Abstract
Interest in evaluating individual cellular populations in the central nervous system has prompted the development of several techniques enabling the enrichment of single cell populations. Herein we detail a relatively inexpensive method to specifically isolate neurons, astrocytes, and microglia from a mixed homogenate utilizing magnetic beads conjugated to cell type specific antibodies. We have used this technique to isolate astrocytes across development and into late adulthood. Finally, we detail the utilization of this technique in novel astrocyte and astrocyte/neuron co-culture paradigms.
Keywords: astrocyte, microglia, neuron, cell-isolation, magnetic cell separation, co-culture
INTRODUCTION
Cell-type specific examination in the central nervous system (CNS) has been of interest for many years. Recent research highlights the specific contributions of different cellular populations to normal and abnormal CNS development, aging and disease (Hansen, Hanson, & Sheng, 2018; Hoye et al., 2018; Kopec, Smith, Ayre, Sweat, & Bilbo, 2018; Lioy et al., 2011; Peferoen, Kipp, van der Valk, van Noort, & Amor, 2014; Yu et al., 2018). To address cell-type specific contributions, several techniques have been developed, including magnetic-activated cell sorting (MACS). Herein we describe the sequential isolation of CNS cell types via MACS. We find that MAC sorting of neural cells is relatively gentle, resulting in cells that retain processes. The retention of cellular processes is important when experiments aim to examine molecular signals important in activity-dependent processes. The collected cells can be used for downstream experiments such as western immunoblotting, quantitative PCR, RNASeq, and proteomics. MAC sorting additionally allows for the investigation of cell-cell communication with direct co-culturing of mixed cellular populations. Directly targeting these populations of interest allows for the isolation and co-culture of mixed cellular populations without the need for passaging and re-plating of cells. The co-culture of different cell-types or mixed genotypes can potentially give researchers greater insight into disease progression.
Within this unit, we detail protocol steps to isolate major CNS cell populations from a whole brain homogenate via magnetic cell separation. Brain regions of interest are enzymatically digested into a single cell suspension and incubated with antibodies against an extracellular protein on the population of interest. These antibodies are conjugated to magnetic beads. When the suspension is passed through a column placed within a strong magnetic field, the targeted, labeled cells remain on the column while the non-targeted population flows through. Targeting one cellular population at a time allows for the sequential isolation of individual CNS cell types. We additionally detail methodology for the isolation and subsequent culture of astrocytes, neurons, and astrocyte/neuron co-cultures from the isolated populations.
BASIC PROTOCOL 1
Sequential isolation of microglia, astrocytes, and neurons
The protocol described herein requires the use of animal materials. Only perform the described protocol with prior approval of the Institutional Animal Care and Use Committee (IACUC), and in accordance of national and local regulations.
Unless otherwise indicated, it is best to keep all solutions at 4°C for the duration of the protocol. This will help prevent activation of detrimental cellular pathways.
Within this protocol, we detail the isolation and collection of cellular populations for RNA isolation. For those interested in MACS for protein isolation, simply substitute snap-freezing the fractions at the steps where addition of RNALater is indicated.
Materials
Experimental animals (sex, age, strain, etc will depend upon the researcher’s needs)
Herein we use male and female wildtype C57BL/6 mice, aged 20–30 days
50mL ACSF (see recipe)
500mL 0.5% BSA in PBS (see recipe)
PBS (Biorad, cat. no 161–0780)
BSA, fatty acid free (MilliporeSigma, cat. no A7030)
Carbogen (95% O2 : 5% CO2 gas tank, Praxair, cat. no MM OXCD5-K)
Carbon dioxide tank (Praxair, cat. no CD M-50)
Sealed induction chamber (Scivenascientific, cat. no RES643)
10mL serological pipettes (Fisher, cat. no 02–707–155)
Water bath (Fisher, cat. no FSGPD05)
Auto-pipette (Waverly, cat. no YF184AE0001107)
Centrifuge (Eppendorf 5804R, Rotor A-4–44)
Worthington Papain Dissociation Kit (Worthington, cat. no LK003178)
50mL Falcon conical tubes (Fisher, cat. no 14–432-22)
70um Falcon Cell Strainer (Fisher, cat. no 08–771-2)
RNA Later Solution (ThermoFisher Scientific, cat. no AM7021)
MidiMACS Separator or QuadroMACS Separator (Miltenyi Biotec, cat. no 130–042-302 and cat. no 130–090-976)
LS Columns (Miltenyi Biotec, cat. no 130–042-401)
MACS Myelin Beads, human, mouse, rat (Miltenyi Biotec, cat. no 130–104-253)
MACS Cd11b+ Microbeads, mouse (Miltenyi Biotec, cat. no 130–093-634)
MACS ACSA-2 MicroBead Kit, mouse (Miltenyi Biotec, cat. no 130–097-678)
MACS Neuron Isolation Kit, mouse (Miltenyi Biotec, cat. no 130–115-389)
Protocol steps
Prepare Worthington Papain Dissociation kit
-
1
Add 32mL EBSS to the albumin-ovomucoid inhibitor and allow the contents to dissolve.
Perform this step only when first opening the Worthington Papain Dissociation kit. Once prepared, the solutions are stable for up to one month.
-
2
Add 5mL EBSS to the papain vial. Mix gently to dissolve the powder.
-
3
Add 500μL EBSS to a DNase vial. Mix gently and allow the powder to fully dissolve. Transfer 250μL of this solution to the vial containing the papain. Store the rest of the DNase solution on ice until needed.
-
4
Equilibrate the papain solution by exposure to 95%O2:5%CO2 without directly bubbling the papain solution.
In order to do so, secure the tubing that is supplying the gas just above the surface of the papain solution. Having the gas disturb the surface of the papain solution is more than sufficient for equilibration. We find direct bubbling of the papain results in loss of solution volume. While you perform the tissue microdissections, keep the solution at room temperature.
Dissociate mouse cortex into single-cell suspension
-
5
Equilibrate the prepared ACSF by exposure to 95%O2:5%CO2 for at least 15 minutes. For this step, directly bubble the solution.
-
6
Anesthetize the animal with CO2 for 1 minute and rapidly decapitate. Remove the brain and dissect the brain region of interest. It is not necessary to remove the meninges for this protocol. Mince the microdissected tissue into 100mm3 sections using scissors. For animals younger than 15 days old, forceps can also be used.
-
7
Transfer the bubbled papain into a 50mL conical tube. Use a modified cap to allow for continuous bubbling of the solution with 95%O2:5%CO2 during the incubation.
Use a pair of scissors or strong forceps to cut or drill a small hole into the conical tube cap that is equal to the size of the tubing, see Figure 1 for visual guide to construction.
-
8
Slowly draw up the minced tissue using a 10mL serological pipette. Allow the tissue to separate from the solution and settle at the bottom of the pipette. Release only the settled tissue into the prepared papain.
-
9
Incubate the tissue/papain mixture for 15–20 minutes in a water bath set to 37°C. Swirl the conical tube every 5 minutes to maximize the tissue’s exposure to the papain. Keep the solution equilibrated with exposure to 95%O2:5%CO2, however do not directly bubble the papain during this step.
-
10
Gently titrate the tissue with 10mL serological pipette (using an auto-pipette) with setting turned to Slow or Medium for 12–15 times up and down until the solution is homogenous. The solution should be a cloudy, light pink in appearance.
The speed of trituration may require optimization for different experimental designs. A fast trituration will result in better dissociation of the tissue but will also give rise to cells with their processes sheared. A slower trituration will be less efficient in dissociation of tissue but will be gentler on the cells.
-
11
Centrifuge the homogenized solution at 300 × g for 3 minutes at room temperature.
