Abstract
Objective
To estimate of the number of children younger than 5 years who were household contacts of people with tuberculosis and were eligible for tuberculosis preventive treatment in 2017.
Methods
To estimate the number of eligible children, we obtained national values for the number of notified cases of bacteriologically confirmed pulmonary tuberculosis in 2017, the proportion of the population younger than 5 years in 2017 and average household size from published sources. We obtained global values for the number of active tuberculosis cases per household with an index case and for the prevalence of latent tuberculosis infection among children younger than 5 years who were household contacts of a tuberculosis case through systematic reviews, meta-analysis and Poisson regression models.
Findings
The estimated number of children younger than 5 years eligible for tuberculosis preventive treatment in 2017 globally was 1.27 million (95% uncertainty interval, UI: 1.24–1.31), which corresponded to an estimated global coverage of preventive treatment in children of 23% at best. By country, the estimated number ranged from less than one in the Bahamas, Iceland, Luxembourg and Malta to 350 000 (95% UI: 320 000–380 000) in India. Regionally, the highest estimates were for the World Health Organization (WHO) South-East Asia Region (510 000; 95% UI: 450 000–580 000) and the WHO African Region (470 000; 95% UI: 440 000–490 000).
Conclusion
Tuberculosis preventive treatment in children was underutilized globally in 2017. Treatment should be scaled up to help eliminate the pool of tuberculosis infection and achieve the End TB Strategy targets.
Résumé
Objectif
Estimer le nombre d'enfants de moins de 5 ans qui étaient en contact avec des membres de la famille atteints de tuberculose et qui étaient éligibles à un traitement préventif de cette maladie en 2017.
Méthodes
Pour estimer le nombre d'enfants éligibles, nous nous sommes procuré, à partir de diverses publications, les valeurs nationales correspondant au nombre de cas signalés de tuberculose pulmonaire confirmée par des analyses bactériologiques en 2017, à la part de la population âgée de moins de 5 ans en 2017 et à la taille moyenne des familles. Nous nous sommes procuré, au moyen d'une revue systématique, d'une méta-analyse et de modèles de régression de Poisson, les valeurs mondiales correspondant au nombre de cas de tuberculose active par foyer avec cas de référence et à la prévalence de l'infection tuberculeuse latente chez les enfants de moins de 5 ans qui étaient en contact avec un membre de la famille atteint de tuberculose.
Résultats
Le nombre estimé d'enfants de moins de 5 ans éligibles à un traitement préventif de la tuberculose dans le monde en 2017 était de 1,27 million (intervalle d'incertitude de 95%, II: 1,24–1,31), soit une couverture mondiale de traitement préventif chez les enfants estimée à 23% au mieux. Par pays, le nombre estimé allait de moins d'un aux Bahamas, en Islande, au Luxembourg et à Malte à 350 000 (II 95%: 320 000–380 000) en Inde. Au niveau des régions, les estimations les plus élevées se retrouvaient dans la Région OMS de l'Asie du Sud-Est (510 000; II 95%: 450 000–580 000) et la Région africaine de l'OMS (470 000; II 95%: 440 000–490 000).
Conclusion
Au niveau mondial, le traitement préventif de la tuberculose chez les enfants était sous-utilisé en 2017. Il faudrait intensifier le recours au traitement afin d'éliminer les foyers de tuberculose et d'atteindre les objectifs de la Stratégie de l'OMS pour mettre fin à la tuberculose.
Resumen
Objetivo
Estimar el número de niños menores de cinco años que tuvieron contacto con personas con tuberculosis en sus hogares y que eran elegibles para el tratamiento preventivo de la tuberculosis en 2017.
Métodos
Para estimar el número de niños elegibles, se obtuvieron valores nacionales para el número de casos notificados de tuberculosis pulmonar bacteriológicamente confirmada en 2017, la proporción de la población menor de 5 años en 2017 y el tamaño promedio del hogar de fuentes publicadas. Se obtuvieron valores globales para el número de casos de tuberculosis activa por hogar con un caso índice y para la prevalencia de infección de tuberculosis latente entre los niños menores de 5 años que estaban en contacto con un caso de tuberculosis en el hogar mediante las revisiones sistemáticas, el metanálisis y los modelos de regresión de Poisson.
Resultados
El número estimado de niños menores de 5 años elegibles para el tratamiento preventivo de la tuberculosis en 2017 a nivel mundial fue de 1,27 millones (intervalo de incertidumbre del 95 %, IU: 1,24-1,31), lo que corresponde a una cobertura mundial estimada de tratamiento preventivo en niños del 23 % en el mejor de los casos. Por país, el número estimado oscila entre menos de uno en las Bahamas, Islandia, Luxemburgo y Malta y 350 000 (95 % UI: 320 000-380 000) en la India. A nivel regional, las estimaciones más elevadas correspondieron a la Región de Asia Sudoriental de la Organización Mundial de la Salud (OMS) (510 000; IC del 95 %: 450 000-580 000) y a la Región Africana de la OMS (470 000; IC del 95 %: 440 000-490 000).
Conclusión
El tratamiento preventivo de la tuberculosis en los niños fue utilizado muy poco a nivel mundial en 2017. El tratamiento debe ampliarse para ayudar a eliminar el conjunto de infecciones de tuberculosis y alcanzar los objetivos de la Estrategia de Fin a la Tuberculosis.
ملخص
الغرض
تقدير عدد الأطفال الذين تقل أعمارهم عن 5 سنوات والذين كانوا يقيمون في منزل واحد مع أشخاص مصابين بالسل، وكانوا مؤهلين للحصول على علاج وقائي من السل في عام 2017.
الطريقة
لتقدير عدد الأطفال المؤهلين، حصلنا على قيم وطنية لعدد الحالات المبلغ عن إصابتها بمرض السل الرئوي المؤكد جرثوميًا في عام 2017، ونسبة السكان الذين تقل أعمارهم عن 5 سنوات في عام 2017، ومتوسط حجم الأسرة من المصادر المنشورة. كما حصلنا على قيم عالمية لعدد حالات الإصابة بالسل النشطة لكل منزل مع حالة كمؤشر، وكذلك قيم لانتشار عدوى السل الكامنة بين الأطفال الذين تقل أعمارهم عن 5 سنوات والذين كانوا يقيمون في منزل واحد من حالة مرض بالسل من خلال المراجعات المنهجية، والتحليل التلوي، ونماذج التحوف لبواسون.
النتائج
العدد التقديري للأطفال الذين تقل أعمارهم عن 5 سنوات، والمؤهلين للعلاج الوقائي من السل في عام 2017 على مستوى العالم، كان 1.27 مليون (فاصل عدم الثقة: 95%، 1.24 إلى 1.31)، وهو ما يتوافق مع تغطية عالمية تقديرية للعلاج الوقائي في الأطفال بنسبة 23% في أحسن الأحوال.حسب البلد ، تراوح العدد التقديري من أقل من واحد في جزر البهاما، وآيسلندا، ولكسمبرغ ومالطة إلى 350000 (فاصل عدم الثقة: 95%، 320000 إلى 380000) في الهند. وعلى المستوى الإقليمي، كانت أعلى التقديرات في منطقة جنوب شرق آسيا التابعة لمنظمة الصحة العالمية (WHO) (510000؛ فاصل عدم الثقة 95%: 450000 إلى 580000) والمنطقة الأفريقية التابعة لمنظمة الصحة العالمية (470000؛ فاصل عدم الثقة 95%: 440000 إلى 490000).
الاستنتاج
لم تتم الاستفادة بالعلاج الوقائي من مرض السل في الأطفال بالشكل الكافي على مستوى العالم في عام 2017. يجب توسيع نطاق العلاج حتى يساعد في القضاء على تصاعد مرض السل وتحقيق أهداف استراتيجية القضاء على السل (End TB Strategy).
摘要
目的
旨在估计 2017 年家人患有结核病且符合结核病预防治疗条件的 5 岁以下儿童人数。
方法
为了估计符合条件的儿童人数,我们从已出版的资料中获取了 2017 年通过细菌学方法确诊的结核病公示案例的全国性数据、2017 年 5 岁以下人口的比例和平均家庭规模数据。通过系统综述、荟萃分析和泊松回归模型,我们获得了全球数据,关于每个家庭活动性结核病病例的数量和家人感染结核病的 5 岁以下儿童感染潜伏性结核病的患病率。
结果
在 2017 年,全球符合结核病预防治疗的 5 岁以下儿童人数估计值为 127 万(95% 不确定区间,UI:1.24–1.31),这与全球儿童预防性治疗覆盖率最高为 23% 的估计值一致。从国家层面来看,估计值范围从冰岛、巴哈马群岛、卢森堡和马耳他中的任一国家的不足一人到印度的 350000 人(95% UI:320000–380000)。从区域角度来看,世卫组织东南亚地区估计值最高(510000;95% UI:450000–580000),其次是世卫组织非洲地区(470000;95% UI:440000-490000)。
结论
2017 年,儿童结核病预防治疗并未在全球范围内充分实施。应扩大治疗范围,以消除感染结核病的可能性并实现“End TB”(消灭结核病)的战略目标。
Резюме
Цель
Оценка по состоянию на 2017 год количества детей младше пяти лет, проживающих в одной семье с больным туберкулезом и нуждающихся в профилактическом лечении.
Методы
Для оценки количества детей, нуждающихся в лечении, авторы получили из опубликованных источников национальные показатели по состоянию на 2017 год о количестве поставленных на диспансерный учет случаев бактериологически подтвержденного туберкулеза легких, сведения о количестве детей младше 5 лет в 2017 году и средние оценки размера семьи. По результатам систематических обзоров, метаанализа и регрессионных моделей Пуассона были получены глобальные сведения о количестве случаев активной формы туберкулеза из расчета на семью с известным источником заболевания и оценки распространенности латентной туберкулезной инфекции среди детей младше 5 лет, проживающих в одной семье с больным туберкулезом.
Результаты
По предварительным оценкам, во всем мире количество детей младше 5 лет, нуждающихся в профилактическом лечении от туберкулеза, составило в 2017 году 1,27 миллиона человек (95%-й интервал неопределенности, ИН: 1,24–1,31), что в лучшем случае соответствует удовлетворению потребности детей в профилактическом лечении приблизительно на 23%. Оценка распределения по странам показала разброс от менее одного ребенка в таких странах, как Багамские острова, Исландия, Люксембург и Мальта, до 350 000 (95%-й ИН: 320 000–380 000) в Индии. В региональном разрезе максимальные оценки были получены для Юго-Восточной Азии по классификации Всемирной организации здравоохранения (ВОЗ) (510 000 человек; 95%-й ИН: 450 000–580 000) и для Африканского региона ВОЗ (470 000; 95%-й ИН: 440 000–490 000).
Вывод
По состоянию на 2017 год во всем мире профилактическое лечение от туберкулеза у детей применяется в недостаточной мере. Необходимо наращивать масштабы лечения, чтобы содействовать исключению резервуаров туберкулезной инфекции и достижению целей стратегии по прекращению эпидемии туберкулеза.
