Abstract
Background.
Impaired working memory appears to play a key role in some of the neurocognitive late effects of pediatric brain tumor treatments, including declines in intellectual and executive functioning. Recent studies of pediatric cancer survivors suggest Cogmed® Working Memory Training is effective at improving working memory, although pediatric brain tumor survivors may demonstrate a less robust response than children with other cancers. The current study sought to determine if an extended course of Cogmed (35 sessions) was both feasible and efficacious for brain tumor survivors and if improvements were observable in near-transfer and far-transfer working memory measures as well as parent rating scores at 6 months post-treatment.
Methods.
Twenty pediatric brain tumor survivors ages 8 to 18 years with working memory deficits completed 35 sessions of Cogmed. Assessments of working memory and academic skills were completed at baseline, completion of training, and 6-month follow-up and parents completed questionnaires at baseline and 6-month follow-up.
Results.
Participants showed significant improvements in working memory at training completion and 6-month follow-up and math achievement at 6-month follow-up. Parents reported executive functioning improvements at follow-up as compared with baseline. Participants’ program-based working memory skills did not change significantly between sessions 25 and 35, suggesting that extended training did not provide additional benefit.
Conclusions.
This study replicates and extends previous research by: (1) demonstrating that brain tumor survivors at high risk for neurocognitive late effects can complete and benefit from working memory training, (2) identifying a point of diminished returns on training time investment, and (3) demonstrating benefits 6 months post-intervention.
Keywords: cancer, Cogmed, cognitive remediation, intervention, pediatric brain tumor, working memory.
Pediatric brain tumors are the most common solid tumors in children.1,2 Although survival rates have increased substantially over the past few decades, there are often neurocognitive sequelae that affect survivors’ learning and development over their lifetimes. Common deficits include intellectual declines, failure to keep pace with developmental expectations, poor attention, difficulties with working memory, slowed processing speed, adaptive skill delays, and motor deficits.3 Children with poor attention and working memory problems have difficulty learning and may do poorly in school, which limits their post-secondary options. Adult survivors of pediatric brain tumors often present with poorer quality of life, particularly in the areas of health and social functioning in adulthood.4 Therapist-delivered interventions aimed at mitigating neurocognitive deficits have had significant barriers to implementation (eg, intensive time and travel requirements) and mixed effectiveness. Although support for pharmacotherapy (eg, stimulant medications) is building,5,6 some brain tumor survivors may have medical contraindications or their parents may be reluctant to use medication. The recent development of computer-delivered cognitive interventions offers promise for this population as it overcomes many of the limitations associated with previous interventions and a growing body of research supports its possible effectiveness.
Children diagnosed with brain tumors are often at higher risk than children with other forms of cancer because of the direct impact to the developing brain from the tumor as well as its treatment.7 Treatments for brain tumors typically involve a combination of surgery, chemotherapy, and/or radiation—all of which can affect the central nervous system (CNS) in negative ways. Conventional cranial radiation therapy appears to be especially deleterious for neurocognitive development.8 Survivors’ difficulties with the acquisition of new information appear to be particularly affected by issues with working memory in those who were treated with radiation compared with children who were treated for solid tumors without CNS-directed therapy (eg, radiation, intrathecal methotrexate, or high-dose methotrexate).9
Working memory is a neurocognitive function that allows individuals to hold information online (in their minds) for brief periods of time, typically a few seconds. It is essential for numerous daily tasks such as the ability to follow directions, remember information, multitask, and stay focused on the task at hand.10,11 It also allows for the processing of incoming information while simultaneously retrieving other information from memory stores. Furthermore, working memory is a measure of learning potential that is not thought to be significantly influenced by socioeconomic status or prior educational experiences.12
Unfortunately, there are few effective interventions that specifically target working memory processes in pediatric brain tumor survivors. Treatments for the neurocognitive sequelae largely have been limited to educational interventions (eg, Individual Education Plans and 504 Accommodation Plans), parent-directed intervention,13 pharmacological interventions, and home-based and clinic-based cognitive rehabilitation.14–16 However, these treatment modalities have drawbacks such as reluctance of parents of survivors to pursue special education or pharmacological intervention5 and poor compliance.16,17 A home-based intervention would be more accessible to a broader range of participants who would not be limited by distance, time, or costs involved with travel to and from the intervention site.
Computerized cognitive training programs, such as Cogmed® Working Memory Training†, are promising new treatment modalities that may overcome some of the limitations associated with existing options by allowing participation from home or school. Cogmed is a computer-based training program for working memory and attention that requires the storage and manipulation of sequences of verbal information (such as repeating numbers in reversed order) and visual-spatial information (such as recalling a sequence and location of objects that light up) offered in game-like tasks. Early Cogmed studies were conducted with children with Attention-Deficit/Hyperactivity Disorder, but more recent work has focused on adults and the nonimpaired as well as children with working memory deficits. There have been more than 80 peer-reviewed publications as of May 2016, including three describing the use of Cogmed for children treated for cancer.18 Computerized working memory interventions have recently shown good feasibility and efficacy in a limited number of trials with pediatric cancer survivors.19–21 However, results from these studies suggest that the magnitude of benefit may be diminished for pediatric brain tumor survivors when compared to other pediatric cancer populations (eg, acute lymphoblastic leukemia) when using a standard course (25 sessions) of Cogmed.19,22 Consequently, one focus of this study was to determine if a more intensive training intervention (35 sessions over a maximum of 12 weeks) would be more efficacious for pediatric brain tumor survivors than the standard dose of Cogmed treatment (25 sessions over the course of 5 to 8 weeks).
