Summary
The purpose of this article is to review the role of extrafollicular and T-cell independent antibody responses in humoral immunity. We consider two interrelated questions: 1) Do T-cell independent antibody responses dominated by IgM and/or IgA play unique functions in immunity and homeostasis; and 2) is it typical for these responses to result in life-long protection? In addressing these questions, we consider the established advantages of T-cell driven responses including the unique role played by germinal center reactions in these responses, and contrast the processes and outcomes of germinal center-centric responses with germinal center- and T-cell independent antibodies. We suggest that T-independent and other extrafollicular responses contribute substantially to highly stable antibody repertoires in both the serum and the intestine, providing relatively constitutive humoral barriers with the collective dual function of protecting against invading pathogens and regulating the composition of non-pathogenic microbial communities.
Keywords: B cells, antibodies, cell differentiation, memory, plasma cells, germinal centers
Introductory remarks
It has become popular for those of us studying antibody responses to focus on antigens that also stimulate a robust T cell response. These proteinaceous antigens, typically classified as T-cell dependent, induce complex cell-cell communication networks between dendritic cells, macrophages, and antigen-reactive T and B cells. The study of such responses is of course highly warranted, as the resulting high-affinity antibodies can play critical and nonredundant roles in life-long protection against a variety of pathogens 1. Furthermore, such responses are highly regulated, and can be influenced in dramatic ways by two separate but related processes that together improve the affinity of the resulting antibodies and switch the class of the antibody from IgM to IgG or other heavy chain classes 2. By contrast, responses to non-proteinaceous antigens that lack molecular attributes needed to activate T cells, T-cell independent antigens that are often enriched for polysaccharide and/or lipopolysaccharide structures, tend to produce mainly low affinity IgM antibodies. It is generally assumed that responses generating high affinity IgG antibodies are always the desired outcome, and for responses to many pathogens including influenza and HIV this appears to be the case 3–7.
In 1989 Herzenberg and Herzenberg proposed that mammalian immune systems consist of multiple layers, with each layer emerging at distinct phases of evolution. Thus, lymphoid and myeloid cells characterized by progressively more sophisticated functions are proposed to have emerged at later stages of evolution, a process reflected by the tendency of many of these cells to develop later in ontogeny 8. Given that the T cell dependent generation of high affinity IgG antibodies relies on both a highly unique and regulated DNA recombination, and a DNA-directed mutational process intended to target antibody variable region genes specifically, it seems fair to consider these processes and the cells and antibodies they produce as relatively sophisticated, for lack of a better metaphor. In contrast, because antigens that fail to induce substantial T cell activation also fail to induce high affinity IgG, such responses and the cells mediating these responses might be considered less sophisticated. But, as we will discuss below, T-independent responses may nonetheless play unique and important roles in a variety of scenarios.
It is the purpose of this article to consider the idea that T-independent antibodies play unique roles in both host protection and barrier surface homeostasis. In this regard, we propose that the evolutionary principles guiding the development of a functional humoral immune system apply equally to T-dependent and T-independent antibodies. We consider that the selection of such a system (or systems) was guided by the following requirements and processes: 1) The generation of a sufficiently diverse antigen-binding repertoire, created by a somatic DNA recombination process. This process is linked to a cell genesis machinery geared to generate sufficient numbers of naïve B cells for producing adequately strong primary responses; 2) A somatic learning process for eliminating self-reactive cells coupled, in principle, to germ-line encoded pattern recognition mechanisms for identifying dangerous agents; and 3) The capacity to adjust the class of the response to achieve maximum protective power together with minimal damage to host tissues. Regulation of antibody class is achieved with systems that distinguish and utilize a variety of cell-extrinsic signals affiliated with different regions of the body or perhaps different classes of micro-organisms. By regulating the class of the response, pools of B-lineage cells can eliminate a variety of dangerous agents by producing pro-inflammatory antibodies while other B cells produce relatively noninflammatory antibodies that contribute to the management of complex beneficial microbial communities. Whereas T-dependent IgG dominated responses can be divided into different pro-inflammatory subclasses affiliated with type 1 or type 2 T cell responses, as we will see below T-independent antibodies can also influence the class of the response, and include both pro-inflammatory and relatively non-inflammatory modes.
An additional and key feature of adaptive humoral immunity is its capacity to provide life-long protection. Indeed, serum antibody concentrations to many T-cell dependent antigens can persist without measurable decay for years in mice and for decades or longer in people 1, 9–12. Because serum antibodies possess exceptionally short half-lives 13, maintenance of serum antibodies requires the generation of plasma cells with exceptionally long lifespans. Thus, while naïve B cells appear to possess a half-life ranging between 3–4 months in mice (but likely much longer in people) 14–16, current evidence suggests that the lifespan of many memory B cells and plasma cells can be substantially longer 9, 11, 12, 16, 17. Indeed, when one considers the lack of detectable decay rates for memory B cell populations in mice and serum antibody titers in mice and people, it is difficult to avoid the conclusion that many of these cells can persist without cell division for the life of the host. How these extended lifespans are achieved remains largely mysterious.