Introduction
The management of latent tuberculosis infection is a critical component of the World Health Organization’s (WHO’s) End TB Strategy. Given that between a quarter and a third of the global population is estimated to be infected with Mycobacteria tuberculosis, 1–3 the Strategy’s ambitious targets and the United Nations’ Sustainable Development Goals cannot be achieved without tackling the reservoir of latent infection.4 The risk of progression from tuberculosis infection to active disease is particularly high in young children, who are also at the greatest risk of severe and disseminated disease.5 As a result, treatment of tuberculosis infection (i.e. tuberculosis preventive treatment) is strongly recommended for children younger than 5 years who are household contacts of people with bacteriologically confirmed pulmonary tuberculosis.6 Accordingly, coverage of tuberculosis preventive treatment is one of the key indicators used to monitor the implementation of the End TB Strategy.7 In 2018, world leaders committed to providing 4 million child household contacts younger than 5 years with tuberculosis preventive treatment by 2022.8
A recent survey of policy and practice on latent tuberculosis infection in countries with a low tuberculosis burden and in African countries found that many lacked recording and reporting systems for infection.9,10 In 2016, WHO started collecting data on the number of children younger than 5 years globally who were household contacts of people with pulmonary tuberculosis and who had started tuberculosis preventive treatment.11 Although 118 countries, including 16 of the 30 countries with a high tuberculosis burden, reported data in 2017,11 there was a lack of clearly defined denominators for assessing coverage of preventive treatment, which makes planning and monitoring difficult.12
Consequently, the aim of this study was to use tuberculosis notification data from 2017 to estimate of the number of children younger than 5 years in individual countries who were household contacts of people with pulmonary tuberculosis and who were eligible for tuberculosis preventive treatment. This information should help countries implement and monitor preventive treatment.
Methods
Countries with a low tuberculosis burden comprised the 113 high-income or upper-middle-income countries in which the estimated annual incidence of tuberculosis disease in 2015 was fewer than 100 cases per 100 000 population, WHO’s 2015 guidelines on the management of latent tuberculosis infection are intended primarily for these countries.13,14 Countries with 100 or more cases per 100 000 population were regarded as having a high tuberculosis burden.
In countries with a high tuberculosis burden, the number of children eligible for tuberculosis preventive treatment was defined as the number younger than 5 years who are household contacts (hereafter referred to as child household contacts) of people with bacteriologically confirmed pulmonary tuberculosis and who do not themselves have active tuberculosis, regardless of whether they have a confirmed tuberculosis infection (in accordance with WHO guidelines on the management of tuberculosis in children).5 In countries with a low tuberculosis burden, the number of children eligible for tuberculosis preventive treatment was defined as the number of children younger than 5 years who are household contacts of people with bacteriologically confirmed pulmonary tuberculosis, who do not themselves have active tuberculosis and who have a confirmed tuberculosis infection, as indicated by a positive result on a standard tuberculin skin test or an interferon-gamma release assay. Consequently, the number of child household contacts eligible for tuberculosis preventive treatment, N, was calculated using:
| (1) |
in countries with a high tuberculosis burden; and
| (2) |
in countries with a low tuberculosis burden; where n was the number of notified cases of bacteriologically confirmed, pulmonary tuberculosis in the country, C was the average number of active tuberculosis cases per household with an index case, h was the average household size, p was the proportion of the national population that was younger than 5 years, T was the proportion of child household contacts who had active tuberculosis, and L was the prevalence of a confirmed latent tuberculosis infection among child household contacts. For countries with a high tuberculosis burden, L was not included in the calculation because eligibility for tuberculosis preventive treatment did not depend on confirmation of infection. We did not estimate numbers for countries or territories with a population under 300 000.
Table 1 details how we derived values for the parameters in these two equations. From the literature, we obtained country-specific values of n and p for 2017, country-specific values of h for different years and a global estimate of T. To obtain a global value for L, we updated a recent systematic review and meta-analysis, and to obtain a global value for C, we carried out a new systematic review of the literature from 1 January 2005 to 11 November 2017.18 For both the updated and new systematic reviews, we used the reference list of Fox et al.’s systematic review,18 which included publications up until 1 October 2011, and supplemented it with papers subsequently published up until 11 November 2017. The new systematic review did not consider publications before 2005 because we judged that earlier publications would not reflect the current situation. The following search string was used in PubMed® for both reviews: (tuberculosis[Title] OR “tuberculosis”[MeSH Terms] OR “mycobacterium tuberculosis”[MeSH Terms] OR “tuberculosis, pulmonary”[MeSH Terms]) AND ((“contact$”[All Fields]) OR (“contact tracing”[MeSH Terms]) OR “disease outbreaks”[MeSH Terms] OR “contact*”[Title] OR “spread”[Title] OR “contact screen*”[All Fields] OR “contact tracing”[Title] OR “disease transmission”[All Fields] OR “case find*”[Title] OR (cluster*[Title] AND analys*[Title]) OR “household*”[All Fields] OR “household contact*”[All Fields] OR (“case finding”[All Fields]) OR (“casefinding”[All Fields]) OR “case detection”[All Fields]).
Table 1. Parameters for estimating the number of child household contacts eligible for tuberculosis preventive treatment.
| Parametera | Value, mean (95% CI) | Source |
|---|---|---|
| Number of notified cases of bacteriologically confirmed pulmonary tuberculosis in 2017 (n) | Country-specific values (Table 4) | WHO tuberculosis burden estimates15 |
| Number of active tuberculosis cases per household with an index case (C) | 1.06 (1.04–1.07) | New systematic review of the literature from January 2005 to November 2017 |
| Average household size (h) | Country-specific valuesb | National censuses, national surveys (e.g. DHSs), statistical yearbooks and official websites of national statistical authorities |
| Proportion of the population aged < 5 years in 2017 (p) | Country-specific valuesb | United Nations 2017 revision of world population prospects16 |
| Proportion of child household contacts (age < 5 years) of a tuberculosis case who had active tuberculosis themselves (T) | 6.1% (1.0–16.3) | Dodd et al., 201417 |
| Prevalence of a confirmed latent tuberculosis infection among children aged < 5 years who were household contacts of a tuberculosis case in countries with fewer than 100 cases per 100 000 population (L) | 27.9% (18.8–39.4) | Updated systematic review of the literature from inception to November 2017 |
CI: confidence interval; DHS: demographic and health survey; WHO: World Health Organization.
a The characters in parentheses represent the parameters in equations in the text.
b Details available from the corresponding author on request.
For the updated and new systematic reviews: (i) household contacts were defined as people living in the same household or people who satisfied the definition of a household contact in the original publication; (ii) an index case was defined as the first identified case of new or recurrent tuberculosis disease in a person of any age in a specific household or as defined in the original publication; (iii) a person was defined as having a tuberculosis infection if the induration 48 to 72 hours after a tuberculin skin test was 10 mm or greater or, if this information was not available, the person satisfied the definition of a tuberculosis infection in the original publication; and (iv) a prevalent tuberculosis case was defined as a case of active disease that was diagnosed at the baseline visit during the study or within 3 months of diagnosis of the index case.
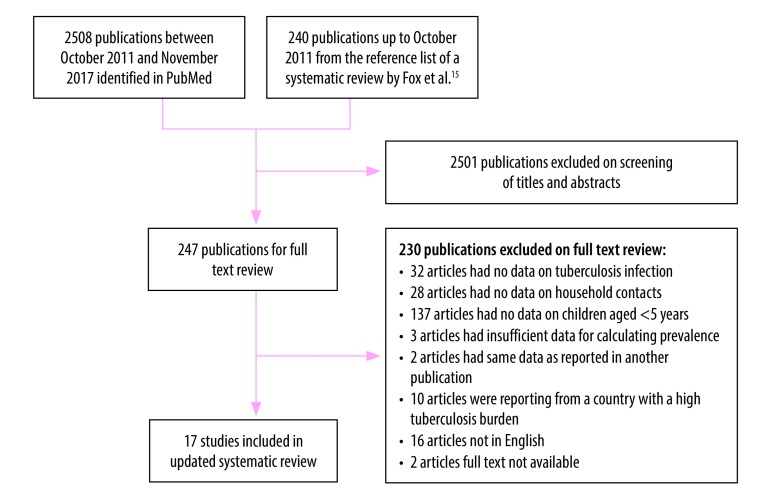
To obtain a global value for L, we included studies in the updated systematic review that reported the prevalence of tuberculosis infection among child contacts in countries with an annual incidence of tuberculosis under 100 cases per 100 000 population at the time of the study, according to WHO estimates.15 If an appropriate WHO estimate was not available, we used estimates from the published literature. We also included studies that reported data on children up to 4 or 6 years of age. The reasons for excluding studies are listed in Fig. 1.
Fig. 1.
Flowchart for the selection of studies on the prevalence of latent tuberculosis infection among child household contacts, countries with a low tuberculosis burden, worldwide, 1964–2017
Notes: We defined a child household contact as a child younger than 5 years living in the same household as a person with active tuberculosis disease. A low tuberculosis burden was defined as fewer than 100 cases per 100 000 population.
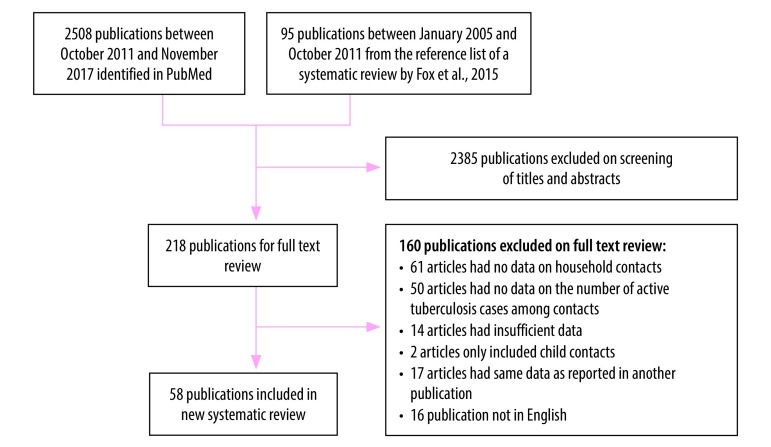
To obtain a global value for C, we included studies in the new systematic review that reported the number of index tuberculosis cases, the number of household contacts and the number of prevalent active tuberculosis cases among household contacts. We excluded studies if: (i) data on contacts other than household contacts were included; (ii) the number of cases or household contacts was less than 10; (iii) only child contacts were included (this would have led to an underestimate of the number of active tuberculosis cases in the household); or (iv) the study was not published in English (Fig. 2).
Fig. 2.
Flowchart for the selection of studies on active tuberculosis cases in households with an index case, worldwide, 2005–2017
One author screened all titles and abstracts for relevance and then reviewed the full text of all potentially eligible articles. For both reviews, we extracted information on the country’s name, the year of the study, the definitions of index cases and household contacts, and the number of household contacts. For the updated systematic review, we obtained information about the number of child household contacts with a confirmed latent tuberculosis infection, the tuberculin skin test cut-off criterion for infection in a child contact, the child’s bacillus Calmette–Guérin (BCG) vaccination status and the age of index cases. For the new systematic review, we extracted information on the age and number of index cases and the number of active tuberculosis cases among household contacts. In evaluating the quality of individual studies, we used a checklist modified from an existing tool to assess issues related to contact investigations and tuberculosis infection.19
Data analysis
The meta-analysis of the prevalence of a confirmed latent tuberculosis infection among child household contacts (L) was conducted using a logistic-normal random-effects model.20 In the primary analysis, we did not consider the different definitions of tuberculosis infection used in the studies. The heterogeneity of study findings was assessed by visual inspection of forest plots and from the results of likelihood-ratio tests. Potential sources of heterogeneity were investigated in subgroup analyses that considered the following factors: (i) whether the index case tested positive or negative on smear microscopy; (ii) the tuberculin skin test cut-off value (i.e. 10 mm or more versus other values); (iii) the year of study publication (i.e. before 2000 or later); (iv) the country’s income status (i.e. whether high- or upper-middle-income);21 and (v) BCG vaccination coverage.