To that end, we undertook a prospective, self-controlled, pilot study to evaluate the effectiveness of computerized training in improving working memory functioning in 20 school-aged pediatric brain tumor survivors who were at least 1 year past completion of all treatments. Participants were evaluated at baseline, postintervention, and at the 6-month follow-up to examine the changes in working memory function, academics, and other behavioral assessments. Specific aims of this study were to: a) assess feasibility and efficacy of a home-based intervention to improve working memory in pediatric brain tumor survivors; b) evaluate whether a higher dose of treatment (10 additional sessions) resulted in improved working memory outcomes; c) demonstrate statistically significant improvements in participants’ performance on near-transfer (a different measure of working memory) and far-transfer (academic measures of mathematics and reading) measures of working memory over baseline testing; and d) demonstrate concomitant improvement on parent ratings of executive function, attention, and adaptive behavior over baseline.
Materials and Methods
Participants
Participants were recruited from the Pediatric Hematology/Oncology clinic at a Midwestern children’s hospital over an 18-month period by conducting a record review of brain tumor patients who had been treated with CNS-directed therapy (surgery, chemotherapy, and radiation), had been off therapy at least 1 year, and were between 8 and 18 years old. These individuals were targeted with the assumption that if Cogmed training benefited those at highest risk for neurocognitive late effects (due to multiple treatments), it likely also would benefit those who had undergone fewer types of treatments. Fifty-five percent of the sample were female and 95% of the sample identified as Caucasian (one person identified as Asian/Caucasian). The majority of participants were diagnosed with medulloblastoma (n = 11), followed by germinoma (n = 4), ependymoma (n=4), and other tumor types (n = 2). The average age at diagnosis was 6 years old (range, 1-14 years old) and the average time since treatment completion was 5 years (range, 1-12 years). Participants had an average score of 7 on the Neurological Predictor Scale [NPS] (range, 1-12), with higher scores denoting more risk factors for neurocognitive impairment associated with cancer treatment.27
Procedures
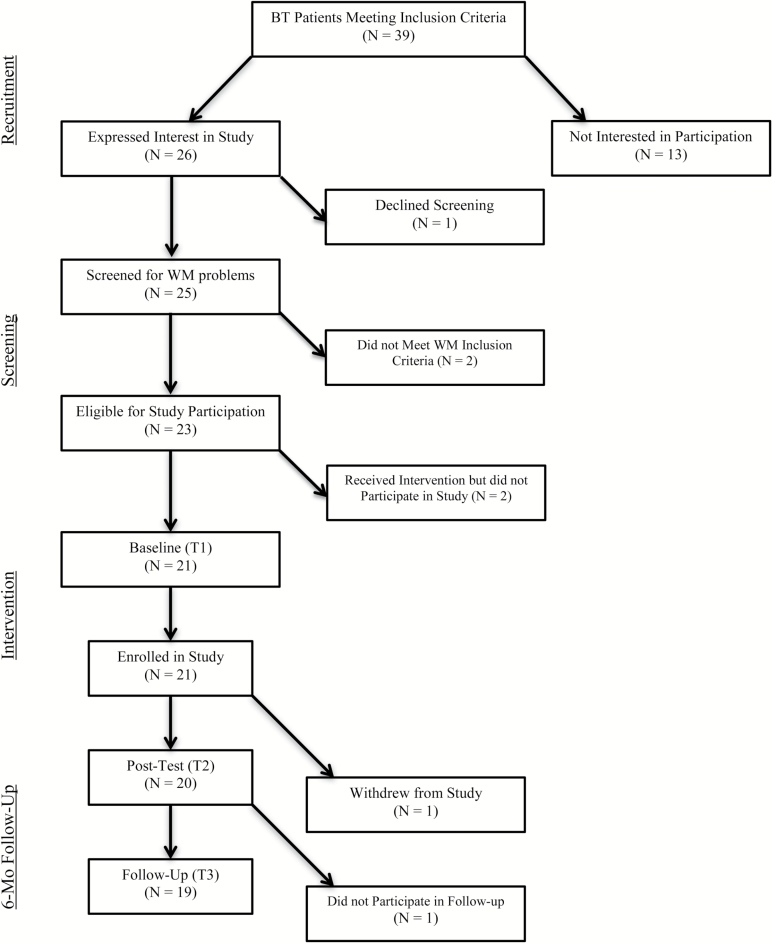
Thirty-nine individuals who had been treated for brain tumors at a large Midwestern children’s hospital who met the treatment and age criteria were sent a letter describing the study and invited to contact study personnel if they were interested or wanted more information. Thirteen of these individuals did not respond to letters or follow-up phone calls. Twenty-six individuals expressed interest in the study, but 1 declined the working memory screening. Exclusion criteria were: 1) a full-scale IQ ≤ 70, 2) motor, visual, or auditory handicap that precluded computer use, 3) photosensitive epilepsy, and 4) insufficient English fluency. See Fig. 1 for a diagram of participant recruitment and enrollment.
Fig. 1.
Participant flow diagram
For the remaining pool of 25 potential participants, an effort was made to minimize family burden of a trip to the study offices to screen for working memory deficits (as some of our potential participant pool did not live close to the hospital and would be required to make at least 3 additional trips if they were enrolled in the study) by offering the option of a prescreening for working memory deficits. This included either parent rating forms (which could be completed by mail) or review of neuropsychological test results from the previous year. Working memory deficits that met inclusion criteria for the study included: 1) a T-score greater than or equal to 75 on the Attention scale of the parent-rated Achenbach Child Behavior Check List (CBCL),23 2) a T-score greater than or equal to 75 on the parent-rated Working Memory scale of the Behavior Rating Inventory of Executive Function (BRIEF),24 or 3) a score of 1 standard deviation below the mean on the Working Memory Index of the Wechsler Intelligence Scale for Children,4thEd (WISC-IV).25 Of the 10 participants who qualified for the study based on the prescreen, 3 met criteria based on parent BRIEF scores and 7 met criteria based on their WISC-IV score. These 10 participants, along with 15 others who did not opt to do the prescreening were then directly assessed with the Automated Working Memory Assessment (AWMA)26 at the baseline visit. For 23 of the 25 potential participants, at least 1 of their AWMA composite scores was more than 1 standard deviation below the mean (including all 10 of the individuals who were prescreened). Two participants did not meet the working memory impairment criteria for participation in the study.
