Abstract
The therapeutic landscape of the management of low- and high-grade infiltrating gliomas continues to evolve. Daily clinical decision making in neuro-oncology clinics across the US is frequently challenging, especially for anaplastic and low grade primary brain tumors. The focus of this review is centered on treatments which are approved by the FDA and/or featured in the NCCN Guidelines. Systemic therapy trials using a variety of agents such as temozolomide, bevacizumab, and procarbazine, lomustine, vincristine (PCV), and lastly trials of local therapies including surgical trials using carmustine impregnated wafers as well as trials investigating the administration of tumor treating fields are evaluated. Pivotal trials on the treatment of the primary brain tumors are discussed in detail along with associated correlative studies.
Keywords: bevacizumab, glioma, lomustine, temozolomide, tumor treating fields
Recent advances and ongoing studies are reshaping the clinical management of infiltrating gliomas. This serves as the impetus to review the key therapeutic trials which have lead us to the current treatment paradigms.(Table 1) Over 20 years ago the successful utilization of nitrosourea-impregnanted wafers administered within tumor resection cavities was demonstrated to positively influence outcomes in patients with GBM. Development of orally administered alkylating agent, temozolomide, served to establish a new standard of care for the management of newly diagnosed high-grade gliomas. The introduction of antiangiogenic agents to neuro-oncology and their subsequent FDA approval (bevacizumab) significantly changed practice patterns. More recently results of the studies of tumor treating fields (TTF) provided new evidence that is now changing management of patients with glioblastoma. In parallel, studies evaluating chemoradiotherapy with either temozolomide or procarbazine, CCNU, and vincristine (PCV) have established a new standard of care for the management of grade II and III gliomas. This review will differ in structure from many others by limiting its scope to a few key clinical trials, and the correlative studies associated with them, which define our current standard of care. It will focus on systemic therapies with the exception of the locally administered BCNU wafers and tumor treating fields (TTF).
Table 1.
Pivotal therapeutic trials for infiltrating gliomas
| AUTHOR | YEAR | PHASE | HISTOLOGY | TREATMENTS | OS | PFS |
|---|---|---|---|---|---|---|
| Newlands1 | 1992 | I | Recurrent glioma, melanoma, renal cancer, breast cancer, colorectal cancer, stomach cancer, other cancers | TMZ | NA | NA |
| Bower2 | 1997 | II | Recurrent AA/GBM | TMZ | 5.8 months | 4.2 months |
| Yung3 | 2000 | II | Recurrent GBM | TMZ procarbazine |
NA | 12.4 weeks 8.32 weeks |
| Stupp5 | 2002 | II | Newly Dx GBM | RT/TMZ+TMZ | 16 months | NA |
| Stupp6 | 2005 | III | Newly Dx GBM | RT/TMZ+TMZ RT alone |
14.6 months 12.1 months |
6.9 months 5.0 months |
| Gilbert11 | 2014 | III | Newly Dx GBM | RT/TMZ+TMZ (5/28 day) RT/TMZ+TMZ (21/28 day) |
16.6 months 14.9 months |
5.5 months 6.7 months |
| Wick15 | 2012 | III | Grade III/IV Newly Dx GBM | RT (60 Gy) TMZ (7day on, 7 day off) |
9.6 months 8.6 months |
NA NA |
| Malmstrom16 | 2012 | III | Newly Dx GBM | RT (60 Gy) RT (34 Gy) TMZ (5/28 days) |
6 months 7.5 months 8.3 months |
NA NA NA |
| Brandes17 | 2003 | NA | Newly Dx GBM | RT (59.4 Gy) RT (59.4Gy)+PCV RT (59.4 Gy)+TMZ (5/28 day) |
11.2 months 12.7 months 14.9 months |
NA NA NA |
| Minniti18 | 2008 | II | Newly Dx GBM | RT (60 Gy)/TMZ+TMZ (5/28 day) | 10.6 months | 7 months |
| Minniti19 | 2009 | II | Newly Dx GBM | RT (30 Gy)+TMZ (5/28 day) | 9.3 months | 6.3 months |
| Perry*20 | 2016 | III | Newly Dx GBM | RT (40 Gy)/TMZ+TMZ (5/28 day) RT (40 Gy) |
9.3 months 7.6 months |
5.3 months 3.9 months |
| Friedman22 | 2009 | II | Recurrent GBM | bev bev+irinotecan |
9.7 months 8.9 months |
4.2 months 5.6 months |
| Kreisl25 | 2009 | II | Recurrent GBM | bev followed by bev+irinotecan | 31 weeks | 16 weeks |
| Taal26 | 2014 | II | Recurrent GBM | bev CCNU bev+CCNU |
8 months 8 months 12 months |
3 months 1 month 4 months |
| Wick*27 | 2015 | III | Recurrent GBM | CCNU bev+CCNU |
8.6 months 9.1 months |
1.5 months 4.2 months |
| Gilbert29 | 2014 | III | Newly Dx GBM | RT/TMZ/bev+TMZ/bev RT/TMZ+TMZ |
15.7 months 16.1 months |
10.7 months 7.3 months |
| Chinot30 | 2014 | III | Newly Dx GBM | RT/TMZ/bev+TMZ/bev RT/TMZ+TMZ |
16.8 months 16.7 months |
10.6 months 6.2 months |
| Brem37 | 1991 | I/II | Recurrent grade III/IV glioma | BCNU wafers | 46 weeks | NA |
| Brem38 | 1995 | III | Recurrent grade III/IV glioma | BCNU wafers Surgery+placebo |
31 weeks 23 weeks |
NA NA |
| Westphal40 | 2003 | III | GBM, AA, AO, AOA | BCNU wafers Surgery+placebo |
11.9 months 13.6 months |
5.9 months 5.9 months |
| Stupp44 | 2012 | III | Recurrent GBM | TTF Physician’s choice chemotherapy |
6.6 months 6.0 months |
2.2 months 2.1 months |
| Stupp45 | 2015 | III | Newly Dx GBM | RT/TMZ+TMZ/TTF RT/TMZ+TMZ |
19.6 months 16.6 months |
7.1 months 4.0 months |
| Van Den Bent48 | 2013 | III | AO | RT+PCV RT alone |
43.2 months 30.6 months |
24.3 months 13.2 months |
| Cairncross46,49 | 2013 | III | AO, AOA | PCV+RT RT alone |
4.7 years 4.6 years |
2.6 years 1.7 years |
| Buckner52 | 2016 | III | O, OA, A | RT+PCV RT alone |
13.3 years 7.8 years |
10.4 years 4.0 years |
| Fisher54 | 2015 | II | O, OA, A | RT/TMZ+TMZ | NA | 4.5 years |
Overall survival, OS; progression free survival, PFS; not available, NA; glioblastoma, GBM; anaplastic astrocytoma, AA; anaplastic oligodendroglioma, AO; anaplastic oligoastrocytoma, AOA; astrocytoma, A, oligodendroglioma, O; oligoastrocytoma, OA; temozolomide, TMZ; bevacizumab, bev; tumor treating fields, TTF; procarbazine/CCNU/ vincristine, PCV; diagnosed, Dx.