The average number of active tuberculosis cases per household with an index case (C) was estimated as follows. For each study, the average number of active tuberculosis cases among contacts in each household was calculated by dividing the number of prevalent active tuberculosis cases among household contacts by the number of index cases, which was assumed to be equal to the number of households. Data were pooled using mixed-effects, Poisson regression models. Subsequently, the average number of tuberculosis cases per household was calculated as the pooled average number of tuberculosis cases among contacts in each household plus one to account for the index case. The heterogeneity of study findings was assessed by visual inspection of forest plots and the effect of the national tuberculosis burden on estimates was assessed in a subgroup analysis. We also conducted a sensitivity analysis by excluding an outlier value for the number of tuberculosis cases per household to assess its influence on the pooled estimate.
We did not evaluate publication bias using statistical tests (e.g. Begg’s test or Egger’s test) or funnel plots because their utility has not been established in the meta-analyses of proportions obtained from observational studies.18,22 We considered uncertainty in: (i) the prevalence of tuberculosis infection in child contacts; (ii) the number of tuberculosis cases per household; and (iii) the proportion of child household contacts with active tuberculosis disease. We ignored uncertainty in population size estimates from the United Nations Population Division. Errors were propagated using a second-order Taylor series expansion.23,24 All statistical analyses were performed using Stata v. 13.1 (StataCorp LP., College Station, United States of America) and R v. 3.4.4 (The R Foundation, Vienna, Austria).
Results
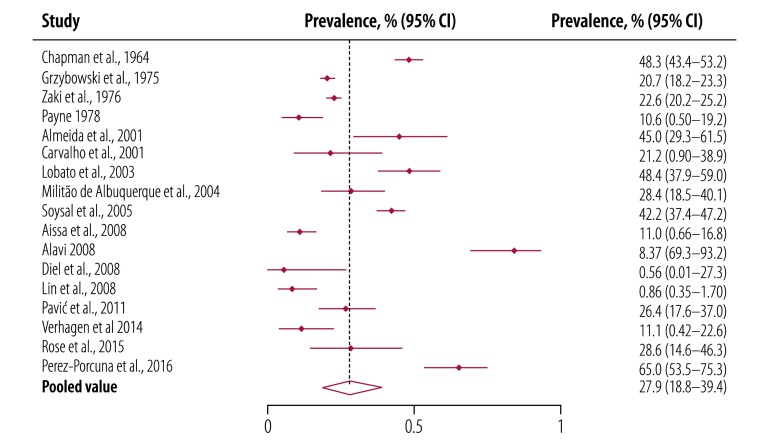
Our systematic review of the prevalence of a latent tuberculosis infection among child household contacts younger than 5 years (L) in countries with a low tuberculosis burden included 17 studies (Fig. 1 and Table 2).25–41 Nine of the 17 (52.9%) were conducted in high-income countries. The presence of a tuberculosis infection was defined as an induration of 10 mm or more on the tuberculin skin test in 11 studies, whereas the other six used different criteria: (i) one used an induration cut-off of 5 mm; (ii) three used multiple induration cut-offs, ranging from 5 to 15 mm depending on BCG vaccination status, the infectiousness of the index case or the study site; (iii) one used a Heaf grade of 2, 3 or 4; and (iv) one did not specify the criterion. The median prevalence of latent tuberculosis infection among child contacts was 26.4% (interquartile range: 11.1–42.2). Twelve studies included children who had received a BCG vaccination, one included only unvaccinated children and BCG vaccination status was not specified in four studies. There was substantial heterogeneity across the studies. The pooled prevalence of latent tuberculosis infection among child contacts younger than 5 years was 27.9% (95% confidence interval, CI: 18.8–39.4; Fig. 3). None of the subgroup analyses found significant differences between subgroups.
Table 2. Systematic review of the prevalence of latent tuberculosis infection among child household contacts,a countries with a low tuberculosis burden,b worldwide, 1964–2017.
| Study reference | Country | Year of study enrolment | Definition of index tuberculosis case | Prevalence of latent tuberculosis infection among child household contacts aged < 5 years, no. infected children/no. all children (%) | Criterion for tuberculosis infection | BCG vaccination status |
|---|---|---|---|---|---|---|
| Chapman et al., 196425 | United States | NA | Pulmonary tuberculosis (no information on bacteriological status) | 200/414 (48.3) | Not defined | Unknown |
| Grzybowski et al., 197526 | Canada | 1966–1971 | Pulmonary or extrapulmonary tuberculosis | 209/1012 (20.7) | Tuberculin skin test induration ≥ 6 mm or ≥ 10 mm, depending on study site | Unknown |
| Zaki et al., 197627 | United States | 1965–1972 | Pulmonary tuberculosis (no information on bacteriological status) | 254/1122 (22.6) | Tuberculin skin test induration ≥ 10 mm | Unknown |
| Payne, 197828 | United Kingdom | 1968–1974 | Pulmonary or extrapulmonary tuberculosis | 9/85 (10.6) | Heaf grade 2, 3 or 4 | No children vaccinated |
| Almeida et al., 200129 | Brazil | 1998 | Smear-positive pulmonary tuberculosis | 18/40 (45.0) | Tuberculin skin test induration ≥ 10 mm | No specific data for children aged < 5 years; 81% of the study population vaccinated |
| Carvalho et al., 200130 | Brazil | 1995–1997 | Smear-positive pulmonary tuberculosis | 7/33 (21.2) | Tuberculin skin test induration ≥ 10 mm | No specific data for children aged < 5 years; 75% of the study population vaccinated |
| Lobato et al., 200331 | United States | 1994 | Pulmonary tuberculosis (smear-positive or -negative) | 45/93 (48.4) | Tuberculin skin test induration ≥ 5 mm | Unknown |
| Militão de Albuquerque et al., 200432 | Brazil | 1997–1999 | Pulmonary tuberculosis (including clinically diagnosed disease) | 21/74 (28.4) | Tuberculin skin test induration ≥ 10 mm | No specific data for children aged < 5 years; 87% of the study population vaccinated |
| Soysal et al., 200533 | Turkey | 2002–2003 | Smear-positive pulmonary tuberculosis | 171/405 (42.2) | Tuberculin skin test induration ≥ 10 mm | No specific data for children aged < 5 years; 79% of the study population vaccinated |
| Aissa et al., 200834 | France | 2004–2005 | Culture-positive pulmonary tuberculosis | 18/164 (11.0) | Tuberculin skin test induration ≥ 10 mm for BCG-vaccinated people; ≥ 15 mm or conversion from negative (i.e. < 5 mm) to positive (i.e. ≥ 10 mm) for non-vaccinated people | No specific data for children aged < 5 years; 98% of the study population vaccinated |
| Alavi, 200835 | Iran (Islamic Republic of) | 2003–2005 | Pulmonary tuberculosis (smear-positive or -negative) | 36/43 (83.7) | Tuberculin skin test induration ≥ 10 mm | No specific data for children aged < 5 years; 51% of the study population vaccinated |
| Diel et al., 200836 | Germany | 2005–2006 | Smear-positive pulmonary tuberculosis | 1/18 (5.6) | Tuberculin skin test induration ≥ 10 mm | No specific data for children aged < 5 years; 86% of the study population vaccinated |
| Lin et al., 200837 | China | 2006–2007 | Smear-positive pulmonary tuberculosis | 7/81 (8.6) | Tuberculin skin test induration ≥ 10 mm | No specific data for children aged < 5 years; 28% of the study population vaccinated |
| Pavić et al., 201138 | Croatia | 2008–2009 | Not defined | 23/87 (26.4) | Tuberculin skin test induration ≥ 10 mm | All children vaccinated |
| Verhagen et al., 201439 | Venezuela (Bolivarian Republic of) | 2010–2011 | Culture-positive pulmonary tuberculosis | 6/54 (11.1) | Tuberculin skin test induration ≥ 10 mm | 76% of children aged < 5 years vaccinated |
| Rose et al., 201540 | Canada | 2008–2010 | Culture-positive pulmonary tuberculosis | 10/35 (28.6) | Tuberculin skin test induration ≥ 5 mm for contacts of a smear-positive tuberculosis case and ≥ 10 mm for contacts of a smear-negative tuberculosis case | 25% of children aged < 5 years vaccinated |
| Perez-Porcuna et al., 201641 | Brazil | 2009–2010 | Pulmonary tuberculosis (smear-positive or -negative) | 52/80 (65.0) | Tuberculin skin test induration ≥ 10 mm | All children vaccinated |
BCG: bacillus Calmette-Guérin; NA: not available.
a We defined a child household contact as a child younger than 5 years living in the same household as a person with active tuberculosis disease.
b We defined a low tuberculosis burden as fewer than 100 cases per 100 000 population.
Fig. 3.
Forest plot of the prevalence of latent tuberculosis infection among child household contacts, countries with a low tuberculosis burden, worldwide, 1964–2017
CI: confidence interval.
Notes: We defined a child household contact as a child younger than 5 years living in the same household as a person with active tuberculosis disease. A low tuberculosis burden was defined as fewer than 100 cases per 100 000 population.
Our systematic review of the number of active tuberculosis cases per household with an index case (C) included 58 studies (Fig. 2 and Table 3).35,37,42–97 Of the 58, 16 (27.6%) were conducted in countries with a low tuberculosis burden. The number of active tuberculosis cases among contacts in each household ranged from 0 to 0.33, except for one study that reported a value of 0.93.35 The pooled number of active tuberculosis cases among contacts in each household was 0.06 (95% CI: 0.04–0.07). Consequently, the average number of active tuberculosis cases per household was 1.06 once the index case had been included. There was no significant difference between countries with a low or high tuberculosis burden (P = 0.33). Furthermore, excluding the one outlier reduced the average number of cases per household by only 0.002.
