revealed that 2.23% in the outcome variance lay between dates, 32.84% between participants, and 64.92% within participants, leaving a substantial amount of variance to be explained by both our repeated, within-participant and individual-difference measures. Fifty-one participants reported at least one conversation about alcohol that coincided with a drinking occasion one day prior.
fMRI Acquisition and Analysis
fMRI data acquisition.
Neuroimaging was performed using a 3-Tesla Siemens Prisma whole-body MRI with a 64-channel head/neck array. Participants completed 4 task runs (2 runs with 458 and 2 runs with 443 volumes) while T2*-weighted images were collected (TR = 1 s, flip angle = 60°, - 30° tilt relative to AC-PC line, TE = 32 ms, 56 axial slices, voxel size = 2.5 × 2.5 × 2.5 mm, slice thickness = 2.5 mm, FOV = 208 mm, multiband acceleration factor = 4). High-resolution, structural T1-weighted images were acquired using an MPRAGE sequence (TI = 1100 ms, 160 axial slices, voxel size = 0.9 × 0.9 × 1). The structural T2-weighted image was collected in-plane (slice thickness = 1 mm, 176 axial slices, voxel size = 1 × 1 × 1).
fMRI data pre-processing.
Functional data were pre-processed using SPM8 (Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). Steps that incorporated tools from AFNI (Cox, 1996) and FSL (Smith et al., 2004) are explicitly identified in the following. The initial 5 volumes per run were not acquired to allow for stabilization of the BOLD (blood oxygen level dependent) signal. AFNI’s 3dDespike was used to despike functional images and FSL’s sinc interpolation to correct slice timing. BOLD data was then re-aligned to the first image. Next, two six-parameter affine steps were performed to register average T2*-weighted images to in-plane T2-weighted images and, subsequently, T1-weighted structural images to T2-weighted images. Following co-registration, high-resolution structural images were segmented into white matter, gray matter, and cerebral spinal fluid to identify voxels used in statistical modeling. The skull-stripped MNI template available in FSL (“MNI152_T1_1mm_brain.nii”) was used to normalize structural T1-weighted images. Finally, we used a Gaussian kernel (8 mm FWHM) to smooth functional images. The first-level fMRI model was constructed using fixed effects models within the general linear model in SPM8, using SPM’s canonical difference of gammas hemodynamic response function. The model included six rigid-body translation and rotation parameters derived from spatial realignment as nuisance regressors. A high-pass with a cutoff of 128 s was applied.
fMRI model.
The first-level fMRI model included separate boxcar functions modeling cue screen periods, message screen periods pooled into separate regressors based on condition (message-consistent and -derogating cognitions, message-consistent and message-derogating emotions, look trials in emotional and cognitive runs; i.e. six regressors), and each of the two rating screens. Fixation periods were pooled into a separate baseline rest regressor.
Neural moderation of conversational influence.
To test whether an individual’s effort (cognitive regulation ROI) and success (valuation ROI) in regulating responses to anti-drinking media was related to susceptibility to risky conversational influence over the following 30 days, we extracted average parameter estimates across voxels within each ROI for each participant from the message screen period (Figure 2) in the message-consistent ((WHY PERSUASIVE and INCREASE NEGATIVE) > LOOK) and message-derogation ((WHY NOT PERSUASIVE and DECREASE NEGATIVE) > LOOK) contrasts in Marsbar (Brett, Anton, & Valabregue, 2002).
Parameter estimates were divided by the grand mean to derive percent signal change. We then re-estimated the conversational influence model separately for each contrast and each ROI, adding an interaction term between lagged conversational valence and neural activity. All control variables with significant effects on drinking in the conversational influence model were included. All variables were grand mean centered. This analysis is based on 51 participants.
Results
Conversations and Drinking Behavior
We first investigated the prevalence of drinking-related conversations and drinking. Conversations occurred, on average, on 47.61% of days (SD = 22.66%, range = 3.57–95.00%) and were positively valenced towards drinking (M = 5.06, SD = 1.54; 7-point scale). Participants reported to have had alcohol a median of twice/week (4 on a 10-point scale; M = 4.90, SD = 1.22) over the last year. They consumed a median of 3–4 drinks per typical drinking occasion during the past year and identified a median of 1 occasion per month as a binge drinking occasion. In the field period, they drank on 40.26% of days, on average (SD = 19.65%, range = 0–90.32%), had a median of 3 drinks (M = 4.33, SD = 3.94, range = 1–35) per occasion, and were around peers who were drinking on 41.35% (SD = 22.60%) of days.
