Abstract
Background
Despite advances in modern therapy, high-grade gliomas continue to portend a dismal prognosis and nearly all patients will experience relapse. Unfortunately, salvage options remain limited. In this study, we assessed outcomes for patients with recurrent gliomas treated with reirradiation.
Methods
We retrospectively identified 48 glioma patients treated with reirradiation between 2013 and 2016. All had radiographic or pathologic evidence of recurrence. Prognostic factors were abstracted from the electronic medical record.
Results
Initial surgery included biopsy in 15, subtotal resection in 21, and gross total resection in 12. Initial chemotherapy included temozolomide (TMZ) in 31, TMZ+dasatinib in 7, TMZ+vorinostat in 3, and procarbazine, lomustine, and vincristine in 2. The median dose of primary radiotherapy was 60 Gy delivered in 30 fractions. Median overall survival (OS) and progression-free survival (PFS) from initial diagnosis were 3.2 and 1.7 years, respectively. A total of 36 patients failed salvage bevacizumab before reirradiation. Salvage surgery was performed before reirradiation in 21 patients. Median time to reirradiation was 1.7 years. Median follow-up was 13.7 months from reirradiation. Concurrent systemic therapy was given in 33 patients (bevacizumab in 27, TMZ in 8, and lomustine in 2). Median PFS and OS after reirradiation were 3.2 and 6.3 months, respectively. Radionecrosis occurred in 4 patients and no radionecrosis was seen in patients receiving concurrent bevacizumab with reirradiation (0% vs 19%, P = .03).
Conclusions
Reirradiation may result in delayed tumor progression with acceptable toxicity. Prospective trials are needed to determine the impact of reirradiation on tumor progression and quality of life.
Keywords: glioma, glioblastoma, radionecrosis, recurrence, reirradiation
Despite advances in modern diagnostics and therapeutics, gliomas continue to confer a poor prognosis. In modern trials of high-grade gliomas, progression-free survival (PFS) remains short at approximately 7 months, with nearly all patients eventually experiencing tumor progression by 36 months.1,2 While the prognosis of low-grade glioma tends to be significantly better than high-grade glioma, at least half of patients with low-grade glioma.3,4 Thus, salvage therapies are commonly used in the majority of glioma patients.
The antiangiogenic agent bevacizumab, a humanized monoclonal vascular endothelial growth factor (VEGF) antibody, is the most common salvage chemotherapeutic option used in the treatment of recurrent glioma. In a seminal study by Kreisl et al, 48 patients with recurrent glioblastoma were treated with bevacizumab every 2 weeks until tumor progression, when they were transitioned to bevacizumab with irinotecan. A radiographic response was achieved in 71% and 6-month PFS was 29% with a median overall survival (OS) of 31 weeks.5 Another study randomized 167 patients to receive bevacizumab alone or in combination with irinotecan after tumor progression, which reported similar response rates in both arms (28% and 39%) and no significant difference in PFS or OS.6 Other agents including single-agent irinotecan, temozolomide (TMZ), procarbazine, vincristine, and lomustine have shown limited efficacy.7–10 Ultimately, these data support the use of bevacizumab as a temporizing measure for patients with recurrent gliomas, but more efficacious agents are desperately needed.
Repeat surgical resection for recurrent gliomas after primary surgery, chemotherapy, and radiotherapy is feasible but carries a risk of morbidity. In a large retrospective study by Ringel et al, 8% of patients had permanent new neurologic deficits after re-resection.11 A retrospective study from Singapore reported better OS (median 25 months) and higher functional outcomes in patients who were selected for re-resection.12 However, other studies have reported less encouraging results.13,14 Furthermore, many patients with recurrent gliomas are not optimal surgical candidates and solely noninvasive therapies must be employed.
Stereotactic radiosurgery and hypofractionated radiotherapy offer similar tumor control to brachytherapy via noninvasive means.15,16 In a report by Fogh et al, 147 patients with recurrent high-grade glioma received a median dose of 35 Gy in 10 fractions. Median survival was 11 months with no significant acute or late toxicity.17 In contrast, radionecrosis rates of up to 36% have been reported in patients receiving doses above 40 Gy.18 Thus, reirradiation appears to have potential for delaying progression in patients with recurrent gliomas but with a narrow therapeutic index. However, as noted in recent ASTRO/ASCO (American Society for Radiation Oncology/American Society of Clinical Oncology) guidelines, there is a paucity of phase 3 reirradiation data to guide clinical practice.19 In this study, we sought to retrospectively assess our efficacy and toxicity outcomes for patients undergoing reirradiation for recurrent gliomas.
Methods
This study was approved by the Mayo Clinic Institutional Review Board. Records of adult patients with a second course of radiotherapy delivered at our institution between 2013 and 2016 were reviewed. To be included, patients needed a prior pathologic diagnosis of a glioma with a previous course of radiotherapy delivering definitive doses. Relapses following initial or salvage treatment courses were defined based on a combination of radiographic progression, clinical progression, changes in systemic therapy, a salvage surgical procedure, or initiation of reirradiation. Toxicities were collected prospectively and stored in the electronic medical record.
While our institution’s approach to reirradiation for recurrent gliomas was not rigidly standardized, it was relatively uniform. Simulation was performed in a head-first supine position and generally utilized a three-point Aquaplast or BrainLab immobilization mask. Axial CT scans were then obtained and fused with pretreatment MRI scans for target delineation. In general, a gross target volume (GTV) was contoured based on the presence of contrast enhancement and the planning target volume (PTV) was a 0-to-2 mm expansion beyond GTV, respecting anatomical boundaries. The majority of patients were treated to a dose of 35 to 40 Gy in 10 total fractions. In special circumstances where overlap with prior radiation fields was minimal, doses were escalated to 50 to 60 Gy in conventional 2 Gy fractions.