*currently only in abstract form.
Glioblastoma
Systemic Therapies
Temozolomide
Temozlomide is an oral alkylating agent which has been a component of the standard of care regimen for newly diagnosed glioblastoma for the past decade. When initially evaluated in advanced solid tumors on a single monthly dosing schedule the toxicities were pronounced. Specifically grade 4 nausea and vomiting as well as grade 3 and 4 leukopenia and thrombocytopenia occurred. When spread over 5 contiguous days on a monthly schedule the regimen became more tolerable. In the phase I study two of the four patients with gliomas exhibited partial responses (PR).1 In the phase II Cancer Research Campaign (CRC) trial for recurrent anaplastic astrocytomas (AA) and glioblastomas a response rate (RR) of 11% was noted. Similar RR was seen in both histologic subtypes.2 When compared to procarbazine, temozolomide for recurrent disease was associated with improved progression free survival (PFS) (12.4 weeks vs. 8.32 weeks) and an improved health-related quality of life (HRQOL).3,4 Another phase II trial evaluated RT with concomitant temozolomide (75mg/m2) followed by temozolomide 5/28 days (200mg/m2) in the newly diagnosed glioblastoma demonstrated safety and tolerability with favorable overall survival (OS), 1 year survival, and 2 year survival.5 This was followed by the phase III EORTC/NCIC trial evaluating this regimen in comparison to RT alone. Common grade III/IV toxicities for this and other phase III trials using temozlomide are listed in Table 2. This trial was conducted at 85 centers in Europe and Canada and enrolled 573 patients. The median age was 56 with a range between 23 and 71. While the trial was for glioblastoma a small percentage (7%) of patients enrolled had other diagnoses. Most (84%) underwent debulking surgeries with 40% having complete radiographic resections. RT was initiated at a median of 5 weeks (range 1.7–12.9 weeks) post-surgery. Due to the previously described PCP during RT in earlier trials patients were prophylaxed with Bactrim or pentamidine during that treatment phase. This was the first randomized trial to demonstrate benefit of a chemotherapy in the treatment of newly diagnosed glioblastoma. OS in the temozolomide arm was 14.6 months compared to 12.1 months in the control arm. Two year survival was 26.5% vs. 10.4%, respectively. Progression free survival (PFS) was also improved at 6.9 months vs. 5.0 months.6 Five year survival was 9.8% compared to 1.9% with PFS of 4.1% vs 1.3%.7 Prognosis was significantly influenced by the promoter methylation status of methyl guanine methyl transferase (MGMT), a suicide enzyme which removes methyl adducts from the O6 guanine position on DNA. While numerous techniques have been used to evaluate MGMT promoter methylation status, in this study methylation-specific PCR (MSP) was utilized on over half of the specimens (n=307/573, 53.6%). Two-hundred and six (36% of the total subjects enrolled) of these samples were evaluable. Decreased activity of the enzyme via methylation of its promoter was associated with an improvement in OS (18.2 months vs. 12.2 months, p<.001). The difference was most pronounced when comparing patients in the chemoradiotherapy arm with MGMT promoter methylation (OS 21.7 months, 2 year survival 46.0%) compared to those with unmethylated promoters (OS 12.7 months, 2 year survival 13.8%). The differences in the radiotherapy alone arm between MGMT promoter methylated (OS 15.3 months, 2 year survival 22.7%) and unmethylated (11.8 months, 2 year survival <2%) were not in this study, however, significant.8 Other studies have demonstrated a higher likelihood of radiographic response or stable disease in MGMT promoter methylated patients (71%) treated with radiotherapy alone when compared to MGMT promoter unmethylated patients (42%, P=.001) treated with radiotherapy alone.9 Of additional interest is the higher incidence of pseudoprogression in patients with methylated MGMT promoter treated with chemoradiotherapy compared to unmethylated patients treated with the same regime.10
Table 2.
Grade III/IV adverse events with temozolomide for newly diagnosed glioblastoma
| Grade III/IV toxicities | EORTC/NCIC 13 investigational arm |
RTOG 0525
18 control arm |
RTOG 082535 control arm during chemoradiotherapy |
RTOG 0825
35 control arm during maintenance therapy |
|---|---|---|---|---|
| Leukopenia | 7% | 6% | 2.3% | 6% |
| Lymphopenia | NA | 15% | 9% | 13.4% |
| Neutropenia | 7% | 7% | 3.7% | 5.1% |
| Thrombocytopenia | 12% | 10% | 7.7% | 11.7% |
| Anemia | 1% | 1% | 0.3% | 1.3% |
| Fatigue | 13% | 3% | 2.7% | 9% |
| Rash/dermatologic | 3% | NA | NA | NA |
| Infection | 7% | NA | NA | NA |
| Nausea/vomiting | 2% | 1% | 0.3% | 1.7% |
NA, not available.
Numerous subsequent trials had evaluated methods to potentially optimize the use of temozolomide including adding second agents or altering dosing schedules. The largest (n=833 randomized, n=1,173 registered) study looking at alternate dosing schedules was the phase III RTOG 0525 trial which compared the treatment regimen employed in the EORTC/NCIC trial with 6 to 12 adjuvant cycles of temozolomide to a dose-dense arm where patients received 21/28 day (75mg/m2/day) for 6 to 12 cycles. There was no significant difference in OS (16.6 months vs. 14.9 months, p=.63) or PFS (5.5 months vs. 6.7 months, p=.06). There was, however, increased grade ≥3 toxicity in the dose-dense arm (34% vs. 53%, p<.001).11 The optimal duration of temozolomide therapy for newly diagnosed glioblastoma remains unclear with some recent abstracts supporting treatment beyond 6 cycles12 and others not.13 Limited data exists to help guide clinical decision making with regards to this question. The phase III EORTC/NCIC trial used 6 cycles. Many subsequent trials used a larger number of cycles, 12 being the most commonly employed. In the RTOG 0525 trial 71% of patients received more than 6 cycles. Of all of the patients without disease progression or toxicity preventing continued treatment (n=261, 78% of the patients on study) a small subset (7%, n=22) discontinued temozolomide after 6 cycles while the majority (71%, n=239) continued beyond 6. There was a non-significant trend toward improved OS (30.2 months [95% CI, 25.5 to 35.4 months] vs 24.9 months [95% CI, 19.2 to 36.2 months]).11 At this time either 6 cycles or 12 cycles of adjuvant temozolomide are deemed to be reasonable by many in the neuro-oncology community.