With these ideas in mind we can formulate some specific questions to set the stage for our discussion: 1) In what scenarios are T-independent antibodies useful and perhaps even desirable? 2) To what extent do T-independent antibodies function to regulate rather than destroy microbes and the communities in which they live? 3) To what extent do T-cell independent pathways of antibody synthesis contribute to long-lived plasma cell pools and highly stable secreted antibody repertoires, and is such stability advantageous?
Basic elements of the germinal center reaction
It has become natural, almost instinctive, for researchers when considering antibody responses to place them in one of several categories. This strategy is understandable, as it provides a framework for thinking about the cell-cell and molecular events responsible for the observed response. These categories are typically based either on the type of antigen involved (T-cell dependent versus T-independent), the class of antibody produced (IgM, IgG or IgA, and so on), or the subtype of B cell believed to be responsible (follicular, B1, etc.) for the response in question. It has become common, understandably, for researchers to focus their attentional primarily on the role of germinal centers (GCs) in each type of response. GCs are unique antigen- and T-cell dependent structures that form in peripheral lymphoid tissues. The focus on GCs has several advantages: GCs appear to be essential for generating high-affinity antibodies to relevant pathogens including influenza and HIV 3–7, and they also appear to be the chief source of self-reactive pathogenic antibodies 18–20.
GCs have been reviewed by multiple writers and from numerous angles 21–25. Hence, we will provide a bird’s eye view of these structures to set the stage for our discussion on the key events in extrafollicular responses and how they contrast with GC-based responses. Very early in responses induced by proteinaceous antigens activated B and T cells congregate at boundaries between T cell rich regions in the spleen or lymph node and B cell enriched follicles. What follows is a series of poorly understood but apparently highly coordinated events. Both B and T cells undergo several rounds of cell division, and within each lineage the resulting daughter cells split off into effector cells and cells that will ultimately initiate or regulate events within GCs. During these early phases in the spleen activated B cells also migrate into the vicinity of the marginal sinus where it appears they must interact with marginal zone macrophages 26, 27. Specialized macrophages in the marginal zone take up antibody-antigen complexes and complement coated antigens with great efficiency 28; this step may reflect a key but underappreciated process whereby macrophages optimize access of activated B cells to antigen in the spleen and also in lymph nodes 29. Ultimately, these cell-cell interactions result in a wave of IgM-secreting plasma cells and the subsequent generation of GCs. At this juncture it should also be emphasized that in the spleen IgM-secreting cells are easily observed outside of B cell rich follicles in the red pulp, where their movement is controlled by the chemokine CXCL12. These plasma cells embody many defining features of extrafollicular antibody responses.
A GC can be divided into two zones, known as the dark and light zones, where B cell receptor specificity is revised and tested. Activated B cells within the GC dark zone are subjected to a somatic hypermutation (SHM) process resulting in the mutation of multiple nucleotides across the variable region coding sequence. Subsequently these cells migrate to the light zone where they must compete effectively within this physical space for at least two signals: a BCR signal provided by antigen-coated follicular dendritic cells (FDCs) 30, and essential additional signals provided by activated follicular helper T cells 31. The ultimate outcome of this Darwinian-like selection process is the generation of memory B cells and plasma cells, each with increased affinity for the immunizing antigen. Whereas it appears that memory B cells re-enter recirculating lymphocyte pools as exceptionally long-lived cells, many of the resulting plasma cells home to the bone marrow where it is theorized they must integrate into specialized microenvironments to gain access to unique cell-extrinsic pro-survival signals 32–34. Here many such cells appear to persist for decades or longer as long-lived non-dividing antibody cells 11. Whereas much remains to be learned about plasma cell survival signals, it is clear that plasma cells cannot survive without the BAFF cytokine family receptor known as BCMA 35, 36.
GCs and long-lived humoral immunity
What is less clear is whether GCs provide unique microenvironmental signals needed for emerging memory B cells and plasma cells to become long-lived. If we focus this question on plasma cells, such GC-unique signals would in theory initiate gene expression networks needed for newborn plasma cells to effectively utilize BCMA-derived signals, and to migrate to appropriate bone marrow niches to access BCMA ligands. Notably however, how B cells and/or newborn plasma cells initiate BCMA expression and become receptive to BCMA-derived signals is unknown. Because even less is known about control of memory B cell lifespan, developing a parallel model for the signals and receptors mediating the exceptionally lengthy lifespan of many memory B cells is an even greater challenge. Nonetheless, an important question at this juncture concerns the relationship, or perhaps lack thereof, between the generation and selection of high affinity B cells and plasma cells, and the implementation of gene expression networks needed for these cells to become long-lived.
