Abstract
Background
Hyperglycemia or diabetes is a well-known side effect of treatment with glucocorticoids. In patients with brain tumors, glucocorticoids are widely used to treat symptoms of peritumoral edema. We conducted a retrospective study of patients with suspected brain tumor to determine the incidence of and risk factors for glucocorticoid-induced diabetes.
Methods
This was a retrospective study of patients referred with suspected brain tumor to a neurological department, using data from a clinical database, electronic medical records, the laboratory system, and the pathology information bank. . Nondiabetic patients with a neuroimaging-verified brain tumor treated with high-dose glucocorticoid and monitored with glucose measurements were included in the study.
Results
Among 809 patients referred with suspected brain tumor, 171 were eligible for the study. Thirty-eight (22%) patients developed glucocorticoid-induced diabetes, defined as 2 glucose measurements ≥200 mg/dl (11.1 mmol/l) within the first week of treatment, and 4 of the patients were treated with insulin. The majority of patients with glucocorticoid-induced diabetes were identified on days 2, 3, and 4, and glucose levels were highest in the afternoon and evening. We were not able to identify any risk factors for glucocorticoid-induced diabetes and glucocorticoid-induced diabetes had no influence on survival in our cohort.
Conclusions
Glucocorticoid-induced diabetes is frequent in the first 7 days of treatment in patients with brain tumors. The results emphasize the need for screening for glucocorticoid-induced diabetes in this group of patients to avoid comorbidity expected to arise from hyperglycemia.
Keywords: brain tumor, diabetes, glucocorticoid, glucocorticoid-induced diabetes
In patients with brain tumors, glucocorticoid therapy is a mainstay in the treatment of peritumoral edema. The beneficial effect is well described and has been so since the 1960s.1 However, treatment with glucocorticoids is associated with multiple side effects, including hyperglycemia and glucocorticoid-induced diabetes.2
The incidence of glucocorticoid-induced hyperglycemia or diabetes in brain cancer patients has been described,2–5 but these studies are hampered by scarce glucose measurements separated by days and weeks or unknown glucose screening density, resulting in potential underestimation.
Hyperglycemia in patients with primary brain tumors has been associated with decreased survival, higher recurrence rate, and malignant degeneration (ie, transformation from a low-grade to a high-grade tumor).3,4 The diagnosis of diabetes in these patients is therefore important, both for prognostic information and in order to initiate treatment that may reduce the risk of catabolism and metabolic derangement, which might ultimately lead to hyperglycemic hyperosmolar syndrome that carries a high risk of mortality. Insulin is recognized as a safe and efficient treatment of glucocorticoid-induced diabetes.
In Denmark the national guidelines for patients with newly diagnosed brain tumor and peritumoral edema recommend 75 to 100 mg of prednisolone or an equivalent dose of another glucocorticoid (approximately 12 sto 16 mg of dexamethasone). However, there are no generally accepted recommendations for detection of hyperglycemia and diabetes during treatment.
In this study of patients with newly diagnosed primary or secondary brain tumors treated with high-dose prednisolone and routinely screened with glucose measurements, we determined the incidence of glucocorticoid-induced diabetes within the first week of initiating treatment. Possible risk factors for glucocorticoid-induced diabetes, as well as the time course of plasma glucose levels during the day, were analyzed and we explored whether hyperglycemia in the first week of glucocorticoid treatment influenced survival.
Materials and Methods
Study Design
We assessed the incidence and risk factors of glucocorticoid-induced diabetes in patients with brain tumors receiving high-dose prednisolone based on data from a local brain cancer database supplemented with data from electronic medical records.
Setting and Subjects
In Denmark an Integrated Brain Cancer Pathway (IBCP) exists for patients with suspected brain cancer. This standardized fast-track pathway involving primary care physicians, neurology departments, neurosurgeons, radiologists, and oncologists is part of an attempt to improve survival in brain cancer patients by speeding up the diagnostic process and initiating treatment as quickly as possible. When fulfilling a minimum of 1 out of 5 inclusion criteria, the patient can be referred to the pathway programme.6
All patients referred according to the IBCP at the Department of Neurology at Nordsjaellands Hospital Hilleroed were prospectively registered in an electronic database. A structured form that included symptoms, glucocorticoid treatment, and performance status (PS) according to the Eastern Cooperative Oncology Group7 was completed during the first admission and subsequently the information was supplemented with data on pathological diagnosis and time of death.