The optimal management of elderly patients with newly diagnosed glioblastoma is still unclear, although recent studies offered new insights on the potential best options for patients over 65. In general, prognosis is poorer in elderly patients and the ability to tolerate treatments lessens with increasing age. Numerous studies have evaluated an array of regimens, often deescalating the aggressiveness of the therapeutic approach. These have included shorter course radiation with or without temozolomide as well as temozolomide alone on an alternate dosing schedule. The role of MGMT methylation in this group of patients has been investigated and shown to have important prognostic and predictive implications. These important studies have been recently reviewed.14 Of particular relevance are the trials in elderly addressing the use of temozolomide alone versus radiotherapy. The randomized NOA-08 trial compared radiotherapy alone (60 Gy) to an alternate dose temozolomide regimen (100mg/m2, 7 days on/7 days off) and found chemotherapy arm to be non-inferior to radiotherapy. OS was 8.6 months in the temozlomide arm and 9.6 months in the radiotherapy. MGMT promoter methylation detected in 35% of patients remained predictive. Patients with MGMT promoter methylation experienced a significant improvement in event free survival (8.4 months vs. 4.6 month) when treated with temozlomide compared to radiotherapy. The inverse scenario was seen in patients without MGMT promoter methylation (3.3 months vs. 4.6 months). These findings help support the use of MGMT promoter methylation status in the clinical decision making process for this patient population.15 In the Nordic trial, which randomized elderly patients to standard radiotherapy (60 Gy) alone vs. hypofractionated radiotherapy (34 Gy in 10 fractions) vs. temozolomide (5/28 days for 6 cycles) the temozolomide arm (OS 8.3 months) and hypofractionated radiotherapy arm (OS 7.5 months) had similar outcomes. Both were superior to standard radiotherapy (OS 6 months). MGMT promoter methylation was predictive of response to temozolomide.16 Combined chemoradiotherapy utilizing temozlomide in conjunction with standard dose and hypofractionated radiotherapy has also been demonstrated to be feasible.17–19 The recently completed and presented, but not yet published, phase III CCTG CE.6/EORTC 26062-26061/TROG 08.02 (NCT00482677) enrolled patients ≥65 years old with an ECOG performance status of 0–1. Patients were randomized to radiotherapy alone (40 Gy in 15 fractions) vs. radiotherapy (40 Gy in 15 fractions) with concomitant temozolomide followed by 12 cycles of adjuvant temozolomide lendsadditional insight into the management of this patient population. In this study there was a clear improvement in OS (9.3 months vs. 7.6 months) and PFS (5.3 months vs. 3.9 months) strongly supporting the utilization of combined chemoradiotherapy with short course radiation in the elderly with reasonable performance status.20 Finally, there is support for very short course radiotherapy (25 Gy in 5 fractions) alone over short course (40 Gy in 15 fractions) in the frail (KPS as low as 50%) elderly.21
Temozolomide remains a mainstay of therapy for newly diagnosed glioblastoma. Its use in anaplastic astrocytoma is also widespread. Its role in the elderly as well as in tumors without MGMT promoter methylation is actively being defined.
Bevacizumab
With the highly angiogenic nature of high grade gliomas and the substantially elevated levels of vascular endothelial growth factor (VEGF) in glioblastomas there has been great interest in targeting the VEGF pathway. Bevacizumab, the humanized monoclonal antibody targeting VEGF has been the best studied agent. It received accelerated FDA approval for recurrent glioblastoma based on the results of two phase II trials. The first, BRAIN trial, randomized 167 patients between bevacizumab alone (10mg/kg every two weeks) and bevacizumab plus irinotecan (125mg/m2 or 340mg/m2 every two weeks, depending on the use of enzyme-inducing anti-epileptic drugs). This trial was not powered to compare bevacizumab vs. bevacizumab plus irinotecan. With statistically similar outcomes many in the neuro-oncology community utilize bevacizumab as a single agent, in accordance with its FDA approval. When compared to historical controls there was a significant improvement in 6 month PFS (PFS6) of 35.1% in the bevacizumab alone arm and 50.2% in the combination therapy arm. This is in contrast to 9–21% with irinotecan alone or other salvage chemotherapy regimens. The radiographic response rates (RR) were also substantially better (28.2% bevacizumab alone, 37.8% bevacizumab plus irinotecan) compared to other salvage regimens (<10%).22 RR and PFS at landmarks of 9, 18, and 26 weeks was predictive of survival.23 Both RR and PFS6 need to be interpreted with caution as the radiographic improvements are at least in part secondary to the mechanism of action of bevacizumab which blocks VEGF and in turn decreases vascular permeability which then leads to decreased extrusion of gadolinium from the vasculature.(Fig. 1) OS was 9.7 months (bevacizumab alone) and 8.9 months (bevacizumab plus irinotecan) in comparison to historical controls of 7.5 months.22 Patients treated on this study were able to substantially decrease their steroid use.24 This is an important point as patients with progressive glioblastoma often require steroids which are associated with a panoply of side effects. In addition steroids dampen immune activity, a concept of growing prominence as investigations into the role of immune modulation and augmentation in the treatment of gliomas continue to progress.
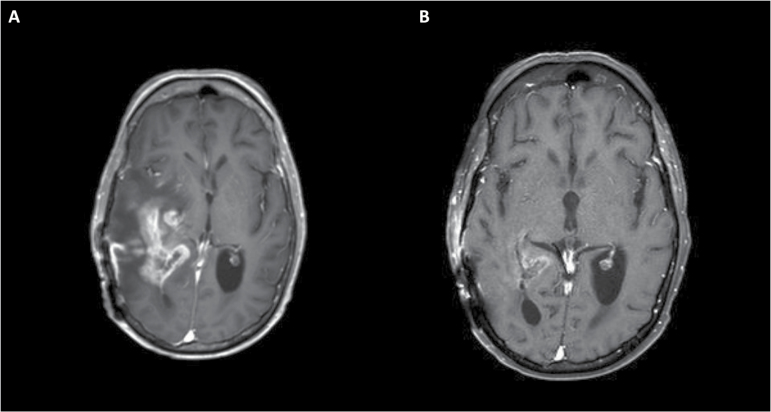
Fig. 1.
Axial T1 post-contrast MRI of a patient with glioblastoma demonstrating substantial enhancement and surrounding edema with associated midline shift (A). Marked decrease in the enhancing lesion and resolution of the midline shift is noted after the initiation of bevacizumab. (B) These findings were associated with notable clinical improvement.