We suggest that because GCs have long been associated with antibody class switching and somatic hypermutation, and because they are typically considered to be a key source for substantial numbers of long-lived plasma and memory B cells, the three processes are easily conflated. Is this appropriate? One possibility is that relatively durable high affinity interactions between antigen receptors on GC B cells and antigen coated FDCs deliver unique inductive signals that provide plasma and memory B cells with what it takes to avoid apoptosis for the long term. While a connection between high affinity receptors and plasma cell differentiation has been considered 37, 38, this idea has been challenged by data indicating the propensity of GCs to produce high-affinity plasma cells instead reflects increased proliferation of high-affinity B cells 39. Weisel et al. recently examined the timing with which the bulk of memory B cells and plasma cells are produced post-immunization 40. Surprisingly, this work revealed that while the vast majority of plasma cells arise coincident with the GC reaction, most memory B cells appear to arise much earlier including before GCs were detected 40. Furthermore, these cells appear to be long-lived cells. This observation is consistent with other work showing that long-lived IgM+ memory B cells can form via a GC-independent pathway 41.
Germinal center-independent responses
For the question of how Ag-responsive B and plasma cells become long-lived, a simple test of the GC-centric model is provided by T-independent antigens. Such antigens generally fail to induce functional GCs. [A closer look at this statement reveals that such antigens can induce GCs, but these structures resolve quickly without producing and selecting for high-affinity cells 42–44.] Until recently, it was widely believed that T-independent antigens fail to engender long-lived responses, instead only producing IgM-secreting plasma cells that die within days of their generation 33. Several papers published 5–7 years ago provide data indicating the contrary. By simply immunizing wild type or T cell deficient mice with polysaccharide or LPS-based antigens, several laboratories reported that antigen-specific plasma cells induced by any of these antigens readily migrate to the bone marrow where they persist for extended periods cells 42, 45, 46. Indeed, we reported that immunization with haptenated LPS induces plasma cells that persist in the bone marrow for upwards of 2 years, approaching the lifespan of the mouse. Moreover, we also detected long-lived marrow plasma cells in experiments with a T-dependent antigen where we blocked early GC responses by giving immunized mice CD40-CD40ligand blocking antibodies. Notably, as expected the plasma cells that emerged despite arresting germinal center responses secreted low-affinity IgM rather than high affinity class switched antibodies 42. Therefore, while IgG-secreting plasma cells clearly dominate responses to immunization with exogenous T-dependent antigens, other pathways may also be at play. Interestingly, we recently reported that, in mice that were not intentionally immunized, upwards of 40% of bone marrow plasma cells secrete IgM, with another 40% or more actively secreting IgA 47. Hence at steady state numbers of IgG-secreting plasma cells in the BM at times may be dwarfed by other plasma cells. Altogether these findings suggest: 1) a meaningful number of the extrafollicular plasma cells observed before GC reactions may survive to integrate into long-lived plasma cell pools, and 2) plasma cells can acquire receptivity to unique pro-survival signals needed to persist in the bone marrow without emerging from a GC.
Evidence is also emerging that many memory B cells arise with little input from GCs. Many but not all of these cells express surface IgM rather than IgG or IgA, and such cells are readily detected in wild type mice and in mice incapable of producing GCs due to mutation of key transcription factors such as BCL6 48–50. As alluded to above, this idea can be taken a step further when considering the early timing with which memory B cells are produced after immunization 40. It is becoming clear based on these observations that B cell memory is not simply the result of GC induction and the subsequent release of exceptionally long-lived IgG (or IgA) memory cells. Instead, it appears that the memory B cell repertoire does indeed consists of multiple layers, not only based on the expression of different IgH isotypes, but also with the potential for distinct activation requirements, homing properties, lifespans and, therefore different functions. The notion of memory B cells with different functions is borne out by the work of Zuccarino-Catania et al., who showed that a single monoclonal B cell population can generate an array of distinct memory B cells in response to a single antigen with differing abilities to yield plasma cells versus GCs following secondary stimulation by antigen 51. It may be worth considering whether initial immunization with a T-independent antigen has similar consequences.
Stable germline-encoded antibody repertoires?
In adults steady naïve B cell populations consist of several types of cells. The vast majority of naïve B cells routinely recirculate through follicles in the spleen and lymph nodes, and hence are commonly termed follicular B cells. However the spleen also contains marginal zone (MZ) B cells, relatively sessile cells that reside adjacent to the marginal sinus of the spleen. Additionally, B1 B cells, so-named because they appear to emerge earlier in ontogeny than follicular and MZ B cells, are found in many peripheral tissues but are also highly enriched for in body cavities such as the peritoneal cavity. It has been suggested that MZ and B1 B cells each have the property of rapidly producing IgM responses, either by quickly generating IgM-secreting plasma cells 52–54, or for B1 cells via a direct pathway resulting in constant low-level IgM secretion 55.
A widely accepted idea is that B1 and marginal zone B cells contribute rarely if at all to GCs 56. Hence the antibodies produced by plasma cells derived from B1 and marginal zone B cells can be considered germline encoded and generally low affinity. While it is difficult to prove that cells within either population never contribute to GCs (see 57), it is clear that these cells rapidly produce large numbers of IgM-secreting cells upon invasion by encapsulated bacteria and that these responses are largely T cell independent 58. What is less clear is whether these cells contribute to long-lived plasma cell pools. As mentioned briefly above, a popular view is that B1 B cells contribute directly to serum antibody pools by constitutively releasing small quantities of antibodies 55, 59, although this view has also been challenged 54. Additionally, Kelsoe and colleagues reported recently that B1 B cells contribute to a stable bone marrow plasma cell population 60. Regardless of whether such antibodies derive from plasma cells or B1 B cells, because both populations can be rather stable, titers of antibodies they produce should also be relatively stable, perhaps with decay rates that match those observed for GC-dominated responses.