Table 3. Systematic review of active tuberculosis cases in households with an index case, worldwide, 2005–2017.
| Study reference | Country | Year of study enrolment | Definition of index tuberculosis case | Eligible age group | No. of index casesa | No. of tuberculosis cases among household contactsb | No. of tuberculosis cases among contacts per householdb | Total no. of tuberculosis cases per household, including the index case |
|---|---|---|---|---|---|---|---|---|
| Becerra et al., 200542 | Peru | 1996–1998 | Culture-positive pulmonary tuberculosis | All ages | 192 | 10 | 0.05 | 1.05 |
| Chee et al., 200543 | Singapore | 2000 | Culture-positive pulmonary tuberculosis | All ages | 679 | 20 | 0.03 | 1.03 |
| Khalilzadeh et al., 200644 | Iran (Islamic Republic of) | 2002–2004 | Smear-positive pulmonary tuberculosis | All ages | 68 | 17 | 0.25 | 1.25 |
| Yeo et al., 200645 | Canada | 1996–2000 | Pulmonary or extrapulmonary tuberculosis | All ages | 39 | 4 | 0.10 | 1.10 |
| Hussain et al., 200746 | Pakistan | 2001–2003 | Smear-positive pulmonary tuberculosis | All ages | 20 | 0 | 0.00 | 1.00 |
| Alavi, 200835 | Iran (Islamic Republic of) | 2007 | Pulmonary tuberculosis (smear-positive or -negative) | All ages | 69 | 64 | 0.93 | 1.93 |
| Hill et al., 200847 | Gambia | 2002–2004 | Smear-positive pulmonary tuberculosis | ≥ 6 months | 317 | 33 | 0.10 | 1.10 |
| Lee et al., 200848 | China, Hong Kong SAR | 2000 | Pulmonary or extrapulmonary tuberculosis | All ages | 1 635 | 29 | 0.02 | 1.02 |
| Lin et al., 200837 | China | 2006–2007 | Smear-positive pulmonary tuberculosis | All ages | 393 | 5 | 0.01 | 1.01 |
| Borrell et al., 200949 | Spain | 2003–2004 | Pulmonary or extrapulmonary tuberculosis | All ages | 717 | 46 | 0.06 | 1.06 |
| del Corral et al., 200950 | Colombia | 2005–2006 | Smear-positive pulmonary tuberculosis | All ages | 366 | 8 | 0.02 | 1.02 |
| Kilicaslan et al., 200951 | Turkey | 1997–2000 | Smear-positive pulmonary tuberculosis | All ages | 1 570 | 92 | 0.06 | 1.06 |
| Machado et al., 200952 | Brazil | 2006–2007 | Pulmonary tuberculosis (including clinically diagnosed disease) | All ages | 76 | 2 | 0.03 | 1.03 |
| Nguyen et al., 200953 | Lao People's Democratic Republic | 2006 | Smear-positive pulmonary tuberculosis | All ages | 72 | 4 | 0.06 | 1.06 |
| Ottmani et al., 200954 | Morocco | 1993–2004 | Smear-positive pulmonary tuberculosis or clinically diagnosed disease | All ages | 200 902 | 44 110 | 0.22 | 1.22 |
| Pai et al., 200955 | India | 2006 | Smear-positive pulmonary tuberculosis | All ages | 54 | 1 | 0.02 | 1.02 |
| Cavalcante et al., 201056 | Brazil | 1999–2004 | Pulmonary or extrapulmonary tuberculosis | All ages | 311 | 26 | 0.08 | 1.08 |
| Lienhardt et al., 201057 | Senegal | 2004–2006 | Smear-positive or culture-positive pulmonary tuberculosis | All ages | 206 | 14 | 0.07 | 1.07 |
| Rakotosamimanana et al., 201058 | Madagascar | 2004–2005 | Smear-positive pulmonary tuberculosis | ≥ 1 year | 85 | 12 | 0.14 | 1.14 |
| Sia et al., 201059 | Philippines | 2001–2008 | Smear-positive pulmonary tuberculosis | All ages | 218 | 20 | 0.09 | 1.09 |
| Becerra et al., 201160 | Peru | 1996–2003 | Multidrug- or extensively drug-resistant tuberculosis | All ages | 693 | 117 | 0.17 | 1.17 |
| Grandjean et al., 201161 | Peru | 2005–2008 | Multidrug-resistant tuberculosis | All ages | 358 | 0 | 0.00 | 1.00 |
| Hussain et al., 201162 | Pakistan | unknown | Smear-positive pulmonary tuberculosis | All ages | 18 | 0 | 0.00 | 1.00 |
| Singla et al., 201163 | India | 2005–2008 | Multidrug-resistant tuberculosis | All ages | 58 | 16 | 0.28 | 1.28 |
| Vella et al., 201164 | South Africa | 2005–2008 | Multidrug- or extensively drug-resistant tuberculosis | ≥ 13 years | 508 | 64 | 0.13 | 1.13 |
| Whalen et al., 201165 | Uganda | 1995–2004 | Smear-positive pulmonary tuberculosis | All ages | 497 | 49 | 0.10 | 1.10 |
| Zhang et al., 201166 | China | 2007 | Smear-positive pulmonary tuberculosis | All ages | 4 695 | 40 | 0.01 | 1.01 |
| Fox et al., 201267 | Viet Nam | 2009–2011 | Smear-positive pulmonary tuberculosis | All ages | 167 | 8 | 0.05 | 1.05 |
| Gyawali et al., 201268 | Nepal | 2009–2010 | Smear-positive pulmonary tuberculosis | ≥ 5 years | 184 | 13 | 0.07 | 1.07 |
| Ntinginya et al., 201269 | United Republic of Tanzania | 2010–2011 | Smear-positive pulmonary tuberculosis | ≥ 5 years | 80 | 5 | 0.06 | 1.06 |
| Shapiro et al., 201270 | South Africa | 2009–2009 | Tuberculosis based on clinical evaluation (with or without sputum smear test or sputum culture) | All ages | 749 | 169 | 0.23 | 1.23 |
| Thind et al., 201271 | South Africa | 2009–2010 | Smear-positive pulmonary tuberculosis | All ages | 732 | 127 | 0.17 | 1.17 |
| Chamie et al., 201372 | Uganda | Unknown | Pulmonary tuberculosis (with or without sputum smear test) | All ages | 61 | 13 | 0.21 | 1.21 |
| Jones-López et al., 201373 | Uganda | 2009–2011 | Smear-positive pulmonary tuberculosis | All ages | 96 | 1 | 0.01 | 1.01 |
| Leung et al., 201374 | China, Hong Kong SAR | 1997–2006 | Multidrug-resistant tuberculosis | All ages | 256 | 12 | 0.05 | 1.05 |
| Puryear et al., 201375 | Botswana | 2009–2011 | Paediatrician-diagnosed tuberculosis | All ages | 163 | 12 | 0.07 | 1.07 |
| Shah et al., 201376 | Pakistan | 2010–2011 | Smear-positive pulmonary tuberculosis | All ages | 3 037 | 490 | 0.16 | 1.16 |
| Singh et al., 201377 | India | 2007–2011 | Smear-positive pulmonary tuberculosis | All ages | 450 | 52 | 0.12 | 1.12 |
| Tao et al., 201378 | Uganda | 2002–2006 | Culture-positive pulmonary tuberculosis | All ages | 277 | 19 | 0.07 | 1.07 |
| Yassin et al., 201379 | Ethiopia | 2010–2011 | Smear-positive pulmonary tuberculosis | All ages | 2 906 | 69 | 0.02 | 1.02 |
| Jia et al., 201480 | China | 2008–2008 | Smear-positive pulmonary tuberculosis | All ages | 1 575 | 92 | 0.06 | 1.06 |
| Jones-López et al., 201481 | Brazil | 2008–2012 | Smear-positive pulmonary tuberculosis | All ages | 124 | 2 | 0.02 | 1.02 |
| Loredo et al., 201482 | Brazil | 2001–2008 | Pulmonary tuberculosis (smear-positive or -negative) | ≥ 15 years | 626 | 51 | 0.08 | 1.08 |
| Thanh et al., 201483 | Viet Nam | 2008–2008 | Smear-positive pulmonary tuberculosis | All ages | 1 091 | 27 | 0.02 | 1.02 |
| Zelner et al., 201484 | Peru | 2009–2012 | Pulmonary tuberculosis (including clinically diagnosed disease) | All ages | 3 466 | 229 | 0.07 | 1.07 |
| Chamie et al., 201585 | Uganda | 2012–2013 | Pulmonary or extrapulmonary tuberculosis | ≥ 18 years | 54 | 1 | 0.02 | 1.02 |
| Grandjean et al., 201586 | Peru | 2010–2013 | Multidrug-resistant tuberculosis | All ages | 213 | 5 | 0.02 | 1.02 |
| Jerene et al., 201587 | Ethiopia | 2013–2014 | Smear-positive pulmonary tuberculosis | All ages | 6 015 | 389 | 0.06 | 1.06 |
| Zellweger et al., 201588 | Ten European countries | 2009–2013 | Not defined | All ages | 1 023 | 17 | 0.02 | 1.02 |
| Guputa et al., 201689 | India | 2013–2014 | Smear-positive pulmonary tuberculosis | All ages | 133 | 6 | 0.05 | 1.05 |
| Javaid et al., 201690 | Pakistan | 2012–2015 | Multidrug-resistant tuberculosis | All ages | 154 | 51 | 0.33 | 1.33 |
| Nair et al., 201691 | India | 2007–2014 | Smear-positive pulmonary tuberculosis | All ages | 280 | 29 | 0.10 | 1.10 |
| Wysocki et al., 201692 | Brazil | 2012–2013 | Pulmonary tuberculosis | All ages | 213 | 9 | 0.04 | 1.04 |
| Armstrong-Hough et al., 201793 | Uganda | 2015–2016 | Pulmonary tuberculosis (microbiological confirmation was required for patients aged ≥ 5 years) | All ages | 293 | 5 | 0.02 | 1.02 |
| Datiko et al., 201794 | Ethiopia | 2011–2013 | Smear-positive pulmonary tuberculosis | All ages | 5 345 | 169 | 0.03 | 1.03 |
| Fox et al., 201795 | Viet Nam | 2014 | Smear-positive pulmonary tuberculosis | All ages | 212 | 4 | 0.02 | 1.02 |
| Mandalakas et al., 201796 | Eswatini | 2013–2015 | Initiation of antituberculosis treatment | All ages | 3 258 | 196 | 0.06 | 1.06 |
| Muyoyeta et al., 201797 | Zambia | 2013–2014 | Bacteriologically confirmed tuberculosis | All ages | 977 | 19 | 0.02 | 1.02 |
SAR: Special Administrative Region.
a We assumed that the number of index cases was equal to the number of households studied.
b We defined household contacts as people living in the same household as the index case or people who satisfied the definition of a household contact in the original publication.
Using the values we obtained for L and C with the values of other parameters from the literature (Table 1), we estimated that the number of child household contacts younger than 5 years who were eligible for tuberculosis preventive treatment in 2017 ranged from less than one in four countries (i.e. Bahamas, Iceland, Luxembourg and Malta) to 350 000 (95% uncertainty interval, UI: 320 000–380 000) in India (Table 4; available at: http://www.who.int/bulletin/volumes/96/8/18-218651). Globally, the estimated number of child contacts eligible for preventive treatment was 1.27 million (95% UI: 1.24 to 1.31). Viewed regionally, the highest estimate was for the WHO South-East Asia Region: 510 000 (95% UI: 450 000–580 000; Table 5).