The second trial for progressive glioblastoma which lead to the accelerated FDA approval was a smaller (n=48) single-arm phase II trial of bevacizumab (10mg/kg every two weeks) followed by bevacizumab plus irinotecan (125mg/m2 or 340mg/m2 every two weeks) at progression (n=19). Median OS was 31 weeks, PFS 16 weeks, PFS6 29%, and RR 35% (MacDonald criteria); results fairly comparable to the BRAIN trial. With the addition of irinotecan after progression on bevacizumab there were no objective responses and no clear benefit in PFS or OS.25 Numerous trials in patients with progressive glioblastoma have explored combining bevacizumab with other systemically administered therapies. Until recently, no combination has provided evidence for improvement in efficacy. The randomized three arm BELOB trial conducted in the Netherlands evaluated bevacizumab alone (10mg/kg every 2 weeks), CCNU alone (110mg/m2 every 6 weeks), and bevacizumab plus CCNU (110mg/m2 initially but subsequently decreased to 90mg/m2 due to toxicity). RR and PFS were higher in the bevacizumab containing arms as would be expected. The improvement in OS (12 months in combined arm, 8 months in bevacizumab alone, 8 months in CCNU alone) and PFS6 (42% combined arm, 16% bevacizumab, 13% CCNU) lend strong support to a combined regimen of bevacizumab and nitrosourea in progressive GBM.26 This combination regimen was compared to CCNU alone in the ongoing EORTC 26101 phase III trial. The recently presented, but not yet published, results of this trial proved discordant with the results of the preliminary phase II trial. While the combination regimen demonstrate improved PFS (4.2 months vs 1.5 months, HR=0.49) as would be expected with bevacizumab, there was no significant improvement of OS (9.1 months vs 8.6 months, HR=0.95, P=.650) with the combination over CCNU alone.27 While there was no direct comparison to bevacizumab alone in this study, the results have potential practice changing implications by diminishing use of the combination regimen and potentially dampening excitement around the use of single agent bevacizumab as well. This should be weighed against the favorable decreases in steroid use and epidemiologic studies supporting improved survival since the FDA approval of and widespread use of bevacizumab in this patient population.28
Pronounced radiographic responses, favorable RR, and improved PFS6 of bevacizumab in recurrent glioblastoma paved the way to studies in newly diagnosed glioblastoma. A number of phase II trials as well as two phase III studies were conducted. RTOG 0825 enrolled 978 subjects and randomized 637 to either radiotherapy with concomitant temozolomide followed by up to 12 cycles of temozolomide (5/28 day) vs. the same regimen with the addition of bevacizumab midway through radiotherapy and continuing on up to and beyond progression. Patients with progressive disease were unblinded and could cross over to receive bevacizumab. There was no significant improvement in OS (15.7 months vs 16.1 months, p=.21) and only a non-significant trend for improved PFS (10.7 months vs. 7.3 months, p=.07) with the addition of bevacizumab. While MGMT promoter methylation was redemonstrated to have prognostic significance (OS 14.3 months in promoter unmethylated, 23.2 months in promoter methylated) it was not predictive of response to bevacizumab.29 The parallel AVAglio trial randomized 921 subjects to radiotherapy with concomitant temozolomide followed by 6 cycles of adjuvant temozolomide, as described in the EORTC/NCIC trial discussed earlier, vs. the same regimen with the addition of bevacizumab. Bevacizumab was given every 2 weeks with conversion to every 3 week schedule after the completion of 6 cycles of adjuvant temozolomide. Unlike the RTOG 0825 trial there was no planned crossover although 31.1% of patients in the control arm received subsequent bevacizumab. Similarly disappointing results for OS were noted (16.8 months vs. 16.7 months, p=.10). The improvement in PFS was significant (10.6 months vs. 6.2 months, p<.001). MGMT promoter methylation was again not predictive of response to bevacizumab.30 Discordance exists between the health-related quality of life (HR-QOL) results between the two trials. The AVAglio trial, utilizing the EORTC QLQ-C30/BN20 questionaires, demonstrated no significant deterioration of HRQOL during the progression free interval with the addition of bevacizumab.31 In addition to patient reported outcome assessment via EORTC QLQ-C30/BN20, the RTOG 0825 trial included the MD Anderson Symptom Inventory-Brain Tumor module (MDASI-BT). Extensive neurocognitive testing was also performed using the Hopkins Verbal Learning Test-Revised (HVLT-R), Trail Making Test (TMT), and Controlled Oral Word Association (COWA). A greater degree of deterioration in performance on neurocognitive testing as well as on patient reported HR-QOL questionaires was seen in the bevacizumab arm.29 The underlying explanation for the deterioration in symptoms and neurocognitive function still requires elucidation. The addition of bevacizumab to the standard of care in the newly diagnosed setting warrants additional investigation. Marked radiographic responses can be seen in some patients due at least in part to the drug’s mechanism of action. These are often associated with improvements in clinical parameters such as decreased steroid requirements.32 Of even greater interest, a subgroup of patients in the AVAglio trial had improved OS with the addition of bevacizumab. The isocitrate dehydrogenase 1 (IDH1) wild type proneural molecular subtype of glioblastoma had a significant improvement in OS (17.1 months vs. 12.8 months, p=.002). Thirty-eight percent (n=349/941) of subjects, a similar percentage as those evaluated in the pivotal MGMT promoter methylation evaluations, retrospectively underwent biomarker analysis via a NanoString platform.33 This is of particular importance as the proneural subgroup does not appear to have any clear improvement in survival with the use of our traditional chemoradiotherapy.34 Similar investigations into the RTOG 0825 study population are ongoing. If distinct subsets of patients with newly diagnosed glioblastoma are found to benefit from upfront bevacizumab, it would argue for universal screening of glioblastoma patients for the predictive biomarker which could have a meaningful clinical application. In light of the lack of improvement in OS in the broad (but not entire) glioblastoma patient population and the retrospective studies supporting no detriment to delaying the initiation of bevacizumab35,36 the optimal timing of this agent will need to be more clearly defined. When contemplating the use of bevacizumab, the clinician will need to weigh the above discussed benefits with the potential toxicities of this agent. These include an increased risk of thromboembolic events, bleeding, wound healing/wound dehiscence, and visceral perforations.29,30 Serious thrombocytopenia and neutropenia also seem to be more common when bevacizumab was used concomitantly with radiotherapy and bevacizumab.29
Locally Administered Therapies
Surgically Implanted BCNU Wafers
