Abstract
Background:
In new onset type 1 diabetes (T1D), overall C-peptide measures such as area under the curve (AUC) C-peptide and peak C-peptide are useful for estimating the extent of β-cell dysfunction, and for assessing responses to intervention therapy. However, measures of the timing of C-peptide responsiveness could have additional value.
Objectives:
We assessed the contribution of the timing of C-peptide responsiveness during oral glucose tolerance tests (OGTTs) to hemoglobin A1c (HbA1c) variation at T1D diagnosis.
Methods:
We analyzed data from 85 individuals <18 years with OGTTs and HbA1c measurements at diagnosis. Overall [AUC and peak C-peptide] and timing measures [30–0 minute C-peptide (early); 60 to 120 minute C-peptide sum-30 minutes (late); 120/30 C-peptide; time to peak C-peptide] were utilized.
Results:
At diagnosis, the mean (±SD) age was 11.2 ± 3.3 years, body mass index (BMI)-z was 0.4 ± 1.1, 51.0% were male. The average HbA1c was 43.54 ± 8.46 mmol/mol (6.1 ± 0.8%). HbA1c correlated inversely with the AUC C-peptide (P < 0.001), peak C-peptide (P < 0.001), early and late C-peptide responses (P < 0.001 each), and 120/30 C-peptide (P < 0.001). Those with a peak C-peptide occurring at ≤60 minutes had higher HbA1c values than those with peaks later (P = 0.003). HbA1c variance was better explained with timing measures added to regression models (R2 = 11.6% with AUC C-peptide alone; R2 = 20.0% with 120/30 C-peptide added; R2 = 13.7% with peak C-peptide alone, R2 = 20.4% with timing of the peak added). Similar associations were seen between the 2-hour glucose and the C-peptide measures.
Conclusions:
These findings show that the addition of timing measures of C-peptide responsiveness better explains HbA1c variation at diagnosis than standard measures alone.
Keywords: C-peptide, glycemia, HbA1c, OGTT, type 1 diabetes
1 |. INTRODUCTION
The area under the curve (AUC) C-peptide and peak C-peptide, commonly employed measures of overall C-peptide levels during oral glucose tolerance tests (OGTTs), have been used as markers of β-cell function in the peri-diagnostic period of type 1 diabetes (T1D). However, it is unclear whether such measures are optimal, as they do not take insulin secretory patterns into account. Measures of the timing of C-peptide responsiveness have been shown to change during the progression to T1D.1 In that report, the early (30–0 minute) C-peptide response progressively declined from approximately 2 years prior to until the time of diagnosis, while the late ([60 + 90 + 120 minute C-peptide]−30 minutes) C-peptide response progressively increased.1 However, to our knowledge, no studies have yet described the timing of C-peptide responses at diagnosis and how this timing may relate to glycemia. Thus, our objective in this report was to examine associations of glycemia, primarily as indicated by HbA1c, with measures of the timing of C-peptide responsiveness at the diagnosis of T1D. Moreover, we sought to determine whether the addition of those timing measures to standard overall C-peptide measures (such as the AUC C-peptide) in multivariable models helps to explain HbA1c variance.
It has been difficult to assess C-peptide responsiveness at diagnosis in the community, as insulin is almost always administered at that time, and may interfere with endogenous insulin secretory patterns. However, data from the Diabetes Prevention Trial-Type 1 (DPT-1) provides the opportunity for such assessments. In that study, a large proportion of individuals were diagnosed by OGTT, prior to the clinical administration of insulin; many also had HbA1c measurements at diagnosis. We have, therefore, utilized these features of DPT-1 to improve our understanding of how glycemia relates to C-peptide responsiveness at diagnosis.
2 |. METHODS
The DPT-1 study has been previously described.2 Participants (relatives of persons with T1D, ages 1–45 years) were monitored with 2-hour OGTTs bi-annually for diagnosis of diabetes. ADA criteria for the diagnosis of diabetes were used for the interpretation of OGTTs.3 Glucose and C-peptide were measured fasting and every 30 minutes. If an OGTT was in the diabetic range, a confirmatory OGTT was performed (unless otherwise contraindicated). The date of diagnosis was based on the first OGTT, which was used for the following analyses.
