Abstract
Leukocyte telomere length (LTL) may be sensitive to psychosocial stressors such as discrimination. An inclusive examination of experiences of discrimination on LTL across racial/ethnic and sex groups is currently lacking. Baseline data were obtained from 369 White and African American patients with coronary artery disease (CAD) in the Mental Stress Ischemia Mechanisms and Prognosis Study. LTL was measured from peripheral blood leukocytes by quantitative polymerase chain reaction and calculated in kilobase pairs. Discrimination was measured using the 10-item Everyday Discrimination Scale (EDS). Responses were rated using 4-point Likert scales ranging from never =1 to often = 4 and summed. Regression models were stratified by race/ethnicity and sex to estimate associations between discrimination and LTL. Each 10-unit increase in experiences of everyday discrimination was associated with an average of .20 fewer kilobase pairs (or 200 base pairs) among both African American women (β = −0.19; 95% CI: −0. 35, −0.04; p-value: 0.02) and White women (β = −0.19; 95% CI: −0.37, −0.01; p-value: 0.04), after adjusting for basic demographic factors. Results were similar after further adjusting for behavioral, disease, and psychosocial risk factors (depression and stress). There were no significant associations between experiences of everyday discrimination and LTL for White men or African American men. Overall, experiences of discrimination were associated with shorter LTL among women and not in men. Discrimination may be a potential source of stress associated with shorter LTL among women with CAD. Future studies should explore longitudinal associations between everyday experiences of discrimination and telomere length and also with adverse cardiovascular outcomes.
Keywords: Leukocyte telomere length, discrimination, psychosocial stressors, women
1. INTRODUCTION
Research suggests that psychosocial stress is associated with earlier onset of age-related diseases (O’Donovan et al., 2012). Telomere length, a biomarker of aging at the cellular level may illuminate potential mechanisms between stress and pathogenesis of disease (O’Donovan et al., 2012; Sanders and Newman, 2013). Telomeres, which are protective caps at the end of chromosomes composed of repeated nucleotides or base pairs of DNA, have a central role in genomic stability and chromosomal structural integrity (Blackburn, 2000, 2001). Telomere shortening is a biologically natural phenomenon that occurs over time and across the lifespan, eventually stripping the chromosome of its protective armor. Subsequently, cells become functionally impaired and are unable to proliferate, which leads to cellular senescence or cell death (Blackburn, 2000, 2001).
However, chronological age accounts for less than 10% of the variance in human telomere length (Blackburn et al., 2015). While genetic factors can influence telomere length, environmental and psychosocial determinants can also play a role. Many studies have found that greater exposure to psychosocial stressors are associated with shorter telomere length including life stress (Epel et al., 2004), low social support (Carroll et al., 2013), low socioeconomic status (Adler et al., 2013; Needham et al., 2013), and adverse, or stressful neighborhood environments (Gebreab et al., 2016). Although the majority of research in this area has focused on adults, associations between psychosocial stressors and shorter telomere length have also been observed among children (Theall et al., 2013). Similar results have also been confirmed in recent meta-analyses in adults and children (Hanssen et al., 2017; Mathur et al., 2016; Pepper et al., 2018; Schutte and Malouff, 2016).
One important, yet relatively understudied source of stress-related cellular aging is everyday discrimination, or interpersonal mistreatment. To date, only a few studies have examined the association between discrimination and telomere length (Chae et al., 2016; Chae et al., 2014; Liu and Kawachi, 2017) and while some significant associations were found, all of these studies focused on racial/ethnic discrimination alone. Research suggests; however, that experiences of discrimination may transcend experiences of mistreatment due to race/ethnicity and may include occurrences of unfair treatment related to age, sex, physical disability, or other characteristics. Further, none of the aforementioned studies explicitly examined experiences of discrimination and telomere length stratified or moderated by sex, failing to consider whether the effect of discrimination on LTL differs for women and men. Thus, an inclusive examination of experiences of discrimination on LTL across racial/ethnic and sex groups is currently lacking.
This study was designed to investigate whether experiences of everyday discrimination are associated with shorter LTL across race/ethnicity and sex among African-American and White men and women with coronary artery disease (CAD). LTL may have particular relevance for this group, as CAD is an age-related disease and shorter LTL has been associated with reduced vascular regenerative capacity and repair and a range of adverse outcomes (e.g., stroke, myocardial infarction, mortality) in this population (Hammadah et al., 2017a).
In the current analysis, we hypothesized that experiences of everyday discrimination would be associated with shorter telomere length among women, and African American women in particular. Research suggests that women may be more physiologically vulnerable to psychosocial stressors (Vaccarino and Bremner, 2017), particularly those of an interpersonal nature, compared to men (Stroud et al., 2002). We expected stronger associations among African American women because research suggests that African American women may experience greater frequency, duration, and intensity of psychosocial stressors (Geronimus et al., 2010) and are more likely to experience everyday discrimination on the basis of multiple subordinate identities (Lewis et al., 2015; Lewis and Van Dyke, 2018). Also, there is evidence that African American women are more likely than their white or male counterparts to be discriminated against in medical encounters (Schulman et al., 1999; Shaw et al., 2008), which may be especially relevant for our study population of patients with coronary artery disease.
In addition to exploring the main association between experience of everyday discrimination and telomere length, we further wanted to investigate whether the possible relationship was independent or explained by other dimensions of stressors including perceived stress and depression. Not only has prior research recommended controlling for other dimensions of stressors in discrimination research on health outcomes (Albert and Williams, 2011), perceived stress and depression have also been associated with shorter telomere length in previous studies and in recent meta-analyses (Epel et al., 2004; Lin et al., 2016; Mathur et al., 2016).