Table 4. Child household contactsa eligible for tuberculosis preventive treatment, by country, 2017.
| Country | No. of notified, bacteriologically confirmed, pulmonary tuberculosis cases15 | Estimated number of child household contactsa eligible for tuberculosis preventive treatment, no. (95% UI) |
|---|---|---|
| Afghanistan | 20 946 | 20 000 (19 000–22 000) |
| Albania | 210 | 12 (8–17) |
| Algeria | 6 575 | 1 100 (720–1 600) |
| Angola | 27 086 | 25 000 (23 000–27 000) |
| Argentina | 6 042 | 430 (270–590) |
| Armenia | 369 | 80 (73–87) |
| Australia | 780 | 33 (21–46) |
| Austria | 379 | 10 (6.5–14) |
| Azerbaijan | 3 125 | 340 (220–470) |
| Bahamas | 16 | 1.0 (0.6–1.3) |
| Bahrain | 80 | 8 (5–11) |
| Bangladesh | 144 817 | 55 000 (50 000–59 000) |
| Belarus | 2 171 | 81 (51–110) |
| Belgium | 563 | 19 (12–26) |
| Belize | 71 | 8.2 (5.2–11) |
| Benin | 2 947 | 2 100 (1 900–2 300) |
| Bhutan | 440 | 160 (140–170) |
| Bolivia (Plurinational State of) | 5 412 | 1 800 (1 700–2 000) |
| Bosnia and Herzegovina | 479 | 18 (11–24) |
| Botswana | 2 098 | 780 (720–850) |
| Brazil | 49 922 | 3 000 (1 900–4 100) |
| Brunei Darussalam | 179 | 21 (13–29) |
| Bulgaria | 694 | 19 (12–26) |
| Burkina Faso | 3 841 | 3 300 (3 000–3 600) |
| Burundi | 4 728 | 3 600 (3 300–3 900) |
| Cambodia | 12 049 | 5 600 (5 100–6 000) |
| Cameroon | 14 515 | 10 000 (9 500–11 000) |
| Canada | 1 144 | 39 (24–53) |
| Cabo Verde | 178 | 67 (61–73) |
| Central African Republic | 5 146 | 3 500 (3 200–3 800) |
| Chad | 5 162 | 4 500 (4 100–4 900) |
| Chile | 2 028 | 120 (77–170) |
| China | 235 547 | 11 000 (6 900–15 000) |
| China, Hong Kong SAR | 2 486 | 74 (47–100) |
| China, Macao SAR | 279 | 13 (8–17) |
| Colombia | 8 627 | 630 (400–860) |
| Comoros | 53 | 38 (35–41) |
| Congo | 3 997 | 2 400 (2 200–2 600) |
| Costa Rica | 313 | 20 (12–27) |
| Côte d'Ivoire | 14 311 | 11 000 (10 000–12 000) |
| Croatia | 287 | 9 (6–13) |
| Cuba | 517 | 21 (13–28) |
| Cyprus | 39 | 1.5 (1.0–2.1) |
| Czechia | 366 | 12 (7–16) |
| Democratic People's Republic of Korea | 40 233 | 9 500 (8 700–10 000) |
| Democratic Republic of the Congo | 98 516 | 85 000 (77 000–92 000) |
| Denmark | 159 | 4.3 (2.7–5.8) |
| Djibouti | 1 072 | 610 (550–660) |
| Dominican Republic | 2 076 | 180 (120–250) |
| Ecuador | 4 299 | 400 (260–550) |
| Egypt | 3 660 | 1 800 (1 600–1 900) |
| El Salvador | 3 029 | 950 (860–1 000) |
| Equatorial Guinea | 893 | 550 (500–600) |
| Eritrea | 770 | 490 (440–530) |
| Estonia | 141 | 3.9 (2.5–5.4) |
| Eswatini | 2 171 | 1 200 (1 100–1 300) |
| Ethiopia | 46 148 | 28 000 (25 000–30 000) |
| Fiji | 141 | 16 (10–22) |
| Finland | 146 | 4.1 (2.6–5.6) |
| France | 2 494 | 85 (54–120) |
| Gabon | 2 301 | 1 100 (1 000–1 200) |
| Gambia | 1 429 | 1 800 (1 700–2 000) |
| Georgia | 1 780 | 390 (360–430) |
| Germany | 3 262 | 74 (46–100) |
| Ghana | 8 359 | 3 700 (3 400–4 000) |
| Greece | 313 | 8 (5–12) |
| Guatemala | 2 760 | 1 400 (1 300–1 500) |
| Guinea | 7 737 | 6 900 (6 300–7 500) |
| Guinea-Bissau | 1 769 | 2 100 (1 900–2 300) |
| Guyana | 342 | 110 (99–120) |
| Haiti | 10 633 | 4 700 (4 300–5 100) |
| Honduras | 2 190 | 880 (800–960) |
| Hungary | 333 | 9 (6–12) |
| Iceland | 8 | 0.35 (0.22–0.48) |
| India | 905 513 | 350 000 (320 000–380 000) |
| Indonesia | 215 586 | 72 000 (66 000–78 000) |
| Iran (Islamic Republic of) | 4 785 | 360 (230–490) |
| Iraq | 2 676 | 700 (440–960) |
| Ireland | 165 | 8 (5–11) |
| Israel | 131 | 11 (7–15) |
| Italy | 2 160 | 55 (35–75) |
| Jamaica | 69 | 4 (3–5) |
| Japan | 11 227 | 290 (180–400) |
| Jordan | 179 | 30 (19–41) |
| Kazakhstan | 9 489 | 3 300 (3 000–3 600) |
| Kenya | 46 875 | 25 000 (23 000–27 000) |
| Kiribati | 189 | 130 (120–140) |
| Kuwait | 373 | 42 (27–58) |
| Kyrgyzstan | 3 171 | 1 500 (1 400–1 700) |
| Lao People's Democratic Republic | 3 876 | 2 000 (1 900–2 200) |
| Latvia | 443 | 13 (8.5–18) |
| Lebanon | 325 | 28 (18–39) |
| Lesotho | 3 670 | 1 800 (1 600–1 900) |
| Liberia | 3 382 | 2 300 (2 100–2 500) |
| Libya | 514 | 68 (43–94) |
| Lithuania | 1 004 | 32 (20–44) |
| Luxembourg | 21 | 0.7 (0.5–1.0) |
| Madagascar | 21 773 | 13 000 (12 000–15 000) |
| Malawi | 6 984 | 4 600 (4 200–4 900) |
| Malaysia | 15 888 | 1 400 (900–2 000) |
| Maldives | 98 | 14 (9–20) |
| Mali | 4 420 | 6 100 (5 500–6 600) |
| Malta | 25 | 0.9 (0.6–1.2) |
| Mauritania | 1 376 | 1 100 (1 000–1 200) |
| Mauritius | 109 | 5.2 (3.3–7.1) |
| Mexico | 14 883 | 1 300 (840–1 800) |
| Mongolia | 1 861 | 690 (630–750) |
| Montenegro | 58 | 2.7 (1.7–3.7) |
| Morocco | 13 635 | 5 500 (5 000–5 900) |
| Mozambique | 31 606 | 21 000 (19 000–23 000) |
| Myanmar | 48 088 | 16 000 (15 000–17 000) |
| Namibia | 5 867 | 3 200 (2 900–3 400) |
| Nepal | 16 966 | 6 900 (6 300–7 500) |
| Netherlands | 367 | 11 (7–15) |
| New Zealand | 167 | 8 (5–10) |
| Nicaragua | 1 676 | 650 (600–710) |
| Niger | 8 288 | 8 800 (8 100–9 600) |
| Nigeria | 75 980 | 53 000 (48 000–57 000) |
| North Macedonia | 152 | 8 (5–11) |
| Norway | 137 | 4.5 (2.8–6.2) |
| Oman | 193 | 33 (21–45) |
| Pakistan | 138 818 | 110 000 (98 000–120 000) |
| Panama | 1 012 | 96 (61–130) |
| Papua New Guinea | 3 944 | 2 400 (2 200–2 700) |
| Paraguay | 1 823 | 740 (670–800) |
| Peru | 19 956 | 6 200 (5 600–6 700) |
| Philippines | 119 712 | 55 000 (51 000–60 000) |
| Poland | 3 944 | 130 (81–180) |
| Portugal | 1 112 | 30 (19–41) |
| Puerto Rico | 30 | 1.1 (0.7–1.5) |
| Qatar | 335 | 23 (14–31) |
| Republic of Korea | 19 972 | 600 (380–820) |
| Republic of Moldova | 1 880 | 220 (200–240) |
| Romania | 8 686 | 280 (180–380) |
| Russian Federation | 40 254 | 1 800 (1 100–2 400) |
| Rwanda | 4 175 | 2 300 (2 100–2 500) |
| Samoa | 13 | 10 (9–10) |
| Sao Tome and Principe | 46 | 25 (23–27) |
| Saudi Arabia | 1 802 | 230 (150–320) |
| Senegal | 10 117 | 13 000 (12 000–14 000) |
| Serbia | 781 | 31 (19–42) |
| Sierra Leone | 9 674 | 7 700 (7 100–8 400) |
| Singapore | 1 238 | 51 (32–69) |
| Slovakia | 134 | 4.6 (2.9–6.3) |
| Slovenia | 89 | 2.9 (1.8–3.9) |
| Solomon Islands | 126 | 84 (76–91) |
| Somalia | 7 691 | 7 400 (6 700–8 000) |
| South Africa | 127 187 | 41 000 (37 000–45 000) |
| South Sudan | 4 333 | 3 600 (3 300–3 900) |
| Spain | 2 735 | 77 (48–100) |
| Sri Lanka | 4 243 | 1 100 (1 000–1 200) |
| Sudan | 7 419 | 6 000 (5 500–6 500) |
| Suriname | 90 | 8 (5–11) |
| Sweden | 273 | 9 (6–13) |
| Switzerland | 348 | 10 (7–14) |
| Syrian Arab Republic | 1 080 | 560 (510–610) |
| Tajikistan | 2 820 | 2 100 (1 900–2 300) |
| Thailand | 36 470 | 5 500 (5 100–6 000) |
| Timor-Leste | 1 954 | 1 600 (1 500–1 800) |
| Togo | 2 142 | 1 300 (1 200–1 400) |
| Trinidad and Tobago | 120 | 6.9 (4.4–9.4) |
| Tunisia | 956 | 91 (57–120) |
| Turkey | 6 162 | 470 (300–650) |
| Turkmenistan | 693 | 110 (69–150) |
| Uganda | 27 039 | 21 000 (19 000–23 000) |
| Ukraine | 16 561 | 1 900 (1 800–2 100) |
| United Arab Emirates | 47 | 2.8 (1.8–3.8) |
| United Kingdom | 2 245 | 82 (52–110) |
| United Republic of Tanzania | 28 542 | 21 000 (19 000–23 000) |
| United States | 5 848 | 230 (150–320) |
| Uruguay | 613 | 30 (19–42) |
| Uzbekistan | 5 705 | 2 600 (2 400–2 900) |
| Vanuatu | 47 | 26 (24–28) |
| Venezuela (Bolivarian Republic of) | 7 189 | 670 (420–910) |
| Viet Nam | 57 246 | 16 000 (14 000–17 000) |
| Yemen | 3 487 | 3 000 (2 800–3 300) |
| Zambia | 16 115 | 11 000 (9 700–12 000) |
| Zimbabwe | 13 263 | 7 600 (7 000–8 300) |
SAR: Special Administrative Region; UI: uncertainty interval.
a We defined a child household contact as a child younger than 5 years living in the same household as a person with active tuberculosis disease.