The centrifuge being used only needs to be able to hold 50 mL and 15 mL conical tubes. Ours can hold 2 × 50mL and 8 × 15 mL simultaneously. It also needs to spin at 300 × g at a controlled temperature.
-
12
Prepare the resuspension buffer as directed in the Worthington Papain Dissociation kit by mixing 2.7mL EBSS, 300μL albumin-ovomucoid inhibitor, and the remaining 150μL DNase.
-
13
Discard the supernatant and immediately resuspend the cell pellet in the resuspension buffer prepared in step 12 and mix well.
-
14
Prepare a discontinuous density gradient by adding 5mL of albumin-inhibitor solution to a new conical tube. Carefully layer the cell suspension from step 13 on top of the albumin solution. Centrifuge at 300 × g for 5 minutes at room temperature.
-
15
Discard the supernatant and immediately suspend the pelleted cells in 8mL PBS/BSA solution. To ensure a single-cell suspension, filter the solution using 70μm BD Falcon filter to remove any non-dissociated tissue.
The filter should be wetted prior to applying the cells. We typically use 2mL to wet the filter for a total volume of 10mL.
-
16
Remove 1mL of filtered solution as “whole cortex” fraction. This fraction is therefore 10% of total dissociated cells. The mixed-cellular population, “whole cortex” fraction can then be used as an input control for subsequent analyses.
-
17
Centrifuge “whole cortex” fraction at 300 × g for 3 minutes at 4°C and discard the supernatant. Add 150μL RNALater to the Whole Cortex fraction and set aside.
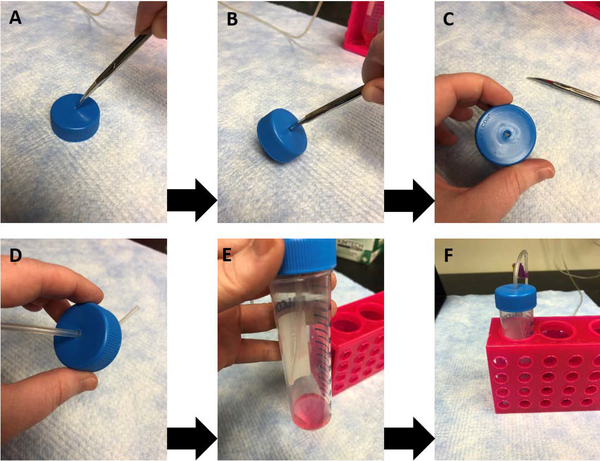
Figure 1.
Creation of the modified conical tube cap for bubbling solutions. Sharp scissors (A) or forceps can be used to drill a small hole in the cap of a conical tube (B, C) to fit the size of tubing. The tubing can be fed through the cap to allow for constant oxygenation of the solutions during papain dissociation (D, E). Note that as demonstrated in (E, F), direct bubbling of the papain solution should be avoided during incubations.
Remove myelin debris
-
18
Centrifuge the remaining 9mL single-cell suspension fraction at 300 × g for 3 minutes at 4°C and discard the supernatant.
-
19
Resuspend the pelleted cells in 150μL PBS/BSA solution. Add 15–20μL anti-myelin microbeads. Incubate the mixture at 4°C for 10 minutes, with a gentle mix every 5 minutes.
These microbeads target myelin basic protein, and therefore will remove myelin debris and mature oligodendrocytes.
-
20
Add 1mL PBS/BSA solution to the conical tube and centrifuge at 300 × g for 3 minutes at 4°C to pellet the cells. This will remove any excess beads from the solution.
-
21
Place the LS column into the MidiMACS or QuadroMACS (Figure 2). Place a fresh 15mL or 50mL conical tube at the bottom. This will be used to collect the non-targeted cells. Apply 2mL PBS/BSA to the column. Pre-wetting the column will prevent any non-labeled cells from becoming stuck in the dry column.
-
22
Remove and discard the resulting supernatant from step 20. Resuspend the pellet in 500μL PBS/BSA solution and apply directly to the prepared LS column. Collect the flow-through into the 50mL conical tube. The flow-through contains the microglia, astrocytes, and neurons.
-
23
Add 3mL PBS/BSA to the column. Continue to collect the flow-through. Repeat this step one more time, for a total of two 3mL solution applications.
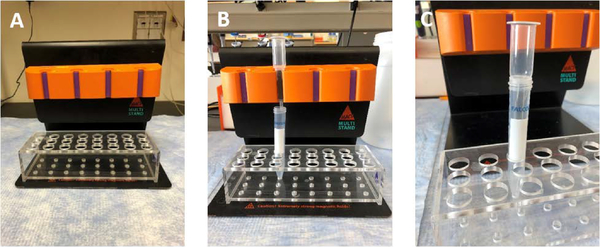
Figure 2.
Images of MACS separators and columns. A) The QuadroMACS is placed on the magnetic stand. B) To separate cellular populations, a column is placed in the individual slots, with a conical tube underneath to collect the flow-through. C) To elute specific cellular populations, remove the column from the separator and use the supplied plunger.
Isolate Microglia Fraction
-
24
Centrifuge the collected flow-through from Step 23 at 300 × g for 3 minutes at 4°C and discard the supernatant. Resuspend the pelleted cells in 150μL PBS/BSA solution.
-
25
Add 10–15μL Cd11b+ microbeads. These microbeads will target microglia populations. Incubate the mixture at 4°C for 10 minutes, with a gentle mix every 5 minutes.
-
26
Add 1mL PBS/BSA solution to the conical tube and centrifuge at 300 xg for 3 minutes at 4°C to pellet the cells. This will remove any excess beads from the solution.
-
27
Place the LS column into the MidiMACS or QuadroMACS. Place a fresh 50mL conical tube at the bottom. This will be used to collect the non-targeted cells. Apply 2mL PBS/BSA to the column. Pre-wetting the column will prevent any non-labeled cells from becoming stuck in the dry column.
-
28
Remove and discard the resulting supernatant from step 26. Resuspend the pellet in 500μL PBS/BSA solution and apply directly to the LS column. Collect the flow-through into the 50mL conical tube. The flow-through contains the astrocytes and neurons, while the microglia will remain on the LS column.
-
29
Add 3mL PBS/BSA to the column. Continue to collect the flow-through. Repeat this step one more time, for a total of two 3mL solution applications.
-
30
Remove the LS column from the magnetic holder, and place into a fresh 15 mL conical tube. Elute the targeted microglial population by adding 5mL PBS/BSA solution. Use the supplied plunger to push the solution through the LS column. This will apply gentle pressure to remove the microglia from the column and result in a Microglia Fraction.
-
31
Centrifuge the Microglia Fraction at 300 × g for 3 minutes at 4°C and discard the supernatant. Add 150μL RNALater to the Microglia Fraction and set aside.
Isolate Astrocyte Fraction
-
32
Centrifuge the collected flow-through 300 × g for 3 minutes at 4°C and discard the supernatant. Resuspend the pelleted flow-through cells in 150μL PBS/BSA solution.
-
33
Add 10–15μL FcR blocking cocktail microbeads from the ACSA-2 MicroBead kit. This cocktail will prevent non-specific binding for the ACSA2 microbeads. Incubate the mixture at 4°C for 10 minutes, with a gentle mix every 5 minutes.
-
34
Add 10–15μL Anti-ACSA2 from the kit. Incubate the mixture at 4°C for 10 minutes, with a gentle mix every 5 minutes. These microbeads target an extracellular protein on astrocytes.
-
35
Add 1mL PBS/BSA solution to the conical tube and centrifuge at 300 xg for 3 minutes at 4°C to pellet the cells. This will remove any excess beads from the solution.
-
36
Place the LS column into the MidiMACS or QuadroMACS. Place a fresh 50mL conical tube at the bottom. This will be used to collect the non-targeted cells. Apply 2mL PBS/BSA to the column. Pre-wetting the column will prevent any non-labeled cells from becoming stuck in the dry column.