Other evidence that B1 B cells contribute to stable antibody repertoires stems from the analysis of antibody responses to the relapsing fever spirochete Borrelia hermsii. By transferring B1 B cells into B and T cell deficient mice before infection with B. hermsii, Alugupalli et al. showed that the resulting response led to exceptionally long-lived protection mediated by germ-line encoded IgM antibodies 61, 62. While the extent to which these results reflect the activity of long-lived memory B cells or plasma cells is uncertain, these results further highlight the need to reconsider the notion that T-dependent GCs are the sole source of either cell type.
A discussion of B1 B cells would be incomplete without at least mentioning natural antibodies. Natural antibodies are spontaneously produced immunoglobulins with broad reactivity against a variety of foreign and self-antigens 63. Natural antibodies have been shown to protect against invasion by viral and bacterial pathogens 64, 65, and thus appear to provide an important layer of protection against a wide variety of potentially harmful agents. B1 B cells are considered the main source of natural antibodies 59, 63. What is unclear is whether the apparent stability of the natural antibody specificity repertoire reflects the continual low-level synthesis of IgM and other Ig isotypes, the constant low-level generation of short-lived plasma cells, or perhaps even long-lived plasma cells derived from B1 B cells.
Origins and function of the IgA repertoire
Are there other circumstances where B1 B cells and other sources of germ-line encoded antibodies contribute to lasting responses? It has also been proposed that B1 B cells contribute heavily to the IgA repertoire at barrier surfaces in the gut 66. Interactions between the intestinal microbiota and immune cells including dendritic cells within the lamina propria of the small intestine (siLP) readily engender the genesis of IgA-producing plasma cells 67, 68. Such cells are mainly produced in Peyer’s patches and mesenteric lymph nodes, but then relocate to the siLP where they secrete dimeric IgA molecules that are actively transported into the intestinal lumen where they bind to a host of commensal bacteria 69. Notably, the bulk of the IgA-producing plasma cell pool in the siLP consists of long-lived cells 70(our unpublished data). When considering that some 50% of these cells appear to stem from T-cell independent processes 71, it may be inferred that T-independent and T-dependent mechanisms for inducing IgA responses are equally facile at generating long-lived plasma cells (our unpublished data).
Here we return to the layered immune system hypothesis. In a recent report, Bunker et al. reported the characterization of the binding properties of numerous monoclonal antibodies derived from IgA+ siLP plasma cells 72. Remarkably some 70% of these antibodies bound a diverse pool of commensal bacteria derived from the small intestine, suggesting that a large fraction of the IgA repertoire targets conserved molecular structures common to a variety of bacterial taxa. By targeting collections of microbes that share particular epitopes, many IgA antibodies may share certain functional hallmarks with pattern recognition receptors. Notably, current information on the structural features of these antibodies suggests that they either arise via completely T- and GC-independent processes, or they arise from GCs but without antigen-mediated selection 72, 73.
Importantly however, it is likely that not all IgA antibodies possess these attributes: Palm et al. reported that IgA responses preferentially target commensal bacteria with colitis-inducing attributes 74, and these antibodies appear to be T-cell dependent. Similarly, unlike much of the IgA synthesis in the gut, we found that the emergence of IgA-secreting plasma cells into the bone marrow and the induction of serum IgA antibodies that target commensal bacteria are each strictly T-cell dependent processes 47. Together these observations suggest that the IgA repertoire consists of multiple stable layers that collectively target and continuously regulate the composition of the bacterial microbiota.