Conversational Influence and Hangover Effects
Next, we estimated the longitudinal effect of peer influence (valenced conversations about alcohol) on future drinking behavior and tested whether this relationship is reciprocal by regressing conversational valence on yesterday’s drinking (hangover effect; Table 1).
Table 1.
Unstandardized fixed effect estimates
| Predictor | Conversational influence model OV: Drinking behavior | Hangover model OV: Conversational valence | Moderation by message-consistent regulation success OV: Drinking Behavior | Moderation by message-derogating regulation success OV: Drinking Behavior |
|---|---|---|---|---|
| Peer Presence | 0.40 [0.08; 0.73], p = .016 | 0.02, [−0.28; 0.33], p = .887 | 0.47 [0.09; 0.85], p = .016 | 0.49 [0.12; 0.87], p = .011 |
| Attitudes | 0.12 [−0.13; 0.36], p = 0.376 | −0.07 [−0.35; 0.20], p = 0.601 | --- | --- |
| Lagged conversational valence (CV) | 0.11 [0.05; 0.17], p = .001 | 0.09 [0.03; 0.14], p = .004 | 0.08 [0.03; 0.14], p = .005 | |
| Typical drinking behavior | 0.47 [0.20; 0.73], p = .002 | 0.32 [−0.03; 0.66], p = .072 | 0.39 [0.12; 0.65], p= .007 | 0.40 [0.11; 0.68], p = .011 |
| Aggregate conversational valence | −0.08 [−0.23; 0.06], p = .296 | 1.04 [0.89; 1.20], p < .001 | --- | --- |
| Gender (0: male, 1: female) | −0.44 [−0.69; −0.19], p = .002 | −0.06 [−0.34; 0.22], p = .683 | −0.48 [−0.74; −0.22], p = .001 | −0.50 [−0.77; −0.24], p = .001 |
| Lagged drinking behavior | --- | −0.24 [−0.43; −0.05], p = .013 | --- | --- |
| Neural Valuation activity (NV) | --- | --- | 0.19 [−1.15; 1.53], p = .788 | −0.45 [−2.13; 1.22], p = .613 |
| Interaction CV × NV | --- | --- | −0.591 [−1.22; 0.03], p = .064 | 0.621 [−0.04; 1.28], p = .066 |
Note. Square brackets show 95% Confidence intervals. OV = outcome variable, --- effects not included in the model, Neural valuation activity represents average activity estimates in clusters within ventral striatum and ventromedial prefrontal cortex. All variables are grand mean centered.
Effects are primarily driven by ventral striatal rather than ventromedial prefrontal cortex activity (see Table 2).
In the conversational influence model, more positive conversations about drinking led to more drinks being consumed the next day. Aggregate conversational valence did not show effects over and above this time-sensitive measure. The hangover model showed negative effects of lagged drinking behavior on conversational valence. Those who drank more talked more negatively about drinking the following day. These results expand on prior work by showing that the relationship between conversational influence and drinking is time-sensitive and not always positive. That is, conversational influence on drinking is not fully explained by an association between frequent drinking and having positive conversations about drinking on average.
Regulation of Neural Valuation Responses to Media Messages Moderates Peer Influence
Next, we tested whether variance in susceptibility to conversational influence on drinking is partially explained by an individual’s success when regulating neural value-related responses to anti-drinking messages. That is, we investigated an interaction term between lagged conversational valence and percent signal change in the valuation ROI in the message-consistent and message-derogation contrasts, respectively (Table 1). We found a (marginally significant) negative interaction between lagged conversational valence and message-consistent changes in brain activity within the valuation ROI (VMPFC/VS), driven by a significant interaction between lagged conversational valence and VS activity (β = −0.75, 95% CI [−1.38; −0.13], p = .020; see Table 2 for full results for VS and VMPFC). Those who showed more value-related neural activity when up-regulating responses consistent with anti-drinking messages at baseline, showed less susceptibility to conversational influence in the 30-day field period. For the message-derogation contrast, we found a marginally significant pattern in the opposite direction, so that those who showed higher increases in neural valuation activity when up-regulating message-derogating responses showed greater susceptibility to conversational influence. This effect was also driven by VS activity (β = 1.02, 95% CI [0.22; 1.83], p = .014; see Table 2).