DPT-1 participants (n = 85) <18.0 years of age at diagnosis with complete OGTT data and a HbA1c measurement performed simultaneously at diagnosis were included in the analysis. C-peptide and glucose assays were performed as previously described.2 Plasma glucose was measured by the glucose oxidase method. C-peptide levels were determined by radioimmunoassay. Measurable C-peptide was considered ≥0.07 nmol/L (≥0.2 ng/mL) because this was the lowest level detectable by that method. HbA1c was measured using the VARIANT (Bio-Rad Laboratories, Hercules, California) instrument. The measurable range for this instrument was 16 to 162 mmol/mol (3.6%−17.0%), with normal levels being 44 mmol/mol (<6.2%). The AUC C-peptide measure was calculated using the trapezoidal rule with the mean values of AUC (divided by 120 minutes) being presented.
Associations between HbA1c and OGTT C-peptide indices at diagnosis were assessed through correlation and multivariable regression. To examine the association of HbA1c with the timing of C-peptide secretion, early and late C-peptide responses were utilized, as previously described.1 The ratio of the 120-minute over 30 minutes C-peptide (120/30 C-peptide) and timing of the peak C-peptide (occurring at ≤60 vs >60 minutes) were used as measures of relative timing.
Multivariable linear regression models were utilized to determine whether adding measures of relative timing of C-peptide responsiveness would better account for HbA1c variance than overall C-peptide indices. Because body mass index (BMI) z-score and age are known to influence C-peptide responses,4 linear regression analyses were performed with BMI z-score and age included as covariates.
The likelihood ratio test was utilized to test the statistical significance of the contribution to the R2 from adding C-peptide covariates to linear regression models. Squared partial correlations were used to examine the individual contributions of the covariates in explaining HbA1c variance. Please note that although the sum of partial correlations and the overall R2 of a model can be similar in multivariable models, they are not identical.
3 |. RESULTS
The mean (±SD) age of participants was 11.2 ± 3.3 years and the BMI z-score was 0.4 ± 1.1; 51% were male. HbA1c values at diagnosis averaged 43.54 ± 8.46 mmol/mol (6.1 ± 0.8%). Metabolic characteristics of the participants at diagnosis are indicated in Table 1.
TABLE 1.
Metabolic characteristics of patients at the time of diagnosis
| Variablea | Value (n = 85) |
|---|---|
| HbA1c at diagnosis (mmol/mol) | 43.54 ± 8.46 (6.1 ± 0.8%) |
| AUC C-peptide (ng/mL) | 2.9 ± 1.6 (1.0 ± 0.5)b |
| Peak C-peptide (ng/mL) | 3.8 ± 2.3 (1.3 ± 0.8)b |
| Early C-peptide response (ng/mL) | 1.4 ± 1.1 (0.5 ± 0.4)b |
| Late C-peptide response (ng/mL) | 1.9 ± 3.0 (0.6 ± 1.0)b |
| 120/30 C-peptide response | 1.3 ± 0.5 |
| % Peak C-peptide at >60 minutes | 68 |
| 0 minute C-peptide (ng/mL) | 1.3 ± 0.9 (0.4 ± 0.3)b |
| 30 minutes C-peptide (ng/mL) | 2.7 ± 1.5 (0.9 ± 0.5)b |
| 60 minutes C-peptide (ng/mL) | 3.0 ± 1.7 (1.0 ± 0.6)b |
| 90 minutes C-peptide (ng/mL) | 3.4 ± 2.0 (1.1 ± 0.7)b |
| 120 minutes C-peptide (ng/mL) | 3.5 ± 2.3 (1.2 ± 0.8)b |
Abbreviations: AUC, area under the curve; HbA1c, hemoglobin A1c.
Values are mean (±SD) unless otherwise indicated.
C-peptide values in parenthesis are reported in SI units (nmol/L).
3.1 |. Associations of HbA1c with overall C-peptide responses
HbA1c was inversely correlated with measures of overall C-peptide responsiveness: AUC C-peptide (r = −0.34; P < 0.001); peak C-peptide (r = −0.37; P < 0.001).
3.2 |. HbA1c associations after partitioning the timing of C-peptide responses
There were significant inverse correlations of HbA1c with both the early and late C-peptide responses (r = −0.34 and −0.38, respectively; P < 0.001 for both), Table 2. These associations are demonstrated in Figure 1A,B, in which HbA1c values are compared among the lowest, the middle and the highest tertiles for the early and late C-peptide distributions-(early-response:−47.70 ± 10.66-for-lowest-vs 38.72 ± 4.26 mmol/mol-for-highest-[6.5 ± 1.0-vs-5.7 ± 0.4%], P < 0.001;-late-response:−47.54 ± 10.46-for-lowest-vs 39.65 ± 5.10 mmol/mol-for-highest-[6.5 ± 1.0-vs-5.8 ± 0.5%], P = 0.001).