Table 5. Child household contactsa eligible for tuberculosis preventive treatment, by region, 2017.
| WHO Region | No. of notified, bacteriologically confirmed, pulmonary tuberculosis cases15 | Estimated number of child household contactsa eligible for tuberculosis preventive treatment, no. (95% UI) |
|---|---|---|
| African | 713 693 | 470 000 (440 000–490 000) |
| Of the Americas | 152 730 | 25 000 (22 000–28 000) |
| South-East Asia | 1 414 408 | 510 000 (450 000–580 000) |
| European | 129 110 | 16 000 (14 000–18 000) |
| Eastern Mediterranean | 210 073 | 150 000 (130 000–170 000) |
| Western Pacific | 487 089 | 95 000 (83 000–110 000) |
| Global | 3 107 103 | 1 270 000 (1 240 000–1 310 000) |
UI: uncertainty interval; WHO: World Health Organization.
a We defined a child household contact as a child younger than 5 years living in the same household as a person with active tuberculosis disease.
Discussion
We estimated that 1.27 million children younger than 5 years who were household contacts of people with bacteriologically confirmed pulmonary tuberculosis were eligible for preventive treatment globally in 2017. According to the WHO Global tuberculosis report 2018, countries reported that 292 182 child contacts received preventive treatment in 2017, which makes the best estimate of the global coverage of preventive treatment in children only 23%.98
Our study has several limitations. First, our estimate of the number of child household contacts was based on the number of notified bacteriologically confirmed tuberculosis cases. However, 3.6 million of the estimated 10.0 million people with incident tuberculosis globally in 2017 were neither reported nor enrolled in tuberculosis care.98 Consequently, our estimates are conservative, there would be substantially more eligible child contacts if all incident tuberculosis cases were considered. Second, we used national values for the average household size and for the proportion of the population younger than 5 years to estimate the number of child contacts. It is possible that the composition of households with a tuberculosis case may have differed from the national average and thus people with tuberculosis may have lived with a different number of children younger than 5 years from the national average. Furthermore, we did not consider people with tuberculosis who lived in a prison or nursing home. Doing so would have reduced the estimated number of child contacts, especially in countries where where number of tuberculosis cases among the prison and nursing home populations was high.the prison and nursing home populations were high. Third, we used the value for the average number of tuberculosis cases per household from our new systematic review for all countries, even though it may have varied between countries.
Fourth, in our updated systematic review, we observed substantial heterogeneity across studies in the prevalence of a latent tuberculosis infection among child household contacts in countries with a low tuberculosis burden. This heterogeneity probably reflects differences between studies in characteristic, such as the study population, setting, incidence of tuberculosis, the tuberculin skin test cut-off used and BCG status. We were unable to identify the source of the heterogeneity because the number of studies included in our subgroup analyses was small. Moreover, our estimates of the number of child household contacts eligible for preventive treatment in these countries were derived using an average value for the prevalence of a confirmed tuberculosis infection among child contacts, whereas the prevalence may have varied between countries. Using country-specific values would have given more accurate estimates. Nevertheless, as countries with a low tuberculosis burden accounted for only 14% of notified tuberculosis cases globally in 2017,14,98 their impact on our global estimate was small.
Fifth, we assumed that children were judged eligible for tuberculosis preventive treatment according to WHO guidelines.5 However, eligibility criteria may have varied between countries according to national policy. Sixth, we used a value for the proportion of child household contacts of a tuberculosis case who had active tuberculosis themselves (T) that was derived from a modelling study in 22 countries with a high tuberculosis burden,17 which together accounted for 80% of the global burden. However, the prevalence of active disease among household contacts in these countries was likely to have been higher than in others. Consequently, by using this proportion, we may have underestimated the number of child household contacts without active tuberculosis disease who were, therefore, eligible for preventive treatment. Our estimates of the number of children eligible for preventive treatment need to be validated using national data on the number of child contacts from well-functioning surveillance systems or surveys. These data could also be used to assess the coverage of preventive treatment directly, which should give more accurate figures than our modelling estimates with their inherent limitations. Nevertheless, in the absence of such data, our estimates should help galvanize efforts to implement, and monitor the progress of, tuberculosis preventive treatment among child contacts.
In conclusion, using our estimate of the number of children younger than 5 years eligible for tuberculosis preventive treatment, we calculated that the coverage of preventive treatment in children in 2017 was only 23%. Despite its proven efficacy, tuberculosis preventive treatment is still being underutilized. As the End TB Strategy targets can only be achieved by addressing the pool of tuberculosis infection, urgent action is needed to scale up the implementation of preventive treatment.
Competing interests:
None declared.
References
- 1.Houben RMGJ, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016. October 25;13(10):e1002152. 10.1371/journal.pmed.1002152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999. August 18;282(7):677–86. 10.1001/jama.282.7.677 [DOI] [PubMed] [Google Scholar]
- 3.Getahun H, Matteelli A, Chaisson RE, Raviglione M. Latent Mycobacterium tuberculosis infection. N Engl J Med. 2015. May 28;372(22):2127–35. 10.1056/NEJMra1405427 [DOI] [PubMed] [Google Scholar]
- 4.Dye C, Glaziou P, Floyd K, Raviglione M. Prospects for tuberculosis elimination. Annu Rev Public Health. 2013;34(1):271–86. 10.1146/annurev-publhealth-031912-114431 [DOI] [PubMed] [Google Scholar]
- 5.Guidance for national tuberculosis programmes on the management of tuberculosis in children. Second edition. Geneva: World Health Organization; 2014. Available from: http://apps.who.int/medicinedocs/documents/s21535en/s21535en.pdf [cited 2018 Dec 20]. [PubMed]
- 6.Latent TB Infection: updated and consolidated guidelines for programmatic management. Geneva: World Health Organization; 2018. Available from: http://apps.who.int/iris/bitstream/10665/260233/1/9789241550239-eng.pdf [cited 2018 Dec 20]. [PubMed]
- 7.Implementing the End TB Strategy: the essentials. Geneva: World Health Organization; 2015. Available from: https://www.who.int/tb/publications/2015/end_tb_essential.pdf [cited 2018 Dec 20].
- 8.United to end tuberculosis: an urgent global response to a global epidemic. Political declaration on the fight against tuberculosis. Co-facilitators’ revised text. New York: United Nations; 26 September 2018. Available from: https://www.un.org/pga/72/wp-content/uploads/sites/51/2018/09/Co-facilitators-Revised-text-Political-Declaraion-on-the-Fight-against-Tuberculosis.pdf [cited 2019 Apr 23].
- 9.Hamada Y, Sidibe A, Matteelli A, Dadu A, Aziz MA, Del Granado M, et al. Policies and practices on the programmatic management of latent tuberculous infection: global survey. Int J Tuberc Lung Dis. 2016. December;20(12):1566–71. 10.5588/ijtld.16.0241 [DOI] [PubMed] [Google Scholar]
- 10.Sulis G, Carvalho ACC, Capone S, Hamada Y, Giorgetti PF, da Silva Martins P, et al. Policies and practices on the programmatic management of LTBI: a survey in the African Region. Int J Tuberc Lung Dis. 2018. February 1;22(2):158–64. 10.5588/ijtld.17.0563 [DOI] [PubMed] [Google Scholar]
- 11.Global tuberculosis report 2017. Geneva: World Health Organization; 2017. Available from: http://apps.who.int/iris/bitstream/10665/259366/1/9789241565516-eng.pdf?ua=1 [cited 2018 Dec 20].
- 12.Getahun H, Matteelli A, Abubakar I, Hauer B, Pontali E, Migliori GB. Advancing global programmatic management of latent tuberculosis infection for at risk populations. Eur Respir J. 2016. May;47(5):1327–30. 10.1183/13993003.00449-2016 [DOI] [PubMed] [Google Scholar]
- 13.Getahun H, Matteelli A, Abubakar I, Aziz MA, Baddeley A, Barreira D, et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J. 2015. December;46(6):1563–76. 10.1183/13993003.01245-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guidelines on the management of latent tuberculosis infection. Geneva: World Health Organization; 2015. Available from: http://apps.who.int/iris/bitstream/10665/136471/1/9789241548908_eng.pdf?ua=1&ua=1 [cited 2018 Dec 20]. [PubMed]
- 15.WHO TB burden estimates for 2017. Geneva: World Health Organization; 2017. Available from: http://www.who.int/tb/country/data/download/en/ [cited 2017 Dec 22].
- 16.2017 revision of world population prospects [website]. New York: United Nations Population Division; 2018. Available from: https://esa.un.org/unpd/wpp/ [cited 2018 Nov 19].
- 17.Dodd PJ, Gardiner E, Coghlan R, Seddon JA. Burden of childhood tuberculosis in 22 high-burden countries: a mathematical modelling study. Lancet Glob Health. 2014. August;2(8):e453–9. 10.1016/S2214-109X(14)70245-1 [DOI] [PubMed] [Google Scholar]
- 18.Fox GJ, Barry SE, Britton WJ, Marks GB. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2013. January;41(1):140–56. [Erratum. Eur Respir J. 2015. August;46(2): 578.] 10.1183/09031936.00070812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag. 2014. August 13;3(3):123–8. 10.15171/ijhpm.2014.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014. November 10;72(1):39. 10.1186/2049-3258-72-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Bank country and lending groups [website]. Washington DC: World Bank; 2018. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups [cited 2018 Nov 19].