Table 2.
Unstandardized fixed effects on drinking behavior
| VMPFC | VS | |||
|---|---|---|---|---|
| Moderation by message-consistent regulation success | Moderation by message-derogating regulation success | Moderation by message-consistent regulation success | Moderation by message-derogating regulation success | |
| Lagged conversational valence (CV) | 0.09 [0.03; 0.15], p = .002 | 0.09 [0.03; 0.15], p = .004 | 0.08 [0.02; 0.14], p = .008 | 0.08 [0.02; 0.14], p = .005 |
| Peer Presence | 0.47 [0.10, 0.86], p = .015 | 0.48 [0.10; 0.86], p = .014 | 0.49 [0.12; 0.86], p = .011 | 0.50 [0.13; 0.88], p = .009 |
| Typical drinking behavior | 0.39 [0.13; 0.66], p= .007 | 0.42 [0.14; 0.70], p = .007 | 0.40 [0.14; 0.66], p= .005 | 0.37 [0.10; 0.65], p = .013 |
| Gender (0: male, 1: female) | −0.47 [−0.74; −0.21], p = .002 | −0.50 [−0.77; −0.23], p = .001 | −0.48 [−0.74; −0.23], p = .001 | −0.49 [−0.76; −0.22], p = .002 |
| Neural Valuation activity (NV) | 0.07 [−1.06; 0.93], p = .899 | −0.45 [−1.68; 0.77], p = .488 | 0.57 [−0.89; 2.02], p = .465 | −0.12 [−2.18; 1.94], p = .910 |
| Interaction CV × NV | −0.28 [−0.77; 0.21], p = .263 | 0.31 [−0.18; 0.81], p = .215 | −0.75 [−1.38; −0.13], p = .020 | 1.02 [0.22; 1.83], p = .014 |
Note. 95% Confidence intervals are in square brackets. ROI = Region of Interest, VMPFC = ventromedial prefrontal cortex, VS = ventral striatum, --- denotes effects not included in the model. All variables are grand mean centered. N = 51
We also examined cognitive regulation efforts (i.e. activity in the cognitive regulation ROI), which we theorize to be a crucial input to the neural value-system. In this model, effects of conversational valence and all control variables were comparable in magnitude and direction to those shown for the neural value model (Table 2). Further, there was a negative trending (non-significant) interaction of conversational valence with neural activity associated with cognitive regulation in the message-consistent contrast (β = −0.67, 95% CI[−1.62; 0.25], p = .163) and a positive trending, non-significant, interaction in the message-derogation contrast (β = 0.66, 95% CI[−0.10; 1.41], p = .096). Note that for all models described here, main results of interest remain unchanged in direction and magnitude when excluding all covariates from the models (see Supplementary Materials). Our results highlight the prominent role of neural value-related activity as a final consequence of cognitive regulation which is related to downstream outcomes.
Discussion
Anti-drinking media campaigns and pro-drinking peer conversations compete for influence on college students’ alcohol consumption behavior (DeJong, 2002; Hendriks et al., 2012). To shed light on the mechanisms that drive and explain how these competing sources of influence are integrated, we propose a “common value” account where competing sources of influence may dynamically interact and serve as inputs to the computation of the subjective value of behaviors within the brain’s value system which impacts behavior. Here, we show that effects of pro-alcohol peer influence on an individual’s drinking behavior depend on a person’s success in cognitively regulating neural value-related responses, particularly within the ventral striatum, to anti-drinking media messages in ways that are consistent with or derogate the media message.
Our findings provide a fine-grained assessment of the relationship between peer influence and drinking behavior in an ecologically valid setting. In line with prior work (Hendriks et al., 2012, 2015), conversations about drinking in our sample of college students were net positive towards alcohol consumption. Conversational valence impacted the number of drinks consumed the next day and this time-sensitive measure of peer influence was a better predictor of drinking than a commonly used measure of average conversational valence over the field period. Further demonstrating the value of this time-sensitive approach, we show an additional ‘hangover effect’. When participants consumed more alcohol, their conversations the next day were more negative. Contrary to the intuition that heavier drinkers generally talk more positively about alcohol, this finding adds nuance to our view of the relationship between conversations and behavior. More broadly, we add specificity to prior work which showed aggregate relationships between conversational valence and drinking (Dorsey et al., 1999; Hendriks et al., 2015).
