Abstract
Background
There has been a substantial increase in the number of imaging studies performed to assess thoracic aortic pathology. We sought to determine the accuracy of transthoracic echocardiography (TTE) compared to transesophageal echocardiography (TEE) for measuring ascending aortic size.
Hypothesis
Transthoracic echocardiography is reasonably accurate for assessing ascending aortic dimension.
Methods
Fifty‐two patients with or without aortic disease underwent both TTE with nonstandard views and TEE. The ascending aorta was measured at 4 levels by 2 blinded observers for each modality. Pearson's correlation coefficients were determined and Bland‐Altman plots and analyses were constructed. Inter‐ and intraobserver variability was determined in a random subgroup of patients.
Results
The mean age of the group was 65.5 years old and 15% had aortic dilation >4.0 cm. A strong positive correlation between the 2 imaging modalities was seen at all levels with the highest correlation for the maximum diameter of the ascending aorta (r = 0.936, P < 0.0001). Interobserver and intraobserver variability showed a good intraclass correlation among readers and among the same reader at all levels.
Conclusions
Transthoracic echocardiography using nonstandard imaging windows is accurate in comparison to TEE for measurement of the ascending aorta at multiple levels in patients with or without aortic pathology. The findings of this study provide support for selected serial follow‐up of patients with aortic disease by TTE only. Copyright © 2008 Wiley Periodicals, Inc.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
In recent years, there has been a substantial increase in the number of imaging studies performed to assess the ascending aorta, spurred by heightened awareness of the association between bicuspid aortic valves, which occurs in 1% to 2% of the general population with thoracic aortic aneurysms.1, 2, 3, 4 Furthermore, the etiology and predictors of acute aortic syndromes are now better understood.5 Concordantly, aortic surgeries are performed more often, at smaller aortic dimensions, and with lower operative mortality, thus averting potentially life‐threatening events.6,7 Determining the timing of aortic surgery is vitally dependent on the absolute and relative aortic size overtime. Thus an increasing need for screening and follow‐up imaging studies to assess thoracic ascending aortic dimensions plays a critical role in the evaluation and management of aortic disease.
Although computed tomography (CT) angiography, magnetic resonance imaging (MRI) angiography, and transesophageal echocardiography (TEE) have evolved rapidly as near equal standards for assessing ascending aortic size and pathology, these studies have limitations related to cost, radiation, invasiveness, and availability. Limited data exist to assess the capability of transthoracic echocardiography (TTE) for measurement of the ascending aorta beyond the aortic root in comparison to CT, MRI, or TEE.8, 9, 10, 11 If TTE were found to have reasonable precision in this regard, gold standard tests could be utilized less frequently, and TTE would allow for a more complete assessment of valvular and aortic structure with a single imaging modality. Therefore, we sought to evaluate the accuracy of TTE compared to TEE for measurement of the ascending aorta.
Methods
Study Design
We prospectively evaluated patients with or without known aortic disease scheduled for a clinically indicated TEE at our institution between December 1, 2006 and December 1, 2008 after approval by the institutional review board. All patients, 18 years and older, undergoing clinically indicated TEE were included in the study. Patients with ascending aortic grafts, those with poor TTE or TEE image quality (2 patients excluded for technically difficult TTE studies), patients who were unstable or in pain, and those in whom any additional delay in management would be detrimental were excluded. All participating patients signed an informed consent for inclusion in the study. Clinical, demographic, and echocardiographic data were extracted from the electronic medical record. All patient data were recorded with patient identifier numbers for confidentiality and a database was secured with access only to the principal investigator and coinvestigators.
Ancillary Transthoracic Echocardiogram
An ancillary TTE dedicated to viewing the ascending aorta was performed with a standard ultrasound machine (Philips iE33; Philips Medical System, Andover, MA or Sequoia C512; Acuson, Mountain View, CA) immediately after completion of the clinically indicated TEE. The sonographer performed a parasternal long‐axis view with movement medial or lateral and up or down relative to standard orientation to optimally see the ascending aorta. The image obtained attempted to visualize the aortic valve and ascending aorta in a single view with the aorta perpendicular to the transducer axis. At least 3 cardiac cycles were obtained for review. Time taken to perform the ancillary TTE was recorded in a limited number of patients. Images were digitally clipped and stored for future review and labeled with a study number.