Biologically Effective Dose (BED) was calculated using the formula nd (1 + d/[α/β]), where n = number of fractions, d = fraction dose, and α/β = tissue repair capacity. Normal Tissue Dose (NTD) was defined as the total dose delivered in 2-Gy fractions with an α/β ratio of 2 Gy. NTD for each course was calculated as BED/2. NTDcumulative was defined as the sum of the NTD for each radiation course. The Kaplan-Meier method was used to report survival estimates. The log-rank test was used to compare Kaplan–Meier estimates. Fisher’s exact test was used to draw correlations between treatment and prognostic factors and the development of late toxicity. The Cox proportional hazards model was used for univariate and multivariate analyses. Statistical analysis was performed using JMP 10.0 (SAS institute, Cary, NC).
Results
Initial Treatment Characteristics
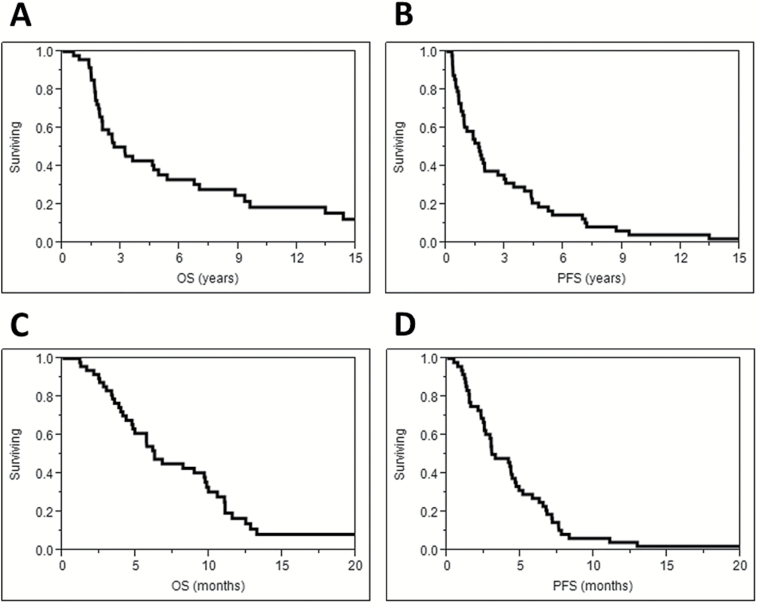
Patient characteristics are summarized in Table 1. The median follow-up from the time of initial diagnosis was 26.0 years for living patients. The median age at initial diagnosis was 55 years. The majority of patients initially had high-grade astrocytic tumors. IDH, 1p/19q, and MGMT status were available for a small number of patients. Initial treatment included TMZ with or without experimental agents for most patients. The median dose for the first course of radiotherapy was 60 Gy delivered in 30 fractions. The median NTD for the first course of radiotherapy (NTD1) was 60 Gy (range 42.8–86.1 Gy). Measured from the date of first diagnosis, OS was 66% at 2 years and 36% at 5 years (Fig. 1A). Median OS was 5.4 years for grade III and 1.9 years for grade IV tumors (P = .007). PFS was 38% at 2 years and 19% at 5 years (Fig. 1B). Median PFS was 2.8 years for grade III and 0.9 years for grade IV tumors (P = .04).
Table 1.
Initial patient characteristics
| N = 48 | n | % | |
|---|---|---|---|
| Age, years | Median (range) | 55 (22–72) | |
| Gender | Male | 31 | 65% |
| Female | 17 | 35% | |
| Initial surgery | Biopsy | 15 | 31% |
| STR | 21 | 44% | |
| GTR | 12 | 25% | |
| Initial histology | Astrocytoma | 38 | 79% |
| Oligodendroglioma | 2 | 4% | |
| Oligoastrocytoma | 7 | 15% | |
| Gliosarcoma | 1 | 2% | |
| Initial grade | II | 8 | 17% |
| III | 14 | 29% | |
| IV | 26 | 54% | |
| IDH status | Mutant | 5 | 10% |
| Wild type | 23 | 48% | |
| Unknown | 20 | 42% | |
| 1p/19q status | Codeleted | 3 | 6% |
| Single deletion | 1 | 2% | |
| Intact | 4 | 8% | |
| Unknown | 40 | 83% | |
| MGMT status | Methylated | 9 | 19% |
| Unmethylated | 6 | 13% | |
| Unknown | 33 | 69% | |
| Initial chemotherapy | TMZ | 31 | 65% |
| TMZ + dasatinib | 7 | 15% | |
| TMZ + vorinostat | 3 | 6% | |
| PCV | 2 | 4% | |
| None | 5 | 10% | |
| RT dose, Gy | Median (range) | 60 (40–76) | |
| RT fractions | Median (range) | 30 (15–36) | |
| Initial GTV volume (cm3) | Median (range) | 69 (14–188) | |
| Initial PTV volume (cm3) | Median (range) | 296 (39–643) | |
STR: subtotal resection, GTR: gross total resection, IDH: isocitrate dehydrogenase, MGMT: O-6-methylguanine-DNA methyltransferase, TMZ: temozolomide, PCV: procarbazine, lomustine, vincristine, RT: radiotherapy, GTV: gross target volume, PTV: planning target volume.
Fig. 1.
Overall survival (A) and progression-free survival (B) from first diagnosis along with overall survival (C) and progression-free survival (D) from reirradiation.