The standard dose of glucocorticoid for patients with brain tumors and symptoms of raised intracranial pressure was 75 to 100 mg of prednisolone. If the patients were unable to take oral prednisolone an equivalent dose of methylprednisolone was administered intravenously. In the IBCP ward it was ward protocol to perform 4 glucose measurements per day for at least 3 days after initiating glucocorticoid treatment as screening for glucocorticoid-induced diabetes. Some had glucose measurements for 7 days and some for 3 days. No patients were treated with intravenous glucose during admission.
Only glucose measurements from our local hospital and neurology department were available for the study. Until approximately 2012 glucose values were noted on a paper record that was subsequently scanned into the medical record. After 2012 a new point-of-care system was introduced and all glucose measurements from fingerprick point-of-care testing are now electronically transferred to the laboratory system. All glucose measurements were available to us.
Within the first week most patients were either transferred to the neurosurgeons or discharged while waiting for surgery. In order to obtain as many glucose values as possible we expanded the period of interest from day 1, first day with glucocorticoid treatment, and 7 days ahead.
From April 2009 to April 2016, 809 patients were enrolled in the IBCP. Patients were eligible for the present study if they fulfilled the inclusion criteria: a space-occupying process using magnetic resonance imaging, treatment with prednisolone ≥75 mg (or an equivalent dose of glucocorticoid) per day, and a minimum of 3 glucose measurements in the first 7 days after initiation of prednisolone. Patients with known diabetes were excluded and the definition of known diabetes was based on information from the patient. If the patients reported on admission that they had diabetes or received antidiabetic medication, they were excluded.
Data Collection
Data on glucocorticoid treatment were collected from electronic medical records and glucose measurements were extracted from the laboratory system. Two types of glucose measurements were made. Venous blood samples were collected on admission and subsequently bedside, fingerprick, capillary point-of-care testing was performed at the IBCP ward (Accu-Chek Inform II, Roche). Both methods are subject to quality assurance and report results as plasma glucose. Data on known diabetes were collected from electronic medical records.
Data on potential risk factors for developing diabetes, such as age, weight, PS, and cancer diagnoses, were selected from the IBCP database, medical records, and the pathology information bank (Patobank). A total of 170 patients out of 171 had a C-reactive protein (CRP) measurement at baseline. The patients were tested by a physiotherapist and an occupational therapist within the first 24 hours after admission and PS was assessed based on their observations.
Trial Registration
The local IBCP database was approved by the Danish Data Protection Agency (I-Suite no. 03811 and local no. NOH-2015-022) and this study by the Danish Health Authority (file number 3-3013-1128/1).
Data Analysis
The primary endpoint was the number of participants developing glucocorticoid-induced diabetes. The World Health Organization (WHO) diagnostic criteria for diabetes are fasting plasma glucose ≥126 mg/dl (7.0 mmol/l), 2-hour plasma glucose (oral glucose tolerance test) ≥200 mg/dl (11.1 mmol/l), or HbA1c > 6.5% (48 mmol/mol).8,9 Fasting glucose measurements, an oral glucose tolerance test, and HbA1c measurements were not available in our retrospective cohort, so we defined glucocorticoid-induced diabetes as 2 random independent glucose measurements ≥200 mg/dl (11.1 mmol/l), which we find is a rational modification of the WHO guidelines.8
Standard statistical tests were used to compare demographic- and diabetes-related parameters. To identify risk factors for developing diabetes, univariate and multivariate logistic regression analyses were done using age, sex, cancer diagnosis, CRP, daily dose of prednisolone, and PS as explanatory variables. Age, CRP, daily dose of prednisolone, and PS were included as continuous covariates in the models. Data on predisposition to diabetes were lacking in many cases and were therefore not included in the analysis. Plasma glucose values were compared over time using linear regression with a natural cubic spline parameterization of the mean.
We analyzed the survival outcome in an illness-death model without recovery. A semi-parametric Markov model was estimated in the form of a stratified Cox proportional hazard model where we allowed for the covariates to have different effects on each transition hazard.10 Equality of the 2 mortality hazards was assessed with a likelihood ratio test under a proportionality assumption.