Measurements
Two staff cardiologists/echocardiographers (G.N., J.B.) made off‐line measurements of the ascending aorta for both the TEE and TTE at four levels—aortic sinus of Valsalva, sinotubular junction (STJ), 1 cm above the STJ, and maximal dimension of the ascending aorta. The largest diameter at the sinus of Valsalva level was recorded, as was the maximal length of the ascending aorta above the ST junction that was measurable. The readers were blinded to patient data, and readings for both TTE and TEE were done in a random order on different days. Aortic sizes were obtained in diastole using an inner wall to inner wall convention with repeated cycles performed at each level as needed to improve accuracy. We considered the inner wall to differ from the inner edge convention by movement of the cursor to include the inner portion or the wall beyond the inner edge. Diastolic measurements were distinguished by the onset of the QRS on the electrocardiogram or if an adequate electrocardiogram was not available, by the closure of the aortic valve and a downward motion of the aortic wall. To assess intraobserver variability, TTE measurements of 10 patients were repeated by the same echocardiographer (J.B.) on a different day, blinded to the measurements made previously. Comparison between TTE and TEE was performed at each level for all patients.
Statistical Analysis
Comparisons were made between TTE and TEE, including Pearson's coefficient of correlation (r) calculation at the 4 aortic levels for all patients, and intraobserver and interobserver correlation for a random subset of 10 patients. We created Bland‐Altman difference plots to evaluate agreement between TTE and TEE at the 4 different levels. These plots depict the mean of the TTE and TEE measurements on the x‐axis, and the difference, TTE − TEE, and its 95% limit of agreement on the y‐axis. A P < 0.05 was considered statistically significant. We used SAS 9.1 (SAS Institute, Cary, NC) and R 2.6.1 (R Foundation, www.r‐project.org/index.html) for all statistical analyses.
Results
Fifty‐two patients satisfied the inclusion and exclusion criteria. The mean age of the group was 65.5 years (40 male and 12 female) with the oldest being 91 years of age. The average body mass index (BMI) was 28.6 kg/m2 with the highest BMI at 40 kg/m2. Indications for TEE included: atrial arrhythmias, 28 (53.8%); rule out endocarditis, 7 (13.4%); stroke/source of embolism, 6 (11.5%); mitral regurgitation, 3 (5.8%); aortic pathology, 3(5.8%); aortic stenosis, 2 (3.8%); and other miscellaneous reason, 3 (5.8%). The aortic valve was normal in 27 (51.9%), mildly stenotic or regurgitant in 15 (28.8%), moderately stenotic or regurgitant in 4 (7.7%), mechanical in 4 (7.7%), and a normally functioning bicuspid valve in 2 (3.9%). Table 1 shows the mean and standard deviation of aortic dimensions at each level measured. Eight patients had maximal aortic dimensions >4.0 cm (15%) (range, 4.1 cm–4.7 cm). Improvement in visualization of the ascending aorta for measurement of aortic dimensions with nonstandard windows was observed in 93% of the patients. Best images of the ascending aorta were obtained with medial and higher intercostal space movement (Figure 1). The average time taken for the ancillary TTE was 3 minutes.
Table 1.
Mean and Standard Deviation of Each Level of Measurement by Transthoracic Echocardiography and Transesophageal Echocardiography
| Measurement Level | TEE | TTE | p |
|---|---|---|---|
| Sinus level, cm, mean (SD) | 3.51 (0.40) | 3.49 (0.43) | 0.7 |
| Sinotubular junction level, cm, mean (SD) | 3.01 (0.40) | 3.04 (0.42) | 0.4 |
| 1 cm above sinotubular junction, cm, mean (SD) | 3.24 (0.45) | 3.28 (0.45) | 0.1 |
| Max diameter of ascending aorta, cm, mean (SD) | 3.58 (0.56) | 3.56 (0.53) | 0.6 |
| Length of proximal aorta, cm, mean (SD) | 7.18 (1.21) | 6.67 (1.19) | 0.02 |
P evaluates differences between transesophageal echocardiography (TEE( and transthoracic echocardiography (TTE( (paired t test)
Figure 1.
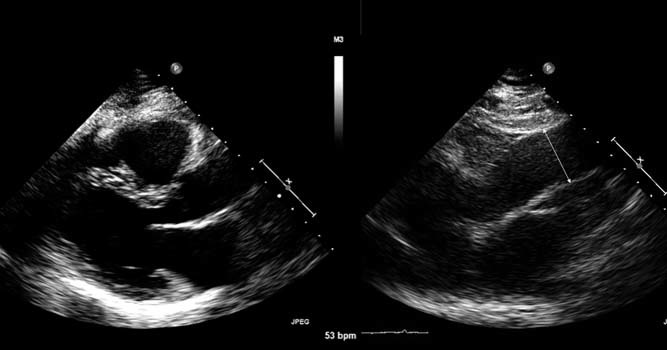
Left panel : Standard transthoracic parasternal long‐axis image of the aortic root. Right panel : Transthoracic parasternal long‐axis image with movement up an interspace for visualization of the ascending aorta and measurement of the inner wall to inner wall of the tubular ascending aorta
The mean difference and 95% limits of agreement between TTE and TEE measurements of the ascending thoracic aorta were small at all levels measured (ranging from a maximum of − 5.8mm to + 5.8mm) (Table 2). A strong positive correlation between the 2 imaging modalities was seen at all levels, with the highest correlation for the maximum diameter of the ascending aorta. Interobserver and intraobserver variability is depicted in Table 3 showing at least a moderate intraclass correlation among readers and among the same reader at all levels.