-
37
Remove and discard the resulting supernatant from step 35. Resuspend the pellet in 500μL PBS/BSA solution and apply directly to the LS column. Collect the flow-through into a new, fresh 50mL conical tube. The flow-through contains the neurons, while the astrocytes will remain on the LS column.
-
38
Add 3mL PBS/BSA to the column. Continue to collect the flow-through. Repeat this step one more time, for a total of two 3mL solution applications.
-
39
Remove the LS column from the magnetic holder, and place into a fresh 15 mL conical tube. Elute the targeted astrocyte population by adding 5mL PBS/BSA solution. Use the supplied plunger to push the solution through the LS column. This will apply gentle pressure to remove the astrocytes from the column and result in an Astrocyte Fraction.
-
40
Centrifuge the Astrocyte Fraction at 300 × g for 3 minutes at 4°C and discard the supernatant. Add 150μL RNALater and set aside
Isolate Neuronal Fraction
-
41
Centrifuge the collected flow-through at 300 × g for 3 minutes at 4°C and discard the supernatant. Resuspend the pelleted cells in 150μL PBS/BSA solution.
-
42
Add 10–15μL Anti-biotin blocking cocktail microbeads from the Neuron Isolation kit. This cocktail will target non-neuronal cellular populations. Incubate the mixture at 4°C for 10 minutes, with a gentle mix every 5 minutes.
-
43
Add 10–15μL Anti-biotin antibodies from the kit. Incubate the mixture at 4°C for 10 minutes, with a gentle mix every 5 minutes.
-
44
Add 1mL PBS/BSA solution to the conical tube and centrifuge at 300 × g for 3 minutes at 4°C to pellet the cells. This will remove any excess beads from the solution.
-
45
Place the LS column into the MidiMACS or QuadroMACS. Place a fresh 50mL conical tube at the bottom. This will be used to collect the non-targeted cells. Apply 2mL PBS/BSA to the column. Pre-wetting the column will prevent any non-labeled cells from becoming stuck in the dry column.
-
46
Remove and discard the resulting supernatant from step 44. Resuspend the pellet in 500μL PBS/BSA solution and apply directly to the LS column. Collect the flow-through into a new, fresh 50mL conical tube. The flow-through contains the Neuron Fraction while the target, non-neuronal cell types will remain on the LS column.
-
47
Add 3mL PBS/BSA to the column. Continue to collect the flow-through (Neuron Fraction). Repeat this step one more time, for a total of two 3mL solution applications.
-
48
Centrifuge the collected flow-through at 300 × g for 3 minutes at 4°C and discard the supernatant. Add 150μL RNALater to the Neuron Fraction and set aside
-
49
Store all fractions in RNALater as manufacturer details.
We have found that pelleted samples can be kept at 4°C in RNALater for up to three weeks. It is recommended that these samples be moved to either −20°C or −80°C for long-term storage.
BASIC PROTOCOL 2
The protocol described herein requires the use of animal materials. Only perform the described protocol with prior approval of the Institutional Animal Care and Use Committee (IACUC), and in accordance of national and local regulations.
Unless otherwise indicated, it is best to keep all solutions at 4°C for the duration of the protocol. This will help prevent activation of detrimental cellular pathways.
Within this protocol, we detail the isolation and collection of neurons and astrocytes for co-culture. Previous techniques for co-culture require utilization of embryonic pups for culturing neurons onto an astrocyte feeder layer. In the protocol below, we detail methodology that circumvents this and allows for direct co-culture of astrocytes and neurons. In our experience, these cultures are stable up to 14–20 days post plating.
ISOLATION AND CO-CULTURE OF NEURONS AND ASTROCYTES
Materials
Experimental animals (sex, age, strain, etc. will depend upon the researcher’s needs)
Herein we use male and female wildtype C57BL/6 mice, aged 0–5 days
Neuron Media (see recipe)
ACSF (see recipe)
0.5% BSA in PBS (see recipe)
PBS (Biorad, cat. no 161–0780)
BSA, fatty acid free (MilliporeSigma, cat. no A7030)
100% EtOH (Fisher, cat. no A405P4)
Bunsen Burner (Humboldt, cat. no 6200.1)
Carbogen (95% O2 : 5% CO2 gas tank, Praxair, cat. no MM OXCD5-K)
10mL serological pipettes (Fisher, cat. no 02–707-155)
Water bath (Fisher, cat. no FSGPD05)
Centrifuge (Eppendorf 5804R, Rotor A-4–44)
24-well tissue culture plate (Fisher, cat. no 353047)
Microscope Cover Glass (Fisher, cat. no 12–545-M)
Nalgene Rapid-Flow Sterile Disposable Filter Units with PES Membrane (Life Tech., cat. no 565–0020)
Poly-L-lysine hydrobromide (0.1 mg/mL in sterile water) (MilliporeSigm, cat. no P2636)
Laminin (MilliporeSigma, cat. no L2020)
Worthington Papain Dissociation Kit (Worthington, cat. no LK003178)
Lab forceps (World Precision Instruments, cat. no 504506)
Lab scissors (Fisher, cat. no 731210)
5mL Luer centric syringe (Fisher, cat. no 14–817-53)
0.2um Syringe Filter, sterile (ThermoScientific, cat. no 726–2520)
50mL Falcon conical tubes (Fisher, cat. no 14–432-22)
Pasteur pipettes (Fisher, cat. no 13–678-20D)
70um Falcon Cell Strainer (Fisher, cat. no 08–771-2)
MidiMACS Separator or QuadroMACS Separator (Miltenyi Biotec, cat. no 130–042-302 and cat. no 130–090-976)
LS Columns (Miltenyi Biotec, cat. no 130–042-401)
MACS Myelin Beads, human, mouse, rat (Miltenyi Biotec, cat. no 130–104-253)
MACS Cd11b+ Microbeads, mouse (Miltenyi Biotec, cat. no 130–093-634)
MACS ACSA2 MicroBead Kit, mouse (Miltenyi Biotec, cat. no 130–097-678)
MACS Neuron Isolation Kit, mouse (Miltenyi Biotec, cat. no 130–115-389)
Protocol steps
Prepare Culture Plates
-
1
Sterilize glass coverslips prior to placing into individual wells in a 24-well plate.
We store our coverslips in 100% EtOH. Prior to plating, each coverglass is briefly exposed to a Bunsen burner. If the BSC is not equipped with a gas-line, you can simply leave the filled culture plate open and allow the EtOH to evaporate.
-
2
Add 500μL 0.1mg/mL poly-l-lysine to each well. Incubate overnight at room temperature. Following incubation, wash glass coverslips three times with sterile water.
-
3
Expose a 200μL pipette tip to Bunsen burner set to a low flame. Once warm, gently press the tip to an inverted culture plate lid such that the tip now has a flat, circular end. Add 1μL laminin to a coverslip, and use the flattened pipette tip to swirl the laminin to ensure coating of the entire coverslip. Repeat on all glass coverslips.
Prepare Worthington Papain Dissociation kit
-
4
Add 32mL EBSS to the albumin-ovomucoid inhibitor and allow the contents to dissolve.
Perform this step only when first opening the Worthington Papain Dissociation kit. Once prepared, the solutions are stable for up to one month.
-
5
Add 5mL EBSS to the papain vial. Mix gently to dissolve the powder.
-
6
Add 500μL EBSS to a DNase vial. Mix gently and allow the powder to fully dissolve. Transfer 250μL of this solution to the vial containing the papain. Store the rest of the DNase solution on ice until needed.
-
7
Equilibrate the papain solution by exposure to 95%O2:5%CO2. While you perform the tissue microdissections, keep the solution at room temperature.