Next, we show that susceptibility to conversational influence on drinking is moderated by neural responses to anti-drinking campaigns. Specifically, people who showed greater neural valuation responses when up-(/down-)regulating message-consistent thoughts were less(/more) susceptible to risky peer influence. Similarly, those who showed more neural activity associated with cognitive regulation during message-consistent(/-inconsistent) processing of anti-drinking ads were directionally, but not significantly, less(/more) affected by pro-alcohol conversations. We propose that activity within the neural value-system is a crucial outcome of cognitive regulation efforts (indexed by cognitive regulation ROI activity). In turn, changes in valuation ROI activity following cognitive regulation affect susceptibility to conversational influence.
These data are consistent with expectancy value theory, which suggests that humans make decisions guided by a weighted sum of the expected positive and negative outcomes of available choice options (Neumann & Morgenstern, 2007; Samuelson, 1937). This signal is encoded within the neural value-system (Bartra et al., 2013; Clithero & Rangel, 2013; Levy & Glimcher, 2012). This framework has been supported across myriad domains including social approval, consumer goods, and monetary value and it has recently been extended to dynamic decision-making processes in the context of competing goals (e.g. drinking with peers while not incurring the negative outcomes shown in anti-drinking media messages; Christopoulos & Schrater, 2015). Our data are not suitable to formally test differential predictions of ‘goods-based’/static theories and dynamic versions of these decision-making models. This is a fruitful direction for future work focused on the relationships uncovered here.
In addition, value-based decision making lies at the heart of both message-based persuasion and peer influence (Falk & Scholz, 2018), as well as what has traditionally been thought of as “self-control” over behaviors like drinking (Berkman, Livingston, & Kahn, 2017). In the context of competing sources of influence (e.g. media and peers) on health behavior, we argue that each source may provide inputs to an individual’s calculation of the value that may be gained by performing a health-related behavior and that these inputs are aggregated and integrated in the neural value signal and updated over time. For instance, anti-drinking media messages may serve to decrease the perceived value of drinking and thus the likelihood of consumption, whereas pro-drinking peer influence may enhance the perceived value and alcohol consumption. Cognitive regulation of the subjective value of one of these inputs (implemented here through a manipulation of message response regulation) should thus impact the calculation of the weighted sum of overall choice value and thereby affect behavior. In line with this framework, we found that those who were more successful in the up-regulation of the perceived value of anti-drinking message-consistent (/-derogating) brain responses showed diminished (/increased) susceptibility to a second, competing source of influence on behavior, namely pro-drinking peer conversations.
Our results were directionally identical for activity extracted from ventral striatum (VS) and ventromedial prefrontal cortex (VMPFC), but VS was a somewhat stronger moderator. In previous work, activity in both regions was used to predict diverse behaviors, but neither region showed a consistent advantage across studies (Berns & Moore, 2012; Falk & Scholz, 2018; Genevsky et al., 2017). Differences in predictive power in our findings may be due to different levels of sampling error or signal variability. An alternative explanation is that VS and VMPFC may encode different aspects of behavioral value. VMPFC is often thought to encode higher-level, calculated processing, reflecting a weighted sum of decision-relevant inputs from a multitude of other brain regions (Falk & Scholz, 2018). VS, in contrast, may encode a lower-level, affective reward perception (Genevsky et al., 2017) and is also sensitive to social rewards (Rademacher et al., 2010). It is possible that in our study, cognitive regulation of low-level affective reactions to anti-drinking ads (encoded in VS) and social feedback in peer conversations each contributed to a common valuation process that impacted downstream behavior. Further understanding the roles of VS and VMPFC is a crucial goal for future work.
Our results show that individuals’ appraisals of media messages are related to their susceptibility to peer influence. This highlights the potential for low-cost, message-based interventions to impact social dynamics which are known to influence health behaviors (Katz & Lazarsfeld, 1955; Rogers, 2003), but are rarely targeted in public health campaigns given high costs of individual- or community-based social interventions. A fruitful future direction may be interventions supporting the cognitive up-regulation of value responses to healthy, but not unhealthy, sources of influence (Berkman et al., 2017). This effort may be guided by tools developed in emotion-regulation research (Gross, 2015). In turn, interventions which down-regulate valuation of alcohol marketing may curb its unhealthy effects, but require further study.