- 22.Hunter JP, Saratzis A, Sutton AJ, Boucher RH, Sayers RD, Bown MJ. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol. 2014. August;67(8):897–903. 10.1016/j.jclinepi.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 23.Ku HH. Notes on the use of propagation of error formulas. J Res Natl Bur Stand. 1966;70C(4):263–73. [Google Scholar]
- 24.Arras KO. An introduction to error propagation: derivation, meaning and examples of equation CY = FX CX FXT. Lausanne: Swiss Federal Institute of Technology Lausanne; 1998. [Google Scholar]
- 25.Chapman JS, Dyerly MD. Social and other factors in intrafamilial transmission of tuberculosis. Am Rev Respir Dis. 1964. July;90:48–60. [DOI] [PubMed] [Google Scholar]
- 26.Grzybowski S, Barnett GD, Styblo K. Contacts of cases of active pulmonary tuberculosis. Bull Int Union Tuberc. 1975;50(1):90–106. [PubMed] [Google Scholar]
- 27.Zaki MH, Lyons HA, Robins AB, Brown EP. Tuberculin sensitivity. Contacts of tuberculosis patients. N Y State J Med. 1976. December;76(13):2138–43. [PubMed] [Google Scholar]
- 28.Payne CR. Surveillance of tuberculosis contacts: experience at Ealing Chest Clinic. Tubercle. 1978. September;59(3):179–84. 10.1016/0041-3879(78)90024-7 [DOI] [PubMed] [Google Scholar]
- 29.Almeida LM, Barbieri MA, Da Paixão AC, Cuevas LE. Use of purified protein derivative to assess the risk of infection in children in close contact with adults with tuberculosis in a population with high Calmette-Guérin bacillus coverage. Pediatr Infect Dis J. 2001. November;20(11):1061–5. 10.1097/00006454-200111000-00011 [DOI] [PubMed] [Google Scholar]
- 30.Carvalho AC, DeRiemer K, Nunes ZB, Martins M, Comelli M, Marinoni A, et al. Transmission of Mycobacterium tuberculosis to contacts of HIV-infected tuberculosis patients. Am J Respir Crit Care Med. 2001. December 15;164(12):2166–71. 10.1164/ajrccm.164.12.2103078 [DOI] [PubMed] [Google Scholar]
- 31.Lobato MN, Royce SE, Mohle-Boetani JC. Yield of source-case and contact investigations in identifying previously undiagnosed childhood tuberculosis. Int J Tuberc Lung Dis. 2003. December;7(12) Suppl 3:S391–6. [PubMed] [Google Scholar]
- 32.Militão de Albuquerque MdeF, Ximenes RA, Campelo AR, Sarinho E, Cruz M, Maia Filho V. Neonatal BCG vaccine and response to the tuberculin test in BCG vaccinated children in contact with tuberculosis patients in Recife, Brazil. J Trop Pediatr. 2004. February;50(1):32–6. 10.1093/tropej/50.1.32 [DOI] [PubMed] [Google Scholar]
- 33.Soysal A, Millington KA, Bakir M, Dosanjh D, Aslan Y, Deeks JJ, et al. Effect of BCG vaccination on risk of Mycobacterium tuberculosis infection in children with household tuberculosis contact: a prospective community-based study. Lancet. 2005. October 22-28;366(9495):1443–51. 10.1016/S0140-6736(05)67534-4 [DOI] [PubMed] [Google Scholar]
- 34.Aissa K, Madhi F, Ronsin N, Delarocque F, Lecuyer A, Decludt B, et al. ; CG94 Study Group. Evaluation of a model for efficient screening of tuberculosis contact subjects. Am J Respir Crit Care Med. 2008. May 1;177(9):1041–7. 10.1164/rccm.200711-1756OC [DOI] [PubMed] [Google Scholar]
- 35.Alavi SM. Pulmonary tuberculosis in household contact of patients with active tuberculosis in Ahwaz, Iran (2003–2005). Pak J Med Sci. 2008;24(6):780–5. [Google Scholar]
- 36.Diel R, Loddenkemper R, Meywald-Walter K, Niemann S, Nienhaus A. Predictive value of a whole blood IFN-gamma assay for the development of active tuberculosis disease after recent infection with Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2008. May 15;177(10):1164–70. 10.1164/rccm.200711-1613OC [DOI] [PubMed] [Google Scholar]
- 37.Lin X, Chongsuvivatwong V, Lin L, Geater A, Lijuan R. Dose-response relationship between treatment delay of smear-positive tuberculosis patients and intra-household transmission: a cross-sectional study. Trans R Soc Trop Med Hyg. 2008. August;102(8):797–804. 10.1016/j.trstmh.2008.04.027 [DOI] [PubMed] [Google Scholar]
- 38.Pavić I, Topić RZ, Raos M, Aberle N, Dodig S. Interferon-γ release assay for the diagnosis of latent tuberculosis in children younger than 5 years of age. Pediatr Infect Dis J. 2011. October;30(10):866–70. 10.1097/INF.0b013e318220c52a [DOI] [PubMed] [Google Scholar]
- 39.Verhagen LM, Maes M, Villalba JA, d’Alessandro A, Rodriguez LP, España MF, et al. Agreement between QuantiFERON®-TB Gold In-Tube and the tuberculin skin test and predictors of positive test results in Warao Amerindian pediatric tuberculosis contacts. BMC Infect Dis. 2014. July 11;14(1):383. 10.1186/1471-2334-14-383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rose W, Read SE, Bitnun A, Rea E, Stephens D, Pongsamart W, et al. Relating tuberculosis (TB) contact characteristics to QuantiFERON-TB-Gold and tuberculin skin test results in the Toronto pediatric TB clinic. J Pediatric Infect Dis Soc. 2015. June;4(2):96–103. 10.1093/jpids/piu024 [DOI] [PubMed] [Google Scholar]
- 41.Perez-Porcuna TM, Pereira-da-Silva HD, Ascaso C, Malheiro A, Bührer S, Martinez-Espinosa F, et al. Prevalence and diagnosis of latent tuberculosis infection in young children in the absence of a gold standard. PLoS One. 2016. October 26;11(10):e0164181. 10.1371/journal.pone.0164181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becerra MC, Pachao-Torreblanca IF, Bayona J, Celi R, Shin SS, Kim JY, et al. Expanding tuberculosis case detection by screening household contacts. Public Health Rep. 2005. May-Jun;120(3):271–7. 10.1177/003335490512000309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chee CB, Teleman MD, Boudville IC, Wang YT. Contact screening and latent TB infection treatment in Singapore correctional facilities. Int J Tuberc Lung Dis. 2005. November;9(11):1248–52. [PubMed] [Google Scholar]
- 44.Khalilzadeh S, Masjedi H, Hosseini M, Safavi A, Masjedi MR. Transmission of Mycobacterium tuberculosis to households of tuberculosis patients: a comprehensive contact tracing study. Arch Iran Med. 2006. July;9(3):208–12. [PubMed] [Google Scholar]
- 45.Yeo IK, Tannenbaum T, Scott AN, Kozak R, Behr MA, Thibert L, et al. Contact investigation and genotyping to identify tuberculosis transmission to children. Pediatr Infect Dis J. 2006. November;25(11):1037–43. 10.1097/01.inf.0000241101.12510.3c [DOI] [PubMed] [Google Scholar]
- 46.Hussain R, Talat N, Shahid F, Dawood G. Longitudinal tracking of cytokines after acute exposure to tuberculosis: association of distinct cytokine patterns with protection and disease development. Clin Vaccine Immunol. 2007. December;14(12):1578–86. 10.1128/CVI.00289-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hill PC, Jackson-Sillah DJ, Fox A, Brookes RH, de Jong BC, Lugos MD, et al. Incidence of tuberculosis and the predictive value of ELISPOT and Mantoux tests in Gambian case contacts. PLoS One. 2008. January 2;3(1):e1379. 10.1371/journal.pone.0001379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee MS, Leung CC, Kam KM, Wong MY, Leung MC, Tam CM, et al. Early and late tuberculosis risks among close contacts in Hong Kong. Int J Tuberc Lung Dis. 2008. March;12(3):281–7. [PubMed] [Google Scholar]
- 49.Borrell S, Español M, Orcau A, Tudó G, March F, Caylà JA, et al. Factors associated with differences between conventional contact tracing and molecular epidemiology in study of tuberculosis transmission and analysis in the city of Barcelona, Spain. J Clin Microbiol. 2009. January;47(1):198–204. 10.1128/JCM.00507-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.del Corral H, París SC, Marín ND, Marín DM, López L, Henao HM, et al. IFNgamma response to Mycobacterium tuberculosis, risk of infection and disease in household contacts of tuberculosis patients in Colombia. PLoS One. 2009. December 14;4(12):e8257. 10.1371/journal.pone.0008257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kilicaslan Z, Kiyan E, Kucuk C, Kumbetli S, Sarimurat N, Ozturk F, et al. Risk of active tuberculosis in adult household contacts of smear-positive pulmonary tuberculosis cases. Int J Tuberc Lung Dis. 2009. January;13(1):93–8. [PubMed] [Google Scholar]
- 52.Machado A Jr, Emodi K, Takenami I, Finkmoore BC, Barbosa T, Carvalho J, et al. Analysis of discordance between the tuberculin skin test and the interferon-gamma release assay. Int J Tuberc Lung Dis. 2009. April;13(4):446–53. [PubMed] [Google Scholar]
- 53.Nguyen TH, Odermatt P, Slesak G, Barennes H. Risk of latent tuberculosis infection in children living in households with tuberculosis patients: a cross sectional survey in remote northern Lao People’s Democratic Republic. BMC Infect Dis. 2009. June 17;9(1):96. 10.1186/1471-2334-9-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ottmani S, Zignol M, Bencheikh N, Laâsri L, Blanc L, Mahjour J. TB contact investigations: 12 years of experience in the National TB Programme, Morocco 1993–2004. East Mediterr Health J. 2009. May-Jun;15(3):494–503. 10.26719/2009.15.3.494 [DOI] [PubMed] [Google Scholar]
- 55.Pai M, Joshi R, Dogra S, Zwerling AA, Gajalakshmi D, Goswami K, et al. T-cell assay conversions and reversions among household contacts of tuberculosis patients in rural India. Int J Tuberc Lung Dis. 2009. January;13(1):84–92. [PMC free article] [PubMed] [Google Scholar]
- 56.Cavalcante SC, Durovni B, Barnes GL, Souza FB, Silva RF, Barroso PF, et al. Community-randomized trial of enhanced DOTS for tuberculosis control in Rio de Janeiro, Brazil. Int J Tuberc Lung Dis. 2010. February;14(2):203–9. [PMC free article] [PubMed] [Google Scholar]
- 57.Lienhardt C, Fielding K, Hane AA, Niang A, Ndao CT, Karam F, et al. Evaluation of the prognostic value of IFN-gamma release assay and tuberculin skin test in household contacts of infectious tuberculosis cases in Senegal. PLoS One. 2010. May 6;5(5):e10508. 10.1371/journal.pone.0010508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rakotosamimanana N, Raharimanga V, Andriamandimby SF, Soares JL, Doherty TM, Ratsitorahina M, et al. ; VACSEL/VACSIS Study Group. Variation in gamma interferon responses to different infecting strains of Mycobacterium tuberculosis in acid-fast bacillus smear-positive patients and household contacts in Antananarivo, Madagascar. Clin Vaccine Immunol. 2010. July;17(7):1094–103. 10.1128/CVI.00049-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sia IG, Orillaza RB, St Sauver JL, Quelapio ID, Lahr BD, Alcañeses RS, et al. Tuberculosis attributed to household contacts in the Philippines. Int J Tuberc Lung Dis. 2010. January;14(1):122–5. [PubMed] [Google Scholar]
- 60.Becerra MC, Appleton SC, Franke MF, Chalco K, Arteaga F, Bayona J, et al. Tuberculosis burden in households of patients with multidrug-resistant and extensively drug-resistant tuberculosis: a retrospective cohort study. Lancet. 2011. January 8;377(9760):147–52. 10.1016/S0140-6736(10)61972-1 [DOI] [PubMed] [Google Scholar]
- 61.Grandjean L, Crossa A, Gilman RH, Herrera C, Bonilla C, Jave O, et al. Tuberculosis in household contacts of multidrug-resistant tuberculosis patients. Int J Tuberc Lung Dis. 2011. September;15(9):1164–9, i. 10.5588/ijtld.11.0030 [DOI] [PubMed] [Google Scholar]