Dissociate mouse cortex into single-cell suspension
-
8
Rapidly decapitate the postnatal day 0–1 animal. Remove the brain, dissect the brain region of interest in bubbled ACSF and remove the meninges. Mince the microdissected tissue into 100mm3 sections using scissors or forceps.
It is not recommended to use CO2 in animals younger than 12 days. Therefore, rapid decapitation is used for euthanasia.
-
9
Sterile-filter the bubbled papain into a 50mL conical tube. Slowly draw up the minced tissue using a 10mL serological pipette. Allow the tissue to separate from the solution and settle at the bottom of the pipette. Release only the settled tissue into the prepared papain.
To sterile-filter the papain, draw up the solution into a 5mL syringe. Apply the sterile-filer to the end and release the papain into the 50mL conical tube.
-
10
Incubate the tissue/papain mixture for 15–20 minutes in a water bath set to 37°C. Swirl the conical tube every 5 minutes to maximize the tissue’s exposure to the papain.
-
11
Gently titrate the tissue with 10mL serological pipette with setting turned to Slow for 5 times up and down. Do not over triturate the tissue at this step.
-
12
Centrifuge the homogenized solution at 300 × g for 3 minutes at room temperature.
-
13
Prepare the resuspension buffer by mixing 2.7mL EBSS, 300μL albumin-ovomucoid inhibitor, and the remaining 150μL DNase.
-
14
Discard the supernatant and immediately resuspend the cell pellet in 1mL resuspension buffer prepared in the step above.
-
15
Fire-polish a glass Pasteur pipette.
The Bunsen burner should be set to a low flame. Expose only the tip while rotating the pipette. A correctly fire-polished pipette should have a smooth, rounded tip that is slightly smaller than an unpolished pipette.
-
16
Triturate the tissue using the fire-polished pipette with setting turned to Slow for 5 times up and down. The solution should now be homogenous, with a cloudy, light pink appearance. Centrifuge at 300 × g for 5 minutes at room temperature.
-
17
Discard the supernatant and immediately suspend the pelleted cells in 4 mL PBS/BSA solution. To ensure that you have a single-cell suspension, filter the solution using a 70μm BD Falcon filter to remove any non-dissociated tissue.
The filter should be wetted prior to applying the cells. We typically use 1 mL to wet the filter for a total volume of 5mL
Remove myelin debris and microglia
-
18
Centrifuge the single-cell suspension at 300 × g for 3 minutes at 4°C and discard the supernatant. Resuspend the pelleted cells in 150μL PBS/BSA solution.
-
19
Add 10–15μL anti-myelin and 10–15μL anti-Cd11b+ MicroBeads. Incubate the mixture at 4°C for 10 minutes, with a gentle mix every 5 minutes.
-
20
Add 1mL PBS/BSA solution to the conical tube and centrifuge at 300 × g for 3 minutes at 4°C to pellet the cells. This will remove any excess beads from the solution.
-
21
Place the LS column into the MidiMACS or QuadroMACS. Place a fresh 50mL conical tube at the bottom. This will be used to collect the non-targeted cells. Apply 2mL PBS/BSA to the column. Pre-wetting the column will prevent any non-labeled cells from becoming stuck in the dry column.
-
22
Remove and discard the supernatant from step 20. Resuspend the pellet in 500μL PBS/BSA solution and apply directly to the LS column. Collect the flow-through into a new, fresh 50mL conical tube. The flow-through contains the astrocytes and neurons.
-
23
Add 3mL PBS/BSA to the column. Continue to collect the flow-through. Repeat this step one more time, for a total of two 3mL solution applications.
Isolate and plate Neuron Fraction
-
24
Centrifuge the collected flow-through at 300 × g for 3 minutes at 4°C and discard the supernatant. Resuspend the pelleted cells in 150μL PBS/BSA solution.
-
25
Add 10–15μL Anti-biotin blocking cocktail microbeads from the Neuron Isolation kit. This cocktail will target non-neuronal cellular populations. Incubate the mixture at 4°C for 10 minutes, with a gentle mix every 5 minutes.
-
26
Add 10–15μL Anti-biotin antibodies from the kit. Incubate the mixture at 4°C for 10 minutes, with a gentle mix every 5 minutes.
-
27
Add 1mL PBS/BSA solution to the conical tube and centrifuge at 300 × g for 3 minutes at 4°C to pellet the cells. This will remove any excess beads from the solution.
-
28
Place a LS column into the MidiMACS or QuadroMACS. Place a fresh 50mL conical tube at the bottom. This will be used to collect the non-targeted cells. Apply 2mL PBS/BSA to the column. Pre-wetting the column will prevent any non-labeled cells from becoming stuck in the dry column.
-
29
Remove and discard the resulting supernatant from step 27. Resuspend the pellet in 500μL Neuronal Media and apply directly to the LS column. Collect the flow-through into a new, fresh 50mL conical tube. The flow-through contains the Neuron Fraction while the targeted, non-neuronal cell types will remain on the LS column.
-
30
Add 3mL Neuronal Media to the column. Continue to collect the flow-through (Neuron Fraction). Repeat this step one more time, for a total of two 3mL media applications.
-
31
Determine the number of neurons collected using a hemacytometer and plate the neurons at 75 × 104 – 300 × 104 plating density in prepared 24-well plates.
[*Copy Editor – add a link to the following protocol for using a hemacytometer: https://doi.org/10.1002/0471142727.nsa03bs38]
-
32One day post-plating, add 2.5uM araC (anti-mitotic cytarabine) to each well. Two days post-plating (one day following araC treatment), perform a full media change.
- Subsequent media changes should be performed every 3–4 days at half-media changes. In our experience, these cells are viable for 14–20 days post-plating. See the above protocol link for information on culture techniques.
Isolation and plating of astrocytes
-
33
5 days post-initial plating of neurons, when the pups are 5–7 postnatal days in age, follow steps 1–17 from above to dissociate into a single-cell suspension and remove myelin debris and microglia.
-
34
Centrifuge the collected flow-through at 300 × g for 3 minutes at 4°C and discard the supernatant. Resuspend the pelleted cells in 150μL PBS/BSA solution.
-
35
Add 10–15μL FcR blocking cocktail microbeads from the ACSA-2 kit. This cocktail will prevent non-specific binding for the ACSA2 microbeads. Incubate the mixture at 4°C for 10 minutes, with a gentle mix every 5 minutes.
-
36
Add 10–15μL Anti-ACSA2 from the kit. Incubate the mixture at 4°C for 10 minutes, with a gentle mix every 5 minutes. These microbeads target an extracellular protein on astrocytes.
-
37
Add 1mL PBS/BSA solution to the conical tube and centrifuge at 300 × g for 3 minutes at 4°C to pellet the cells. This will remove any excess beads from the solution.
-
38
Place the LS column into the MidiMACS or QuadroMACS. Place a fresh 50mL conical tube at the bottom. This will be used to collect the non-targeted cells. Apply 2mL PBS/BSA to the column. Pre-wetting the column will prevent any non-labeled cells from becoming stuck in the dry column.
-
39
Remove and discard the resulting supernatant from step 37. Resuspend the pellet in 500μL PBS/BSA solution and apply directly to the LS column. Collect the flow-through into a new, fresh 50mL conical tube. The flow-through contains the neurons, while the astrocytes will remain on the LS column.
-
40
Add 3mL PBS/BSA to the column. Continue to collect the flow-through. Repeat this step one more time, for a total of two 3mL solution applications.
-
41
Remove the LS column from the magnetic holder, and place into a fresh 15mL conical tube. Elute the targeted astrocyte population by adding 5mL Neuronal Media. Use the supplied plunger to push the solution through the LS column. This will apply gentle pressure to remove the astrocytes from the column and result in an Astrocyte Fraction.