After reaching our final accrual goal of approximately 20 survivors of pediatric brain tumors between the ages of 8 and 18, we offered remaining potential participants the opportunity only to receive the intervention. The 2 participants who pursued this option were given the Cogmed intervention, but did not participate in the baseline and follow-up assessments and their data were not used in the analyses. Therefore, of the 23 participants who were eligible for study participation, 21 were formally enrolled in the study.
During the baseline/preintervention visit, study requirements were discussed at length with potential participants and their parent(s) and Cogmed tasks were demonstrated for them on a computer. All participants and their parents completed Institutional Review Board-approved assenting and consenting procedures. Participants underwent a brief battery of tests to screen for working memory deficits and establish a baseline of functioning that included the AWMA, academic achievement testing in untimed reading comprehension and applied math, and parent-report questionnaires (attempts to obtain teacher ratings were discontinued due to poor rate of return and inconsistent teacher raters over the course of the study for most participants). At the end of the baseline meeting, participants were sent home with a Cogmed CD-ROM (which contained the intervention) and encouraged to engage in additional discussion with their parents about the implications of participating in this study (eg, substantial time commitment and impact on their daily schedule) before committing to the intervention. We implemented this strategy of delaying when patients could commit to the study to a time when the investigators were not present to hopefully increase participants’ sense of being the ‘driver’ in the decision-making process (and therefore taking more ownership in completing the study). We instituted this plan after observing that some of our initial participants may have felt coerced by well-meaning parents during the meeting with study investigators and (possibly as a result) were also much more difficult to coach through the sessions. This policy appeared to work very well as all agreed to take part and all but 1 of the participants enrolled completed all 35 sessions of the intervention (1 completed only 25 sessions because the last 10 sessions overlapped with returning back to high school). One participant, whose family lived out of state, was unable to return for the 6-month follow up assessment.
Once participation was confirmed, eligible participants began intervention with Cogmed. This intervention features 12 game-like computer tasks developed to improve visual-spatial and verbal working memory. The tasks are adaptive, meaning that the level of difficulty of each trial increases and decreases based on whether or not the participant responds correctly. Hence, participants were consistently tested at their working memory limits throughout training.
Participants in this study were asked to complete 35 training sessions over 8 to 12 weeks and were contacted weekly by telephone (at a convenient time for the participant) in order to check progress, enhance motivation, and troubleshoot problems (eg, technical difficulties, check for adverse events, etc.) Participants also received intermittent emails from their coaches reminding them to do their training, updating them of their improvements, providing positive reinforcement, or reviewing strategies that had been discussed during phone calls. Two researchers on this study (BCG and JP) were trained as Cogmed Qualified Coaches, having completed specific training. Participants and their parent(s) also completed questionnaires assessing their satisfaction with the program midway (after 17 sessions) and at the end of training. Upon completion of their Cogmed training (typically within 1 week) and 6 months following their completion date (typically within 1 to 2 weeks), participants returned to the clinic for follow-up testing.
Participants earned gift cards several times during the study in order to maintain motivation. Gift cards in the amount of $25 were given to participants upon completion of 17 and 35 sessions of Cogmed. Gift cards in the amount of $50 were given to participants at the 6-month follow-up appointment and parents also were given $25 gift cards at this appointment. Participants were allowed to request desired gift cards, as opposed to having to select from prepurchased cards, which ensured that the rewards were optimized for each participant.
Measures
The Automated Working Memory Assessment (AWMA)26 is a computerized assessment examining both visual-spatial and verbal working memory. The North American version of this test was used. The following subtests were administered at the baseline and 6-month follow-up assessments: Word Recall, Digit Recall, Backwards Digit Recall, Counting Recall, Dot Matrix, Mr. X, and Spatial Recall. A slightly different combination of subtests was administered at the assessment immediately after completion of training in order to assess generalizability and minimize test-retest interference (although information on test reliability from the test manual suggests little change would be expected between 2 testing points separated by 4 weeks).25 These tests included: Digit Recall, Non-word Recall, Listening Recall, Backwards Digit Recall, Dot Matrix, Mr. X, Mazes Memory, and Odd One Out. This battery provided information about near-transfer effects of working memory training.
The Woodcock-Johnson Tests of Achievement, 3rdEd. (WJ-III)28 is a standardized battery of academic achievement tests. Participants were administered the Applied Problems and Passage Comprehension subtests in order to assess their untimed math problem solving and reading comprehension abilities, respectively. Alternate forms (to reduce test-retest interference) were administered during the baseline (version A) and 6-month assessments (version B) in order to identify far-transfer effects of working memory training (applying training to tasks that are dissimilar to the original training).
The Achenbach Child Behavior Checklist (CBCL)29 is a parent-report questionnaire that asks parents to rate their child’s emotional and behavior problems. Parents were administered this questionnaire during the baseline and 6-month follow-up assessments.
The Behavior Rating Inventory of Executive Function (BRIEF)30 is a parent-report questionnaire that asks about the child’s executive functioning in the home. Executive functioning encompasses numerous cognitive functions, including working memory, and has broadly been defined as the ability to exert effortful control over behaviors.31 Parents were administered this questionnaire during the baseline and 6-month follow-up assessment.
The Adaptive Behavior Assessment System, 2ndEd. (ABAS-II)32 is a parent-report questionnaire that asks about several aspects of children’s adaptive functioning including social skills, practical skills, and conceptual skills. Parents completed this questionnaire during the baseline and 6-month assessments.