2. MATERIALS AND METHODS
2.1. Study Design
The Mental Stress Ischemia Mechanisms and Prognosis Study (MIPS) is a prospective cohort study designed to investigate mechanisms and prognosis of mental stress-induced ischemia among patients with stable coronary artery disease (CAD). Patients were recruited from Emory University-affiliated hospitals and clinics and were eligible for the study if they were between 30–79 years of age and had documented CAD, including any of the following: 1) abnormal coronary angiography or intravascular ultrasound demonstrating atherosclerosis with at least luminal irregularities, 2) previous percutaneous or surgical coronary revascularization, 3) documented myocardial infarction (MI), or 4) positive exercise or pharmacological nuclear stress test or electrocardiographic exercise stress test (Hammadah et al., 2017b). Patients were excluded from participating in the study if they were either pregnant; were hospitalized in the previous week for unstable angina, decompensated heart failure, or myocardial infarction; had severe psychiatric conditions such as schizophrenia; a history of alcohol or substance abuse; active malignancy or end stage renal disease; or other severe medical problems expected to shorten life expectancy to less than 45 years.
Between June 2011 and August 2014, 695 patients were enrolled in MIPS. During the baseline visits, patients underwent clinical evaluations including mental and physical stress testing and questionnaire assessments such as the Beck Depression Inventory and the Perceived Stress Scale. Blood samples for telomere length were collected during the baseline clinic visits. The Institutional Review Board at Emory University approved the MIPS protocol. Written informed consent was obtained from all patients. More detailed information on the MIPS objectives and study design has been described elsewhere (Hammadah et al., 2017b).
2.2. Participants
Analyses were restricted to patients who indicated that their race/ethnicity was either Non-Hispanic White or African American (n=645). Of these, 199 were missing information on everyday discrimination as the Everyday Discrimination Scale was added after the start of the study, and 76 participants did not have telomere assays because of technical difficulties in sample drawing or processing, or because the patient refused. Further, we removed observations with missing covariates of interest including income (n=15), education (n=4), and smoking history (n=2). Thus, there were 369 patients in the final analytic dataset. Participants included in the analytic dataset were not significantly different than participants who were excluded due to missing observations across demographic characteristics with the exception of age (Supplemental Table 1). Specifically, included participants were slightly younger than those with missing data (mean: 62.2; SD: 9.3 vs. 63.9; SD: 9.8; p-value: 0.01).
2.3. Measurements
2.3.1. Telomere Length
Leukocyte telomere length (LTL) was measured from peripheral blood leukocytes collected during the baseline examination. All LTL assays were performed by Dr. Blackburn’s laboratory at the University of California, San Francisco using methods by Cawthon (2009) and Lin et al. (2010). LTL was measured as the ratio of telomeric product/single copy gene (T/S) by quantitative PCR analyses using a serially diluted standard DNA and the standard curve method. Reference DNA (pooled samples of leukocyte genomic DNA from 100 female donors) was included in each PCR run so that the quantity of targeted templates in each research sample could be determined relative to the reference DNA sample by the standard curve method. In each batch (8 plates in total), the T/S ratio of each control DNA were divided by the average T/S for the same DNA from 10 runs to get a normalizing factor. The average normalizing factor for all 8 plates were used to correct the participant DNA samples to get the final T/S ratio. The T/S ratio for each sample was measured twice. When the duplicate T/S value and the initial value varied by more than 7%, the sample was run a third time and the two closest values were reported. The T/S ratios represent the average length of telomeres. We then converted the T/S ratios to kilobase pairs using the following equation: kilobase pairs = [3274 + 2413 * (T/S)]/1000. More detailed information on the LTL measurement in MIPS has been described previously (Hammadah et al., 2017a).
2.3.2. Experiences of Everyday Discrimination
Discrimination was measured using the 10-item Everyday Discrimination Scale (EDS). The EDS was adapted from the original 9-item scale developed for use in the Detroit Area Study (Williams et al., 1997). Occurrences of unfair treatment during the previous 12 months were assessed without reference to race/ethnicity, age, sex, or other demographic characteristics. Specifically, participants were asked how often during their day-to-day experiences: 1) They were treated with less courtesy than other people; 2) They were treated with less respect than other people; 3) They received poorer service than other people at restaurants or stores; 4) People acted as if they were not smart; 5) People acted as if they were afraid of them; 6) People acted as if they were dishonest; 7) People acted as if they were better than them; 8) They were called names or insulted; 9) They were threatened or harassed; and 10) People ignored them, or acted as if they were not there. Responses were rated using 4-point Likert scales ranging from never (1) to often (4) which were summed and ranged from 0 to 40 on an ordinal scale. The Cronbach α for the EDS in the MIPS study was 0.90.
2.3.3. Covariates
Demographic information was obtained using standardized questionnaires. Previous medical history (diabetes, hypertension, previous MI) were obtained by study nurses or physicians through medical history, clinical examinations and by reviewing medical records. Demographics included race/ethnicity (Non-Hispanic White or African American), sex, educational attainment, income, and marital status. Educational attainment was assessed as highest year of education (grade school, some high school, high school or GED, some college, bachelor’s degree, master’s, degree, or other) and recoded as either high school or less or greater than high school. Income was dichotomized as whether family income was < $20,000 or ≥ $20,000. Marital status included 1) married, partnered living as married; or 2) single, separated, divorced, or widowed. Participants’ height and weight were objectively measured during the clinic visit and used to calculate body mass index (BMI, kg/m2).
Depressive symptomology was assessed using the Beck Depression Inventory Second Edition (BDI-II) (Beck AT et al., 1996). The BDI-II includes 21 questions which asks participants to rate their feelings, cognitions, and physical symptoms (e.g., sadness, pessimism, guilt, fatigue) during the past two weeks. Each item contains a 4-point Likert scale to indicate the severity of each feeling, from 0 (not at all) to 3 (extreme form of each symptom) (Beck AT et al., 1996). Responses across the items were summed so that higher scores indicated greater symptoms of depression.