Recurrences and Salvage Therapies
Recurrence and salvage treatment characteristics are summarized in Table 2. The median time to first recurrence was 11.8 months. Median follow-up after reirradiation was 13.7 months for surviving patients. Most patients had a trial of salvage chemotherapy or surgical resection prior to reirradiation. Only 13% of patients underwent reirradiation at the time of first relapse. The most common salvage chemotherapy agents given were bevacizumab, TMZ, and lomustine. Salvage surgery was attempted in 46% of patients and resulted in gross total resection in 52% of those undergoing an operation. All 8 patients with an initial diagnosis of low-grade glioma had pathologic (5) or radiographic (3) evidence (ie, contrast enhancement) of high-grade glioma prior to reirradiation. The median time from initial diagnosis to the start of salvage reirradiation was 20.9 months. The most common doses used were 35 Gy in 10 fractions (48%) and 40 Gy in 10 fractions (25%). The median NTD for reirradiation (NTD2) was 48.2 Gy (range, 37.5–73.2 Gy) and the median cumulative NTD was 108.1 Gy (range, 85.1–134.3 Gy). Concurrent chemotherapy was administered with reirradiation in most patients, most commonly with bevacizumab using standard treatment schedules (10 mg/kg given every 2 weeks). In the 27 patients receiving concurrent bevacizumab, the medication was initiated a median of 50 days prior to starting reirradiation, with all patients receiving a dose less than 2 weeks prior to (n = 26), or one week after (n = 1), starting reirradiation.
Table 2.
Recurrence and reirradiation characteristics
| n | % | ||
|---|---|---|---|
| Time to first recurrence, months | Median (range) | 11.8 (3.0–191.5) | |
| Time from diagnosis to reRT, months | Median (range) | 20.9 (4.2–277.9) | |
| Number of recurrences before reRT | 1 | 6 | 13% |
| 2 | 11 | 23% | |
| 3 | 15 | 31% | |
| 4 | 9 | 19% | |
| 5 | 7 | 15% | |
| Prior salvage chemotherapy | Yes | 42 | 88% |
| No | 6 | 13% | |
| Salvage chemotherapy agents | Bevacizumab | 36 | 86% |
| Lomustine | 27 | 64% | |
| TMZ | 26 | 62% | |
| Dasatinib | 4 | 10% | |
| Carmustine | 2 | 5% | |
| Procarbazine | 2 | 5% | |
| PCV | 2 | 5% | |
| Irinotecan | 1 | 2% | |
| Etoposide | 1 | 2% | |
| TRC105 | 1 | 2% | |
| Prior salvage surgery | None | 27 | 56% |
| 1 | 17 | 35% | |
| 2 | 4 | 8% | |
| Type of salvage surgeryA | Biopsy | 2 | 8% |
| GTR | 13 | 52% | |
| STR | 10 | 40% | |
| Chemotherapy with reRT | None | 15 | 31% |
| Bevacizumab | 23 | 48% | |
| Bevacizumab + lomustine | 2 | 4% | |
| Bevacizumab + TMZ | 2 | 4% | |
| TMZ | 6 | 13% | |
| Reirradiation dose (Gy) / fractions | 28 Gy / 5 fx | 1 | 2% |
| 30 Gy / 5 fx | 1 | 2% | |
| 30 Gy / 6 fx | 1 | 2% | |
| 31.5 Gy / 9 fx | 2 | 4% | |
| 33 Gy / 9 fx | 1 | 2% | |
| 35 Gy / 10 fx | 23 | 48% | |
| 40 Gy / 10 fx | 12 | 25% | |
| 40 Gy / 15 fx | 4 | 8% | |
| 45 Gy / 10 fx | 1 | 2% | |
| 50 Gy / 25 fx | 1 | 2% | |
| 60 Gy / 30 fx | 1 | 2% | |
| Reirradiation PTV volume (cm3) | Median (range) | 49 (3–265) | |
reRT: reirradiation, TMZ: temozolomide, PCV: procarbazine, lomustine, vincristine, GTR: gross total resection, STR: subtotal resection, PTV: planning target volume.
Abecause 25 surgical procedures took place in 21 patients, the denominator is 25.