A phase I/II trial of surgical resection with associated implantation of BCNU-impregnanted polyanhydride biodegradable polymer implantable wafers was conducted in 21 patients with recurrent malignant gliomas. The majority of patients received the maximum of 8 wafers implanted in the walls of the resection cavity. The highest dosing level of BCNU was 12.7mg per wafer. The polymer was found to release drug for ~3 weeks. Median overall survival (OS) after implantation of wafers was 46 weeks (mean 48 weeks). 1 year survival post-wafer implantation was 38%.37 The second treatment dose (7.7mg BCNU per wafer) was utilized in the subsequent phase III trials. At this dose, the local side-effects such as brain necrosis and edema were rare, and there was minimal, if any, systemic toxicity.37–39 While all BCNU doses were well tolerated the mean OS was superior in the second dosing cohort (64 weeks) compared to the third (32 weeks).37 Higher dosing levels are associated with both increased local toxicity as well as detectable serum levels.39
Two phase III trials of surgery with associated implantation of BCNU-impregnated prolifeprosan 20 wafers lead to FDA approval of these wafers in 1997 for patients with recurrent glioblastoma. The first of these trials randomized 222 patients with recurrent gliomas to resection with implantation of non-BCNU impregnated wafers vs. resection with the implantation of BCNU-impregnated wafers. This trial was for patients with grade III and IV gliomas although over 16% of patients had other diagnoses, predominantly grade 2 oligodendrogliomas. Median OS was 23 weeks vs 31 weeks (HR=0.67, p=0.006). In a multiple regression model stratifying for pathology found similar results (HR=0.69, p=0.01). In the glioblastoma patients six month survival also favored those treated with BCNU-impregnated wafers (64% vs. 44%, p=0.02). No substantial differences regarding toxicities between the treatment arms were noted. Nor was there any difference in the change of performance status between the two groups.38 In another pivotal phase III trial 240 patients with newly diagnosed high-grade gliomas underwent surgery with implantation of non-BCNU impregnated wafers vs. surgery with implantation of BCNU-impregnated wafers. Median OS in the intention to treat (ITT) analysis was 11.9 months vs. 13.6 months (P=.03) in the entire study cohort (grade III and IV gliomas). There was a higher percentage of non-glioblastoma patients with better prognoses (15.8% vs. 11.7%) in the BCNU-impregnated wafer arm.40 The survival advantage in the ITT analyses persisted at 3 years with 25% in the BCNU impregnated wafer arm compared to 6% in the control arm (P=.01).41 In the glioblastoma subset, however, the improvement in OS (11.4 months vs. 13.5 months) was not significant (P=.1). In the investigational arm there was a higher incidence of cerebrospinal fluid (CSF) leak (0.8% vs. 5%) and increased intracranial pressure (1.7% vs. 9.7%).40 Another retrospective analyses did not support an increased risk of infection despite the reported increase in the risk of CSF leak.42 In clinical practice, the incidence of CSF leak and increased intracranial following BCNU wafers placement is low as shown by Atenello and colleagues.43 While there was increase in intracranial hypertension in the BCNU-wafer arm there was a favorable delay in decline of Karnovsky performance score (KPS) and neuroperformance measures in the BCNU-wafer arm.40 The results of this study led to the FDA approval of BCNU wafers for newly diagnosed GBM in 2003. Lack of significant benefit in the glioblastoma population (as opposed to the ITT population also including other tumor histologies) has dampened some enthusiasm for this treatment. The studies described above were initiated in the pre-temozolomide era. The use of BCNU-wafers has diminished with the advent of effective systemic therapies. Additionally, prior treatment with BCNU-wafers is an exclusion criteria for many neuro-oncologic clinical trials do, in part, to difficulty with interpretation of radiographic imaging. This in turn, has influenced a more limited pattern of use in academic medical centers.
Tumor Treating Fields
TTF are a novel therapeutic modality, which employ four specially designed arrays directly applied to the scalp to deliver medium frequency electrical fields to the tumor. The primary mechanism of action is the disruption of mitosis and the subsequent apoptosis of the tumor cells. This device initially received FDA approval for recurrent supratentorial glioblastoma based on the randomized phase III EF-11 trial. In this trial 237 patients were randomized between TTF and physicians’ choice systemic therapy. In the physicians’ choice arm 31% received bevacizumab and 25% received nitrosorueas. Almost half of the patients in the control arm received regimens with suboptimal evidence for efficacy. The TTF arm was found to be comparable to the control arm with respect to OS (6.6 months vs. 6.0 months, p=.27), PFS6 (21% vs. 16.3%, p=.13), and RR (14% vs. 9.6%, p=.19).44 The utilization of systemic therapies without substantial efficacy in the control arm has led to dampened enthusiasm for the use of TTF in the recurrent setting.
More recently (October 2015), the FDA approved TTF for the treatment of newly diagnosed supratentorial glioblastoma. This approval was based on the results of the randomized phase III EF-14 trial which was stopped early after an interim analysis.45 This trial enrolled 695 patients at 83 sites internationally. Patients were treated with radiotherapy and concomitant temozolomide followed by adjuvant temozolomide (5/28 days) in the control arm. In the investigational arm TTF were added to the adjuvant temozlomide in patients without evidence of progression after radiotherapy. This would have excluded both pseudoprogressors and true progressors. TTF were continued for up to 24 months or until second progression. The randomization was 2:1 in favor of the investigational arm. In an analysis of the first 315 patients there was a substantial improvement in the PFS (7.1 months vs. 4.0 months, p=.0014). PFS was the primary endpoint in the intent to treat (ITT) analysis. Secondary endpoints: OS (19.6 months vs. 16.6 months, p=.034) and 2 year survival (43% vs 29%) were also superior in the TTF arm in the ITT analyses. This was achieved without any substantial increase in toxicity other than local site reactions, the majority of which were grade 1 and 2 (43% of patients). How this novel treatment modality will be incorporated into existing treatment paradigms is yet to be seen. Complete analyses of the full patient cohort are still pending. Results of these analyses will potentially influence the likelihood of more widespread adoption of this therapeutic modality. Its limited toxicity profile may lend it well towards combinations with other more toxic modalities. However, practical difficulties with using this treatment modality will also need to be considered. These difficulties include the necessity of regularly shaving one’s head, near continuous utilization of the device (>18 hours per day recommended), the prolonged course of treatment (potentially beyond disease progression), logistical issues when travelling both locally as well as further afield. These points should be weighed by both the clinician and the patients in the decision making process when this treatment modality is being contemplated.