Data were analyzed using SPSS (version 19.0 and 22.0) and R (version 3.2.2). A two-sided P value below .05 was considered statistically significant.
Results
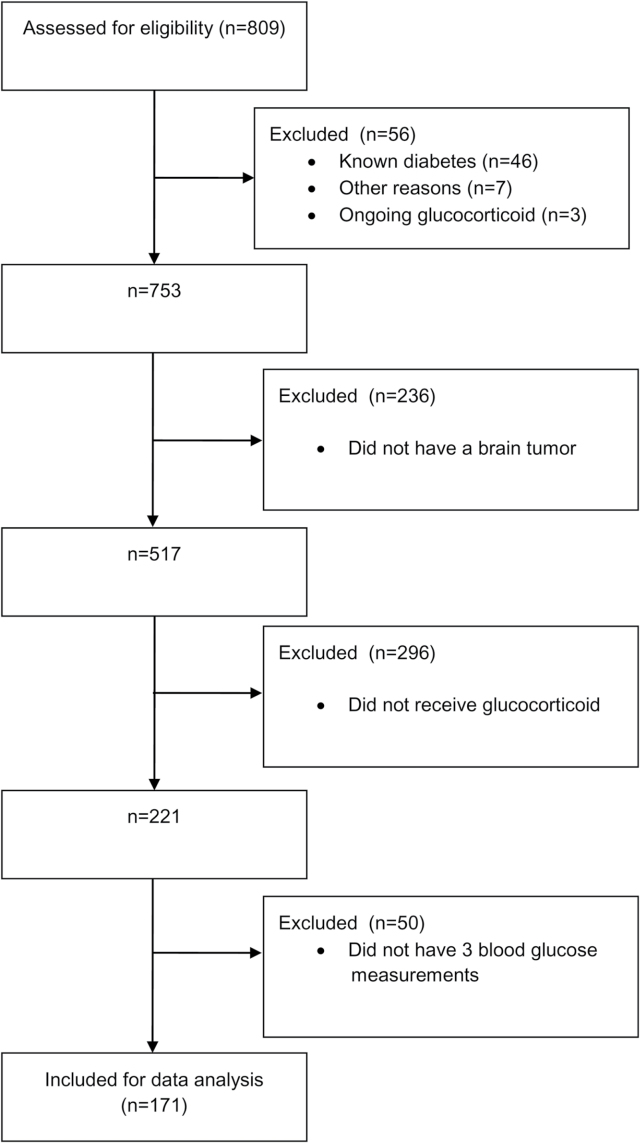
Out of 809 patients referred to the IBCP, 171 were eligible for the study. Patients with preexisting diabetes were excluded and the main reasons for not being eligible were no tumor detected, no glucocorticoid treatment, and/or less than 3 glucose measurements (Fig. 1).
Fig. 1.
Flow diagram of inclusion of patients.
Sixty-eight percent (n = 117) of the patients had primary brain cancer (both low-grade and high-grade tumors), 23% (n = 39) had secondary brain cancer from different primary cancer types, and 9% (n = 15) had an unclassified brain tumor (Table 1). The majority of patients (n = 144) had a fixed dose of glucocorticoid in the 7-day study period. The rest had minor changes but no rapid reductions in dose. In the first 7 days from prescription of glucocorticoid, a median of 10 (IQR 7–14) glucose measurements were made. The frequency of glucose measurements were highest at day 2, 3, and 4 and most often performed in the morning before 11:00 and in the afternoon and evening after 17:00.
Table 1.