Consequences of long-lived extrafollicular responses
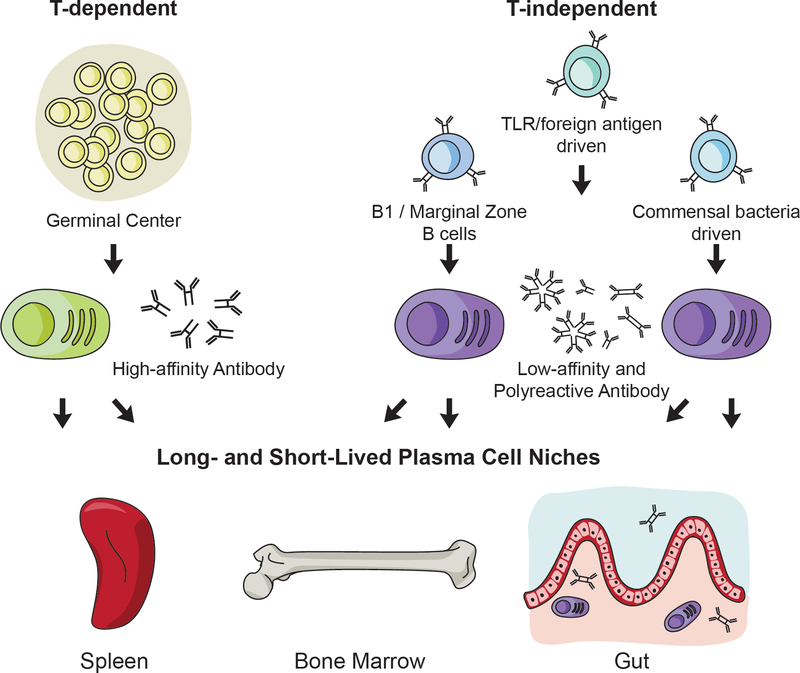
Based on these collective ideas and observations we propose that stable antibody repertoires derived from long-lived plasma cells arise from multiple pathways including GC- and T-independent processes (Figure 1). From a practical standpoint, these ideas suggest that the capacity of a particular vaccine to induce durable antibody titers is not necessarily a consequence of the degree to which it also drives T cell activation. Participation of activated T cells is essential for GC responses and affinity maturation, and they can optimize class switching, but whether the resulting plasma and memory B cells are long-lived is likely to be a separate process influenced by the adjuvant used and other factors. Further support for this viewpoint stems from the study of mice lacking the transcription factor Zbtb20, which cannot generate lasting responses to protein-based antigen but can produce long-lived plasma cells when immunized with adjuvants that stimulate Toll-like receptors 75.
Figure 1. Multiple pathways feed the long-lived plasma cell pool.
T-dependent plasma cells that arise from GC reactions typically produce high-affinity class switched antibody. T-independent plasma cell responses can arise from multiple sources of stimulation including from B cells primed to respond rapidly like B1 and marginal zone B cells, from TLR or foreign antigens that stimulate B cells in the absence of a GC, or from commensal bacteria in the mucosal tissues. The resulting antibody from T-independent plasma cell responses is typically low-affinity and not class-switched, with the exception of mucosal derived plasma cells that switch to IgA. All of these sources of plasma cells are capable of seeding the long-lived plasma cell niches that include the spleen, bone marrow, and gut.
It has been proposed that the production of IgM+ memory B cells is essential for long-lived durable protection 48, 49. These IgM+ memory cells are induced in response to both T-dependent and -independent antigens. The extent to which non-switched memory B cells respond to secondary challenge by entering GC reactions versus differentiating directly in plasma cells is unclear. However, the advantage of having an IgM+ memory B cell pool with broad reactivity and in some cases polyreactivity would include the ability to quickly secrete protective antibody without the necessity of lengthy cell-cell interactions needed to form a GC. This idea is consistent with aforementioned evidence for T-independent polyreactive or natural IgM conferring protection to bacterial and viral pathogens 64, 65. The advantages of long-lived polyreactive IgM secretion may come with increased risk for autoimmunity. People with Hyper-IgM, most commonly caused by mutations in CD40/CD40L resulting in a lack of T-dependent antibody, have increased frequencies of autoimmune-arthritis, -thrombocytopenia, and -anemia 76.
If we accept the possibility that IgM+ memory B cells and IgM-secreting plasma cells play unique roles in immunity, then there appears to be every reason to consider the possibility that stable titers of IgM antibodies are every bit as advantageous as stable IgG titers. It may also be useful to apply this viewpoint to the secreted IgA repertoire in the intestinal lumen. Indeed, in the gut such stability might also prove useful during severe infection, which can cause disruption of primary responses 77. Hence, such infections would be less likely to provide opportunities for other potentially pathogenic microbes because IgA targeting of these taxa would remain unchanged.
Opposing models for inducing long-lived B-lineage cells
Currently we are faced with two dramatically differing views of how stimulation with antigen and other signals result in the production of B-lineage cells with markedly increased lifespans. Each view builds on the idea that the survival of antigen-experienced cells requires their ability to utilize unique cell-extrinsic pro-survival factors. The more classic view, derived from a focus on plasma cells, holds that cells must position themselves in unique microenvironments to gain access to these factors. Hence plasma cell survival has been proposed to hinge on physical access to the BAFF family cytokine APRIL, which in turn may be available only within specialized niches in the bone marrow. Whether a parallel model will emerge for memory B cells is unclear, though it should be noted that memory B cells apparently do not rely on access to either BAFF or APRIL, in contrast to plasma cells and naïve B cells 78, 79. Alternatively, deployment of gene expression and biochemical processes needed for subsequent survival may be occur much earlier in differentiation, and these pathways may be needed for cells to utilize APRIL and other signals optimally. It should be stressed that while these views are not mutually exclusive, there has been relatively little work directed towards the latter possibility. We suggest that, while T-cell dependent GCs play essential roles in the selection of high-affinity B and plasma cells, it is likely that B cell-T cell interactions are not essential for facilitating access or utilization of needed pro-survival signals for memory B cells or plasma cells.
Acknowledgments
Acknowledgements: We thank Kelly Owens for her artistic contributions. This work was supported by NIH grants R01AI139123 and R01AI113543 to D.A, F32AI114089 to J.R.W., and T32CA009140 to B.T.G.