Limitations
Our novel daily mobile electronic diary approach to study conversational influence has many advantages, but is subject to known limitations (Shiffman, 2009; Wray et al., 2014) such as testing effects due to repeated reporting and a potential bias towards conscientious participants. This may limit ecological validity. Further, our findings relating neural activity to behavior are correlational. We controlled several alternative explanations through covariates (e.g. attitudes), but causal inferences require an experimental design in the field period. Further, our interpretation of the neural data is based on meta-analytic brain mapping evidence and a priori hypotheses, but still constitutes a reverse inference (Poldrack, 2011). To formally test the common-value account proposed here, future work may identify value-related neural activity independently using a localizer task and test value-related responses to media and social influence within the same fMRI scan. Finally, our work focused on college students, a crucial population for alcohol research (Hingson et al., 2009). Yet, differences in drinking, sensitivity to social influence, and brain development across the lifespan, require additional work to understand variance in effects across populations.
Conclusion
Taken together, our results provide new insights into how information from competing sources of influence is reconciled in decisions about health behavior. Cognitive regulation of neural valuation responses to anti-drinking media messages was linked to the degree to which alcohol consumption was affected by pro-drinking peer influence. Our time-sensitive, ecologically valid model of conversational influence on drinking in college students provides a more nuanced view of peer influence than previously reported and complements our mechanistic account of how neural valuation responses moderate these effects. Together with prior work in communication and social neuroscience, these results support a framework where competing sources of influence (e.g. media and peers) constitute separate inputs to the perceived value to be gained by performing a behavior. Cognitive regulation of subjective valuation in response to each source may impact the integration of different sources of influence into a final weighted sum, the perceived value of a behavior, which ultimately influences health behaviors.
Supplementary Material
Funding Sources:
The National Institutes of Health (New Innovator Award - NIH 1DP2DA03515601; PI Falk), an FDA Center for Tobacco Products pilot grant (PI Falk & Doré), and HopeLab supported this work.
References
- Bartra O, McGuire JT, & Kable JW (2013). The valuation system: A coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage, 76, 412–427. 10.1016/j.neuroimage.2013.02.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman ET, Livingston JL, & Kahn LE (2017). Finding The “Self” in Self-Regulation: The Identity-Value Model. Psychological Inquiry, 28(2–3), 77–98. 10.1080/1047840X.2017.1323463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns G, & Moore SE (2012). A neural predictor of cultural popularity. Journal of Consumer Psychology, 22(1), 154–160. 10.1016/j.jcps.2011.05.001 [DOI] [Google Scholar]
- Borsari B, & Carey KB (2003). Descriptive and injunctive norms in college drinking: a meta-analytic integration. Journal of Studies on Alcohol, 64(3), 331–341. 10.15288/jsa.2003.64.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle SC, LaBrie JW, Froidevaux NM, & Witkovic YD (2016). Different digital paths to the keg? How exposure to peers’ alcohol-related social media content influences drinking among male and female first-year college students. Addictive Behaviors, 57(Supplement C), 21–29. 10.1016/j.addbeh.2016.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton J-L, & Valabregue J-BP (2002). Region of interest analysis using an SPM toolbox. NeuroImage, 16(2). [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, … Ochsner KN (2014). Cognitive Reappraisal of Emotion: A Meta-Analysis of Human Neuroimaging Studies. Cerebral Cortex, 24(11), 2981–2990. 10.1093/cercor/bht154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulos V, & Schrater PR (2015). Dynamic Integration of Value Information into a Common Probability Currency as a Theory for Flexible Decision Making. PLOS Computational Biology, 11(9), e1004402 10.1371/journal.pcbi.1004402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua HF, Ho SS, Jasinska AJ, Polk TA, Welsh RC, Liberzon I, & Strecher VJ (2011). Self-related neural response to tailored smoking-cessation messages predicts quitting. Nature, 20(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clithero JA, & Rangel A (2013). Informatic parcellation of the network involved in the computation of subjective value. Social Cognitive and Affective Neuroscience, 9(9), 1289–1302. 10.1093/scan/nst106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins RL, & Marlatt GA (1981). Social modeling as a determinant of drinking behavior: Implications for prevention and treatment. Addictive Behaviors, 6(3), 233–239. 10.1016/0306-4603(81)90021-6 [DOI] [PubMed] [Google Scholar]