Overall Survival After Reirradiation
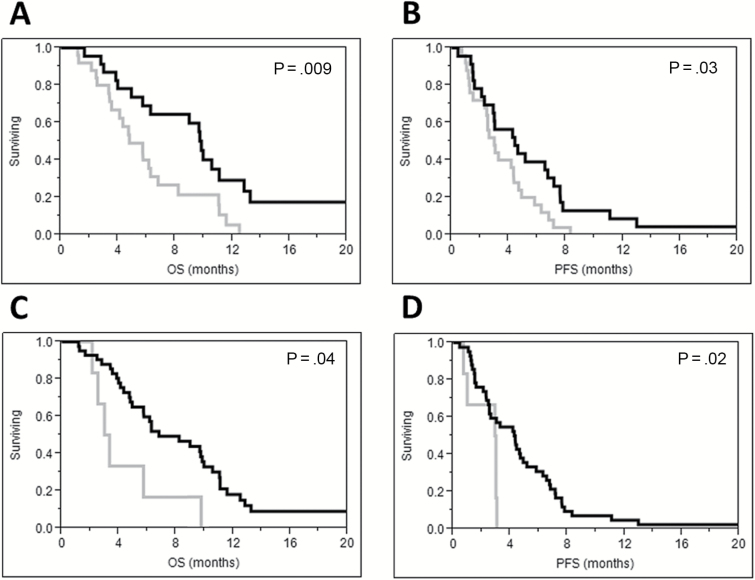
Overall survival after reirradiation was 54% at 6 months, 17% at 12 months, and 8% at 24 months (Fig. 1C). Median OS was 9.0 months for grade III and 4.9 months for grade IV tumors (P = .08). Initial treatment with TMZ (HR = 2.76; 95% CI, 1.09–9.29; P = .03), prior salvage bevacizumab (HR = 2.20; 95% CI, 1.08–4.89; P = .03) and prior salvage lomustine (HR = 2.7; 95% CI, 1.32–5.94; P = .01) were significantly associated with worse OS. Time to reirradiation greater than 22 months (HR = 0.56; 95% CI, 0.29–1.08; P = .08) and reirradiation dose ≥35 Gy (HR = 0.37; 95% CI, 0.15–1.11; P = .07) were of borderline significance. For grade IV patients treated with reirradiation after bevacizumab failure, median OS was lower than bevacizumab naïve patients (4.9 months vs 10.6 months, P = .02). Comparing Kaplan-Meier survival curves with the log-rank test revealed a significant association between a greater time from diagnosis to reirradiation (Fig. 2A) and doses ≥35 Gy (Fig. 2C) with better OS. On multivariate analysis including time to reirradiation, initial TMZ, prior salvage bevacizumab and prior salvage lomustine, time to reirradiation greater than 22 months was associated with better OS (HR = 0.47; 95% CI, 0.22–0.95; P = .04). When the variable “initial TMZ” was replaced with reirradiation dose ≥35 Gy on multivariate analysis, time to reirradiation remained the only variable significantly associated with OS (HR = 0.38; 95% CI, 0.19–0.73; P = .004).
Fig. 2 .
Overall survival (A) and progression-free survival (B) were higher for patients with greater than 22 months between courses of radiotherapy (black) compared with patients with 22 months or less between courses (grey). Overall survival (C) and progression-free survival (D) were higher when doses of ≥35 Gy were delivered (black) compared with lower doses (grey).
Progression-free Survival After Reirradiation
Progression occurred after reirradiation in 41 of 48 patients at a median time of 3.3 months (range, 0.5–30.6 months) after treatment. Progression-free survival after reirradiation was 27% at 6 months and 4% at 12 months (Fig. 1D). Median PFS after reirradiation was 4.3 months for grade III and 2.6 months for grade IV tumors (P = .16). For grade IV patients with a history of progression on bevacizumab, median PFS was lower than bevacizumab-naïve patients (2.5 vs 6.7 months, P = .04). High-grade primary tumors (HR = 2.29; 95% CI, 1.07–5.70; P = .03), treatment with initial TMZ (HR = 2.52; 95% CI, 1.12–6.76; P = .02), ≤22 months between primary diagnosis and reirradiation (HR = 0.53; 95% CI, 0.29–0.98; P = .04), reirradiation dose <35 Gy (HR = 0.35; 95% CI, 0.14–0.97; P = .04), reirradiation PTV sizes ≤75 cc (HR = 0.49; 95% CI, 0.25–0.91; P = .02), and no radionecrosis (HR = 0.34; 95% CI, 0.10–0.87; P = .02) were associated with lower PFS on univariate analysis. Kaplan–Meier PFS curves compared using the log-rank test based on duration between diagnosis and reirradiation and reirradiation dose are shown in Fig. 2B and Fig. 2D. On multivariate analysis for PFS including the variables high-grade primary tumor, time to reirradiation, reirradiation dose, and PTV size, we found that reirradiation dose ≥35 Gy (HR = 0.27; 95% CI, 0.11–0.77; P = .02) and reirradiation PTV volumes >75 cc (HR = 0.43; 95% CI, 0.21–0.84; P = .01) were associated with improved PFS. To further investigate the potential interaction between PTV size and radionecrosis, an exploratory multivariate analysis was performed including the following variables: time to reirradiation, reirradiation dose, PTV volume, and presence of radionecrosis. With this model, time to reirradiation >22 months (HR = 0.45; 95% CI, 0.23–0.85; P = .01) and reirradiation doses ≥35 Gy (HR = 0.31; 95% CI, 0.12–0.90; P = .03) were statistically significant and statistical significance was lost for PTV volume >75 cc (P = .09) and radionecrosis (P = .19). Unfortunately, with only 48 events, multivariate analysis was limited to 4 variables and further exploration was not possible.
Toxicity of Reirradiation
Acute radiation toxicity is summarized in Table 3. The most common toxicities included fatigue, headaches, alopecia, and radiation dermatitis. Three patients experienced grade 3 toxicity; 2 were weakness unchanged from baseline and 1 was treatment-related grade 3 fatigue. No grade 4 or 5 acute toxicities were recorded. Late toxicities were rare and included increasing seizures in 1 patient, leukoencephalopathy in 1 patient, and radionecrosis in 4 patients. Radionecrosis was grade 2 in 2 patients and grade 3 in 2 patients. The time from reirradiation to radionecrosis was a median of 2.6 months (range, 1.7–4.8 months). Treatment included dexamethasone in 2 and bevacizumab in 3 (1 patient received both treatments). Symptoms of radionecrosis included seizures in 2 patients, aphasia in 2, fatigue in 1, motor symptoms in 1, and imbalance in 1. All patients had their symptoms stabilize or improve with treatment. Due to subsequent progressive disease, 2 of the patients never experienced full resolution of symptoms. The 2 patients with resolution of symptoms were symptomatic from radionecrosis for a total duration of 2.9 and 1.4 months. No patient required surgery for treatment of radionecrosis. All cases of radionecrosis had reirradiation PTV volumes >75 cc (P = .007). Radionecrosis was more common in bevacizumab-naïve patients (25%) compared with patients with a history of prior salvage bevacizumab (3%; P = .04). No cases of radionecrosis were seen in patients receiving concurrent bevacizumab with reirradiation (0% vs 19%, P = .03). Two of the 3 patients receiving reirradiation doses greater than 45 Gy developed radionecrosis (P = .02). Three of the 4 patients with radionecrosis were treated with doses less than 3.5 Gy per fraction (P = .008). The dose and fractionation schedules used for the 4 patients developing radionecrosis included 60 Gy in 30 fractions, 50 Gy in 25 fractions, 40 Gy in 15 fractions, and 35 Gy in 10 fractions. No threshold for NTD2 (P = .92) and NTDcumulative (P = .82) correlated with the risk of radionecrosis. Similarly, time to reirradiation did not correlate with radionecrosis (P = .88).