Baseline characteristics of the patients included in the study
| All | No diabetes | Glucocorticoid induced diabetes | |
|---|---|---|---|
| Incidence (95% CI) | N = 171 100% |
n = 133 78% (72%–85%) |
n = 38, 4 patients treated with insulin 22% (16%–28%) |
| Age, years, mean (min-max) | 64 (23–101) | 63 (23–101) | 66 (30–92) |
| Sex (% female) | 50 | 51 | 45 |
| Type of cancer | |||
| • Primary brain tumor | 117 (68%) | 93 (70%) | 24 (63%) |
| • Secondary brain tumor (metastasis) | 39 (23%) | 30 (23%) | 9 (24%) |
| • Unclassified | 15 (9%) | 10 (7%) | 5 (13%) |
| Performance status | |||
| • 0 | 62 (36%) | 49 (37%) | 13 (34%) |
| • 1 | 62 (36%) | 50 (38%) | 12 (32%) |
| • 2 | 27 (16%) | 20 (15%) | 7 (18%) |
| • 3 | 11 (7%) | 7 (5%) | 4 (10%) |
| • 4 | 4 (2%) | 3 (2%) | 1 (3%) |
| • Unclassified | 5 (3%) | 4 (3%) | 1 (3%) |
| Smoking | |||
| • Never smoker | 80 (47%) | 58 (44%) | 22 (58%) |
| • Smoking now or previously | 87 (51%) | 71 (53%) | 16 (42%) |
| • Missing | 4 (2%) | 4 (3%) | 0 (0%) |
| Antihypertensive therapy | 47 (28%) | 33 (25%) | 14 (37%) |
| Lipid-lowering therapy | 31 (18%) | 19 (14%) | 12 (32%) |
| Symptomatic atherosclerosis | 14 (8%) | 10 (8%) | 4 (11%) |
| Liver disease | 1 (1%) | 1 (1%) | 0 |
| Pancreatic disease | 0 (0%) | 0 | 0 |
| Number of glucose measurements in the 7 days observation period, median (IQR) | 10 (7–14) | 9 (6–12) | 15 (11–18) |
| Cumulative prednisolone dose, mg, mean in the 7 days observation period (min-max) | 674 (150–2150) | 668 (150–2150) | 693 (475–1050) |
Overall, 38 (22%; 95% CI, 16%–28%) out of 171 patients developed glucocorticoid-induced diabetes with 2 glucose measurements ≥200 mg/dl (11.1 mmol/l), and 4 of the patients were treated with insulin. The majority of patients with glucocorticoid-induced diabetes were identified on days 2, 3, and 4.
In univariate and multivariate logistic regression analyses we were not able to identify any significant risk factors (age, sex, cancer diagnosis, CRP, daily dose of prednisolone or PS) for glucocorticoid-induced diabetes (data not shown).
All plasma glucose values were classified according to time-of-day measurement as: 07:00–09:00, 09:01–11:00, 11:01–13:00, 13:01–15:00, 15:01–17:00, 17:01–19:00, or 19:01–24:00. Comparing the average plasma glucose values across time of day for the 2 groups (no diabetes and glucocorticoid-induced diabetes), we found that the glucose levels were highest in the afternoon and evening. Mean plasma glucose from 07:00 to 09:00 seemed to be almost identical in the two groups and the difference was greatest in the afternoon and evening.
During the 7-year study period from 2009 to 2016, 125 (73%) of the 171 patients died. Patients with primary brain tumor (n = 78) lived for a mean of 14 months (range, 1–58), patients with secondary brain tumor (n = 36) for 9 months (range, 0–43) and patients with unclassified tumors for 5 months (range, 1–30). Mean follow-up time was 44 months (range, 2–87).
Development of glucocorticoid-induced diabetes had no significant influence on survival in our cohort even when adjusting for the covariates (P = .235). Both age and type of cancer significantly affected the transition hazards to death for patients with and without diabetes, but the effect of type of cancer disappeared when simultaneously adjusting for age. CRP showed a significant effect on the mortality hazard for patients with glucocorticoid-induced diabetes (P = .003), where hazard increased by 5.27% (95% CI, 1.80%–8.90%) for every unit increase in CRP.
Discussion
Screening and Incidence
Few studies have assessed the incidence of glucocorticoid-induced diabetes in brain cancer patients and the definition of hyperglycemia or diabetes varies, as well as the screening intensity. Some of the studies on hyperglycemia also include diabetic patients and therefore describe the well-known dysregulation in diabetic patients during glucocorticoid treatment rather than new onset glucocorticoid-induced hyperglycemia.
We excluded patients who reported to have diabetes and/or received antidiabetic medication on admission. In Denmark it is estimated that around 5% of the population between 20 and 79 years have undiagnosed diabetes. In our study it would correspond to 8 potential patients with undiagnosed diabetes out of 171 included in the study.
We found that 22% of the patients with no known diabetes developed glucocorticoid-induced diabetes defined as 2 glucose measurements ≥200 mg/dl (11.1 mmol/l) and 4 patients were treated with insulin.