- Collins RL, & Muraven M (2007). Ecological momentary assessment of alcohol consumption In Stone A, Shiffman S, Atienza A, & Nebeling L (Eds.), The Science of Real-Time Data Capture: Self-Reports in Health Research (p. 189ff). USA: Oxford University Press. [Google Scholar]
- Cooper N, Tompson S, O’Donnell MB, & Falk EB (2015). Brain activity in self- and value-related regions in response to online antismoking messages predicts behavior change. Journal of Media Psychology, 27(3), 93–109. 10.1027/1864-1105/a000146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–173. 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- David C, Cappella JN, & Fishbein M (2006). The social diffusion of influence among adolescents: Group interaction in a chat room environment about antidrug advertisements. Communication Theory, 16(1), 118–140. 10.1111/j.1468-2885.2006.00008.x [DOI] [Google Scholar]
- DeJong W (2002). The role of mass media campaigns in reducing high-risk drinking among college students. Journal of Studies on Alcohol, Supplement, (s14), 182–192. 10.15288/jsas.2002.s14.182 [DOI] [PubMed] [Google Scholar]
- Doré BP, Cooper N, Scholz C, O’Donnell MB, & Falk EB (in press). Cognitive regulation of ventromedial prefrontal activity evokes lasting change in the perceived self-relevance of persuasive messaging. Human Brain Mapping. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsey AM, Scherer CW, & Real K (1999). The College Tradition of “Drink ‘Til You Drop”: The Relation Between Students’ Social Networks and Engaging in Risky Behaviors. Health Communication, 11(4), 313–334. 10.1207/S15327027HC1104_1 [DOI] [Google Scholar]
- Falk EB, Berkman ET, & Lieberman MD (2012). From neural responses to population behavior: Neural focus group predicts population-level media effects. Psychological Science, 23(5), 439–445. 10.1177/0956797611434964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk EB, & Scholz C (2018). Persuasion, Influence, and Value: Perspectives from Communication and Social Neuroscience. Annual Review of Psychology, 69(1), 329–356. 10.1146/annurev-psych-122216-011821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genevsky A, Yoon C, & Knutson B (2017). When Brain Beats Behavior: Neuroforecasting Crowdfunding Outcomes. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 37(36), 8625–8634. 10.1523/JNEUROSCI.1633-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore FM, Bloem PJ, Patton GC, Ferguson J, Joseph V, Coffey C, … Mathers CD (2011). Global burden of disease in young people aged 10–24 years: a systematic analysis. The Lancet, 377(9783), 2093–2102. 10.1016/S0140-6736(11)60512-6 [DOI] [PubMed] [Google Scholar]
- Gross JJ (2015). Emotion Regulation: Current Status and Future Prospects. Psychological Inquiry, 26(1), 1–26. 10.1080/1047840X.2014.940781 [DOI] [Google Scholar]
- Heatley MK, & Crane J (1990). The blood alcohol concentration at post-mortem in 175 fatal cases of alcohol intoxication. Medicine, Science , and the Law, 30(2), 101–105. 10.1177/002580249003000203 [DOI] [PubMed] [Google Scholar]
- Hendriks H, & de Bruijn G-J (2015). What Do Dutch College Students Talk about When They Talk about Alcohol? Health Behavior and Policy Review, 2(3), 232–242. 10.14485/HBPR.2.3.8 [DOI] [Google Scholar]
- Hendriks H, de Bruijn G-J, & van den Putte B (2012). Talking about alcohol consumption: Health campaigns, conversational valence, and binge drinking intentions: Talking about alcohol consumption. British Journal of Health Psychology, 17(4), 843–853. 10.1111/j.2044-8287.2012.02080.x [DOI] [PubMed] [Google Scholar]
- Hendriks H, van den Putte B, & de Bruijn G-J (2015). Subjective Reality: The Influence of Perceived and Objective Conversational Valence on Binge Drinking Determinants. Journal of Health Communication, 20(7), 859–866. 10.1080/10810730.2015.1018570 [DOI] [PubMed] [Google Scholar]