Anaplastic Gliomas
Procarbazine/CCNU/Vincristine
The recent fully mature results of two phase III cooperative group trials, one conducted in the US and Canada (RTOG 9402), the other in Europe (EORTC 26951), have helped redefine the management of grade III gliomas. (Table 3) Preliminary analyses had demonstrated a significant improvement in PFS but not in OS.46,47 In EORTC 26951 368 patients with anaplastic oligodendroglioma were enrolled with a median follow up of 140 months. They were randomized to receive radiation alone to 59.4 Gy or radiation followed by CCNU (110mg/m2) day #1, procarbazine (75mg/m2) day #8–21, and vincristine (1.4mg/m2) day #8 and #29 on 6 week cycles for 6 cycles. OS was 30.6 months vs 43.2 months (HR 0.75, 95%CI .6-.95). In the 1p19q co-deleted patients there was a significant improvement in OS in the combined modality arm. Retrospective evaluation of the IDH1 mutation (n=179) found it to be prognostic with OS of 8.4 years in the mutated patients and 1.4 years in the non-mutated.48 In RTOG 9402 patients with both anaplastic oligodendroglioma and anaplastic oligoastrocytoma (a group excluded from EORTC 26951) were included. Two hundred and ninety-one patients were enrolled with a median follow up of 5 years. In this study patients were also randomized to RT alone vs. RT plus PCV. A lower dose of RT was given (50.4 Gy) and chemotherapy was administered prior to radiation. Patients received only 4 six week cycles. The dose of CCNU was slightly higher (130mg/m2). OS in the overall group was not significantly different. However, there was a significant improvement (HR 0.59, 95%CI .37-.95) in OS in the 1p19q co-deleted group treated with PCV plus radiation.49 The findings from these studies provide clear support for the combination of radiation and chemotherapy in newly diagnosed anaplastic gliomas, particularly those with 1p19q co-deletion. Questions remain regarding the optimal chemotherapy regimen. There has been extensive use of temozolomide in these patients due to its efficacy in grade IV gliomas and better tolerability compared to PCV. Retrospective data further supports superiority of a combined radiochemotherapy approach and demonstrates trends towards possible improved outcomes with PCV over temozolomide in these patients.50
Table 3.
Trials for grade III and high-risk grade II gliomas
| Trial | Phase | Tumor histology | Criteria for high-risk | Treatment regimen | OS | PFS | Prolonged survival |
|---|---|---|---|---|---|---|---|
| RTOG 9802 52 , 53 | III |
Grade II
O, OA, A |
≤39 years and subtotal resection/biopsy or ≥40 years |
RT (54 Gy) followed by PCV(X6)* vs RT (54 Gy) alone |
13.3 years vs 7.8 years |
10.4 years vs 4.0 years |
10 year survival
62% vs 41% |
| RTOG 0424 54 | II |
Grade II
O, OA, A |
3 of the following features:
≥40 years old, astrocytic features in the histology, bihemispheric tumor, ≥6cm preoperative tumor size, preoperative ECOG performance status >1 |
RT (50.4 Gy) with concomitant TMZ followed by adjuvant TMZ (X12)** | NA | NA |
3 year survival
73.1% |
| RTOG 9402 46 , 49 | III |
Grade III
AO, AOA |
Not applicable | PCV (X4) followed by RT (50.4 Gy) vs RT (50.4 Gy) |
Overall group
4.6 years vs 4.7 years 1p19q codeleted population 14.7 years vs 7.3 years |
2.6 years vs 1.7 years |
NA |
| EORTC 2695147 47 , 48 | III |
Grade III
AO |
Not applicable | RT (59.4 Gy) followed by PCV (X6) vs RT (59.4 Gy) |
Overall group
43.2 months vs 30.6 months 1p19q codeleted population Not yet reached vs 112 months |
156 months vs 50 months |
5 year survival
43.4% vs 37.0% |
OS, overall survival; PFS, progression free survival; O, oligodendroglioma; OA, oligoastrocytoma; A, astrocytoma; RT, radiotherapy; PCV, procarbazine, CCNU, vincristine; TMZ, temozolomide; NA, not available; AO, anaplastic oligodendroglioma; AOA, anaplastic oligoastrocytoma.
*PCV [CCNU 110mg/m2 D#1, vincristine 1.4mg/m2 D#8 and D#29, procarbazine 60mg/m2 D#8–21] was administered over 8 week cycles; **TMZ 75mg/m2 during RT followed by 150-200mg/m2 /day for 5/28 days; ***PCV [CCNU 130mg/m2 D#1, vincristine 1.4mg/m2 D#8 and D#29, procarbazine 60mg/m2 D#8–21] was administered over 6 week cycles, ****PCV [CCNU 110mg/m2 D#1, vincristine 1.4mg/m2 D#8 and D#29, procarbazine 60mg/m2 D#8–21] was administered over 6 week cycles.
Ongoing trials are attempting to further clarify the role of chemotherapy in newly diagnosed grade III gliomas. The CODEL and CATNON paired trials are evaluating this in 1p19q co-deleted and non-deleted patients respectively. In CODEL the two current treatment arms include radiotherapy followed by PCV vs radiotherapy with concomitant temozolomide followed by adjuvant temozolomide. In CATNON the arms are radiotherapy alone, radiotherapy with concomitant temozolomide, radiotherapy followed by temozolomide, and radiotherapy with concomitant temozolomide followed by temozolomide. Finally, NOA-04 is looking at sequential radiotherapy, PCV, and temozolomide as single treatment modalities at time of study enrollment, followed by crossover to another treatment modality at progression. This differs substantially from RTOG 9402 and EORTC 26951 where the combination of radiotherapy and chemotherapy is used initially. Preliminary reports have revealed no significant differences thus far in OS, PFS, or time to progression after two treatment modalities.51 The results of these studies support radiotherapy and chemotherapy in combination in the newly diagnosed setting for anaplastic oligodendrogliomas, particularly those with 1p19q codeletion. Variability can be seen with regards to the timing of radiotherapy in relation to the chemotherapy as well as the chemotherapy regimen employed. While temozolomide or PC (without the vincristine) can be employed in our practices we advocate for PCV as the level one evidence supports its use.
High-Risk Low-Grade Gliomas
For a long-time the optimal management of low grade infiltrating gliomas had not been well defined. Management ranged from watchful waiting to aggressive multimodality treatment involving surgery, radiation, and chemotherapy. Over the recent past, the analyses of mature data from clinical trials for high-risk low grade gliomas support the utilization of combined radiation and chemotherapy for this subset of patients.