Perceived stress was measured using the Cohen’s Perceived Stress Scale (PSS) (Cohen et al., 1983). Participants were asked to rate their feelings about situations and experiences during the past month across 10 items using 5-point Likert scale ranging from Never (0) to Very Often (4) (e.g., nervous and stressed, confident about handling personal problems, not able cope with things, angered because of things outside of control). Positively stated items were reverse coded, and items were summed so that higher scores indicated greater perceptions of stress.
2.4. Statistical Analysis
Descriptive statistics were calculated for the MIPS sample by race/ethnicity and sex. Group differences were tested using chi-squared tests for categorical variables and analysis of variance (ANOVA) for continuous variables. Additionally, because we were interested in determing whether associations were stronger among women and especially African American women, we also ran race and sex stratified models. Using linear regression models, we tested for differences in slopes across sex and race for discrimination on telomere length by including interaction terms of discrimination-by-sex, and discrimination-by-race, race-by-sex, and discrimination-by-sex-by-race. Adjusted models further included covariates that were considered a priori. Model 1 was adjusted for age. Model 2 was further adjusted for income, education and marital status. Model 3 further included cardiovascular risk factors and medical history, including smoking, BMI, diabetes, hypertension, and previous MI. Model 4 added additional adjustments for psychosocial factors (depression and perceived stress) as potential mediators. The significance level for main effects was set at p < 0.05 while the statistical significance of interaction effects was set at p < 0.10. Also, to minimize any potential batch effect in telomere length, we deemed important to include telomere plate as a random effect in our models to account for any correlation of values related to how the samples were run or prepared in the laboratory. Thus, we further included plate effect as a random intercept across all models. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, North Carolina, USA).
3. RESULTS
Table 1 presents descriptive characteristics of the analytic sample by race/ethnicity and sex. Of the 369 participants, 13.5% were White women, 54.7% were White men, 11.7% were African American women, and 20.1% were African American men (Table 1). African American race and female sex was significantly associated with younger age, lower income, marital status of single/widowed/or divorced, diabetes, and hypertension. African American race and male sex was associated with sigificiantly higher discrimination scores and younger age. There were also significant differences across sex within race worth noting such that White women were significantly associated with higher discrimination scores, lower income, history of smoking, and with higher depressive and stress scores compared to White men. Bivariate correlations of LTL with discrimination, demographic, clinical, and psychosocial covariates are described in Table 2.
Table 1.
Descriptive Characteristics of Participants Stratified by Race/Ethnicity and Sex (N=369) in the Mental Stress Ischemia Prognosis Study (MIPS).
| Women | Men | |||||
|---|---|---|---|---|---|---|
| White | African American | p-value | White | African American | p-value | |
| Total, n (%) | 50 (13.5) | 43 (11.7) | 202 (54.7) | 74 (20.1) | ||
| Telomere Length, kilobase pairs, mean (SD) | 5.2 (0.05) | 5.6 (0.1) | <.0001 | 5.2 (0.03) | 5.3 (0.04)† | 0.13 |
| Discrimination Score, mean (SD) | 14.9 (4.3) | 15.4 (4.9) | 0.55 | 13.5 (3.8)* | 16.2 (6.6) | 0.001 |
| Age, mean (SD) | 65.3 (8.8) | 58.9 (8.0) | 0.0003 | 63.5 (9.2) | 58.0 (9.7) | 0.0002 |
| Income < $20,000, n (%) | 10 (20.0) | 18 (41.9) | 0.02 | 16 (7.9)* | 21 (28.4) | <.0001 |
| Education, High School or Less, n (%) | 16 (32.0) | 13 (30.2) | 0.85 | 39 (19.3) | 29 (39.2) | 0.001 |
| History of Smoking, n (%) | 22 (44.0) | 18 (41.9) | 0.84 | 82 (40.6)* | 25 (33.8)* | 0.30 |
| Married/living with partner, n (%) | 26 (52.0) | 13 (30.2) | 0.03 | 157 (77.7) | 41 (55.4) | 0.0003 |
| Diabetes, n (%) | 12 (24.0) | 19 (44.2) | 0.04 | 56 (27.7) | 30 (40.5) | 0.04 |
| Hypertension, n (%) | 36 (72.0) | 40 (93.0) | 0.01 | 145 (71.8) | 70 (94.6) | <.0001 |
| Previous Myocardial Ischemia, n (%) | 18 (36.0) | 21 (48.8) | 0.21 | 67 (33.2) | 34 (46.0) | 0.05 |
| BMI, kg/m2, mean (SD) | 29.3 (6.7) | 32.5 (7.3) | 0.03 | 29.5 (4.8) | 30.4 (4.9) | 0.21 |
| BDI total score, mean (SD) | 9.4 (6.9) | 10.0 (9.4) | 0.76 | 6.7 (6.9) * | 9.9 (10.1) | 0.01 |
| PSS total score, mean (SD) | 13.8 (8.3) | 15.1 (7.4) | 0.43 | 10.8 (6.7)* | 13.7 (7.7) | 0.003 |
Abbreviations: BDI: Beck depression inventory; BMI: Body mass index; MIPS: Mental stress ischemia mechanisms and prognosis study; PSS: Cohen’s perceived stress scale; SD: Standard deviation.
p < 0.05 between women and men within race
p <0.0001 between women and men within race
Table 2:
Bivariate Correlations of Leukocyte Telomere Length with Discrimination, Demographic, Clinical, and Psychosocial Covariates, Mental Stress Ischemia Prognosis Study (MIPS).