Table 3.
Acute toxicity
| None | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Aphasia | 45 | 94% | 3 | 6% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Confusion | 44 | 92% | 4 | 8% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Weakness | 43 | 90% | 1 | 2% | 2 | 4% | 2A | 4% | 0 | 0% | 0 | 0% |
| Vision changes | 43 | 90% | 3 | 6% | 2 | 4% | 0 | 0% | 0 | 0% | 0 | 0% |
| Gait instability | 47 | 98% | 0 | 0% | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% |
| Seizures | 45 | 94% | 3 | 6% | 2 | 4% | 0 | 0% | 0 | 0% | 0 | 0% |
| Dysphagia | 47 | 98% | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Headache | 34 | 71% | 13 | 27% | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% |
| Alopecia | 35 | 73% | 11 | 23% | 2 | 4% | 0 | 0% | 0 | 0% | 0 | 0% |
| Dermatitis | 41 | 85% | 7 | 15% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Fatigue | 11 | 23% | 22 | 46% | 14 | 29% | 1 | 2% | 0 | 0% | 0 | 0% |
| Nausea | 45 | 94% | 3 | 6% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
Aunchanged from baseline.
Discussion
In this series, we report outcomes after reirradiation in a heavily pretreated patient population with recurrent gliomas. The favorable OS from time of diagnosis suggests a highly selected group of longer-term survivors nearing the end of the course of their disease. We found that radiotherapy delayed progression of disease by approximately 3 months and resulted in modest survival with minimal toxicity. Overall, these findings support the use of salvage reirradiation in patients with recurrent gliomas.
Patients with recurrent gliomas have limited efficacious salvage options. Most commonly, salvage therapy includes treatment with bevacizumab, which results in a median PFS of 4 to 6 months.5,6,20 In patients naïve to the agent, salvage TMZ results in a similar prolongation of PFS.21,22 Other agents appear to have lower efficacy.7,9,23–27 When second-line therapies become ineffective, further salvage agents delay tumor progression by less than 2 months and survival is limited.5,28–30 Thus, alternative treatment options with the potential to more effectively delay progression are needed.
Salvage reirradiation has been reported by several prior groups to have reasonable efficacy (Table 4).16–18,31–37 In a study by Fogh et al, 147 patients with recurrent high-grade gliomas were treated with hypofractionated stereotactic reirradiation to a median dose of 35 Gy in 10 fractions.17 While our study had a median time from initial diagnosis to reirradiation of 21 months, Fogh et al treated patients earlier in their course of progressive disease at a median time of 8 months. In contrast to our heavily pretreated population, 57% of their cohort had salvage surgery and none received salvage chemotherapy prior to reirradiation. Likely because of these differences, our median survival of 6.3 months is expectedly shorter than their reported survival of 11 months. Our finding of worse survival in patients who have already progressed on bevacizumab and/or lomustine is not surprising. In a comparable cohort of patients with recurrence while on bevacizumab, median PFS was improved from 1.7 to 2.6 months and median OS was improved from 3.3 to 7.2 months when reirradiation was delivered compared with those transitioning to further salvage chemotherapy.38 Thus, our PFS and OS compare favorably with prior reports of comparably pretreated patients.
Table 4.
Summary of the literature
| Citation | N | Time Period | Median time to reRT, months (range) | Median RT dose/ fx (range) | PTV volume | RN | Time to progression | Median OS after reRT, months | 1-year OS |
|---|---|---|---|---|---|---|---|---|---|
| Kim 1997 | 20 | 1988–1991 | 38 (8–234) | 36 Gy/20 fx (30.6–59.4 Gy) | GTV + 5mm | 15% | N/R | 9 | 26% |
| Shepherd 1997 | 36 | 1989–1994 | 29 (5–174) | 20–50 Gy/4–10 fx | GTV + 2mm | 34% | N/R | 11 | 45% |
| Hudes 1999 | 20 | 1994–1996 | 3.1 (0.7–45.5) | 21–35 Gy/7–10 fx | N/R | 0% | N/R | 10.5 | 20% |
| Cho 1999 | 46 | 1991–1998 | 13 (1–228) | 17 Gy to 50% (9–40 Gy) | GTV | 13% | 86% overall | 12 | 50% |
| Cho 1999 | 25 | 1991–1998 | 13 (1–228) | 37.5 Gy/15 (20–45 Gy/10–20 fx) | GTV | 4% | 86% overall | 12 | 50% |
| Selch 2000 | 21 | 1997–1999 | 11 (3–99) | 25 Gy/5 fx | N/R | 0% | Median 5 months | 6.7 | 15% |
| Voynov 2002 | 10 | 1995–2000 | 18 (2.1–251) | 30 Gy/6 fx | GTV | 20% | N/R | 10 | 50% |
| Combs 2005 | 172 | 1990–2004 | 10A (N/R) | 36 Gy/18 fx | GTV + 5-10mm | 1% | N/R | 8A | 23%A |
| Fogh 2010 | 147 | 1994–2008 | 8 (4–205) | 35 Gy/10 fx | GTV | 0% | 30% at 3 months | 11A | 50% |
| Hundsberger 2013 | 14 | 2009–2011 | 40.9 (6.1–387.9) | 41.6 Gy/16 fx (39–55 Gy) | GTV + 10–25mm | 7% | N/R | 9.1 | 35% |
| Ciammella 2013 | 15 | 2007–2012 | 10.8 (6–54) | 25 Gy/5 to 70% isodose line | GTV + 3–5mm | 13% | N/R | 9.5 | 40% |
reRT: reirradiation, RT: radiotherapy, RN: radionecrosis, PTV: planning target volume, OS: overall survival, N/R: not reported, fx: fractions.