Procarbazine/CCNU/Vincristine
The RTOG 9802 trial for high-risk low-grade gliomas including grade II oligodendrogliomas, oligoastrocytomas, and astrocytomas demonstrated improved OS with the combination of radiotherapy and chemotherapy. High-risk was defined as ≤39 years old with subtotal resection/biopsy or ≥40 years old. In this phase III trial 251 patients were randomized to radiotherapy (54 Gy in 30 fractions) followed by 6 cycles of PCV vs. radiotherapy alone. The PCV cycles were similar to those used for anaplastic gliomas except that they were 8 weeks long, essentially adding an additional two weeks without active chemotherapy on the second half of each cycle. OS, PFS, and 10 year survival were all significantly better in the combined modality arm (Table 2)52 Cognitive function assessed by crude yet easily reproducible mini-mental status exam revealed no substantial decline in the average scores over time and no difference between the two arms.53 Over 3/4th of patients in the radiotherapy alone arm received salvage chemotherapy. These data indicate that early treatment decision making in this group of patients influences outcomes in meaningful ways. One can begin to appreciate that watchful waiting in the setting of presumed low-grade gliomas may not be the best option for higher risk patients.
Temozolomide
RTOG0424 evaluated a different chemotherapy regimen in conjunction with radiotherapy in patients with high-risk gliomas (grade II oligodendrogliomas, oligoastrocytomas, or astrocytomas). A different set of high-risk criteria were employed which necessitated at least 3 of the following features: ≥40 years old, astrocytic features in the histology, bihemispheric tumor, ≥6cm preoperative tumor size, and preoperative ECOG performance status >1. In this phase II trial (n=129) patients received radiotherapy (50.4 Gy) with concomitant temozolomide followed by 12 additional cycles of temozolomide (150–200mg/m2 for 5/28 days). Three year survival was increased when compared to a historical control (73.1% vs. 54%, p<.001). The 3 year PFS was 59.2% and the 5 year survival was 57.1%. A substantial percentage of patients experienced grade 3 (43%) and 4 (10%) adverse events.54 This study lends further support for combined chemoradiotherapy in high-risk low grade astrocytomas. At this time we lack evidence supporting superiority of one chemotherapy regimen over another in this patient population.
Conclusions
An understanding of the pivotal trials which underlie the standard of care for infiltrating gliomas is essential for both the clinician caring for patients and the researcher working on future advances in the field. In this review we have taken a somewhat unconventional approach removing discussion of the preclinical science as well as treatments without formal indications for treating infiltrating gliomas as these have been well covered in other publications.53–55 This approach has allowed us to focus on the later phase clinical trials and emphasize some of their nuances. The framework provided will allow readers to place important new advances in the field of neuro-oncology within the context of what has come before. The review will also provide clinicians with a quick access to clinically relevant information. Many of the discoveries discussed are practice-changing, and detailed knowledge of the pivotal trials is necessary to successfully educate patients and other non-neuro-oncology providers, and to practice evidence-based medicine. While some of the data is still maturing and interpretation of the studies’ results often differs among the clinicians, we are now very close to being able to practice more personalized neuro-oncology than ever before.
Funding
No funding was used to support this work.
Conflict of interest statement. Dr Lukas has served on an advisory board for Novocure.
Dr Mrugala has served on an advisory board for Novocure
References
- 1. Newlands ES, Blackledge GR, Slack JA, et al. Phase I trial of temozolomide (CCRG 81045, M&B 39831, NSC 362856). Br J Cancer. 1992;65(2):287–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bower M, Newlands ES, Bleehen NM, et al. Multicenter CRC phase II trial in recurrent or progressive high-grade glioma. Cancer Chemother Pharmacol. 1997;40(6):484–488. [DOI] [PubMed] [Google Scholar]
- 3. Yung WK, Albright RE, Olson J, et al. A phase II study of temozolomide vs procarbazine in patients with glioblastoma multiforme in first relapse. Br J Cancer. 2000;83(5):588–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Osoba D, Brada M, Yung WK, Prados MD. Health-related quality of life in patients with anaplastic astrocytoma during treatment with temozolomide. Eur J Cancer. 2000;36(14):1788–1795. [DOI] [PubMed] [Google Scholar]
- 5. Stupp R, Dietrich PY, Ostermann Kraljevic S, et al. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol. 2002;20(5):1375–1382. [DOI] [PubMed] [Google Scholar]
- 6. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 7. Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomized phase III study: 5-year analysis of the EORTC/NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 8. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 9. Rivera AL, Peloski CE, Gilbert MR, et al. MGMT promoter methylation is predictive of response to radiotherapy and prognostic in the absence of adjuvant alkylating chemotherapy for glioblastoma. Neuro Oncol. 2010;12(2):116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brandes AA, Francheshi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant chemoradiotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26(13):2192–2117. [DOI] [PubMed] [Google Scholar]
- 11. Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruzevick J, Silgard E, Graham C, Mrugala M. Is more chemotherapy better? Prolonged survival in patients with newly diagnosed glioblastoma receiving more than six cycles of adjuvant temozolomide. ATPS-74. Neuro Oncol. 2015;17(Suppl 5):v34 [Google Scholar]
- 13. Blumenthal DT, Stupp R, Zhang P, et al. The impact of extended adjuvant temozolomide in newly-diagnosed glioblastoma: a secondary analysis of EORTC and NRG Oncology/RTOG. ATCT-08. Neuro Oncol. 2015;17(Suppl 5):v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jordan JT, Gerstner ER, Batchelor TT, Cahill DP, Plotkin SR. Glioblastoma care in the elderly. Cancer. 2016;122(2):189–197. [DOI] [PubMed] [Google Scholar]
- 15. Wick W, Platten M, Meisner C, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–715. [DOI] [PubMed] [Google Scholar]
- 16. Malmstrom A, Gronberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. [DOI] [PubMed] [Google Scholar]
- 17. Brandes AA, Vastola F, Basso U, et al. A prospective study on glioblastoma in the elderly. Cancer. 2003;97(3):657–662. [DOI] [PubMed] [Google Scholar]
- 18. Minniti G, De Sanctis V, Muni R, et al. Radiotherapy plus concomitant and adjuvant chemotherapy with temozolomide for glioblastoma in elderly patients. J Neurooncol. 2008;88(1):97–103. [DOI] [PubMed] [Google Scholar]
- 19. Minniti G, De Sanctis V, Muni R, et al. Hypofractionated radiotherapy followed by adjuvant chemotherapy with temozolomide in elderly patients with glioblastoma. J Neurooncol. 2009;91(1):95–100. [DOI] [PubMed] [Google Scholar]