The Neurological Predictor Scale (NPS)27 is a cumulative index of a child’s exposure to neurocognitive risk factors during cancer treatment and served as a proxy for treatment severity. This measure has been shown to predict composite intellectual functioning, short-term memory, and abstract reasoning abilities among children diagnosed with brain tumors. Parents completed this questionnaire at the baseline assessment.
A Satisfaction Survey was administered to participants and their parents following the completion of session 17 and the completion of session 35. This questionnaire was designed for a similar study19 and used with permission of the authors. It inquired about the ease of using computer hardware and software, any experience of pain or discomfort associated with the training, and satisfaction with the training.
Data Analysis
Descriptive statistics were used to summarize the characteristics of the sample. We used a linear mixed model approach to assess the efficacy of Cogmed on near-transfer measures of working memory (eg, the AWMA26) by comparing results from the 6-month follow-up assessment to results from the baseline assessment. Quantitative analysis of parent-report and far-transfer (academic achievement) outcomes utilized Wilcoxon signed rank tests, as the distribution of differences between pairs was non-normally distributed. For all quantitative analyses, False Discovery Rate corrections were used to test statistical significance.33 All statistical analyses were made using SPSS version 22.34
Results
Was Cogmed feasible for brain tumor survivors who had been treated with multiple treatment modalities?
Nineteen participants completed all 35 training sessions; 1 participant was allowed to stop after 25 sessions because he was returning to high school and could not make the time commitment to continue. Results from satisfaction surveys completed by participants and their parents at the conclusion of training (ie, following session 35) support the general feasibility of this intervention with this population. A summary of the Satisfaction Survey results can be found in Table 1. The majority of participants were able to easily navigate the hardware associated with this intervention (ie, computer, mouse, or track pad) as well as the software (ie, downloading the program, syncing with the database, and accessing the games). The majority of participants (n = 10; 66.7%) enjoyed the training sessions, did not experience pain or discomfort while completing the training sessions (n = 12; 80%), and were satisfied with their participation (n = 11; 73.3%). Parents also reported that their children did not experience significant difficulties using the hardware and software needed to complete the sessions. The majority of parents were satisfied with their child’s participation in the intervention (n = 14; 82.4%).
Table 1.
Satisfaction survey responses at session 35
| Parent Report* | |||||
|---|---|---|---|---|---|
| Never n (%) | Rarely n (%) | Sometimes n (%) | Often n (%) | Always n (%) | |
| Did not feel like doing training session? | 3 (17.6%) | 7 (41.2%) | 5 (29.4%) | 1 (5.9%) | 1 (5.9%) |
| How often did child ask for help? | 13 (76.5%) | 3 (17.5%) | |||
| Did child have pain or discomfort? | 13 (76.5%) | 1 (5.9%) | 1 (5.9%) | 1 (5.9%) | |
| Did child experience frustration? | 1 (5.9%) | 8 (47.1%) | 5 (29.4%) | 1 (5.9%) | 1 (5.9%) |
| Did child feel bored? | 5 (29.4%) | 7 (41.2%) | 3 (17.6%) | 1 (5.9%) | |
| Did child enjoy the training sessions? | 1 (5.9%) | 7 (41.2%) | 5 (29.4%) | 3 (17.6%) | |
| Very Satisfied n (%) |
Somewhat Satisfied n (%) |
Neither Satisfied nor Dissatisfied n (%) |
Somewhat Dissatisfied n (%) |
Very Dissatisfied n (%) |
|
| Satisfaction with child’s participation? | 12 (70.6%) | 2 (11.8%) | 1 (5.9%) | 1(5.9%) | |
| Child Report ** | |||||
| Never n (%) |
Rarely n (%) |
Sometimes n (%) | Often n (%) |
Always n (%) |
|
| Did not feel like doing training session? | 2 (13.3%) | 1 (6.7%) | 9 (60%) | 3 (20%) | |
| How often did you ask for help? | 12 (80%) | 1 (6.7%) | 1 (6.7%) | 1 (6.7%) | |
| Did you have pain or discomfort? | 9 (60%) | 3 (20%) | 2 (13.3%) | 1 (6.7%) | |
| Did you experience frustration? | 5 (33.3%) | 8 (53.3%) | 2 (13.3%) | ||
| Did you feel bored? | 4 (26.7%) | 2 (13.3%) | 5 (33.3%) | 3 (20%) | 1 (6.7%) |
| Did you enjoy the training sessions? | 1 (6.7%) | 2 (13.3%) | 2 (13.3%) | 4 (26.7%) | 6 (40%) |
| Very Satisfied n (%) |
Somewhat Satisfied n (%) |
Neither Satisfied nor Dissatisfied n (%) |
Somewhat Dissatisfied n (%) |
Very Dissatisfied n (%) |
|
| Satisfaction with participation? | 5 (33.3%) | 6 (40%) | 3 (20%) | 1 (6.7%) | |
* Seventeen parents returned the satisfaction survey at session 35, only sixteen parents completed the majority of the questions.
** Fifteen children completed the satisfaction survey at session 35.
Does a higher dose of Cogmed (35 sessions) benefit pediatric BT survivors?
A paired sample t-test evaluated whether or not there was a significant difference between participants’ Cogmed Training Index score at 25 sessions (mean score = 86.9) as compared with their score at 35 sessions (mean score = 90.5) and no significant difference was reported (P = 0.261). In summary, extending training an additional 10 sessions did not yield significant improvements in program-based performance, while likely adding another 8 to 10 hours of intervention time.
Did participants demonstrate statistically significant improvements in near-transfer measures (AWMA) and far-transfer measures (WJ-III) of working memory at 6 months as compared with baseline testing?