Footnotes
Conflicts of Interest Statement: The authors have no conflicts of interest.
References
- 1.Amanna IJ, Carlson NE and Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007; 357: 1903–15. [DOI] [PubMed] [Google Scholar]
- 2.Kinoshita K and Honjo T. Linking class-switch recombination with somatic hypermutation. Nature reviews Molecular cell biology. 2001; 2: 493–503. [DOI] [PubMed] [Google Scholar]
- 3.Angeletti D, Gibbs JS, Angel M, et al. Defining B cell immunodominance to viruses. Nat Immunol. 2017; 18: 456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khurana S, Verma N, Yewdell JW, et al. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Science translational medicine. 2011; 3: 85ra48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheid JF, Mouquet H, Feldhahn N, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009; 458: 636–40. [DOI] [PubMed] [Google Scholar]
- 6.Escolano A, Steichen JM, Dosenovic P, et al. Sequential Immunization Elicits Broadly Neutralizing Anti-HIV-1 Antibodies in Ig Knockin Mice. Cell. 2016; 166: 1445–58 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu M, Yang G, Wiehe K, et al. Polyreactivity and autoreactivity among HIV-1 antibodies. J Virol. 2015; 89: 784–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herzenberg LA. Toward a layered immune system. Cell. 1989; 59: 953–4. [DOI] [PubMed] [Google Scholar]
- 9.Slifka MK, Antia R, Whitmire JK and Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998; 8: 363–72. [DOI] [PubMed] [Google Scholar]
- 10.Manz RA and Radbruch A. Plasma cells for a lifetime? Eur J Immunol. 2002; 32: 923–7. [DOI] [PubMed] [Google Scholar]
- 11.Manz RA, Thiel A and Radbruch A. Lifetime of plasma cells in the bone marrow. Nature. 1997; 388: 133–4. [DOI] [PubMed] [Google Scholar]
- 12.Ahuja A, Anderson SM, Khalil A and Shlomchik MJ. Maintenance of the plasma cell pool is independent of memory B cells. Proc Natl Acad Sci U S A. 2008; 105: 4802–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vieira P and Rajewsky K. The half-lives of serum immunoglobulins in adult mice. Eur J Immunol. 1988; 18: 313–6. [DOI] [PubMed] [Google Scholar]
- 14.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA and Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001; 167: 6834–40. [DOI] [PubMed] [Google Scholar]
- 15.Allman DM, Ferguson SE, Lentz VM and Cancro MP. Peripheral B cell maturation. II. Heat-stable antigen(hi) splenic B cells are an immature developmental intermediate in the production of long-lived marrow-derived B cells. J Immunol. 1993; 151: 4431–44. [PubMed] [Google Scholar]
- 16.Jones DD, Wilmore JR and Allman D. Cellular Dynamics of Memory B Cell Populations: IgM+ and IgG+ Memory B Cells Persist Indefinitely as Quiescent Cells. J Immunol. 2015; 195: 4753–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chernova I, Jones DD, Wilmore JR, et al. Lasting antibody responses are mediated by a combination of newly formed and established bone marrow plasma cells drawn from clonally distinct precursors. J Immunol. 2014; 193: 4971–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shlomchik M, Mascelli M, Shan H, et al. Anti-DNA antibodies from autoimmune mice arise by clonal expansion and somatic mutation. J Exp Med. 1990; 171: 265–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shlomchik MJ, Marshak-Rothstein A, Wolfowicz CB, Rothstein TL and Weigert MG. The role of clonal selection and somatic mutation in autoimmunity. Nature. 1987; 328: 805–11. [DOI] [PubMed] [Google Scholar]
- 20.Shlomchik MJ, Nemazee DA, Sato VL, Van Snick J, Carson DA and Weigert MG. Variable region sequences of murine IgM anti-IgG monoclonal autoantibodies (rheumatoid factors). A structural explanation for the high frequency of IgM anti-IgG B cells. J Exp Med. 1986; 164: 407–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manser T Textbook germinal centers? J Immunol. 2004; 172: 3369–75. [DOI] [PubMed] [Google Scholar]
- 22.Shlomchik MJ and Weisel F. Germinal centers. Immunol Rev. 2012; 247: 5–10. [DOI] [PubMed] [Google Scholar]
- 23.Victora GD and Nussenzweig MC. Germinal centers. Annual review of immunology. 2012; 30: 429–57. [DOI] [PubMed] [Google Scholar]
- 24.Song S and Matthias PD. The Transcriptional Regulation of Germinal Center Formation. Frontiers in immunology. 2018; 9: 2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen CD, Okada T and Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007; 27: 190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coffey F, Alabyev B and Manser T. Initial clonal expansion of germinal center B cells takes place at the perimeter of follicles. Immunity. 2009; 30: 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nikbakht N, Shen S and Manser T. Cutting edge: Macrophages are required for localization of antigen-activated B cells to the follicular perimeter and the subsequent germinal center response. J Immunol. 2013; 190: 4923–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kellermayer Z, Fisi V, Mihalj M, Berta G, Kobor J and Balogh P. Marginal Zone Macrophage Receptor MARCO Is Trapped in Conduits Formed by Follicular Dendritic Cells in the Spleen. J Histochem Cytochem. 