Afor grade IV gliomas.
Acute toxicity in our cohort was minimal and transient, with only 1 patient experiencing acute grade 3 toxicity related to radiotherapy (fatigue). Late toxicity was similarly modest, with only 8% of patients developing symptomatic radionecrosis at a median of 2.6 months from reirradiation with subsequent resolution of symptoms in half of patients. The risk of radionecrosis in the setting of reirradiation varies widely in the literature. Using conventional fractionation to a dose of 36 Gy, Combs et al reported a <1% risk of radionecrosis in 173 patients.35 While hypofractionated radiotherapy to doses of 35 Gy in 3.5-Gy fractions produces a similarly low risk of radionecrosis, doses greater than 40 Gy and/or 5 to 6 Gy per fraction are associated with a significant risk of radionecrosis.17,18,32,39 While we found a greater risk of radionecrosis in patients receiving doses above 45 Gy, these courses were typically conventionally fractionated, so we found no clear association between radionecrosis risk and higher fraction sizes. In fact, 3 of the 4 patients developing radionecrosis were treated with more definitive dose and fractionation schedules for marginal recurrences. As a consequence, our risk of radionecrosis correlated significantly with PTV size, consistent with prior studies.18 Ultimately, our low rate of radionecrosis, particularly with modest PTV sizes and moderately hypofractionated regimens, is consistent with prior literature.
We found no association between the use of concurrent chemotherapy and PFS or OS, consistent with several prior studies.17,40,41 Although a randomized clinical trial reported improved PFS with the addition of concurrent and adjuvant APG101, a CD95 ligand-binding fusion protein, this agent is not currently used in routine clinical practice.42 In particular, as shown in Table 3, concurrent use of bevacizumab with reirradiation did not improve PFS or OS. However, a few smaller studies have shown better PFS and OS in patients receiving concurrent bevacizumab.43,44 The benefit of adding reirradiation to bevacizumab will remain unclear until results from RTOG 1205 are reported.45 We found a significantly lower risk of radionecrosis in patients receiving concurrent bevacizumab, which has been previously reported.36,46 Although radionecrosis can be difficult to differentiate from tumor progression, misclassification in this study was avoided through retrospective analysis of serial imaging. While bevacizumab is commonly used for the treatment of pseudoprogression and radionecrosis, it has not yet been formally evaluated in a prospective manner as a prophylactic agent.47–50 Thus, these data add to the retrospective literature suggesting a preventive effect of bevacizumab on developing radionecrosis and should be viewed as hypothesis-generating.
While the optimal dose of reirradiation has yet to be established, doses of at least 35 Gy were associated with improved PFS but not OS in the current study. Hudes et al reported a significant improvement in tumor responses when doses of at least 30 Gy were delivered.32 While Fogh et al did not evaluate dose-response in relation to PFS, OS was not associated with dose.17 Other studies have similarly not shown a relationship between dose and OS.51,52 Therefore, our findings are consistent with the existing literature and suggest that if 10-fraction regimens are used, at least 35 Gy should be delivered to optimize tumor control. The prognostic value of PTV size in relation to PFS in this series was likely confounded by high incidence of radionecrosis in patients with larger PTV sizes. As a result, statistical significance for both factors was lost on multivariate analysis for PFS when both were included. Thus, it seems likely that dose contributes the most to PFS and OS in this group of patients with limited efficacious options.
Because of the retrospective nature of this study, there are several inherent limitations. At our institution, patients treated with reirradiation tend to be a heterogeneous group with a variety of salvage treatments employed before consideration of reirradiation, which has the potential to mask the influence of initial histology, patterns of failure, prior or concurrent systemic therapy and prior surgery on outcomes. Furthermore, although the general approach to reirradiation at our institution was relatively uniform, it was not standardized. At the time of tumor progression, the majority of patients underwent a transition in therapy or enrolled in hospice. Therefore, no patients were classified as having pseudoprogression, which may be under-reported. In addition, despite showing a delay in tumor progression, we did not prospectively assess quality of life or patient-reported outcomes. Thus, the delay in tumor progression may or may not translate into a quality-of-life benefit. Lastly, the impact of concurrent chemotherapy was not standardized and should be the subject of future investigations.
In conclusion, reirradiation for recurrent glioma is feasible and outcomes compare favorably to salvage chemotherapy after progression on bevacizumab. Toxicity appears to be minimal, particularly in patients receiving concurrent bevacizumab and those with limited recurrence volumes treated with hypofractionated, stereotactic radiation. Further research is needed to prospectively evaluate the impact of reirradiation on tumor progression and quality of life.
Funding
None used.
Conflict of interest statement. None identified.