-
42
Determine the number of astrocytes collected using a hemacytometer, and plate on top of the neurons at 75 × 104 – 300 × 104 plating density. Any remaining astrocytes may be plated as described in Alternate Protocol 2.
ALTERNATE PROTOCOL 2
ISOLATION AND CULTURE OF ASTROCYTES
Introductory paragraph
In addition to co-culture of neurons and astrocytes, MACS sorting can be utilized to culture astrocytes-alone in serum-free conditions. Traditional astrocyte cultures are maintained in serum-containing media. However, recent research has highlighted the experimental limitations to this in vitro technique. Here we detail methodology to culture astrocytes that bypasses the need for serum-containing media.
Materials
Experimental animals (sex, age, strain, etc. will depend upon the researcher’s needs)
Herein we use male and female wildtype C57BL/6 mice, aged 0–5 days
Astrocyte Media (see recipe)
ACSF (see recipe)
0.5% BSA in PBS (see recipe)
PBS (Biorad, cat. no 161–0780)
BSA, fatty acid free (MilliporeSigma, cat. no A7030)
100% Ethanol (Fisher, cat. no A405P4)
Bunsen Burner (Humboldt, cat. no 6200.1)
Carbogen (95% O2 : 5% CO2 gas tank, Praxair, cat. no MM OXCD5-K)
Water bath (Fisher, cat. no FSGPD05)
10mL serological pipette (Fisher, cat. no 02–707-155)
Centrifuge (Eppendorf 5804R, Rotor A-4–44)
24-well tissue culture plate (Fisher, cat. no 353047)
Microscope Cover Glass (Fisher, cat. no 12–545-M)
0.1 mg/mL in sterile water Poly-l-ornithine hydrobromide (MilliporeSigma, cat. no P3655)
Laminin (MilliporeSigma, cat. no L2020)
Lab forceps (World Precision Instruments, cat. no 504506)
Lab scissors (Fisher, cat. no 731210)
Worthington Papain Dissociation Kit (Worthington, cat. no LK003178)
5mL Luer centric syringe (Fisher, cat. no 14–817-53)
0.2um Syringe Filter, sterile (ThermoScientific, cat. no 726–2520)
Pasteur pipettes (Fisher, cat. no 13–678-20D)
50mL Falcon conical tubes (Fisher, cat. no 14–432-22)
70um Falcon Cell Strainer (Fisher, cat. no 08–771-2)
MidiMACS Separator or QuadroMACS Separator (Miltenyi Biotec, cat. no 130–042-302 and cat. no 130–090-976)
LS Columns (Miltenyi Biotec, cat. no 130–042-401)
MACS Myelin Beads, human, mouse, rat (Miltenyi Biotec, cat. no 130–104-253)
MACS Cd11b+ Microbeads, mouse (Miltenyi Biotec, cat. no 130–093-634)
MACS ACSA2 MicroBead Kit, mouse (Miltenyi Biotec, cat. no 130–097-678)
Protocol steps
All steps should be performed in a Biosafety Cabinet using aseptic technique
Prepare Culture Plates
-
1
Sterilize glass coverslips prior to placing into individual wells in a 24-well plate.
We store our coverslips in 100% EtOH. Prior to plating, each coverglass is briefly exposed to a Bunsen burner. If the BSC is not equipped with a gas line, you can simply leave the filled culture plate open to allow the EtOH to evaporate.
-
2
Add 500μL poly-l-ornithine to each well. Incubate overnight at room temperature. Following incubation, wash glass coverslips three times with sterile water.
-
3
Expose a 200μL pipette tip to Bunsen burner set to a low flame. Once warm, gently press the tip to an inverted culture plate lid such that the tip now has a flat, circular end.
-
4
Add 1μL laminin to a coverslip and use the flattened pipette tip to swirl the laminin to ensure coating of the entire coverslip. Repeat on all glass coverslips.
Prepare Worthington Papain Dissociation kit
-
5
Add 32mL EBSS to the albumin-ovomucoid inhibitor in a BSC and allow the contents to dissolve.
Perform this step only when first opening the Worthington Papain Dissociation kit. Once prepared, the solutions are stable for up to one month.
-
6
Add 5mL EBSS to the papain vial. Mix gently to dissolve the powder.
-
7
Add 500μL EBSS to a DNase vial. Mix gently and allow the powder to fully dissolve. Transfer 250μL of this solution to the vial containing the papain. Store the rest of the DNase solution on ice until needed.
-
8
Equilibrate the papain solution by exposure to 95%O2:5%CO2. While you perform the tissue microdissections, keep the solution at room temperature.
Dissociate mouse cortex into single-cell suspension
-
9
Rapidly decapitate the postnatal day 3–5 animal. Remove the brain, dissect the brain region of interest and remove the meninges. Mince the microdissected tissue into 100mm3 sections using scissors or forceps.
It is not recommended to use CO2 on animals younger than 12 days. Therefore, rapid decapitation is used for euthanasia.
-
10
Sterile-filter the bubbled papain into a 50mL conical tube. Slowly draw up the minced tissue using a 10mL serological pipette. Allow the tissue to separate from the solution and settle at the bottom of the pipette. Release only the settled tissue into the prepared papain.
To sterile-filter the papain, draw up the solution into a 5mL syringe. Apply the sterile-filer to the end, and release the papain into the 50mL conical tube.
-
11
Incubate the tissue/papain mixture for 15–20 minutes in a water bath set to 37°C. Swirl the conical tube every 5 minutes to maximize the tissue’s exposure to the papain.
-
12
Gently titrate the tissue with 10mL serological pipette (using an auto-pipette) with setting turned to Slow for 5 times up and down. Do not over triturate the tissue at this step.
-
13
Centrifuge the homogenized solution at 300 × g for 3 minutes at room temperature.
-
14
Prepare the resuspension buffer by mixing 2.7mL EBSS, 300μL albumin-ovomucoid inhibitor, and the remaining 150μL DNase.
-
15
Discard the supernatant and immediately resuspend the cell pellet in 1mL resuspension buffer prepared in the step above.
-
16
Fire-polish a glass Pasteur pipette.
The Bunsen burner should be set to a low flame. Expose only the tip while rotating the pipette. A correctly fire-polished pipette should have a smooth, rounded tip that is slightly smaller than an unpolished pipette (see Figure 3 for reference).
-
17
Triturate the tissue using the fire-polished pipette with setting turned to Slow for 5 times up and down. The solution should now be homogenous, with a cloudy, light pink appearance. Centrifuge at 300 × g for 5 minutes at room temperature.
-
18
Discard the supernatant and immediately suspend the pelleted cells in 4 mL PBS/BSA solution. To ensure that you have a single-cell suspension, filter the solution using a 70μm BD Falcon filter to remove any non-dissociated tissue. The filter should be wetted prior to applying the cells. We typically use 1 mL to wet the filter for a total volume of 5mL.
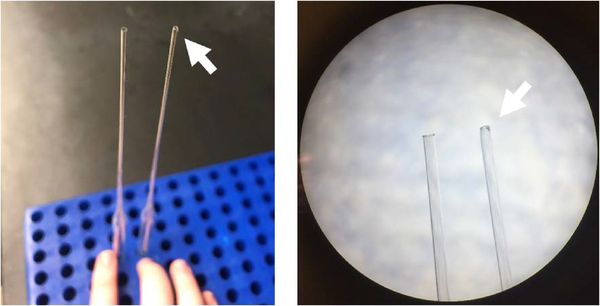
Figure 3.
Comparison of pasteur and fire-polished pasteur pipettes. Fire-polished pipettes (arrows) are rounded and have a smaller opening.
Remove myelin debris and microglia
-
19
Centrifuge single-cell suspension at 300 × g for 3 minutes at 4°C and discard the supernatant. Resuspend the pelleted cells in 150μL PBS/BSA solution.