2014; 62: 436–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez SF, Lukacs-Kornek V, Kuligowski MP, et al. Complement-dependent transport of antigen into B cell follicles. J Immunol. 2010; 185: 2659–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki K, Grigorova I, Phan TG, Kelly LM and Cyster JG. Visualizing B cell capture of cognate antigen from follicular dendritic cells. J Exp Med. 2009; 206: 1485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Victora GD, Schwickert TA, Fooksman DR, et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010; 143: 592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cassese G, Arce S, Hauser AE, et al. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J Immunol. 2003; 171: 1684–90. [DOI] [PubMed] [Google Scholar]
- 33.Radbruch A, Muehlinghaus G, Luger EO, et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006; 6: 741–50. [DOI] [PubMed] [Google Scholar]
- 34.Zehentmeier S, Roth K, Cseresnyes Z, et al. Static and dynamic components synergize to form a stable survival niche for bone marrow plasma cells. Eur J Immunol. 2014; 44: 2306–17. [DOI] [PubMed] [Google Scholar]
- 35.O’Connor BP, Raman VS, Erickson LD, et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. 2004; 199: 91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peperzak V, Vikstrom I, Walker J, et al. Mcl-1 is essential for the survival of plasma cells. Nat Immunol. 2013; 14: 290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phan TG, Paus D, Chan TD, et al. High affinity germinal center B cells are actively selected into the plasma cell compartment. J Exp Med. 2006; 203: 2419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paus D, Phan TG, Chan TD, Gardam S, Basten A and Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J Exp Med. 2006; 203: 1081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan TD, Gatto D, Wood K, Camidge T, Basten A and Brink R. Antigen affinity controls rapid T-dependent antibody production by driving the expansion rather than the differentiation or extrafollicular migration of early plasmablasts. J Immunol. 2009; 183: 3139–49. [DOI] [PubMed] [Google Scholar]
- 40.Weisel FJ, Zuccarino-Catania GV, Chikina M and Shlomchik MJ. A Temporal Switch in the Germinal Center Determines Differential Output of Memory B and Plasma Cells. Immunity. 2016; 44: 116–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaji T, Ishige A, Hikida M, et al. Distinct cellular pathways select germline-encoded and somatically mutated antibodies into immunological memory. J Exp Med. 2012; 209: 2079–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bortnick A, Chernova I, Quinn WJ 3rd, Mugnier M, Cancro MP and Allman D. Long-lived bone marrow plasma cells are induced early in response to T cell-independent or T cell-dependent antigens. J Immunol. 2012; 188: 5389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Vinuesa CG, Cook MC, Ball J, et al. Germinal centers without T cells. J Exp Med. 2000; 191: 485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lentz VM and Manser T. Cutting edge: germinal centers can be induced in the absence of T cells. J Immunol. 2001; 167: 15–20. [DOI] [PubMed] [Google Scholar]
- 45.Foote JB, Mahmoud TI, Vale AM and Kearney JF. Long-term maintenance of polysaccharide-specific antibodies by IgM-secreting cells. J Immunol. 2012; 188: 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taillardet M, Haffar G, Mondiere P, et al. The thymus-independent immunity conferred by a pneumococcal polysaccharide is mediated by long-lived plasma cells. Blood. 2009; 114: 4432–40. [DOI] [PubMed] [Google Scholar]
- 47.Wilmore JR, Gaudette BT, Gomez Atria D, et al. Commensal Microbes Induce Serum IgA Responses that Protect against Polymicrobial Sepsis. Cell host & microbe. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pape KA, Taylor JJ, Maul RW, Gearhart PJ and Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 2011; 331: 1203–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor JJ, Pape KA and Jenkins MK. A germinal center-independent pathway generates unswitched memory B cells early in the primary response. J Exp Med. 2012; 209: 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toyama H, Okada S, Hatano M, et al. Memory B cells without somatic hypermutation are generated from Bcl6-deficient B cells. Immunity. 2002; 17: 329–39. [DOI] [PubMed] [Google Scholar]
- 51.Zuccarino-Catania GV, Sadanand S, Weisel FJ, et al. CD80 and PD-L2 define functionally distinct memory B cell subsets that are independent of antibody isotype. Nat Immunol. 2014; 15: 631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayakawa K, Hardy RR, Honda M, Herzenberg LA and Steinberg AD. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci U S A. 1984; 81: 2494–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oliver AM, Martin F, Gartland GL, Carter RH and Kearney JF. Marginal zone B cells exhibit unique activation, proliferative and immunoglobulin secretory responses. Eur J Immunol. 1997; 27: 2366–74. [DOI] [PubMed] [Google Scholar]