| Overall | White Women | African American Women | White Men | African American Men | |
|---|---|---|---|---|---|
| Discrimination | 0.03 | −0.21 | −0.18 | 0.02 | 0.08 |
| Age | −0.35 | −0.11 | −0.59* | −0.30† | −0.27* |
| Race/Ethnicity | 0.24† | -- | -- | -- | -- |
| Sex | −0.17* | -- | -- | -- | -- |
| Marital Status | −0.08 | 0.05 | −0.03 | 0.06 | −0.13 |
| Income | −0.17 | −0.24 | −0.16 | 0.02 | −0.19 |
| Education | 0.06 | −0.08 | 0.03 | 0.11 | 0.19 |
| Smoking History | −0.08 | −0.10 | 0.20 | −0.14* | −0.10 |
| Diabetes | 0.01 | 0.03 | −0.20 | −0.05 | −0.01 |
| Hypertension | 0.002 | −0.02 | −0.43* | −0.08 | 0.09 |
| Previous Ml | −0.02 | −0.13 | 0.10 | −0.08 | −0.04 |
| BMI, kg/m2 | 0.06 | −0.11 | 0.06 | −0.05 | −0.01 |
| BDI | 0.09 | 0.21 | 0.11 | −0.02 | 0.09 |
| PSS | 0.08 | −0.16 | −0.05 | 0.04 | 0.18 |
Abbreviations: BDI: Beck depression inventory; BMI: Body mass index; MIPS: Mental stress ischemia mechanisms and prognosis study; PSS: Cohen’s perceived stress scale; SD: Standard deviation.
Pearson correlations for continuous covariates and Spearman correlations for categorical covariates. Race/ethnicity coded as 0=White, 1 = African American; Sex coded as 0=Female, 1=Male; Marital status coded as 0=Single/Separated/Divorded/Widowed, 1=Married/Living with Partner; Education coded as 0= High School or less, 1 = Greater than High School; Smoking History coded as 0=Never, 1=Current/Former; Diabetes, Hypertension, and Previous MI coded as 0=No, 1=Yes. Discrimination, age, BMI, Depression, and Stress are continuous variables.
p < 0.05
p < 0.0001
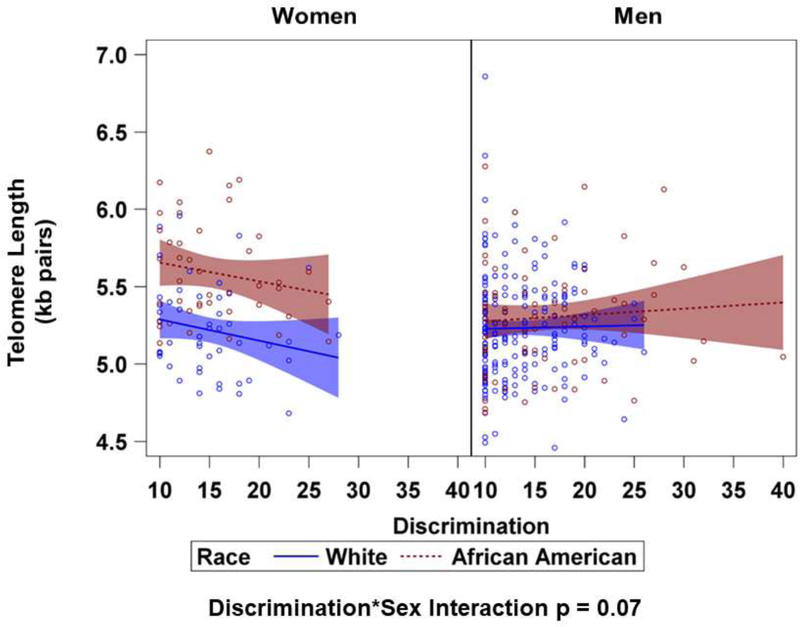
In a multivariable model including discrimination, race, sex, their interaction terms, there was a steeper and negative slope for discrimination on telomere length for White and African American women compared to White and African American men (Figure 1) and the interaction term of discrimination-by-sex was p = 0.07 (Supplemental Table 2). However, after adjusting for age and in subsequent models, there was no significant difference in the effect of discrimination on LTL between women and men.
Figure 1. Regression Slopes and 95% Confidence Intervals for Discrimination across Sex and Race on Telomere Length (kilobase pairs).
Linear regression models were used to test for differences in slopes across sex and race for discrimination on telomere length by including interaction terms of discrimination-by-sex, discrimination-by-race, race-by-sex, and discrimination-by-sex-by-race. The interaction term of discrimination-by-sex was p = 0.07.
In linear regression analyses stratified by sex and race/ethnicity (Table 3) and after adjusting for age alone, each 10-unit increase in experiences of everyday discrimination were significantly associated with shorter LTL among African American women (β = −0.17; 95% CI: −0. 33, −0.04; p-value: 0.03). After further adjusting for basic demographic factors including income, education, and marital status (model 2), experiences of everyday discrimination were significantly associated with shorter LTL among both African American women (β = −0.19; 95% CI: −0. 35, −0.04; p-value: 0.02) and White women (β = −0. 19; 95% CI: −0.37, −0.01; p-value: 0.04). Specifically, greater experiences of discrimination were associated with an average of .20 fewer kilobase pairs (or 200 base pairs) in both groups. Results were similar for both African American women and White women after further adjusting for behavioral and disease risk factors in model 3, and also after adjusting for psychosocial factors in model 4. There were no significant associations between discrimination and LTL in either White or African American men in any of the regression models and effect estimates were close to the null. Alternatively, a one standard deviation increase in discrimination score for each race sex group on LTL {(β for LTL/10) * SD of discrimination score}, would change LTL by −0.09 kilobase pairs (90 base pairs) for White Women, −10 kilobase pairs (100 base pairs) for American American women, −03 kilobase pairs (30 base pairs) for White men, and −0.07 kilobase pairs (70 base pairs) among African American men.
Table 3.