Welch et al conducted a retrospective study on 988 glioblastoma multiforme patients and found 34 (3.4%) patients developed diabetes during glucocorticoid treatment.11 However, the definition of glucocorticoid-induced diabetes in this study is unclear.
One small prospective study with high-dose glucocorticoid treatment has previously been published.12 In that study of 35 patients with hematological diseases treated with 100 mg prednisolone per day, 40.6% had developed diabetes at 8 weeks, defined as a fasting plasma glucose ≥126 mg/dl (7 mmol/l). No patients were treated with antidiabetic medicine.
No study like the present describes the incidence within the first 7 days of glucocorticoid treatment with a screening intensity like ours.
We might have underestimated the incidence of glucocorticoid-induced diabetes because the screening instruction was not strictly followed. Following the instructions in the Department of Neurology would have given 4 glucose measurements a day for at least 3 days in every patient, but that standard was not achieved. A median number of 10 (IQR 7–14) glucose measurements were made in 7 days from start of glucocorticoid treatment. We can only speculate that the nurses and doctors might have discontinued glucose measuring 4 times a day if a patient presented strictly normal measurements for 1 to 2 days, and some patients may have been absent from the department for various reasons.
Risk Factors
We did not identify any risk factors for glucocorticoid-induced diabetes. This is in contrast to earlier studies in which mean daily dexamethasone dose, age, and preexisting diabetes was associated with hyperglycemia in brain cancer patients.3,4 In cancer patients, dose and regime of glucocorticoid, age, body mass index, and level of HbA1c have been identified as risk factors.12–14 The reasons for not finding any risk factors for developing diabetes in our population may be low sample size (type 2 statistical errors), and relatively low age and high performance status of the patients. The dose of prednisolone did not vary much and therefore dose of glucocorticoid would not be identified as a risk factor in our study. Finally, the very heterogeneous tumor diagnoses despite similar initial clinical presentation in the patients with a space-occupying intracranial lesion may explain the lack of significant associations.
Time Course and Blood Glucose Values During the Day
The time course of development of diabetes is important to uncover in order to focus blood glucose screening. No earlier studies with brain cancer patients have addressed the issue of the timing of screening for glucocorticoid-induced diabetes.
As expected from the pharmacokinetic profile of prednisolone (half-life 2–4 hours), when administered in the morning the blood glucose values were highest in the afternoon and evening and lowest in the morning before administration. However, glucose levels in the morning were overlapping in the 2 groups, illustrating that a normal morning glucose level is no guarantee for euglycemia during the rest of the day.
Survival
When patients with preexisting diabetes are part of survival analysis the results may not be applicable to survival in glucocorticoid-induced diabetes as it is two different types of diabetes15 and because patients with preexisting diabetes already have a shorter life expectancy from cardiovascular death than patients with glucocorticoid-induced diabetes and nondiabetic patients.16
In the present study, excluding patients with known diabetes, we found no significant influence of glucocorticoid-induced hyperglycemia during the first week of glucocorticoid treatment on survival.
In contrast, persistent outpatient hyperglycemia was associated with decreased survival in a retrospective study; patients with grade III and IV astrocytomas and persistent hyperglycemia had a median survival of 5 months compared to patients without hyperglycemia who had a median survival of 11 months. Hyperglycemia was secondary to preexisting diabetes in 7 patients (25%) and secondary to dexamethasone use in 21 patients (75%).5 Derr et al found that in patients with grade IV tumors (glioblastoma multiforme) with and without preexisting diabetes, the higher the mean glucose level, the shorter the survival period.4 Among patients with low-grade gliomas, those with persistent outpatient hyperglycemia had a 5-year survival rate of 43% versus 84% in the group without hyperglycemia, a result that remained statistically significant after controlling for various other factors known to influence survival.3
In our study, the fact that the population was very heterogeneous in tumor diagnoses—ranging from patients expected to live for less than a year (grade IV tumors) to some that will be considered cured after surgery (meningioma)—impairs the possibility to assess the influence of glucocorticoid-induced diabetes on survival.
CRP values were only obtained systematically for all patients on admission, but not later during the admission and the association between CRP at baseline and survival needs to be further studied in subsequent prospective studies.