To test whether Cogmed training had near-transfer effects on a different measure of working memory (AWMA26), linear mixed models were run to examine the effect of working memory training on AWMA26 performance. All linear mixed models in Table 2 were adjusted for gender, age at diagnosis, time since completion treatments, and total NPS scores (as a proxy for neurocognitive risk factors). Results indicated that intervention with Cogmed had significant near-transfer effects on other measures of working memory at 6 months following the termination of the intervention. Participants demonstrated significantly improved scores on 2 of the 3 verbal working memory tasks, including Digit Recall (P < .01) and Word Recall (P < .05). Significant improvements were also seen on 4 of the 5 visual-spatial working memory tasks, including Dot Matrix (P < .01), Block Recall (P < .01), Mr. X (P < .05), and Spatial Recall (P < .05). In summary, participants showed improvements on other (non-Cogmed) measures of visual and verbal working memory 6 months after completing the intervention.
Table 2.
Automated Working Memory Assessment standard score changes from baseline to 6-month follow-up
| Subtests | Adjusted Effect Size^ | ||
|---|---|---|---|
| Beta Coefficient | Standard Error | P value | |
| Digit Recall | 8.7 | 3.0 | .009** |
| Word Recall | 9.3 | 4.1 | .035* |
| Counting Recall | -2.1 | 2.7 | .457 |
| Backwards Digit Recall | 7.8 | 3.8 | .052 |
| Dot Matrix | 16.8 | 4.5 | .002** |
| Block Recall | 8.7 | 2.7 | .005** |
| Mr. X | 7.0 | 2.5 | .013* |
| Spatial Recall | 12.3 | 4.3 | .010* |
^ Covariates controlled for in models were age, gender, time since completion of treatments and total NP score since baseline. A positive coefficient means that improvement in the predictor variable is associated with an increase in the outcome variables.
* P<.05 after correction for false discovery rate.
** P<.01 after correction for false discovery rate.
A Wilcoxon signed rank test revealed that Cogmed also had far-transfer effects on measures of participants’ academic achievement (WJ-III) (Table 3).28 Participants’ scores on tests of academic achievement in applied math (P < .05) were significantly improved (over baseline) at 6 months following the termination of the intervention. This effect was not observed, however, for an academic test of untimed reading comprehension (P = .80).
Table 3.
Results from non-parametric paired sample t-tests for parent questionnaire and academic achievement outcomes from baseline to 6-month follow-up
| Measure | Baseline Median (Range) | 6-Month Follow-Up Median (Range) | P value |
|---|---|---|---|
| Behavior Rating Inventory of Executive Function (BRIEF) | |||
| Inhibit | 47(40,91) | 42(40,63) | .00** |
| Shift | 51(36,81) | 43(38,85) | .02* |
| Initiate | 56(41,82) | 45(37,88) | .01* |
| Emotional Control | 48(36,85) | 46(36,74) | .37 |
| Working Memory | 62(40,80) | 56(40,81) | .00** |
| Plan/Organization | 60(37,82) | 49(38,87) | .01* |
| Organization of Materials | 52(34,68) | 47(34,65) | .10 |
| Monitor | 55(37,72) | 44(31,72) | .00** |
| Child Behavior Check List(CBCL) | |||
| Anxious/Depressed | 50(50,76) | 50(50,81) | .28 |
| Withdrawn/Depressed | 54(50,81) | 51(50,69) | .05 |
| Somatic Complaints | 54(50,94) | 50(50,85) | .03* |
| Social problems | 56(50,78) | 51(50,72) | .12 |
| Thought Problems | 51(50,73) | 51(50,72) | .92 |
| Attention Problems | 57(50,71) | 51(50,71) | .01* |
| Rule breaking Behavior (n=18) | 50(50,64) | 50(50,60) | .87 |
| Aggressive behavior | 50(50,64) | 50(50,65) | .07 |
| Adaptive Behavior Assessment System, 2 nd Ed.(ABAS-II) | |||
| Conceptual | 90(57,117) | 99(63,120) | .08 |
| Social | 95.5(70,119) | 100(72,119) | .02* |
| Practical | 89.5(52,112) | 96(52,120) | .87 |
| Woodcock-Johnson Tests of Achievement, 3 rd Ed (WJ-III). | |||
| Passage Comprehension | 88(47,110) | 89(50, 142) | .80 |
| Applied Problems | 92(68, 116) | 95(68, 124) | .016* |
* P< .05.
** P<.001.
(P values corrected for false discovery rate).
Did Cogmed improve parent ratings of participants’ executive function, behavior, and emotional adjustment or adaptive behaviors?
Wilcoxon signed rank tests were used to examine the effect of Cogmed training on parent reports of participants’ executive functioning (BRIEF30), behavioral and emotional adjustment (CBCL29) and adaptive skills (ABAS-II32) (Table 3). Parents rated significant improvements (compared with baseline) in several aspects of their children’s executive functioning including subscales measuring working memory, (P < .001 [BRIEF Working Memory], inhibitory control (P < .001) [BRIEF Inhibit], self-monitoring (P < .001) [BRIEF Monitor] and planning/organization (P < .05) [BRIEF Plan/Organization]. Parents also reported significant reductions (compared with baseline) in participants’ symptoms of somatic complaints (P < .05) and attention problems (P < .05) on the CBCL,29 as well as improved social skills (P < .05) at the 6-month follow-up (ABAS-II32). The original goal of obtaining teacher input was not realized due to an initial low response rate, a high proportion of training interventions taking place during the summer months, and teachers often changing over the course of time the participants were enrolled in the study (and therefore having different raters for different time points of the study).