Table 2.
Mean and 95% Limits of Agreement Between TTE and TEE Measurements
| Level | TTE‐TEE, cm | ||
|---|---|---|---|
| Mean Difference | 95% Limits of Agreement | ||
| Sinus of Valsalva | −0.015 | −0.588 | 0.558 |
| Sinotubular junction | 0.027 | −0.391 | 0.444 |
| 1 cm above sinotubular junction | 0.046 | −0.363 | 0.455 |
| Maximum diameter of ascending aorta | −0.013 | −0.410 | 0.383 |
Abbreviations: TEE, transesophageal echocardiography; TTE, transthoracic echocardiography
Table 3.
Inter‐ and Intraobserver Correlation Coefficient
| Level | Interobserver Correlation Coefficient | P Interobserver | Intraobserver Correlation Coefficient | P Intraobserver |
|---|---|---|---|---|
| Sinus of Valsalva | 0.8661 | 0.001 | 0.9202 | 0.0002 |
| Sinotubular junction | 0.8064 | 0.005 | 0.8167 | 0.004 |
| 1 cm above sinotubular junction | 0.8723 | 0.001 | 0.5398 | 0.1 |
| Maximum diameter of ascending aorta | 0.9722 | <0.0001 | 0.8532 | 0.002 |
Correlation coefficient between TTE and TEE for the sinus level was 0.765, sinotubular junction was 0.872, 1 cm above sinotubular junction was 0.896, and maximum diameter of the ascending aorta was 0.936. There is a strong positive correlation between measurements by TTE and TEE, most notably for the maximal diameter of the ascending aorta. The majority of aortic sizes recorded are within a 0.3 cm difference. Correlation curves and Bland‐Altman difference plots for analysis of agreement between the 2 measurements for the maximum diameter of the ascending aorta are depicted in Figure 2. The mean length and standard deviation (SD) of the ascending aorta that was measurable beyond the ST junction was different between the 2 echocardiographic techniques (6.7 cm [SD 1.2] for TTE vs 7.2 [SD 1.2] cm for TEE, P = 0.03).
Figure 2.
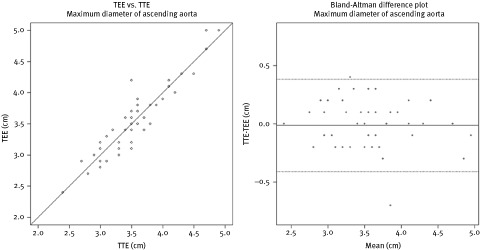
Scatterplot showing the correlation between transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) measurements for the maximal dimension of the ascending aorta. Bland‐Altman difference plots showing the mean differences between TTE and TEE measurements for the maximal dimension of the ascending aorta
Discussion
This is the first prospective study comparing the accuracy of TTE and TEE for the measurement of ascending thoracic aortic dimensions in patients with and without known aortic disease. Our study demonstrates that TTE and TEE measurements of the proximal to mid ascending aorta are strongly correlated at all levels. Nontraditional parasternal long‐axis windows with a higher interspace and medial position during TTE improve visualization of the ascending aorta and can be performed with little added time to a standard study.
Ascending aortic ectasia or aneurysms occur as a result of several pathophysiologic abnormalities, including degenerative, congenital or familial, inflammatory, and infectious etiologies. Although hypertension and atherosclerosis are the most common aortic pathologies, they generally effect older individuals and have a more predictable natural course. Congenital bicuspid aortic valves occur with a prevalence of 1% to 2% in the general population with a high‐degree of heritable transmission.1,2,12 The existence of a bicuspid aortic valve and associated ascending aortopathy has garnered increasing attention with approximately 20% of patients undergoing aortic valve surgery for this disorder, requiring concomitant aortic repair.4 Similar to Marfan syndrome, the aortopathy associated with bicuspid aortic valves is now well established to have an underlying connective tissue disorder with medial degeneration.13 Unlike degenerative diseases, these connective tissue diseases more often affect young and middle‐aged patients and often present first with acute aortic syndromes.