-
20
Add 15–20μL anti-myelin and anti-Cd11b+ MicroBeads. Incubate the mixture at 4°C for 10 minutes, with a gentle mix every 5 minutes.
-
21
Add 1mL PBS/BSA solution to the conical tube and centrifuge at 300 × g for 3 minutes at 4°C to pellet the cells. This will remove any excess beads from the solution. Remove and discard the supernatant. Resuspend the pellet in 500μL PBS/BSA solution and apply directly to the LS column. Collect the flow-through into a new, fresh 50mL conical tube. The flow-through contains the astrocytes and neurons.
-
22
Add 3mL PBS/BSA to the column. Continue to collect the flow-through. Repeat this step one more time, for a total of two 3mL solution applications.
Isolation and plating of astrocytes
-
23
Centrifuge the collected flow-through at 300 × g for 3 minutes at 4°C and discard the supernatant. Resuspend the pelleted cells in 150μL PBS/BSA solution.
-
24
Add 10–15μL FcR blocking cocktail microbeads from the ACSA-2 Microbead kit. This cocktail will prevent non-specific binding for the ACSA-2 microbeads. Incubate the mixture at 4°C for 10 minutes, with a gentle mix every 5 minutes.
-
25
Add 10–15μL Anti-ACSA2 from the kit. Incubate the mixture at 4°C for 10 minutes, with a gentle mix every 5 minutes. These microbeads target an extracellular protein on astrocytes.
-
26
Add 1mL PBS/BSA solution to the conical tube and centrifuge at 300 × g for 3 minutes at 4°C to pellet the cells. This will remove any excess beads from the solution. Remove and discard the resulting supernatant.
-
27
Resuspend the pellet in 500μL PBS/BSA solution and apply directly to the LS column. Collect the flow-through into a new, fresh 50mL conical tube. The flow-through contains the neurons, while the astrocytes will remain on the LS column.
-
28
Add 3mL PBS/BSA to the column. Continue to collect the flow-through. Repeat this step one more time, for a total of two 3mL solution applications.
-
29
Remove the LS column from the magnetic holder, and place into a fresh 15 mL conical tube. Elute the targeted astrocyte population by adding 5mL Astrocyte Media. Use the supplied plunger to push the solution through the LS column. This will apply gentle pressure to remove the astrocytes from the column and result in an Astrocyte Fraction.
-
30
Determine the number of astrocytes collected using a hemacytometer, and plate at 75 × 104 – 300 × 104 plating density.
REAGENTS AND SOLUTIONS
Artificial Cerebrospinal Fluid (ACSF)
Stock Composition:
120mM NaCl (FisherScientific, cat. no S671)
3mM KCl (FisherScientific, cat. no BP366)
26.2 mM NaHCO3 (MilliporeSigma, cat. no S6014)
11.1 mM Glucose (MilliporeSigma, cat. no G8270)
2mM MgCl2 (MilliporeSigma, M8266)
0.2 mM CaCl2 (MilliporeSigma, C1016)
20mM CNQX (Tocris, cat. no 0910)
20nM AP5 (Tocris, cat. no 0106)
For 1 liter stock ACSF:
7 g NaCl
0.223 g KCl
2.2 g NaHCO3
2 g Glucose
Bring up to 1 liter with water
Stock ACSF should be made without MgCl2, CaCl2, CNQX, or AP5 and stored at 4°C. At these conditions, ACSF is stable for up to a month.
ACSF should be made fresh every day, with addition of MgCl2, CaCl2, AP5, and CNQX.
For 50 mL ACSF:
50 uL 1M MgCl2 (0.95 g in 10ml)
10 uL 1M CaCl2 (1.11 g in 10mL)
50 uL 20mM AP5 (10mg AP5 in 2.6 mL water)
50uL 20mM CNQX (50mg CNQX in 10.76mL DMSO [MilliporeSigma D5879])
Bring up to 50mL with stock ACSF
ACSF must be carbogenated for at least 15 minutes before use.
0.5% BSA in PBS
Composition:
1x PBS (Biorad, cat. no 161–0780)
BSA, fatty acid free (MilliporeSigma, cat. no A7030)
For 500mL:
2.5g BSA to 500mL 1x PBS
PBS/BSA should be stored at 4°C. In our experience, PBS/BSA has a shelf life of 3–4 weeks.
Neuron Media
Composition:
Neurobasal Media (ThermoScientific, cat. no 21103049)
1x B27 (ThermoScientific, cat. no. 17504–044)
2mM glutamine
500U penicillin/streptomycin
For 500mL:
10 mL 50x B27
2.5 mL 2M glutamine
500uL pen/strep
Bring up to 500 mL with Neurobasal Media
Neuron media should be prepared in a BioSafety Cabinet and sterile-filtered prior to use. In our experience, the media has a shelf life of 2–3 weeks.
Media must be warmed to 37°C before use.
Astrocyte Media
Composition:
50% Minimal Earl’s Media (ThermoFisher, cat. no 51200038)
50% Neurobasal Media (ThermoScientific, cat. no 21103049)
1mM sodium pyruvate (ThermoFisher, cat. no 11360070)
2mM glutamine (Gibco, cat. no 21051–024)
1x B27 (ThermoScientific, cat. no. 17504–044)
500U penicillin/streptomycin (ThermoFisher, cat. no 15140122)
For 500mL:
10 mL 50x B27
2.5mL 100 mM sodium pyruvate
2.5 mL 2M glutamine (2.92 g in 100mL water)
500uL pen/strep
Bring up to 500 mL with Neurobasal Media
Astrocyte media should be prepared in a BioSafety Cabinet and sterile-filtered prior to use. In our experience, the media has a shelf life of 2–3 weeks.
Media must be warmed to 37°C before use.
COMMENTARY
BACKGROUND INFORMATION
Several techniques have been developed to ‘capture’ unique cell populations from neural tissue. These include laser-capture microdissection (LCM), fluorescent activated sorting (FACS), and more recently translation ribosomal affinity purification (TRAP). Each technique offers unique advantages and limitations. To date, FACS is the method most frequently utilized. FACS is regularly performed in fluorescent cell populations driven by cell type specific fluorescent reporter genes but can also be performed in immunolabeled fresh or fixed tissue (Cahoy et al., 2008; Guez-Barber et al., 2012). One drawback to FAC sorting is that cellular processes are shorn off in the flow cytometer as cells move through a rapidly flowing stream of fluid during the separation process. This limits the utility of this technique if total cell protein is the final outcome measure. Additionally, this technique requires a flow activated cell sorter, an expensive piece of equipment that not all researchers may access. LCM is commonly used in fixed tissue to capture cellular populations of interest. The user “traces” the cells, which are dissected with a laser and subsequently collected (Decarlo, Emley, Dadzie, & Mahalingam, 2011). The specific isolation of the chosen cells gives researchers high specificity in cellular population and brain regions. This technique is particularly useful in human postmortem tissue, where tissue is limited in quantity. However, similar to FACs, this technique requires specialized equipment. Additionally, this technique requires extensive training and expertise, as the individual user identifies cells for tracing and microdissection based on their morphological appearance; a limitation that may be circumvented by immunolabeling cells prior to LCM (Chabrat, Doucet-Beaupre, & Levesque, 2015). Recently TRAP has become an attractive option (Ayata et al., 2018; Doyle et al., 2008; Heiman et al., 2008; Hoye et al., 2018; Yu et al., 2018). Fluorescently-tagged ribosomes are expressed in a single cell population using a cell-type specific promoter. The ribosomes are immunoprecipitated along with mRNA that is actively translated. While this technique has the major advantage of negating the need for enzymatic digestion to create a single cell suspension, limitations include a ‘snap shot’ of the RNA pool that is undergoing active translation rather than the total RNA pool. To evaluate different cell types, multiple mouse lines driving cell type specific fluorescently tagged ribosomes must be generated (Hoye et al., 2018). In contrast, the MACS technique is relatively fast and inexpensive, allowing for the isolation of multiple cellular populations from a single brain. The development of commercially available kits has increased the popularity of this technique. Limitations to this particular technique are similar to those we have already discussed, in that enzymatic digestion is utilized to generate single-cell suspensions, which causes cellular stress and may alter gene and protein expression. MACS, thus far, has not be performed in fixed tissue and requires an antibody targeted to extracellular proteins. Furthermore, depending on the desired downstream application, MACS may require larger inputs to capture an adequate number of cells. Simple pooling of animals may overcome this limitation. Overall, we find this technique to be relatively gentle in allowing for the retention of cellular processes important for some downstream applications, including evaluation of extra somatic proteins and ‘capturing’ cell populations for cell culture.