- 62.Hussain R, Talat N, Ansari A, Shahid F, Hasan Z, Dawood G. Endogenously activated interleukin-4 differentiates disease progressors and non-progressors in tuberculosis susceptible families: a 2-year biomarkers follow-up study. J Clin Immunol. 2011. October;31(5):913–23. 10.1007/s10875-011-9566-y [DOI] [PubMed] [Google Scholar]
- 63.Singla N, Singla R, Jain G, Habib L, Behera D. Tuberculosis among household contacts of multidrug-resistant tuberculosis patients in Delhi, India. Int J Tuberc Lung Dis. 2011. October;15(10):1326–30. 10.5588/ijtld.10.0564 [DOI] [PubMed] [Google Scholar]
- 64.Vella V, Racalbuto V, Guerra R, Marra C, Moll A, Mhlanga Z, et al. Household contact investigation of multidrug-resistant and extensively drug-resistant tuberculosis in a high HIV prevalence setting. Int J Tuberc Lung Dis. 2011. September;15(9):1170–5, i. 10.5588/ijtld.10.0781 [DOI] [PubMed] [Google Scholar]
- 65.Whalen CC, Zalwango S, Chiunda A, Malone L, Eisenach K, Joloba M, et al. Secondary attack rate of tuberculosis in urban households in Kampala, Uganda. PLoS One. 2011. February 14;6(2):e16137. 10.1371/journal.pone.0016137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang X, Wei X, Zou G, Walley J, Zhang H, Guo X, et al. Evaluation of active tuberculosis case finding through symptom screening and sputum microscopy of close contacts in Shandong, China. Trop Med Int Health. 2011. December;16(12):1511–7. 10.1111/j.1365-3156.2011.02869.x [DOI] [PubMed] [Google Scholar]
- 67.Fox GJ, Nhung NV, Sy DN, Lien LT, Cuong NK, Britton WJ, et al. Contact investigation in households of patients with tuberculosis in Hanoi, Vietnam: a prospective cohort study. PLoS One. 2012;7(11):e49880. 10.1371/journal.pone.0049880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gyawali N, Gurung R, Poudyal N, Amatya R, Niraula SR, Jha P, et al. Prevalence of tuberculosis in household contacts of sputum smears positive cases and associated demographic risk factors. Nepal Med Coll J. 2012. December;14(4):303–7. [PubMed] [Google Scholar]
- 69.Ntinginya EN, Squire SB, Millington KA, Mtafya B, Saathoff E, Heinrich N, et al. Performance of the Xpert® MTB/RIF assay in an active case-finding strategy: a pilot study from Tanzania. Int J Tuberc Lung Dis. 2012. November;16(11):1468–70. 10.5588/ijtld.12.0127 [DOI] [PubMed] [Google Scholar]
- 70.Shapiro AE, Variava E, Rakgokong MH, Moodley N, Luke B, Salimi S, et al. Community-based targeted case finding for tuberculosis and HIV in household contacts of patients with tuberculosis in South Africa. Am J Respir Crit Care Med. 2012. May 15;185(10):1110–6. 10.1164/rccm.201111-1941OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thind D, Charalambous S, Tongman A, Churchyard G, Grant AD. An evaluation of ‘Ribolola’: a household tuberculosis contact tracing programme in North West Province, South Africa. Int J Tuberc Lung Dis. 2012. December;16(12):1643–8. 10.5588/ijtld.12.0074 [DOI] [PubMed] [Google Scholar]
- 72.Chamie G, Wandera B, Luetkemeyer A, Bogere J, Mugerwa RD, Havlir DV, et al. Household ventilation and tuberculosis transmission in Kampala, Uganda. Int J Tuberc Lung Dis. 2013. June;17(6):764–70. 10.5588/ijtld.12.0681 [DOI] [PubMed] [Google Scholar]
- 73.Jones-López EC, Namugga O, Mumbowa F, Ssebidandi M, Mbabazi O, Moine S, et al. Cough aerosols of Mycobacterium tuberculosis predict new infection: a household contact study. Am J Respir Crit Care Med. 2013. May 1;187(9):1007–15. 10.1164/rccm.201208-1422OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leung EC, Leung CC, Kam KM, Yew WW, Chang KC, Leung WM, et al. Transmission of multidrug-resistant and extensively drug-resistant tuberculosis in a metropolitan city. Eur Respir J. 2013. April;41(4):901–8. 10.1183/09031936.00071212 [DOI] [PubMed] [Google Scholar]
- 75.Puryear S, Seropola G, Ho-Foster A, Arscott-Mills T, Mazhani L, Firth J, et al. Yield of contact tracing from pediatric tuberculosis index cases in Gaborone, Botswana. Int J Tuberc Lung Dis. 2013. August;17(8):1049–55. 10.5588/ijtld.12.0933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shah SA, Qayyum S, Abro R, Baig S, Creswell J. Active contact investigation and treatment support: an integrated approach in rural and urban Sindh, Pakistan. Int J Tuberc Lung Dis. 2013. December;17(12):1569–74. 10.5588/ijtld.13.0169 [DOI] [PubMed] [Google Scholar]
- 77.Singh J, Sankar MM, Kumar S, Gopinath K, Singh N, Mani K, et al. Incidence and prevalence of tuberculosis among household contacts of pulmonary tuberculosis patients in a peri-urban population of South Delhi, India. PLoS One. 2013. July 26;8(7):e69730. 10.1371/journal.pone.0069730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tao L, Zalwango S, Chervenak K, Thiel B, Malone LL, Qiu F, et al. Tuberculosis Research Unit (TBRU). Genetic and shared environmental influences on interferon-γ production in response to Mycobacterium tuberculosis antigens in a Ugandan population. Am J Trop Med Hyg. 2013. July;89(1):169–73. 10.4269/ajtmh.12-0670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yassin MA, Datiko DG, Tulloch O, Markos P, Aschalew M, Shargie EB, et al. Innovative community-based approaches doubled tuberculosis case notification and improve treatment outcome in Southern Ethiopia. PLoS One. 2013. May 27;8(5):e63174. 10.1371/journal.pone.0063174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jia Z, Cheng S, Ma Y, Zhang T, Bai L, Xu W, et al. Tuberculosis burden in China: a high prevalence of pulmonary tuberculosis in household contacts with and without symptoms. BMC Infect Dis. 2014. February 6;14(1):64. 10.1186/1471-2334-14-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jones-López EC, Kim S, Fregona G, Marques-Rodrigues P, Hadad DJ, Molina LP, et al. Importance of cough and M. tuberculosis strain type as risks for increased transmission within households. PLoS One. 2014. July 2;9(7):e100984. 10.1371/journal.pone.0100984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Loredo C, Cailleaux-Cezar M, Efron A, de Mello FC, Conde MB. Yield of close contact tracing using two different programmatic approaches from tuberculosis index cases: a retrospective quasi-experimental study. BMC Pulm Med. 2014. August 7;14(1):133. 10.1186/1471-2466-14-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thanh TH, Ngoc SD, Viet NN, Van HN, Horby P, Cobelens FG, et al. A household survey on screening practices of household contacts of smear positive tuberculosis patients in Vietnam. BMC Public Health. 2014. July 11;14(1):713. 10.1186/1471-2458-14-713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zelner JL, Murray MB, Becerra MC, Galea J, Lecca L, Calderon R, et al. Bacillus Calmette-Guérin and isoniazid preventive therapy protect contacts of patients with tuberculosis. Am J Respir Crit Care Med. 2014. April 1;189(7):853–9. 10.1164/rccm.201310-1896OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chamie G, Wandera B, Marquez C, Kato-Maeda M, Kamya MR, Havlir DV, et al. Identifying locations of recent TB transmission in rural Uganda: a multidisciplinary approach. Trop Med Int Health. 2015. April;20(4):537–45. 10.1111/tmi.12459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grandjean L, Gilman RH, Martin L, Soto E, Castro B, López S, et al. Transmission of multidrug-resistant and drug-susceptible tuberculosis within households: a prospective cohort study. PLoS Med. 2015. June 23;12(6):e1001843–, discussion e1001843.. 10.1371/journal.pmed.1001843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jerene D, Melese M, Kassie Y, Alem G, Daba SH, Hiruye N, et al. The yield of a tuberculosis household contact investigation in two regions of Ethiopia. Int J Tuberc Lung Dis. 2015. August;19(8):898–903. 10.5588/ijtld.14.0978 [DOI] [PubMed] [Google Scholar]
- 88.Zellweger JP, Sotgiu G, Block M, Dore S, Altet N, Blunschi R, et al. ; TBNET. Risk assessment of tuberculosis in contacts by IFN-γ release assays. A Tuberculosis Network European Trials group study. Am J Respir Crit Care Med. 2015. May 15;191(10):1176–84. 10.1164/rccm.201502-0232OC [DOI] [PubMed] [Google Scholar]
- 89.Gupta M, Saibannavar AA, Kumar V. Household symptomatic contact screening of newly diagnosed sputum smears positive tuberculosis patients – an effective case detection tool. Lung India. 2016. Mar-Apr;33(2):159–62. 10.4103/0970-2113.177445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Javaid A, Khan MA, Khan MA, Mehreen S, Basit A, Khan RA, et al. Screening outcomes of household contacts of multidrug-resistant tuberculosis patients in Peshawar, Pakistan. Asian Pac J Trop Med. 2016. September;9(9):909–12. 10.1016/j.apjtm.2016.07.017 [DOI] [PubMed] [Google Scholar]
- 91.Nair D, Rajshekhar N, Klinton JS, Watson B, Velayutham B, Tripathy JP, et al. Household contact screening and yield of tuberculosis cases – a clinic based study in Chennai, South India. PLoS One. 2016. September 1;11(9):e0162090. 10.1371/journal.pone.0162090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wysocki AD, Villa TC, Arakawa T, Brunello ME, Vendramini SH, Monroe AA, et al. Latent tuberculosis infection diagnostic and treatment cascade among contacts in primary health care in a city of Sao Paulo State, Brazil: cross-sectional study. PLoS One. 2016. June 10;11(6):e0155348. 10.1371/journal.pone.0155348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Armstrong-Hough M, Turimumahoro P, Meyer AJ, Ochom E, Babirye D, Ayakaka I, et al. Drop-out from the tuberculosis contact investigation cascade in a routine public health setting in urban Uganda: a prospective, multi-center study. PLoS One. 2017. November 6;12(11):e0187145. 10.1371/journal.pone.0187145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Datiko DG, Yassin MA, Theobald SJ, Cuevas LE. A community-based isoniazid preventive therapy for the prevention of childhood tuberculosis in Ethiopia. Int J Tuberc Lung Dis. 2017. September 1;21(9):1002–7. 10.5588/ijtld.16.0471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fox GJ, Anh NT, Nhung NV, Loi NT, Hoa NB, Ngoc Anh LT, et al. Latent tuberculous infection in household contacts of multidrug-resistant and newly diagnosed tuberculosis. Int J Tuberc Lung Dis. 2017. March 1;21(3):297–302. 10.5588/ijtld.16.0576 [DOI] [PubMed] [Google Scholar]
- 96.Mandalakas AM, Ngo K, Alonso Ustero P, Golin R, Anabwani F, Mzileni B, et al. BUTIMBA: intensifying the hunt for child TB in Swaziland through household contact tracing. PLoS One. 2017. January 20;12(1):e0169769. 10.1371/journal.pone.0169769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Muyoyeta M, Kasese NC, Milimo D, Mushanga I, Ndhlovu M, Kapata N, et al. Digital CXR with computer aided diagnosis versus symptom screen to define presumptive tuberculosis among household contacts and impact on tuberculosis diagnosis. BMC Infect Dis. 2017. April 24;17(1):301. 10.1186/s12879-017-2388-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Global tuberculosis report 2018. Geneva: World Health Organization; 2018. Available from: http://apps.who.int/iris/bitstream/handle/10665/274453/9789241565646-eng.pdf?ua=1 [cited 2018 Nov 19].