Strengths and Limitations
This is to our knowledge the first study of the early incidence of glucocorticoid-induced diabetes in patients with an intracranial tumor. We had the advantage of using a database for patients in the IBCP, where data are systematically collected, giving valid data on tumor diagnoses, glucocorticoid initiation, and PS. Compared to earlier studies, the number of glucose measurements was reasonably high and distributed over the day.
As a consequence of the retrospective design, we missed information on possible confounders, such as familial predisposition for diabetes, body mass index, former diabetes (gestational or glucocorticoid-induced), and HbA1c values.
Implications
The high incidence of glucocorticoid-induced diabetes during the first 7 days of treatment warrants attention to strict screening with special focus on glucose measurements in the afternoon and evening since a low fasting blood glucose value is no guarantee for euglycemia during the rest of the day.
Funding
Helen Rudes Foundation founded the salary of Helga Schultz, which was the only cost of the study.
Conflict of interest statement. The authors declare no potential conflicts of interest.
Unpublished papers cited: H. Schultz, unpublished manuscript.
Acknowledgment
Physiotherapist Monique Mesot Liljehut is thanked for her effort evaluating performance status on all the patients.
References
- 1. Kaal EC, Vecht CJ. The management of brain edema in brain tumors. Curr Opin Oncol. 2004;16(6):593–600. [DOI] [PubMed] [Google Scholar]
- 2. Hempen C, Weiss E, Hess CF. Dexamethasone treatment in patients with brain metastases and primary brain tumors: do the benefits outweigh the side-effects?Support Care Cancer. 2002;10(4):322–328. [DOI] [PubMed] [Google Scholar]
- 3. Chaichana KL, McGirt MJ, Woodworth GF, et al. . Persistent outpatient hyperglycemia is independently associated with survival, recurrence and malignant degeneration following surgery for hemispheric low grade gliomas. Neurol Res. 2010;32(4):442–448. [DOI] [PubMed] [Google Scholar]
- 4. Derr RL, Ye X, Islas MU, et al. . Association between hyperglycemia and survival in patients with newly diagnosed glioblastoma. J Clin Oncol. 2009;27(7):1082–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McGirt MJ, Chaichana KL, Gathinji M, et al. . Persistent outpatient hyperglycemia is independently associated with decreased survival after primary resection of malignant brain astrocytomas. Neurosurgery. 2008;63(2):286–291; discussion 291. [DOI] [PubMed] [Google Scholar]
- 6. Laursen EL, Rasmussen BK. A brain cancer pathway in clinical practice. Dan Med J. 2012;59(5):A4437. [PubMed] [Google Scholar]
- 7. Oken MM, Creech RH, Tormey DC, et al. . Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655. [PubMed] [Google Scholar]
- 8. World Health Organization and International Diabetes Federation. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 9. World Health Organization. Use of Glycolated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus, 2011. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 10. Andersen PK, Keiding N. Multi-state models for event history analysis. Stat Methods Med Res. 2002;11(2):91–115. [DOI] [PubMed] [Google Scholar]
- 11. Welch MR, Grommes C. Retrospective analysis of the effects of steroid therapy and antidiabetic medication on survival in diabetic glioblastoma patients. CNS Oncol. 2013;2(3):237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gonzalez-Gonzalez JG, Mireles-Zavala LG, Rodriguez-Gutierrez R, et al. . Hyperglycemia related to high-dose glucocorticoid use in noncritically ill patients. Diabetol Metab Syndr. 2013;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schultz H, Engelholm SA, Harder E, Pedersen-Bjergaard U, Kristensen PL. Glucocorticoid-induced diabetes in patients with metastatic spinal cord compression: a prospective study of incidence, risk factors and time course. Submitted manuscript 2016.
- 14. Lee SY, Kurita N, Yokoyama Y, et al. . Glucocorticoid-induced diabetes mellitus in patients with lymphoma treated with CHOP chemotherapy. Support Care Cancer. 2014;22(5):1385–1390. [DOI] [PubMed] [Google Scholar]
- 15. Simmons LR, Molyneaux L, Yue DK, et al. . Steroid-induced diabetes: is it just unmasking of type 2 diabetes?ISRN Endocrinol. 2012;2012:910905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu L, Simon B, Shi J, et al. . Impact of diabetes mellitus on risk of cardiovascular disease and all-cause mortality: evidence on health outcomes and antidiabetic treatment in United States adults. World J Diabetes. 2016;7(18):449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]