Once aortic disease is detected, careful long‐term surveillance is vital to avert valvular and vascular complications by initiation of lifestyle adjustments and medical therapy. Recent studies on the molecular basis of Marfan syndrome‐associated aortic aneurysms have demonstrated the slowing of progression of dilation with angiotensin II‐receptor blockers and angiotensin converting enzyme inhibition.14,15 In addition, several innovations in surgical repair of the aorta have resulted in a reduced operative mortality and therefore a lower threshold for intervention. Altogether, the increasing options for aortic disease management, including medical and surgical treatment, have fostered an increased requirement for monitoring with cardiac imaging modalities.
It is well established that CT, MRI, and TEE visualize the thoracic aorta with excellent accuracy and have traditionally been used for follow‐up of patients with ascending aortic pathology. However, older studies using M‐mode and 2‐dimensional echocardiography demonstrated that TTE is a reliable noninvasive test to estimate primarily the aortic root diameter compared to contrast angiography in normal infants and adults.16, 17, 18 DeMaria studied 44 adult patients (12 patients with aortic aneurysms and 32 without aneurysms) and showed that using a superior intercostal space, an overall good correlation was noted between TTE 2‐dimensional imaging and contrast angiography measurements of the aorta (r = 0.94).16 Many initial descriptions of acute aortic dissection or aortic aneurysms seen by TTE have also been reported, including detection with nonstandard windows such as the right parasternal long‐axis and suprasternal notch views.19 A study of 44 patients with aortic aneurysms (including atherosclerotic, Marfan syndrome, and bicuspid aortic valve etiologies) found TTE and multidetector CT angiography correlated strongly at all levels of the ascending aorta including the aortic arch (Pearson's correlation = 0.976, P < 0.0001 for the ascending aorta).8
Because the early reports determined the normative dimensions of the aortic root based on M‐mode conventions, there is no widely accepted method for measurement of the ascending aorta by 2‐dimensional imaging. In 1978, the American Society of Echocardiography (ASE) recommended aortic root M‐mode measurements should be made at end‐diastole using the leading edge to leading edge methodology.20 Subsequent recommendations for chamber quantification from the ASE in 2005 stated that 2‐dimensional measurements of the aortic root should be made rather than M‐mode due to an underestimation with the latter technique.21 The committee stated that an inner edge to inner edge technique is acceptable in order to be consistent with other vascular imaging modalities (CT and MRI). However, the leading edge convention could also be used because most of the normative data exist for this method, and given the improved resolution of TTE with current technology the difference is likely negligible. Because neither of these techniques are validated in comparison to TEE, we chose to use an inner wall to inner wall method by TTE that is congruent with what is usually done by TEE.
Limitations
Because the study included unselected patients, few patients (15%) had aortic ectasia or aortic aneurysms and separate statistical analysis for this group was not performed. In addition, although TTE correlated well with TEE for the maximal diameter in this study, it is possible that the range of view of the ascending aorta by TTE in some patients might not visualize the region of maximal dilation. For conditions that might involve the entire thoracic aorta, CT or MRI is required and TTE would not be adequate. However, for conditions where the ascending aorta is predominantly involved, such as with bicuspid aortopathy, TTE might be adequate.22 Finally, adequate views of the ascending aorta are not possible in all patients and determining end‐diastole can often be problematic.
Conclusion
Transthoracic echocardiography using nonconventional imaging windows appears accurate in comparison to TEE for measurement of the ascending aorta. The additional time and effort to optimize visualization of the ascending aorta is minimal. The findings of this study provide support for selected serial follow‐up of patients with aortic disease by TTE only. Further prospective validation of this approach, particularly in patients with aortic pathology, is warranted.