References
- 1. Stupp R, Mason WP, van den Bent MJ et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 2. Stupp R, Taillibert S, Kanner AA et al. . Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314(23):2535–2543. [DOI] [PubMed] [Google Scholar]
- 3. Youland RS, Schomas DA, Brown PD et al. . Changes in presentation, treatment, and outcomes of adult low-grade gliomas over the past fifty years. Neuro Oncol. 2013;15(8):1102–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shaw EG, Wang M, Coons SW et al. . Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low-grade glioma: initial results of RTOG 9802. J Clin Oncol. 2012;30(25):3065–3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kreisl TN, Kim L, Moore K et al. . Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Friedman HS, Prados MD, Wen PY et al. . Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 7. Brada M, Stenning S, Gabe R et al. . Temozolomide versus procarbazine, lomustine, and vincristine in recurrent high-grade glioma. J Clin Oncol. 2010;28(30):4601–4608. [DOI] [PubMed] [Google Scholar]
- 8. Ruiz J, Case D, Enevold G et al. . A phase II trial of thalidomide and procarbazine in adult patients with recurrent or progressive malignant gliomas. J Neurooncol. 2012;106(3):611–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Friedman HS, Petros WP, Friedman AH et al. . Irinotecan therapy in adults with recurrent or progressive malignant glioma. J Clin Oncol. 1999;17(5):1516–1525. [DOI] [PubMed] [Google Scholar]
- 10. Prados MD, Lamborn K, Yung WK et al. ; North American Brain Tumor Consortium. A phase 2 trial of irinotecan (CPT-11) in patients with recurrent malignant glioma: a North American Brain Tumor Consortium study. Neuro Oncol. 2006;8(2):189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ringel F, Pape H, Sabel M et al. ; SN1 study group. Clinical benefit from resection of recurrent glioblastomas: results of a multicenter study including 503 patients with recurrent glioblastomas undergoing surgical resection. Neuro Oncol. 2016;18(1):96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen MW, Morsy AA, Liang S et al. . Re-do craniotomy for recurrent grade IV glioblastomas: impact and outcomes from the National Neuroscience Institute Singapore. World Neurosurg. 2016;87:439–445. [DOI] [PubMed] [Google Scholar]
- 13. Barker FG 2nd, Chang SM, Gutin PH et al. . Survival and functional status after resection of recurrent glioblastoma multiforme. Neurosurgery. 1998;42(4):709–720; discussion 720–723. [DOI] [PubMed] [Google Scholar]
- 14. Strömblad LG, Anderson H, Malmström P et al. . Reoperation for malignant astrocytomas: personal experience and a review of the literature. Br J Neurosurg. 1993;7(6):623–633. [DOI] [PubMed] [Google Scholar]
- 15. Shrieve DC, Alexander E 3rd, Wen PY et al. . Comparison of stereotactic radiosurgery and brachytherapy in the treatment of recurrent glioblastoma multiforme. Neurosurgery. 1995;36(2):275–282; discussion 282–284. [DOI] [PubMed] [Google Scholar]
- 16. Cho KH, Hall WA, Gerbi BJ et al. . Single dose versus fractionated stereotactic radiotherapy for recurrent high-grade gliomas. Int J Radiat Oncol Biol Phys. 1999;45(5):1133–1141. [DOI] [PubMed] [Google Scholar]
- 17. Fogh SE, Andrews DW, Glass J et al. . Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin Oncol. 2010;28(18):3048–3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shepherd SF, Laing RW, Cosgrove VP et al. . Hypofractionated stereotactic radiotherapy in the management of recurrent glioma. Int J Radiat Oncol Biol Phys. 1997;37(2):393–398. [DOI] [PubMed] [Google Scholar]
- 19. Sulman EP, Ismaila N, Armstrong TS et al. . Radiation therapy for glioblastoma: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation Oncology Guideline. J Clin Oncol. 2017;35(3):361–369. [DOI] [PubMed] [Google Scholar]
- 20. Vredenburgh JJ, Desjardins A, Herndon JE 2nd et al. . Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13(4):1253–1259. [DOI] [PubMed] [Google Scholar]
- 21. Lamborn KR, Yung WK, Chang SM et al. ; North American Brain Tumor Consortium. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10(2):162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prados MD, Yung WK, Fine HA et al. ; North American Brain Tumor Consortium study. Phase 2 study of BCNU and temozolomide for recurrent glioblastoma multiforme: North American Brain Tumor Consortium study. Neuro Oncol. 2004;6(1):33–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cloughesy TF, Filka E, Kuhn J et al. . Two studies evaluating irinotecan treatment for recurrent malignant glioma using an every-3-week regimen. Cancer. 2003;97(9 Suppl):2381–2386. [DOI] [PubMed] [Google Scholar]
- 24. Chamberlain MC. Salvage chemotherapy with CPT-11 for recurrent glioblastoma multiforme. J Neurooncol. 2002;56(2):183–188. [DOI] [PubMed] [Google Scholar]
- 25. Nagpal S, Recht CK, Bertrand S et al. . Phase II pilot study of single-agent etirinotecan pegol (NKTR-102) in bevacizumab-resistant high grade glioma. J Neurooncol. 2015;123(2):277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reardon DA, Desjardins A, Peters K et al. . Phase II study of metronomic chemotherapy with bevacizumab for recurrent glioblastoma after progression on bevacizumab therapy. J Neurooncol. 2011;103(2):371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reardon DA, Desjardins A, Peters KB et al. . Phase II study of carboplatin, irinotecan, and bevacizumab for bevacizumab naïve, recurrent glioblastoma. J Neurooncol. 2012;107(1):155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iwamoto FM, Abrey LE, Beal K et al. . Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology. 2009;73(15):1200–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Norden AD, Young GS, Setayesh K et al. . Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70(10):779–787. [DOI] [PubMed] [Google Scholar]