Adjusted Estimates for Experiences of Everyday Discrimination (per 10-unit increase) on Leukocyte Telomere Length (N=369) in the Mental Stress Ischemia Prognosis Study (MIPS) by Race/Ethnicity and Sex.
| White Women | African American Women | White Men | African American Men | |
|---|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| Model 1 | ||||
| Discrimination | −0.16 (−0.35, 0.03) | −0.17 (−0.33, −0.01)* | −0.08 (−0.21, 0.04) | −0.02 (−0.14, 0.11) |
| Age | −0.01 (−0.01, 0.004) | −0.02 (−0.03, −0.01)† | −0.01 (−0.02, −0.01)† | −0.01 (−0.02, −0.0004)* |
| Model 2 | ||||
| Discrimination | −0.19 (−0.37, −0.01)* | −0.19 (−0.35, −0.04)* | −0.07 (−0.19, 0.06) | −0.04 (−0.17, 0.08) |
| Age | −0.003 (−0.01, 0.01) | −0.02 (−0.03, −0.01)† | −0.01 (−0.02, −0.01)† | −0.01 (−0.02, −0.002)* |
| Income < $20,000 | 0.27 (0.05, 0.50)* | 0.13 (−0.04, 0.30) | −0.06 (−0.24, 0.12) | 0.16 (−0.04, 0.36) |
| Education HS or Less | −0.02 (−0.20, 0.15) | 0.04 (−0.13, 0.20) | −0.11 (−0.22, −0.002)* | −0.21 (−0.36, −0.06)* |
| Single/Sep/Div/Wid | −0.09 (−0.25, 0.07) | −0.05 (−0.23, 0.13) | −0.06 (−0.17, 0.06) | −0.05 (−0.22, 0.11) |
| Model 3 | ||||
| Discrimination | −0.19 (−0.39, 0.01) | −0.20 (−0.34, −0.06)* | −0.08 (−0.20, 0.04) | −0.08 (−0.21, 0.05) |
| Age | −0.004 (−0.01, 0.006) | −0.01 (−0.02, −0.01)* | −0.01 (−0.02, −0.01)† | −0.02 (−0.03, −0.004)* |
| Income < $20,000 | 0.28 (0.02, 0.53)* | 0.08 (−0.08, 0.24) | −0.05 (−0.22, 0.13) | 0.15 (−0.05, 0.36) |
| Education HS or Less | −0.02 (−0.20, 0.17) | 0.08 (−0.08, 0.23) | −0.09 (−0.20, 0.01) | −0.22 (−0.38, −0.06)* |
| Single/Sep/Div/Wid | −0.09 (−0.28, 0.09) | −0.08 (−0.25, 0.10) | −0.05 (−0.16, 0.06) | −0.05 (−0.22, 0.11) |
| Smoking History | −0.03 (−0.19, 0.13) | 0.11 (−0.04, 0.25) | −0.10 (−0.19, −0.01)* | −0.03 (−0.21, 0.14) |
| Diabetes | −0.03 (−0.22, 0.16) | −0.10 (−0.25, 0.05) | −0.01 (−0.11, 0.09) | 0.03 (−0.14, 0.19) |
| Hypertension | −0.05 (−0.24, 0.14) | −0.49 (−0.82, −0.16)* | −0.02 (−0.12, 0.07) | 0.05 (−0.29, 0.40) |
| Previous Ml | −0.04 (−0.23, 0.15) | 0.07 (−0.07, 0.21) | −0.09 (−0.18, 0.003) | −0.12 (−0.29, 0.05) |
| BMI | 0.001 (−0.01, 0.02) | 0.01 (−0.004, 0.02) | 0.0005 (−0.01, 0.01) | −0.008 (−0.02, 0.01) |
| Model 4 | ||||
| Discrimination | −0.21 (−0.39, −0.02)* | −0.20 (−0.35, −0.04)* | −0.08 (−0.21, 0.05) | −0.11 (−0.25, 0.04) |
| Age | −0.002 (−0.01, 0.01) | −0.01 (−0.02, −0.01)* | −0.01 (−0.02, −0.01)† | −0.01 (−0.03, −0.003)* |
| Income < $20,000 | 0.27 (0.04, 0.50)* | 0.08 (−0.08, 0.24) | −0.05 (−0.22, 0.13) | 0.15 (−0.05, 0.36) |
| Education HS or Less | −0.06 (−0.23, 0.12) | 0.08 (−0.08, 0.24) | −0.09 (−0.20, 0.01) | −0.23 (−0.39, −0.06)* |
| Single/Sep/Div/Wid | −0.09 (−0.26, 0.08) | −0.08 (−0.25, 0.10) | −0.05 (−0.16, 0.06) | −0.03 (−0.20, 0.14) |
| Smoking History | 0.004 (−0.14, 0.15) | 0.11 (−0.04, 0.25) | −0.10 (−0.19, −0.01) | −0.02 (−0.19, 0.15) |
| Diabetes | 0.02 (−0.15, 0.19) | −0.10 (−0.26, 0.06) | −0.01 (−0.11, 0.09) | 0.02 (−0.14, 0.19) |
| Hypertension | −0.06 (−0.23, 0.10) | −0.49 (−0.83, −0.15)* | −0.02 (−0.12, 0.07) | 0.03 (−0.32, 0.38) |
| Previous Ml | −0.01 (−0.18, 0.16) | 0.07 (−0.07, 0.21) | −0.09 (−0.18, 0.003) | −0.11 (−0.28, 0.05) |
| BMI | 0.003 (−0.01, 0.02) | 0.01 (−0.01, 0.02) | 0.001 (−0.01, 0.01) | −0.01 (−0.03, 0.01) |
| BDI | 0.02 (0.01, 0.03)* | −0.0004 (−0.01, 0.01) | −0.001 (−0.01, 0.01) | −0.003 (−0.01, 0.01) |
| PSS | −0.01 (−0.02, −0.003)* | 0.0003 (−0.01, 0.01) | 0.001 (−0.01, 0.01) | 0.01 (−0.01, 0.02) |
Abbreviations: BDI: Beck depression inventory; BMI: Body mass index; CI: Confidence interval; MIPS: Mental stress ischemia mechanisms and prognosis study; PSS: Cohen’s perceived stress scale.
p < 0.05
p < 0.0001
4. DISCUSSION
Although we hypothesized that associations would be most pronounced for African American women, we found that reports of everyday discrimination were significantly associated with fewer LTL among both African American and White women, but not among men in stratified models after adjusting for basic demographic factors. Results were similar after further adjusting for lifestyle/disease factors and psychosocial factors. Similar to prior studies of healthy populations (Brown et al., 2016; Fitzpatrick et al., 2011; Hunt et al., 2008), in our sample of CAD patients, African American women had longer mean LTL compared to African American men, White women and White men. Nonetheless, the association between discrimination and LTL was significant for African American and White women, and remained statistically significant after adjusting for lifestyle and disease risk factors as well as possible alternative psychosocial and mediating pathways, including depression and stress. Across stratified regression models, there were no significant associations between discrimination and LTL for either White or African American men.