- 20. Perry JR, Laperierre N, O’Callaghan CJ, et al. A phase III randomized controlled trial of short-course radiotherapy with or without concomitant and adjuvant temozolomide in elderly patients with glioblastoma (CCTG CE.6, EORTC 26062-26061, TROG 08.02, NCT00482677). J Clin Oncol. 2016;34(Suppl):Abstract LBA2. [Google Scholar]
- 21. Roa W, Kepka L, Kumar N, et al. International Atomic Energy Agency Randomized Phase III Study of Radiation Therapy in Elderly and/or Frail Patients With Newly Diagnosed Glioblastoma Multiforme. J Clin Oncol. 2015;33(35):4145–4150. [DOI] [PubMed] [Google Scholar]
- 22. Friedman H, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 23. Prados M, Cloughesy T, Samant M, et al. Response as a predictor of survival in patients with recurrent glioblastoma treated with bevacizumab. Neuro Oncol. 2011;13(1):143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vredenburgh JJ, Cloughesy T, Samant M, et al. Corticosteroid use in patients with glioblastoma at first or second relapse treated with bevacizumab in the BRAIN study. Oncologist. 2010;15(12):1329–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizuamb plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial):a randomised controlled phase II trial. Lancet Oncol. 2014;15(9):943–953. [DOI] [PubMed] [Google Scholar]
- 27. Wick W, Brandes AA, Gorlia T, et al. Phase III trial exploring the combination of bevacizumab and lomustine in patients with first recurrence of glioblastoma: the EORTC 26101 trial. Neuro Oncol. 2015;17(Suppl 5):v1. Abstract LB-05. [Google Scholar]
- 28. Johnson DR, Leeper HE, Uhm JH. Glioblastoma survival in the United States improved after Food and Drug Administration approval of bevacizumab: a population-based analysis. Cancer. 2013;119(19):3489–3495. [DOI] [PubMed] [Google Scholar]
- 29. Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 31. Taphoorn MJ, Henriksson R, Bottomley A, et al. Health-related quality of life in a randomized phase III study of bevacizumab, temozolomide, and radiotherapy in newly diagnosed glioblastoma. J Clin Oncol. 2015;33(19):2166–2175. [DOI] [PubMed] [Google Scholar]
- 32. Prados MD. “We will know it when we see it;” bevacizumab and glioblastoma. Neuro Oncol. 2014;16(4):469–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sandmann T, Bourgon R, Garcia J, et al. Patients with proneural glioblastoma may derive overall survival benefit from the addition of bevacizumab to first-line radiotherapy and temozolomide: retrospective analysis of the AVAglio trial. J Clin Oncol. 2015;33(25):2735–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Piccioni DE, Selfridge J, Mody RR, et al. Deferred use of bevacizumab is not associated with diminished efficacy. Neuro Oncol. 2014;16(6):815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hamza MA, Mandel JJ, Conrad CA, et al. Survival outcome of early versus delayed bevacizumab treatment in patients with recurrent glioblastoma. J Neurooncol. 2014;119(1):135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brem H, Mahaley MS, Jr, Vick NA, et al. Interstitial chemotherapy with drug polymer implants for the treatment of recurrent gliomas. J Neurosurg. 1991;74(3):441–446. [DOI] [PubMed] [Google Scholar]
- 38. Brem H, Piantadosi S, Burger PC, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet. 1995;345(8956):1008–1012. [DOI] [PubMed] [Google Scholar]
- 39. Olivi A, Grossman SA, Tatter S, et al. Dose escalation of carmustine in surgically implanted polymers in patients with recurrent malignant glioma: a New Approaches to Brain Tumor Therapy CNS Consortium trial. J Clin Oncol. 2003;21(9):1845–1849. [DOI] [PubMed] [Google Scholar]
- 40. Westphal M, Hilt DC, Bortey E, et al. A phase III trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma Neuro Oncol. 2003;5(2):79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Westphal M, Ram Z, Riddle V, et al. Gliadel wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial. Acta Neurochir (Wien). 2006;148(3):269–275. [DOI] [PubMed] [Google Scholar]
- 42. Chaichana KL, Kone L, Bettegowda C, et al. Risk of surgical site infection in 401 consecutive patints with glioblastoma with and without carmustine wafer implantation. Neurol Res. 2015;37(8):717–726. [DOI] [PubMed] [Google Scholar]
- 43. Attenello FJ, Mukherjee D, Datoo G, et al. Use of Gliadel (BCNU) wafer in the surgical treatment of malignant glioma: a 10-year institutional experience. Ann Surg Oncol. 2008;15(10):2887–2893. [DOI] [PubMed] [Google Scholar]
- 44. Stupp R, Wong ET, Kanner AA, et al. Novo TTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomized phase III trial of a novel treatment modality. Eur J Cancer. 2012;48(14):2192–2202. [DOI] [PubMed] [Google Scholar]
- 45. Stupp R, Taillibert S, Kanner AA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized trial. JAMA. 2015;314(23):2535–2543. [DOI] [PubMed] [Google Scholar]
- 46. Intergroup Radiation Therapy Oncology Group Trial 9402 , Cairncross G, Berkey B, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Trial 9402. J Clin Oncol. 2006;24(18):2707–2714. [DOI] [PubMed] [Google Scholar]
- 47. Van den Bent MJ, Carpentier JF, Brandes AA, et al. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organization for Research and Treatment of Cancer phase III trial. J Clin Oncol. 2006;24(18):2715–2722. [DOI] [PubMed] [Google Scholar]
- 48. Van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31(3):344–350. [DOI] [PubMed] [Google Scholar]
- 49. Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long term results of RTOG 9402. J Clin Oncol. 2013;31(3):337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lassman AB, Iwamoto FM, Cloughesy TF, et al. International restrospective study of over 1000 adults with anaplastic oligodendroglial tumors. Neuro Oncol. 2011;13(6):649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wick W, Roth P, Wiestler B, et al. Long term analysis of the NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide. J Clin Oncol. 2015;33(Suppl). Abstract #2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Buckner JC, Shaw EG, Pugh SL, et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma.. N Engl J Med. 2016;374(14):1344–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Prabhu RS, Won M, Shaw EG, et al. Effect of the addition of chemotherapy to radiotherapy on cognitive function in patients with low-grade glioma: secondary analysis of RTOG 98-02. J Clin Oncol. 2014;32(6):535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fisher BJ, Hu C, Macdonald DR, et al. Phase II study of temozolomide-based chemoradiation for high-risk low-grade gliomas: preliminary results of Radiation Therapy Oncology Group 0424. Int J Radiat Oncol Biol Phys. 2015;91(3):497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mrugala MM. Advances and challenges in the treatment of glioblastoma: a clinician’s perspective. Discov Med. 2013;15(83):221–230. [PubMed] [Google Scholar]
- 56. Lukas RV, Nicholas MK. Update in the treatment of high-grade gliomas. Neurol Clin. 2013;31(3):847–867. [DOI] [PubMed] [Google Scholar]
- 57. Ahluwalia MS, Chang SM. Medical therapy of gliomas. J Neurooncol. 2014;119(3):503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]