Within this unit we also detail protocols for the culture of astrocytes alone and co-culture of astrocytes and neurons. The traditional, most commonly used astrocyte culture technique was initially reported in the 1980s (McCarthy & de Vellis, 1980). While this system has garnered a wealth of information for the field, drawbacks have been highlighted (for review, see (Lange, Bak, Waagepetersen, Schousboe, & Norenberg, 2012). The most prominent includes the lack of translation from cultured astrocytes to their in vivo counterparts, with different gene and protein expression (Cahoy et al., 2008; Doyle et al., 2008), biophysical properties, and a distinct lack of morphological complexity (Cahoy et al., 2008; Doyle et al., 2008; Foo et al., 2011). Many of these features have been attributed to the presence of serum in the culture media (Foo et al., 2011). Serum contains high levels of glutamate, which is excitotoxic to neurons and results in their death within the culture dish (Ye & Sontheimer, 1998). Astrocytes and microglia remain, and microglia are shaken off prior to performing experiments on the astrocytes. Immunopanning of astrocytes has, to date, been the only proposed alternative (Foo et al., 2011). Immunopanning is performed by plating a dissociated cell suspension onto antibody-coated plates (Foo et al., 2011). Following a period of recovery, the cells are enzymatically lifted off the plate. The targeted cell populations bound to the antibodies remain on the plate, while non-targeted cell populations are removed. Typically, a series of plates is necessary to remove non-astrocytic cells such as microglia, oligodendrocytes, and neurons with a final plate targeting astrocytes. Herein, we detail an alternative method for obtaining pure astrocyte cultures without the need for serum, resulting in astrocytes with a significantly more complex morphology than traditional astrocyte cultures (Fig 5C). We additionally detail a novel method to directly co-culture mixed cell populations. Typical co-culture protocols require a series of culturing and passaging of astrocytes prior to plating neurons on top, resulting in co-culture of cells that differ in age (this negates the ability to culture different cell populations from littermates). Furthermore, neuronal cultures are often performed in embryonic animals to reduce non-neuronal contamination. This results in the additional loss of the breeding mother. Magnetic separation allows for co-cultures in post-natal littermates, minimizing the number of breeding animals needed. Collectively, we have found that MACS separation is a relatively easy, fast, and inexpensive technique with a wide array of downstream applications.
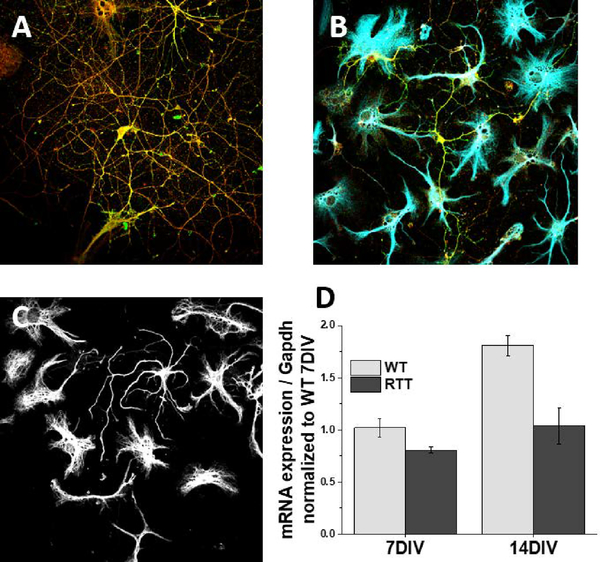
Figure 5.
Representative images of A) neurons alone, B) neuron/astrocyte co-cultures, and C) astrocytes alone. For all above, the cells were collected at 14DIV, fixed, and immunofluorescence performed. A) Neurons are visualized with presynaptic Vglut1 and postsynaptic marker PSD95 for synaptic quantification. B) Astrocytes visualized with Gfap (blue) are plated on top of neurons (green). C) Astrocytic filaments are visualized with Gfap (white) demonstrate complex morphology of serum-free astrocyte cultures. D) qPCR analysis of Kcnj10 expression in wildtype (WT) and Mecp2 deficient (RTT) astrocytes reveals loss of Kir4.1 in Rett syndrome is due to loss of mecp2 in astrocytes, reproduced with permission from Kahanovitch et al.
CRITICAL PARAMETERS
This protocol can be utilized for a variety of experimental designs from brain regions, ages, and genotypes to RNA to protein to culture work. In our experience, a single mouse cortex is sufficient to capture approximately 1–2 × 106 astrocytes, and RNA that is suitable for RNA-Seq analysis. However, experiments designed to isolate cellular populations from a smaller region of interest may require pooling of animals.
TROUBLESHOOTING
| Potential Problem | Common Cause | Solution |
|---|---|---|
| Low yield of targeted population | Incomplete dissociation | •Longer incubation with papain •Better trituration |
| Small brain region of interest | •Combine and pool multiple animals | |
| Magnetic Beads | •Ensure beads are in-date •Increase incubation time •Ensure the total number of cells is not clogging the LS column |
|
| Low cell viability | Dissociation | •Decrease time in papain •Ensure papain solution is equilibrated •Decrease trituration speed/time |
| Low cell viability (culture specific) | Low plating density | •Neurons do not survive on their own well, so ensure the plating density is sufficient |
| Media | •Ensure media is properly made •Use fresh media •Ensure proper media change schedule |
|
| Culture plate | •Increase incubation time for poly-lysine or -ornithine coating •Ensure the coverglass was washed 3X |
|
| Incubator | •Ensure incubator is set to proper conditions |
UNDERSTANDING RESULTS
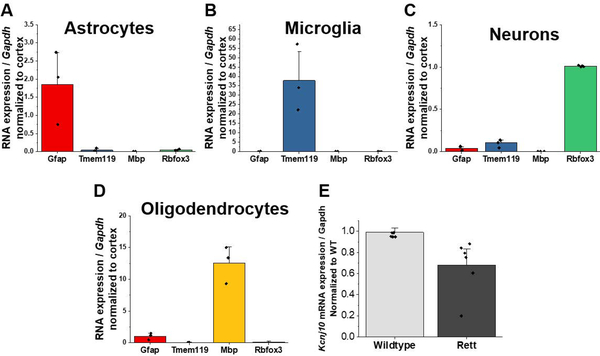
Following MACS separation of CNS cell populations, the isolated populations can be used for a variety of downstream applications. We demonstrate in Figure 4 the expected purity of each individual population. To determine the purity of the isolated populations, we use the “whole cortex” fraction as an input control to show enriched or depleted gene expression in purified cell populations (Holt & Olsen, 2016). For example, qPCR analysis of the astrocyte-fraction reveals 1-fold enriched expression of Gfap compared to whole cortex, with no expression of microglial (Tmem119), oligodendrocytic (Mbp), or neuronal (Rbfox3) gene expression (Fig 4A). We can conclude, therefore, that we have specifically isolated only astrocytes in our astrocyte-fraction. This holds true for microglia and neuron qPCR analysis as well (Fig 4B, C). In contrast, we observe equal Gfap expression as whole cortex in the oligodendrocyte-fraction (from the myelin-removal steps), suggesting astrocyte contamination in this fraction (Fig 4D). For this reason, our current protocol does not include collecting the myelin-removal fraction. For those interested, however, it is possible to optimize the protocol to isolate pure oligodendrocytes. These isolated populations can then be used to examine cell-type specific gene expression changes in vivo. For example, we have used MACS to examine astrocytic gene expression in a mouse model of Rett syndrome (RTT) (Kahanovitch et al., 2018). Examination of isolated astrocytes reveals roughly 30% decrease in Kir4.1 mRNA expression in Mecp2 deficient astrocytes compared to their wildtype littermates (Fig 4E).