Our findings suggest that there may be stronger associations between everyday discrimination and telomere length for women than for men with CAD, irrespective of racial/ethnic background. Although these findings are preliminary and should be considered exploratory given the limited number of women in our sample, it is possible that there are sex-specific differences in the mechanisms through which psychosocial stressors, such as discrimination, affect physiological processes. Research suggests that women are more vulnerable to psychosocial stress, having greater activation of stress processes (Bangasser and Valentino, 2012; Hallman et al., 2001) and thereby biological effects that may be detrimental to cardiovascular health, especially women with CAD (Vaccarino and Bremner, 2017). There are also known sex differences in psychological responses to stress. For example, research suggests that irrespective of race, women ruminate about stressors more than men (Nolen-Hoeksema, 2012; Shull et al., 2016) and this may be particularly true for stressors that are interpersonal in nature, such as discrimination. Rumination, in turn, has been known to impact physiological pathways that ultimately influence disease (Gerin et al., 2012; Gianferante et al., 2014; Johnson et al., 2012). Additional research on the physiological and psychological pathways linking discrimination to telomere length is needed.
The fact that our results were statistically significant in African American women but were not observed among African American men in stratified models is consistent with prior research. Across indicators, studies have observed more pronounced discrimination and health associations among African American women compared to African American men for a range of outcomes including CRP (Cunningham et al., 2012), kidney function (Beydoun et al., 2017), psychological distress (Banks et al., 2006), hypertension (Roberts et al., 2008), and health-related quality-of-life (Coley et al., 2017). Some studies suggest that this may be due to African American women having different experiences of, reactions to, and/or coping differently with experiences of discrimination compared to African American men (Coley et al., 2017; Cunningham et al., 2012). African American women also experience combined effects of discrimination as they occupy more than one social disadvantage status on the basis of race and on the basis of sex (intersectionalities) (Lewis et al., 2015; Lewis and Van Dyke, 2018). At least one other study has found that multiple types of discrimination combine, or intersect, to affect health among individuals with multiple subordinate social identities (Grollman, 2014). Our results are also similar to findings by Chae et al. (2016; 2014), who did not find a significant main effect between racial discrimination and LTL among African American men.
In our study, there are several limitations worth noting. First, we only measured discrimination at the individual-level. Institutional-level discrimination might also adversely affect the health of women and African Americans by creating disadvantage through systemic policies and practices, services, opportunities, and social norms that restrict equal opportunities in employment and education (socioeconomic), residential segregation and selection, and also in health care access and treatment (Williams, 1999). By limiting our investigation to an individual-level measure of discrimination alone, we may have underestimated the association between discrimination and telomere length, particularly among African American men and women. This is a topic worthy of exploration in future research. Second, although MIPS is a prospective cohort study, telomere length was only collected during the baseline examination, limiting a longitudinal examination of associations between discrimination and telomere length. Since our data are cross-sectional, we are constrained from assumptions of temporality and causality. Also, we had a relatively small sample size of women across race/sex subgroups which may have limited the power for some analyses and statistical power to detect significant interactions, especially the 3-way interaction between discrimination, race, and sex. However, we were able to show some significant associations between discrimination and leukocyte telomere length in our race and sex stratified analyses despite our small sample of women. We believe this offers support for studying and presenting these results although they should be interpreted with some caution given our small sample of women. Ideally these preliminary findings will provide a foundation for other studies to investigate not only racial differences in the association between discrimination and leukocyte telomere length, but also examine differences by sex. Finally, since we studied a sample of patients with CAD, whether these associations can be generalizable to healthy populations is unknown, but serves as rationale for further exploration.
Nonetheless, there are several strengths of our study. To our knowledge, no previous study has investigated the association between experiences of discrimination and LTL in CAD patient samples across sex and racial/ethnic groups. Previous studies on discrimination and LTL have limited their investigations by race/ethnicity and focusing on racial discrimination, leaving an important gap in this sphere of research on whether the effect of discrimination on LTL differs for women and men, and unfair treatment related to characteristics other than race. Importantly, by stratifying our analyses by race/ethnicity and sex, and including an inclusive examination of discrimination, we bring to light associations among women that have been previously obscured. This study furthers the growing literature linking discrimination with adverse physiological associations, particularly among women.
4.1. Conclusion
In a cohort of African American and White CAD patients, experiences of discrimination were associated with shorter LTL among women and not in men. Future studies with with larger sample sizes and prospective designs are warranted to determine whether discrimination-linked telomere shortening is a potential mechanism of sex-specific disparities in cardiovascular outcomes. Future studies should explore these associations and broaden the investigation to other race/ethnic groups. These findings, albeit preliminary, raise important questions and bring attention to possible sex-specific differences in stress physiology that require further exploration, particularly given the relatively poor long-term outcomes for young women with CAD.
Supplementary Material
HIGHLIGHTS.
Discrimination is an understudied source of stress-related cellular aging.
Investigations across racial/ethnic and sex groups is currently lacking.
Discrimination was associated with shorter LTL among women and not in men.
Women may be more vulnerable to psychosocial stressors such as discrimination.