- 54.Fairfax KA, Corcoran LM, Pridans C, et al. Different kinetics of blimp-1 induction in B cell subsets revealed by reporter gene. J Immunol. 2007; 178: 4104–11. [DOI] [PubMed] [Google Scholar]
- 55.Tumang JR, Frances R, Yeo SG and Rothstein TL. Spontaneously Ig-secreting B-1 cells violate the accepted paradigm for expression of differentiation-associated transcription factors. J Immunol. 2005; 174: 3173–7. [DOI] [PubMed] [Google Scholar]
- 56.Forster I and Rajewsky K. Expansion and functional activity of Ly-1+ B cells upon transfer of peritoneal cells into allotype-congenic, newborn mice. Eur J Immunol. 1987; 17: 521–8. [DOI] [PubMed] [Google Scholar]
- 57.Song H and Cerny J. Functional heterogeneity of marginal zone B cells revealed by their ability to generate both early antibody-forming cells and germinal centers with hypermutation and memory in response to a T-dependent antigen. J Exp Med. 2003; 198: 1923–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin F, Oliver AM and Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001; 14: 617–29. [DOI] [PubMed] [Google Scholar]
- 59.Holodick NE, Tumang JR and Rothstein TL. Immunoglobulin secretion by B1 cells: differential intensity and IRF4-dependence of spontaneous IgM secretion by peritoneal and splenic B1 cells. Eur J Immunol. 2010; 40: 3007–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reynolds AE, Kuraoka M and Kelsoe G. Natural IgM is produced by CD5- plasma cells that occupy a distinct survival niche in bone marrow. J Immunol. 2015; 194: 231–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alugupalli KR, Gerstein RM, Chen J, Szomolanyi-Tsuda E, Woodland RT and Leong JM. The resolution of relapsing fever borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. J Immunol. 2003; 170: 3819–27. [DOI] [PubMed] [Google Scholar]
- 62.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T and Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004; 21: 379–90. [DOI] [PubMed] [Google Scholar]
- 63.Holodick NE, Rodriguez-Zhurbenko N and Hernandez AM. Defining Natural Antibodies. Frontiers in immunology. 2017; 8: 872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zinkernagel RM and Hengartner H. Protective ‘immunity’ by pre-existent neutralizing antibody titers and preactivated T cells but not by so-called ‘immunological memory’. Immunol Rev. 2006; 211: 310–9. [DOI] [PubMed] [Google Scholar]
- 65.Ochsenbein AF, Fehr T, Lutz C, et al. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 1999; 286: 2156–9. [DOI] [PubMed] [Google Scholar]
- 66.Bunker JJ, Flynn TM, Koval JC, et al. Innate and Adaptive Humoral Responses Coat Distinct Commensal Bacteria with Immunoglobulin A. Immunity. 2015; 43: 541–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Macpherson AJ, Yilmaz B, Limenitakis JP and Ganal-Vonarburg SC. IgA Function in Relation to the Intestinal Microbiota. Annual review of immunology. 2018; 36: 359–81. [DOI] [PubMed] [Google Scholar]
- 68.Macpherson AJ, Koller Y and McCoy KD. The bilateral responsiveness between intestinal microbes and IgA. Trends in immunology. 2015; 36: 460–70. [DOI] [PubMed] [Google Scholar]
- 69.Macpherson AJ, McCoy KD, Johansen FE and Brandtzaeg P. The immune geography of IgA induction and function. Mucosal immunology. 2008; 1: 11–22. [DOI] [PubMed] [Google Scholar]
- 70.Lemke A, Kraft M, Roth K, Riedel R, Lammerding D and Hauser AE. Long-lived plasma cells are generated in mucosal immune responses and contribute to the bone marrow plasma cell pool in mice. Mucosal immunology. 2016; 9: 83–97. [DOI] [PubMed] [Google Scholar]
- 71.Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H and Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000; 288: 2222–6. [DOI] [PubMed] [Google Scholar]
- 72.Bunker JJ, Erickson SA, Flynn TM, et al. Natural polyreactive IgA antibodies coat the intestinal microbiota. Science. 2017; 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yeap LS, Hwang JK, Du Z, et al. Sequence-Intrinsic Mechanisms that Target AID Mutational Outcomes on Antibody Genes. Cell. 2015; 163: 1124–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Palm NW, de Zoete MR, Cullen TW, et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014; 158: 1000–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Y and Bhattacharya D. Adjuvant-specific regulation of long-term antibody responses by ZBTB20. J Exp Med. 2014; 211: 841–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jesus AA, Duarte AJ and Oliveira JB. Autoimmunity in hyper-IgM syndrome. Journal of clinical immunology. 2008; 28 Suppl 1: S62–6. [DOI] [PubMed] [Google Scholar]
- 77.Fonseca DM, Hand TW, Han SJ, et al. Microbiota-Dependent Sequelae of Acute Infection Compromise Tissue-Specific Immunity. Cell. 2015; 163: 354–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Benson MJ, Dillon SR, Castigli E, et al. Cutting edge: the dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J Immunol. 2008; 180: 3655–9. [DOI] [PubMed] [Google Scholar]
- 79.Scholz JL, Crowley JE, Tomayko MM, et al. BLyS inhibition eliminates primary B cells but leaves natural and acquired humoral immunity intact. Proc Natl Acad Sci U S A. 2008; 105: 15517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]