REFERENCES
- 1. Tutar E, Ekici F, Atalay S, et al. The prevalence of bicuspid aortic valve in newborns by echocardiography. Am Heart J 2005; 150: 513–515. [DOI] [PubMed] [Google Scholar]
- 2. Robert WC. The congenitally bicuspid aortic valve: a study of 85 autopsy cases. Am J Cardiol 1970; 26: 72–83. [DOI] [PubMed] [Google Scholar]
- 3. Tzemos N, Therrien J, Yip J, et al. Outcomes in adults with bicuspid aortic valves. JAMA 2008; 300: 1317–1325. [DOI] [PubMed] [Google Scholar]
- 4. Novaro GM, Tiong IY, Pearce GL, et al. Features and predictors of ascending aortic dilation in association with a congenital bicuspid aortic valve. Am J Cardiol 2003; 93: 99–101. [DOI] [PubMed] [Google Scholar]
- 5. Hagan PG, Nienaber CA, Isselbacher EM, et al. In International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000; 283: 897–903. [DOI] [PubMed] [Google Scholar]
- 6. Zehr KJ, Orszulak TA, Mullany CJ, et al. Surgery for aneurysms of the aortic root: a 30‐year experience. Circulation 2004; 110: 1364–1371. [DOI] [PubMed] [Google Scholar]
- 7. Svensson LG, Kim KH, Lytle BW, et al. Relationship of aortic cross‐sectional area to height ratio and the risk of aortic dissection in patient with bicuspid aortic valves. J Thorac Cardiovasc Surg 2003; 126: 892–893. [DOI] [PubMed] [Google Scholar]
- 8. Tamborini G, Galli CA, Maltagliati A, et al. Comparison of feasibility and accuracy of transthoracic echocardiography versus computed tomography in patients with known ascending aortic aneurysm. Am J Cardiol 2006; 98: 966–969. [DOI] [PubMed] [Google Scholar]
- 9. Iliceto S, Ettorce G, Francioso G. Diagnosis of aneurysm of the thoracic aorta. Comparison between two non invasive techniques: two‐dimensional echocardiography and computed tomography. Eur Heart J 1984; 7: 545–555. [DOI] [PubMed] [Google Scholar]
- 10. Friedman BJ, Waters J, Kwan OL. Comparison of magnetic resonance imaging and echocardiography in determination of cardiac dimensions in normal subjects. J Am Coll Cardiol 1985; 6: 1369–1376. [DOI] [PubMed] [Google Scholar]
- 11. Dinsmore RE, Liberthson RR, Wismer GL. Magnetic resonance imaging of thoracic aortic aneurysms: comparison with other imaging methods. Am J Roentgenol 1986; 146: 309–314. [DOI] [PubMed] [Google Scholar]
- 12. Cripe L, Andelfinger G, Martin L, et al. Bicuspid aortic valve is heritable. J Am Coll Cardiol 2004; 44: 138–143. [DOI] [PubMed] [Google Scholar]
- 13. Nataatmadja M, West M, West J, et al. Abnormal extracellular matrix protein transport associated with increased apoptosis of vascular smooth muscle cells in Marfan syndrome and bicuspid aortic valve thoracic aortic aneurysms. Circulation 2003; 108(suppl 1): II329–II334. [DOI] [PubMed] [Google Scholar]
- 14. Brooke BS, Habashi JP, Judge DP, et al. Angiotensin II blockade and aortic root dilation in Marfan's syndrome. N Engl J Med 2008; 358: 2787–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ahimastos AA, Aggarwal A, D'Orsa KM, et al. Effect of perindopril on large artery stiffness and aortic root diameter in patients with Marfan syndrome: a randomized controlled trial. JAMA 2007; 298: 1539–1547. [DOI] [PubMed] [Google Scholar]
- 16. DeMaria AN, Bommer W, Neumann A. Identification and localization of aneurysms of the ascending aorta by cross‐sectional echocardiography. Circulation 1979; 59: 755–761. [DOI] [PubMed] [Google Scholar]
- 17. Marx GR, Goldberg SJ, Allen HD. Two methods for measurement of ascending aortic diameter by 2D echocardiography as compared with cineangiography. Am Heart J 1986; 112: 172–173. [DOI] [PubMed] [Google Scholar]
- 18. Roman MJ, Devereux RB, Kramer‐Fox R, et al. Two‐dimensional echocardiographic aortic root dimensions in normal children and adults. Am J Cardiol 1989; 64: 507–512. [DOI] [PubMed] [Google Scholar]
- 19. D'Cruz IA, Jain DP, Hirsch L. Echocardiographic diagnosis of dilatation of the ascending aorta using right parasternal scanning. Radiology 1978; 129: 465–469. [DOI] [PubMed] [Google Scholar]
- 20. Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M‐mode echocardiography: results of a survey of echocardiographic measurements. Circulation 1978; 58: 1072–1083. [DOI] [PubMed] [Google Scholar]
- 21. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, Developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005; 18: 1440–1463. [DOI] [PubMed] [Google Scholar]
- 22. Fazel SS, Mallidi HR, Lee RS, et al. The aortopathy of bicuspid aortic valve disease has distinctive patterns and usually involves the transverse aortic arch. J Thorac Cardiovasc Surg 2008; 136: 1604. [DOI] [PubMed] [Google Scholar]