- Hendriks H, van den Putte B, de Bruijn G-J, & de Vreese CH (2014). Predicting Health: The Interplay Between Interpersonal Communication and Health Campaigns. Journal of Health Communication, 19(5), 625–636. 10.1080/10810730.2013.837552 [DOI] [PubMed] [Google Scholar]
- Hingson RW, Zha W, & Weitzman ER (2009). Magnitude of and trends in alcohol-related mortality and morbidity among U.S. college students ages 18–24. Journal of Studies on Alcohol and Drugs, (s16), 12–20. 10.15288/jsads.2009.s16.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong M, & Bae RE (2017). The Effect of Campaign-Generated Interpersonal Communication on Campaign-Targeted Health Outcomes: A Meta-Analysis. Health Communication, 0(0), 1–16. 10.1080/10410236.2017.1331184 [DOI] [PubMed] [Google Scholar]
- Johnston LD (2010). Monitoring the Future: National Survey Results on Drug Use, 1975–2008: Volume II: College Students and Adults Ages 19.-. DIANE Publishing. [Google Scholar]
- Katz E, & Lazarsfeld PF (1955). Personal Influence, The part played by people in the flow of mass communications. Glencoe: Free Press. [Google Scholar]
- Klucharev V, Munneke MAM, Smidts A, & Fernandez G (2011). Downregulation of the Posterior Medial Frontal Cortex Prevents Social Conformity. Journal of Neuroscience, 31(33), 11934–11940. 10.1523/JNEUROSCI.1869-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsche E, Knibbe R, Gmel G, & Engels R (2005). Why do young people drink? A review of drinking motives. Clinical Psychology Review, 25(7), 841–861. 10.1016/j.cpr.2005.06.002 [DOI] [PubMed] [Google Scholar]
- LaBrie JW, Hummer JF, & Pedersen ER (2007). Reasons for Drinking in the College Student Context: The Differential Role and Risk of the Social Motivator. Journal of Studies on Alcohol and Drugs, 68(3), 393–398. 10.15288/jsad.2007.68.393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DJ, & Glimcher PW (2012). The root of all value: a neural common currency for choice. Current Opinion in Neurobiology, 22(6), 1027–1038. 10.1016/j.conb.2012.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JGA, Nussey DH, Wilson AJ, & Réale D (2011). Measuring individual differences in reaction norms in field and experimental studies: a power analysis of random regression models: Power analysis of random regression models. Methods in Ecology and Evolution, 2(4), 362–374. 10.1111/j.2041-210X.2010.00084.x [DOI] [Google Scholar]
- Neumann J. von, & Morgenstern O (2007). Theory of Games and Economic Behavior. Princeton University Press. [Google Scholar]
- NIAAA. (2003). Recommended Alcohol Questions | National Institute on Alcohol Abuse and Alcoholism (NIAAA). Retrieved from https://www.niaaa.nih.gov/research/guidelines-and-resources/recommended-alcohol-questions
- Poldrack RA (2011). Inferring mental states from neuroimaging data: from reverse inference to large-scale decoding. Neuron, 72(5), 692–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher L, Krach S, Kohls G, Irmak A, Gründer G, & Spreckelmeyer KN (2010). Dissociation of neural networks for anticipation and consumption of monetary and social rewards. NeuroImage, 49(4), 3276–3285. 10.1016/j.neuroimage.2009.10.089 [DOI] [PubMed] [Google Scholar]
- Real K, & Rimal RN (2007). Friends Talk to Friends About Drinking: Exploring the Role of Peer Communication in the Theory of Normative Social Behavior. Health Communication, 22(2), 169–180. 10.1080/10410230701454254 [DOI] [PubMed] [Google Scholar]
- Rogers EM (2003). Diffusion of innovations (5th ed.). New York, NY: Free Press. [Google Scholar]
- Samuelson PA (1937). A Note on Measurement of Utility. The Review of Economic Studies, 4(2), 155–161. 10.2307/2967612 [DOI] [Google Scholar]
- Shiffman S (2009). Ecological momentary assessment (EMA) in studies of substance use. Psychological Assessment, 21(4), 486–497. 10.1037/a0017074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, … others. (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage, 23, S208–S219. 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Wray TB, Merrill JE, & Monti PM (2014). Using Ecological Momentary Assessment (EMA) to Assess Situation-Level Predictors of Alcohol Use and Alcohol-Related Consequences. Alcohol Research : Current Reviews, 36(1), 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung AWK (2018). An Updated Survey on Statistical Thresholding and Sample Size of fMRI Studies. Frontiers in Human Neuroscience, 12 10.3389/fnhum.2018.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Schirmer J, & Mitchell JP (2011). Social influence modulates the neural computation of value. Psychological Science, 22(7), 894–900. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.