Discussion
This study examined the feasibility of utilizing an extended (35 session) working memory training program among pediatric brain tumor survivors at the highest risk for neurocognitive late effects (who had undergone surgery, radiation and chemotherapy treatments). To our knowledge, this is the first study to date examining computerized working memory interventions exclusively with pediatric brain tumor patients (ie, not combined with other pediatric cancer survivors). This study provides further support that Cogmed training is feasible for childhood cancer survivors and effective at improving their working memory skills.19–20 Factors contributing to the high completion rate of this study’s participants may have been: (1) insisting that potential participants take a minimum of 24 hours to think about and to discuss the implications of their participation with parents, (2) allowing some students to delay starting the intervention (and baseline testing) until school breaks when they would have fewer demands on their time, and (3) in some cases, agreements to allow participants to opt out of chores or other expectations to compensate for the additional time and effort required for them to do working memory training. The majority of participants in this study felt this training was beneficial and would recommend it to other survivors and no participants reported experiencing adverse side effects from the training.
The efficacy of Cogmed was examined by looking at near-transfer and far-transfer effects 6 months after completing the intervention. Near-transfer improvements on other measures of working memory (AWMA26) were seen for both verbal and visual-spatial tasks 6 months after cessation of the intervention. The intervention produced improvements on many dimensions of the AWMA assessment that are not likely to be due to chance, with scores typically improving on the order of 10 points (ie, slightly under one standard deviation). It is important to note that for the brain tumor population, working memory scores would be expected to be maintained or to decline, not to improve, over time.
In an effort to assess real-world improvements in functioning among the study participants, far-transfer effects were examined in the domains of academic, adaptive, and executive functioning. Far-transfer effects were found as participants’ scores on an academic math test were significantly improved over baseline (prior to the intervention). This is consistent with previous research indicating improvements in math skills following Cogmed.35,36 However, no differences in participants’ academic reading scores were observed. This difference may be attributable to the nature of the working memory tasks in the Cogmed training. Specifically, the majority of the games are visual-spatial in nature, which may facilitate improvements in areas of academics that are also visual-spatial in nature (like math). The structured nature of the Cogmed tasks may have provided a framework for participants to improve their performance on an equally structured task of mathematics (eg, questions are read to participants and visual cues are provided). In contrast, the unstructured nature of the reading comprehension task (eg, participants were asked to read passages to themselves and identify a missing word), may have precluded improvements in this domain. Furthermore, the working memory demands of the math problem-solving task (often involving multiple steps) are likely greater than what is required for the reading comprehension task (typically limited to reading only one or two sentences).
Parent reports of their children’s executive and adaptive functioning and emotional/behavioral adjustment 6 months after completing the intervention were also areas of far-transfer improvements. In the domain of executive functioning, parents reported significant improvements in their children’s emotional and behavioral control, ability to transition/shift between activities, planning and organizational skills, ability to monitor their behavior, and working memory. Executive functioning skills, including working memory, are mediated by the frontal lobes of the brain.51 It may be that the benefits derived from computerized working memory training programs are due to strengthening connections between the frontal lobes and other parts of the brain. Hence, improvements in one area of executive functioning may lead to improvements in other skills that are also subsumed under this group. Parents also reported decreases in their children’s somatic complaints (eg, aches and pains) as well as improved attention. Attention and working memory are closely related, yet distinct, neurocognitive functions37; hence, attentional improvements were an expected outcome of this training given its development as an intervention for AD/HD. Why there were parent-reported improvements in somatic complaints is less clear. Perhaps increased freedom from external distractions also increased freedom from internal distractions, which led to a decrease in physical complaints.
Finally, parents reported significant improvements in their children’s social skills following Cogmed training. Children with working memory deficits often experience social-skills deficits as they may not pay attention to subtle, and sometimes not subtle, social cues.38 Social-skills deficits have been reported for brain tumor survivors, who are more likely to be described as socially withdrawn or isolated, excluded by others, or victims of teasing or bullying as compared with peers.39,40 Improvements in working memory and attention may lead to improvements in social skills if survivors are better able to pick up on social cues and follow conversations more easily.
Both the diversity of neurocognitive domains that were improved by this intervention and the fact that improvements were noted 6 months after the intervention highlight the importance of working memory in daily life and the functional impairments that could result from deficits in this area. Previous research indicated that pediatric brain tumor survivors may not receive maximal benefit from the standard regimen of 25 Cogmed sessions.19 Hence, this study sought to determine whether extending this regimen by 10 additional sessions further enhanced and extended the benefits of this intervention. Results showed that participants did not demonstrate a statistically significant improvement in their program-based working memory from session 25 to 35. The nominal improvement that participants were able to achieve during these 10 sessions likely does not outweigh the time investment required (8 to 10 additional hours). Since this study did not feature a control group, it is unknown whether these additional sessions played a role in the improvements observed in multiple domains over time. However, an abstract published after our study was completed reported that the number of Cogmed training sessions completed by childhood cancer survivors (20 with acute lymphoblastic leukemia and 10 with brain tumors) was a significant predictor of working memory improvement on performance-based cognitive measures41 (as opposed to use of the Cogmed Training Index used for our study). Although the studies differ in meaningful ways (including cancer diagnoses, reasons for giving extra sessions [ie, demonstrated lack of improvement versus providing for all participants] and range of sessions [ie, 21 to 30 versus 35]), the results suggest additional work is still needed to identify factors that may require additional working memory training for some survivors.