Figure 4.
Quantitative PCR demonstrating sequential isolation of A) astrocytes, B) microglia, and C) neuronal populations. Normalization to a mixed cellular population (whole cortex) allows for the examination of enrichment or depletion of cell-type specific gene markers. As demonstrated in D), oligodendrocytes display enrichment of Gfap, and therefore astrocyte contamination. E) MACs-isolated astrocytes from wildtype and Mecp2-deficient animals was utilized to demonstrate that astrocytes exhibit a decrease in Kcnj10 mRNA expression, reproduced with permission from Kahanovitch et al.
Similarly, a variety of experimental designs can be implemented using MACS to isolate and culture specific CNS populations. Within this protocol, we detail neuron/astrocyte co-culture and astrocyte-alone culture paradigms. Figure 5 shows representative immunofluorescent images of neurons alone, astrocyte/neuron co-cultures and astrocytes alone from 14 days in vitro. A higher neuronal plating density (Fig 5A) results in a greater number of connections for neurons, whereas a lower density (Fig 5B) may be more suitable for morphology assessments. We have utilized serum-free astrocyte cultures described within this unit to demonstrate that the loss of Kir4.1 in RTT astrocytes is due specifically to the loss of MeCP2 in astrocytes (Fig 5D). Again using MACS to culture astrocytes from wildtype and RTT littermate pups, qPCR analysis demonstrated that wildtype astrocytes exhibit an increase in Kir4.1 expression over time, which is lost in Mecp2 deficient astrocytes (Kahanovitch et al., 2018).
TIME CONSIDERATIONS
We find that the sequential isolation of CNS populations is easily performed within three hours. The addition or subtraction of cellular populations of interest will result in a modification of that timing. In totality, between preparing coverslips to plating of cells also takes 3 hours. We find the best results with overnight incubations of poly-lysine or poly-ornithine.
ACKNOWLEDGMENTS
This work was funded by the National Institutes of Health NINDS R01NS075062 (M.L.O.) and F31NS100259 (L.M.H.).
LITERATURE CITED
- Ayata P, Badimon A, Strasburger HJ, Duff MK, Montgomery SE, Loh YE, … Schaefer A (2018). Epigenetic regulation of brain region-specific microglia clearance activity. Nat Neurosci, 21(8), 1049–1060. doi: 10.1038/s41593-018-0192-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, … Barres BA (2008). A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci, 28(1), 264–278. doi:28/½64 [pii]; 10.1523/JNEUROSCI.4178-07.2008 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabrat A, Doucet-Beaupre H, & Levesque M (2015). RNA Isolation from Cell Specific Subpopulations Using Laser-capture Microdissection Combined with Rapid Immunolabeling. J Vis Exp(98). doi: 10.3791/52510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decarlo K, Emley A, Dadzie OE, & Mahalingam M (2011). Laser capture microdissection: methods and applications. Methods Mol Biol, 755, 1–15. doi: 10.1007/978-1-61779-163-5_1 [DOI] [PubMed] [Google Scholar]
- Doyle JP, Dougherty JD, Heiman M, Schmidt EF, Stevens TR, Ma G, … Heintz N (2008). Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell, 135(4), 749–762. doi: 10.1016/j.cell.2008.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo LC, Allen NJ, Bushong EA, Ventura PB, Chung WS, Zhou L, … Barres BA (2011). Development of a method for the purification and culture of rodent astrocytes. Neuron, 71(5), 799–811. doi:S0896–6273(11)00649–0 [pii]; 10.1016/j.neuron.2011.07.022 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guez-Barber D, Fanous S, Harvey BK, Zhang Y, Lehrmann E, Becker KG, … Hope BT (2012). FACS purification of immunolabeled cell types from adult rat brain. J. Neurosci. Methods, 203(1), 10–18. doi:S0165–0270(11)00520–6 [pii]; 10.1016/j.jneumeth.2011.08.045 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen DV, Hanson JE, & Sheng M (2018). Microglia in Alzheimer’s disease. J Cell Biol, 217(2), 459–472. doi: 10.1083/jcb.201709069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman M, Schaefer A, Gong S, Peterson JD, Day M, Ramsey KE, … Heintz N (2008). A translational profiling approach for the molecular characterization of CNS cell types. Cell, 135(4), 738–748. doi: 10.1016/j.cell.2008.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt LM, & Olsen ML (2016). Novel Applications of Magnetic Cell Sorting to Analyze Cell-Type Specific Gene and Protein Expression in the Central Nervous System. PLoS ONE, 11(2), e0150290. doi: 10.1371/journal.pone.0150290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoye ML, Regan MR, Jensen LA, Lake AM, Reddy LV, Vidensky S, … Miller TM (2018). Motor neuron-derived microRNAs cause astrocyte dysfunction in amyotrophic lateral sclerosis. Brain, 141(9), 2561–2575. doi: 10.1093/brain/awy182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahanovitch U, Cuddapah VA, Pacheco NL, Holt LM, Mulkey DK, Percy AK, & Olsen ML (2018). MeCP2 Deficiency Leads to Loss of Glial Kir4.1. eNeuro, 5(1). doi: 10.1523/eneuro.0194-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec AM, Smith CJ, Ayre NR, Sweat SC, & Bilbo SD (2018). Microglial dopamine receptor elimination defines sex-specific nucleus accumbens development and social behavior in adolescent rats. Nat Commun, 9(1), 3769. doi: 10.1038/s41467-018-06118-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange SC, Bak LK, Waagepetersen HS, Schousboe A, & Norenberg MD (2012). Primary cultures of astrocytes: their value in understanding astrocytes in health and disease. Neurochem Res, 37(11), 2569–2588. doi: 10.1007/s11064-012-0868-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioy DT, Garg SK, Monaghan CE, Raber J, Foust KD, Kaspar BK, … Mandel G (2011). A role for glia in the progression of Rett’s syndrome. Nature, 475(7357), 497–500. doi:nature10214 [pii]; 10.1038/nature10214 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KD, & de Vellis J (1980). Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol, 85(3), 890–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peferoen L, Kipp M, van der Valk P, van Noort JM, & Amor S (2014). Oligodendrocyte-microglia cross-talk in the central nervous system. Immunology, 141(3), 302–313. doi: 10.1111/imm.12163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZC, & Sontheimer H (1998). Astrocytes protect neurons from neurotoxic injury by serum glutamate. Glia, 22(3), 237–248. doi: [DOI] [PubMed] [Google Scholar]
- Yu X, Taylor AMW, Nagai J, Golshani P, Evans CJ, Coppola G, & Khakh BS (2018). Reducing Astrocyte Calcium Signaling In Vivo Alters Striatal Microcircuits and Causes Repetitive Behavior. Neuron, 99(6), 1170–1187.e1179. doi: 10.1016/j.neuron.2018.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]