Sex-specific differences in stress physiology deserves further exploration.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health (P01HL101398, P20HL113451–01, P01HL086773–06A1, R56HL126558–01, R01HL109413, R01HL109413–02S1, R01HL125246, R01HL088726, UL1TR000454, KL2TR000455, K24HL077506, K24 MH076955, K23HL127251, T32HL130025A, and K12HD085850).
ROLE OF THE FUNDING SOURCE
The authors of this article are solely responsible for the content of this paper. The funding agency had no role in the design and conduct of this study, in the collection, analysis, interpretation of the data, or in the preparation, review or approval of this manuscript.
Abbreviations and Acronyms:
- BDI
Beck Depression Inventory
- BMI
Body Mass Index
- CAD
Coronary Artery Disease
- CRP
C-reactive protein
- EDS
Everyday Discrimination Scale
- IL-6
Interleukin-6
- LTL
Leukocyte Telomere Length
- MIPS
Mental Stress Ischemia Mechanisms and Prognosis Study
- PSS
Cohen’s Perceived Stress Scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adler N, Pantell MS, O’Donovan A, Blackburn E, Cawthon R, Koster A, Opresko P, Newman A, Harris TB, Epel E, 2013. Educational attainment and late life telomere length in the Health, Aging and Body Composition Study. Brain Behav Immun 27:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MA, Williams DR, 2011. Invited commentary: Discrimination--an emerging target for reducing risk of cardiovascular disease? Am J Epidemiol 173:1240–3. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Valentino RJ, 2012. Sex differences in molecular and cellular substrates of stress. Cell Mol Neurobiol 32:709–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks KH, Kohn-Wood LP, Spencer M, 2006. An Examination of the African American Experience of Everyday Discrimination and Symptoms of Psychological Distress. Community Mental Health Journal 42:555–70. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK, 1996. BDI-II. Beck Depression Inventory, Second Edition ed. The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Beydoun MA, Poggi-Burke A, Zonderman AB, Rostant OS, Evans MK, Crews DC, 2017. Perceived Discrimination and Longitudinal Change in Kidney Function Among Urban Adults. Psychosom Med 79:824–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn EH, 2000. Telomere states and cell fates. Nature 408:53–6. [DOI] [PubMed] [Google Scholar]
- Blackburn EH, 2001. Switching and signaling at the telomere. Cell 106:661–73. [DOI] [PubMed] [Google Scholar]
- Blackburn EH, Epel ES, Lin J, 2015. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 350:1193–8. [DOI] [PubMed] [Google Scholar]
- Brown L, Needham B, Ailshire J, 2016. Telomere Length Among Older U.S. Adults. J Aging Health:898264316661390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JE, Diez Roux AV, Fitzpatrick AL, Seeman T, 2013. Low social support is associated with shorter leukocyte telomere length in late life: multi-ethnic study of atherosclerosis. Psychosom Med 75:171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM, 2009. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res 37:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae DH, Epel ES, Nuru-Jeter AM, Lincoln KD, Taylor RJ, Lin J, Blackburn EH, Thomas SB, 2016. Discrimination, mental health, and leukocyte telomere length among African American men. Psychoneuroendocrinology 63:10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae DH, Nuru-Jeter AM, Adler NE, Brody GH, Lin J, Blackburn EH, Epel ES, 2014. Discrimination, racial bias, and telomere length in African-American men. Am J Prev Med 46:103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R, 1983. A global measure of perceived stress. Journal of health and social behavior 24:385–96. [PubMed] [Google Scholar]
- Coley SL, Mendes de Leon CF, Ward EC, Barnes LL, Skarupski KA, Jacobs EA, 2017. Perceived discrimination and health-related quality-of-life: gender differences among older African Americans. Qual Life Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham TJ, Seeman TE, Kawachi I, Gortmaker SL, Jacobs DR, Kiefe CI, Berkman LF, 2012. Racial/ethnic and gender differences in the association between self-reported experiences of racial/ethnic discrimination and inflammation in the CARDIA cohort of 4 US communities. Soc Sci Med 75:922–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM, 2004. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A 101:17312–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kronmal RA, Kimura M, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Hardikar S, Aviv A, 2011. Leukocyte telomere length and mortality in the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci 66:421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebreab SY, Riestra P, Gaye A, Khan RJ, Xu R, Davis AR, Quarells RC, Davis SK, Gibbons GH, 2016. Perceived neighborhood problems are associated with shorter telomere length in African American women. Psychoneuroendocrinology 69:90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerin W, Zawadzki MJ, Brosschot JF, Thayer JF, Christenfeld NJ, Campbell TS, Smyth JM, 2012. Rumination as a mediator of chronic stress effects on hypertension: a causal model. Int J Hypertens 2012:453465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Hicken MT, Pearson JA, Seashols SJ, Brown KL, Cruz TD, 2010. Do US Black Women Experience Stress-Related Accelerated Biological Aging?: A Novel Theory and First Population-Based Test of Black-White Differences in Telomere Length. Hum Nat 21:19–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianferante D, Thoma MV, Hanlin L, Chen X, Breines JG, Zoccola PM, Rohleder N, 2014. Post-stress rumination predicts HPA axis responses to repeated acute stress. Psychoneuroendocrinology 49:244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grollman EA, 2014. Multiple disadvantaged statuses and health: the role of multiple forms of discrimination. Journal of health and social behavior 55:3–19. [DOI] [PubMed] [Google Scholar]
- Hallman T, Burell G, Setterlind S, Oden A, Lisspers J, 2001. Psychosocial risk factors for coronary heart disease, their importance compared with other risk factors and gender differences in sensitivity. J Cardiovasc Risk 8:39–49. [DOI] [PubMed] [Google Scholar]
- Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Abdelhadi N, Alkhoder A, Obideen M, Pimple PM, Levantsevych O, Kelli HM, Shah A, Sun YV, Pearce B, Kutner M, Long Q, Ward L, Ko YA, Hosny Mohammed K, Lin J, Zhao J, Bremner JD, Kim J, Waller EK, Raggi P, Sheps D, Quyyumi AA, Vaccarino V, 2017a. Telomere Shortening, Regenerative Capacity, and Cardiovascular Outcomes. Circ Res 120:1130–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Shah AJ, Sun Y, Pearce B, Garcia EV, Kutner M, Bremner JD, Esteves F, Raggi P, Sheps DS, Vaccarino V, Quyyumi AA, 2017b. The Mental Stress Ischemia Prognosis Study: Objectives, Study Design, and Prevalence of Inducible Ischemia. Psychosom Med 79:311–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssen LM, Schutte NS, Malouff JM, Epel ES, 2017. The Relationship Between Childhood Psychosocial Stressor Level and Telomere Length: A Meta-Analysis. Health Psychol Res 5:6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt SC, Chen W, Gardner JP, Kimura M, Srinivasan SR, Eckfeldt JH, Berenson GS, Aviv A, 2008. Leukocyte telomeres are longer in African Americans than in whites: the National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging cell 7:451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JA, Lavoie KL, Bacon SL, Carlson LE, Campbell TS, 2012. The effect of trait rumination on adaptation to repeated stress. Psychosom Med 74:258–62. [DOI] [PubMed] [Google Scholar]
- Lewis TT, Cogburn CD, Williams DR, 2015. Self-reported experiences of discrimination and health: scientific advances, ongoing controversies, and emerging issues. Annu Rev Clin Psychol 11:407–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TT, Van Dyke ME, 2018. Discrimination and the Health of African Americans: The Potential Importance of Intersectionalities. Current Directions in Psychological Science:0963721418770442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Epel E, Cheon J, Kroenke C, Sinclair E, Bigos M, Wolkowitz O, Mellon S, Blackburn E, 2010. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. J Immunol Methods 352:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PY, Huang YC, Hung CF, 2016. Shortened telomere length in patients with depression: A meta-analytic study. J Psychiatr Res 76:84–93. [DOI] [PubMed] [Google Scholar]
- Liu SY, Kawachi I, 2017. Discrimination and Telomere Length Among Older Adults in the United States. Public Health Rep:33354916689613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur MB, Epel E, Kind S, Desai M, Parks CG, Sandler DP, Khazeni N, 2016. Perceived stress and telomere length: A systematic review, meta-analysis, and methodologic considerations for advancing the field. Brain Behav Immun 54:158–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham BL, Adler N, Gregorich S, Rehkopf D, Lin J, Blackburn EH, Epel ES, 2013. Socioeconomic status, health behavior, and leukocyte telomere length in the National Health and Nutrition Examination Survey, 1999–2002. Soc Sci Med 85:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, 2012. Emotion regulation and psychopathology: the role of gender. Annu Rev Clin Psychol 8:161–87. [DOI] [PubMed] [Google Scholar]
- O’Donovan A, Tomiyama AJ, Lin J, Puterman E, Adler NE, Kemeny M, Wolkowitz OM, Blackburn EH, Epel ES, 2012. Stress appraisals and cellular aging: a key role for anticipatory threat in the relationship between psychological stress and telomere length. Brain Behav Immun 26:573–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper GV, Bateson M, Nettle D, 2018. Telomeres as integrative markers of exposure to stress and adversity: a systematic review and meta-analysis. R Soc Open Sci 5:180744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CB, Vines AI, Kaufman JS, James SA, 2008. Cross-sectional association between perceived discrimination and hypertension in African-American men and women: the Pitt County Study. Am J Epidemiol 167:624–32. [DOI] [PubMed] [Google Scholar]
- Sanders JL, Newman AB, 2013. Telomere length in epidemiology: a biomarker of aging, age-related disease, both, or neither? Epidemiol Rev 35:112–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman KA, Berlin JA, Harless W, Kerner JF, Sistrunk S, Gersh BJ, Dube R, Taleghani CK, Burke JE, Williams S, Eisenberg JM, Escarce JJ, 1999. The effect of race and sex on physicians’ recommendations for cardiac catheterization. N Engl J Med 340:618–26. [DOI] [PubMed] [Google Scholar]
- Schutte NS, Malouff JM, 2016. The Relationship Between Perceived Stress and Telomere Length: A Meta-analysis. Stress Health 32:313–19. [DOI] [PubMed] [Google Scholar]
- Shaw LJ, Shaw RE, Merz CN, Brindis RG, Klein LW, Nallamothu B, Douglas PS, Krone RJ, McKay CR, Block PC, Hewitt K, Weintraub WS, Peterson ED, American College of Cardiology-National Cardiovascular Data Registry, I., 2008. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology-National Cardiovascular Data Registry. Circulation 117:1787–801. [DOI] [PubMed] [Google Scholar]
- Shull A, Mayer SE, McGinnis E, Geiss E, Vargas I, Lopez-Duran NL, 2016. Trait and state rumination interact to prolong cortisol activation to psychosocial stress in females. Psychoneuroendocrinology 74:324–32. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Salovey P, Epel ES, 2002. Sex differences in stress responses: social rejection versus achievement stress. Biol Psychiatry 52:318–27. [DOI] [PubMed] [Google Scholar]
- Theall KP, Brett ZH, Shirtcliff EA, Dunn EC, Drury SS, 2013. Neighborhood disorder and telomeres: connecting children’s exposure to community level stress and cellular response. Soc Sci Med 85:50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Bremner JD, 2017. Behavioral, emotional and neurobiological determinants of coronary heart disease risk in women. Neurosci Biobehav Rev 74:297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, 1999. Race, socioeconomic status, and health. The added effects of racism and discrimination. Ann N Y Acad Sci 896:173–88. [DOI] [PubMed] [Google Scholar]
- Williams DR, Yan Y, Jackson JS, Anderson NB, 1997. Racial Differences in Physical and Mental Health: Socio-economic Status, Stress and Discrimination. J Health Psychol 2:335–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.