- 30. Quant EC, Norden AD, Drappatz J et al. . Role of a second chemotherapy in recurrent malignant glioma patients who progress on bevacizumab. Neuro Oncol. 2009;11(5):550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim HK, Thornton AF, Greenberg HS et al. . Results of re-irradiation of primary intracranial neoplasms with three-dimensional conformal therapy. Am J Clin Oncol. 1997;20(4):358–363. [DOI] [PubMed] [Google Scholar]
- 32. Hudes RS, Corn BW, Werner-Wasik M et al. . A phase I dose escalation study of hypofractionated stereotactic radiotherapy as salvage therapy for persistent or recurrent malignant glioma. Int J Radiat Oncol Biol Phys. 1999;43(2):293–298. [DOI] [PubMed] [Google Scholar]
- 33. Selch MT, DeSalles AAF, Solberg TD et al. . Hypofractionated stereotactic radiotherapy for recurrent malignant gliomas. Journal of Radiosurgery. 2000;3(1):3–12. [Google Scholar]
- 34. Voynov G, Kaufman S, Hong T et al. . Treatment of recurrent malignant gliomas with stereotactic intensity modulated radiation therapy. Am J Clin Oncol. 2002;25(6):606–611. [DOI] [PubMed] [Google Scholar]
- 35. Combs SE, Thilmann C, Edler L et al. . Efficacy of fractionated stereotactic reirradiation in recurrent gliomas: long-term results in 172 patients treated in a single institution. J Clin Oncol. 2005;23(34):8863–8869. [DOI] [PubMed] [Google Scholar]
- 36. Hundsberger T, Brügge D, Putora PM et al. . Re-irradiation with and without bevacizumab as salvage therapy for recurrent or progressive high-grade gliomas. J Neurooncol. 2013;112(1):133–139. [DOI] [PubMed] [Google Scholar]
- 37. Ciammella P, Podgornii A, Galeandro M et al. . Hypofractionated stereotactic radiation therapy for recurrent glioblastoma: single institutional experience. Radiat Oncol. 2013;8:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Torcuator RG, Thind R, Patel M et al. . The role of salvage reirradiation for malignant gliomas that progress on bevacizumab. J Neurooncol. 2010;97(3):401–407. [DOI] [PubMed] [Google Scholar]
- 39. Laing RW, Warrington AP, Graham J et al. . Efficacy and toxicity of fractionated stereotactic radiotherapy in the treatment of recurrent gliomas (phase I/II study). Radiother Oncol. 1993;27(1):22–29. [DOI] [PubMed] [Google Scholar]
- 40. Arcicasa M, Roncadin M, Bidoli E et al. . Reirradiation and lomustine in patients with relapsed high-grade gliomas. Int J Radiat Oncol Biol Phys. 1999;43(4):789–793. [DOI] [PubMed] [Google Scholar]
- 41. VanderSpek L, Fisher B, Bauman G et al. . 3D conformal radiotherapy and cisplatin for recurrent malignant glioma. Can J Neurol Sci. 2008;35(1):57–64. [DOI] [PubMed] [Google Scholar]
- 42. Wick W, Fricke H, Junge K et al. . A phase II, randomized, study of weekly APG101+reirradiation versus reirradiation in progressive glioblastoma. Clin Cancer Res. 2014;20(24):6304–6313. [DOI] [PubMed] [Google Scholar]
- 43. Cuneo KC, Vredenburgh JJ, Sampson JH et al. . Safety and efficacy of stereotactic radiosurgery and adjuvant bevacizumab in patients with recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2012;82(5):2018–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Niyazi M, Ganswindt U, Schwarz SB et al. . Irradiation and bevacizumab in high-grade glioma retreatment settings. Int J Radiat Oncol Biol Phys. 2012;82(1):67–76. [DOI] [PubMed] [Google Scholar]
- 45. Radiation Therapy Oncology Group Study 1205: Randomized phase II trial of concurrent bevacizumab with reirradiation versus bevacizumab alone as treatment for recurrent glioblastoma. ClinicalTrials.gov Identifier: NCT01730950 http://clinicaltrials.gov/show/NCT01730950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gutin PH, Iwamoto FM, Beal K et al. . Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2009;75(1):156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Beal K, Abrey LE, Gutin PH. Antiangiogenic agents in the treatment of recurrent or newly diagnosed glioblastoma: analysis of single-agent and combined modality approaches. Radiat Oncol. 2011;6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gonzalez J, Kumar AJ, Conrad CA et al. . Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys. 2007;67(2):323–326. [DOI] [PubMed] [Google Scholar]
- 49. Levin VA, Bidaut L, Hou P et al. . Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79(5):1487–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Torcuator R, Zuniga R, Mohan YS et al. . Initial experience with bevacizumab treatment for biopsy confirmed cerebral radiation necrosis. J Neurooncol. 2009;94(1):63–68. [DOI] [PubMed] [Google Scholar]
- 51. Aktan M, Koc M, Kanyilmaz G. Survival following reirradiation using intensity-modulated radiation therapy with temozolomide in selected patients with recurrent high grade gliomas. Ann Transl Med. 2015;3(20):304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vordermark D, Kölbl O, Ruprecht K et al. . Hypofractionated stereotactic re-irradiation: treatment option in recurrent malignant glioma. BMC Cancer. 2005;5:55. [DOI] [PMC free article] [PubMed] [Google Scholar]