Although this study provides important insight into the treatment of neurocognitive deficits among pediatric brain tumor survivors with working memory deficits, some limitations need to be addressed. The sample size of this study is small (N = 20) and the diversity of participants is extremely limited, making it difficult to generalize the findings more broadly. Results may not apply to pediatric brain tumor survivors who do not have working memory deficits. The design of this study did not feature a control group and participants, parents, and study personnel were not blinded to intervention status (as all participants received the intervention). Hence, parental reports of children’s outcomes must be interpreted with caution as the parents and study personnel all knew the participants were receiving an active treatment and may have anticipated and been biased toward improvements in functioning. This limitation is somewhat mitigated by the fact that parents did not endorse across-the-board improvements in all areas of functioning and those areas that did improve were logically related to improvements in working memory. Another drawback was a lack of information from multiple respondents as efforts to obtain teacher questionnaires were unsuccessful. As a result, it is unclear the extent to which participants’ improvements may have translated into improved academic outcomes at school. The literature would benefit from larger clinical studies that feature a double-blind, placebo-controlled design,21 following children for a longer period of time and collecting information about functioning from multiple respondents across multiple settings. Such a study would provide additional clarity regarding the efficacy of the intervention, the duration of effects, and the extent of the functional improvements derived therein. Finally, it important to note that “brain training” of any sort likely does not result in a permanent improvement of the impairments. Instead, periodic retraining as well as behavioral changes to apply and support the training to the individual’s daily life is much more likely to create lasting and meaningful results.42
Research has long identified the neurocognitive late effects experienced by pediatric survivors of brain tumors.6 More recently, specific domains of neurocognitive functioning have been identified as central to this overall decline (eg, working memory and attention)43 and biological mechanisms for this decline have begun to be identified (eg, degeneration of or failure to develop white matter tracts).44 Preliminary research examining computerized working memory interventions with pediatric cancer survivors has been promising in terms of feasibility and efficacy. The current study replicates and extends previous research by (1) limiting participants only to brain tumor survivors who were treated with the most intensive treatment regimens, (2) demonstrating that survivors who are most at risk for neurocognitive late effects are able to both complete this training and benefit from it, (3) identifying a point of diminished returns on the participant’s investment (as extending the training by ten sessions did not yield significant working memory improvements for our group), (4) demonstrating benefits of Cogmed training on other measures of working memory performance (AWMA26), (5) extending tracking to 6 months after intervention, and (6) evaluating potential impact of computerized working memory training on far-transfer measures of academic achievement as well as parent ratings of executive functions, behavior, and emotional functioning and adaptive skills.
Funding
Children’s Hospitals Foundation [RF#47549 to BCG]
Acknowledgements
The authors are grateful for the participation of the children and parents who made this study possible through their motivation and persistence. Statistical support for this study was provided by Yi Lu, M.S., Michael Finch, PhD and Andrew Flood, PhD. Sarah Wimmer, RN, BSN was instrumental in helping us with participant accrual. We also would like to thank Pearson for providing the Cogmed software. Finally, many thanks to Children’s Hospitals & Clinics of Minnesota Foundation for funding this study.
Conflict of interest statement. None declared. Pearson Education, Inc. did not have any input into study design, data analysis, or in the presentation of findings.
Cogmed and Cogmed Working Memory Training are trademarks, in the U.S. and/or other countries, of Pearson Education, Inc. or its affiliate(s).
References
- 1. Ostrom QT, Gittleman H, Liao P., et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007–2011. Neuro-Oncol. 2014;16(suppl 4): iv1–iv63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xu J, Margol A, Asgharzadeh S, Erdreich-Epstein A. Pediatric Brain Tumor Cell Lines. J of Cell Biochem. 2015;116(2):218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Robinson K E, Kuttesch J F, Champion J E, et al. A quantitative meta-analysis of neurocognitive sequelae in survivors of pediatric brain tumors. Pediatr Blood Cancer. 2010;55(3):525–531. [DOI] [PubMed] [Google Scholar]
- 4. Zeltzer LK, Lu Q, Leisenring W, et al. Psychosocial outcomes and health related quality of life in adult childhood cancer survivors: A report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev, 2008;17(2):435–446. [DOI] [PubMed] [Google Scholar]
- 5. Conklin HM, Kahn RB, Reddick WE, et al. Acute Neurocognitive Response to Methylphenidate Among Survivors of Childhood Cancer: A Randomized, Double-Blind, Cross-Over Trial. J Pediatr Psychol, 2007;32(9):1127–1139. [DOI] [PubMed] [Google Scholar]
- 6. Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol, 2004;5(7):399–408. [DOI] [PubMed] [Google Scholar]
- 7. Nelson MB, Compton P, Patel SK, Jacob E, Harper R. Central Nervous System Injury and Neurobehavioral Function in Children with Brain Tumors. Cancer Nurs, 2013;36(2):E31–E47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Dijk IWEM, Cardous-Ubbink MC, van der Pal HJH, et al. Dose-Effect Relationships for Adverse Events After Cranial Radiation Therapy in Long-term Childhood Cancer Survivors. Int J Radiat Oncol, Biol, and Phys, 2013;85(3):768–775. [DOI] [PubMed] [Google Scholar]
- 9. Conklin HM, Ashford JM, Howarth RA, et al. Working Memory Performance among Childhood Brain Tumor Survivors. J Int Neuropsychol Soc, 2012;18(6):996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dunning DN, Holmes J, Gathercole SE. Does working memory training lead to generalized improvements in children with low working memory? A randomized controlled trial. Dev Sci, 2013;16(6):1467–7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reeves CB, Palmer SL, Reddick WE., et al. Attention and memory functioning among pediatric patients with medulloblastoma. J Pediatr Psychol, 2006;31(3):272–280. [DOI] [PubMed] [Google Scholar]
- 12. Alloway TP, Banner GE, Smith P. Working memory and cognitive styles in adolescents’ attainment. Br J Educ Psychol, 2008;80(4):567–581. [DOI] [PubMed] [Google Scholar]
- 13. Patel SK, Ross P, Cuevas M, et al. Parent- directed intervention for children with cancer-related neurobehavioral late effects: a randomized pilot study. J Pediatr Psychol, 2014;39(9):1013–27. Epub 2014 Jun 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smithson EF, Phillips R, Harvey DW, Morrall MCHJ. The use of stimulant medication to improve neurocognitive and learning outcomes in children diagnosed with brain tumours: A systematic review. Eur J Cancer, 2013;49(14):3029–3040. [DOI] [PubMed] [Google Scholar]
- 15. Thompson SJ, Leigh L, Christensen R, et al. Immediate neurocognitive effects of methylphenidate on learning-impaired survivors of childhood cancer. J Clin Oncol, 2001;19(6):1802–1808. [DOI] [PubMed] [Google Scholar]
- 16. Butler RW, Copeland DR. Attentional processes and their remediation in children treated for cancer: A literature review and the development of a therapeutic approach. J Int Neuropsychol Soc, 2002;8(1):115–124. [PubMed] [Google Scholar]
- 17. Butler RW, Copeland DR, Fairclough DL, et al. A multicenter, randomized clinical trial of a cognitive remediation program for childhood survivors of a pediatric malignancy. J Consult Clin Psychol, 2008;76(3):367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pearson. (2016) Cogmed Working Memory Training Published Research Available at http://www.cogmed.com/published-research Accessed May 9, 2016.
- 19. Hardy KK, Willard VW, Allen TM, Bonner MJ. Working memory training in survivors of pediatric cancer: a randomized pilot study. Psycho-Oncol, 2013;22(8):1856–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cox LE, Ashford JM, Clark KN, et al. Feasibility and acceptability of a remotely administered computerized intervention to address cognitive late effects among childhood cancer survivors. Neuro-Oncol Practice, 2015;2(npu 036):78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Conklin HM, Ogg RJ, Ashford JM, et al. Computerized cognitive training for the amelioration of cognitive late effects among childhood cancer survivors: A randomized controlled study. J Clin Oncol, 2015;33(JCO 2015):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hardy KK, Willard VW, Bonner MJ. Computerized cognitive training in childhood survivors of cancer: A Pilot study. J Pediatr Oncol Nurs, 2011;28(1):27–33. [DOI] [PubMed] [Google Scholar]
- 23. Achenbach TM, Rescorla LA. Manual for the ASEBA Preschool forms and Profiles. Burlington: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- 24. Gioia G, Isquith PK, Guy SC, Kenworthy L. Test Review: Behavior Rating Inventory of Executive Function. Child Neuropsychol, 2000;6(3):235–238. [DOI] [PubMed] [Google Scholar]
- 25. Wechsler D. The Wechsler intelligence scale for children—fourth edition. London: Pearson Assessment; 2004. [Google Scholar]
- 26. Alloway TP. Automated Working Memory Assessment: Manual. Pearson, 2007. [Google Scholar]
- 27. Micklewright JL, King TZ, Morris RD, Krawiecki N. Quantifying pediatric neuro-oncology risk factors: development of the neurological predictor scale. J Child Neurol. 2008;23:455–458. [DOI] [PubMed] [Google Scholar]
- 28. Woodcock RW, McGrew RS, Mather N. Woodcock-Johnson Tests of Achievement, 3rd Ed. Itasca: Riverside Publishing; 2001. [Google Scholar]
- 29. Achenbach TM, Edelbrock C. Child behavior checklist.Burlington: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- 30. Gioia GA. BRIEF: Behavior rating inventory of executive function: Professional manual.Psychological Assessment Resources; 2000. [Google Scholar]
- 31. Zelazo PD, Carlson SM, Kesek A. The development of executive function in childhood. In: Nelson CA, Luciana M, eds. Handbook of Developmental Cognitive Neuroscience.Cambridge: MIT Press; 2008:553–574. [Google Scholar]
- 32. Oakland T, Harrison PL, eds. Adaptive behavior assessment system-II: Clinical use and interpretation.Academic Press; 2011. [Google Scholar]
- 33. Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological), 1995;57(1):289–300. [Google Scholar]
- 34. IBM Corp. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp; 2013. [Google Scholar]
- 35. Bergman-Nutley S., Klingberg T. Effect of working memory training on working memory, arithmetic and following instructions. Psychol Res, 2014;78(6):869–877. [DOI] [PubMed] [Google Scholar]
- 36. Holmes J, Gathercole SE. Taking working memory training from the laboratory into schools. Educational Psychology: An International Journal of Experimental Educational Psychology, 2014;34(4):440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Otero TM, Barker LA. The frontal lobes and executive functioning. In Goldstein S., Naglieri JA. (eds). The Handbook of Executive Functioning.New York: Springer; 2013:29–44. [Google Scholar]
- 38. Kofler MJ, Rapport MD, Bolden JD, Sarver DE, Raiker JS, Alderson RM. Working memory deficits and social problems in children with ADHD. J Abnorm ChildPsychol, 2011;39(6):805–817. [DOI] [PubMed] [Google Scholar]
- 39. Patenaude AF, Kupst MJ. Psychosocial functioning in pediatric cancer. J Pediatr. Psychol,2005;30(1):9–27. [DOI] [PubMed] [Google Scholar]
- 40. Sally CG, Hewitt LL, Patenaude AG. Temperament and social behavior in pediatric brain tumor survivors and comparison peers. J Pediatr Psychol, 2015;40(3):297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ashford JM, Clark KN, Martin-Elbahesh K, et al. Predicting response to computerized working memory training among childhood cancer survivors. J Int Neuropsych Soc, 2014;20(Suppl S1):58. [Google Scholar]
- 42. Rabipour S, Raz A. Training the brain: fact and fad in cognitive and behavioral remediation. Brain Cognition. 2012;79(2):159–79. Epub 2012 Mar 30. [DOI] [PubMed] [Google Scholar]
- 43. Reddick WE, White HA, Glass JO, et al. Developmental model relating white matter volume to neurocognitive deficits in pediatric brain tumor survivors. Cancer, 2003;97(10):2512–2519. [DOI] [PubMed] [Google Scholar]
- 44. Khong PL, Kwong DL, Chan GC, Sham JST, Chan FL, Gaik-Cheng O, et al. Diffusion-tensor imaging for the detection of treatment-induced white matter injury in children with medulloblastoma: A pilot study. Am J Neuroradiol, 2003;24(4):734–740. [PMC free article] [PubMed] [Google Scholar]