TABLE 2.
Correlation coefficients and R2 for associations of HbA1c with C-peptide indices at diagnosis
| Correlation (r) | R2 (%) | P-value | |
|---|---|---|---|
| AUC C-peptide | −0.34 | 11.6 | <0.001 |
| Peak C-peptide | −0.37 | 13.7 | <0.001 |
| Early C-peptide response | −0.34 | 11.6 | <0.001 |
| Late C-peptide response | −0.38 | 14.4 | <0.001 |
| 120/30 C-peptide | −0.40 | 16.0 | <0.001 |
Abbreviations: AUC, area under the curve; HbA1c, hemoglobin A1c.
FIGURE 1.
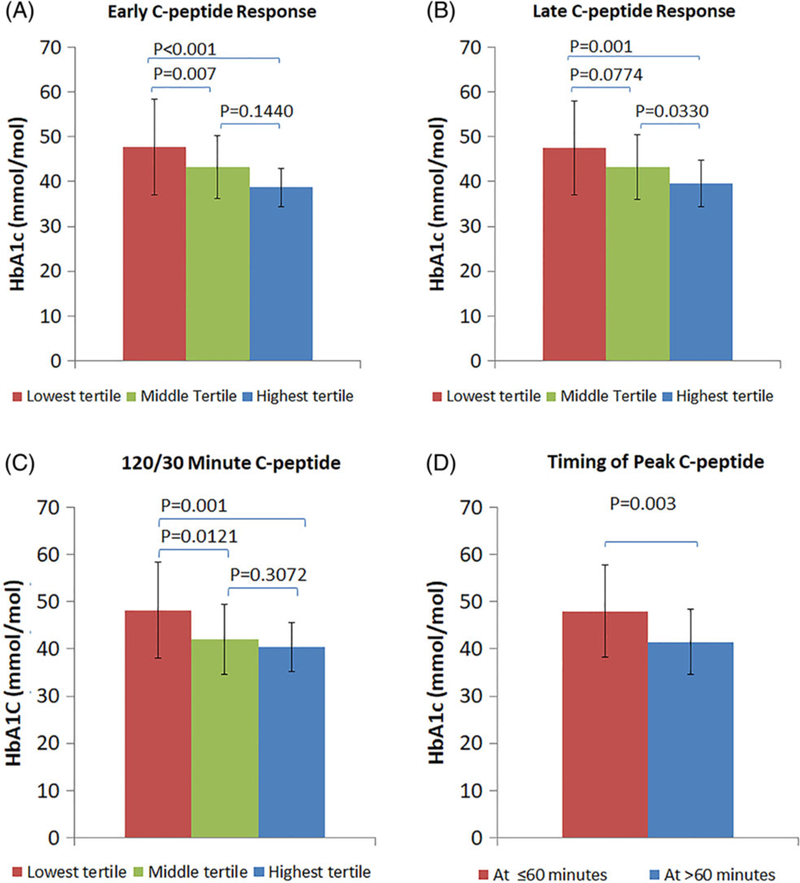
(A) Comparison of HbA1c values (mean ± SD) among the lowest, middle and highest tertiles of the early C-peptide response. (B) Comparison of HbA1c values (mean ± SD) among the lowest, middle and highest tertiles of the late C-peptide response. (C) Comparison of HbA1c values (mean ± SD) among the lowest, middle and highest tertiles of the 120/30 minutes C-peptide. (D) Comparison of HbA1c values (mean ± SD) between the peak C-peptide occurring at ≤60 minutes and the peak C-peptide occurring at >60 minutes
3.3 |. Associations of HbA1c with the relative timing of C-peptide responses
We examined HbA1c associations with the relative timing of C-peptide responsiveness. There was an inverse correlation between HbA1c and the 120/30 C-peptide (r = −0.40; P < 0.001). HbA1c values were significantly greater in the lowest 120/30 C-peptide tertile than in the highest tertile (48.20 ± 10.16 vs 40.36 ± 5.25 mmol/mol [6.6 ± 0.9 vs 5.8 ± 0.5%], P = 0.001) (Figure 1C).
Also apparent was the influence of the relative timing of the C-peptide response when we examined the association of HbA1c with the timing of the peak C-peptide (Figure 1D). Those with a peak C-peptide at ≤60 minutes (n = 27) had higher HbA1c values than those with a peak C-peptide at >60 minutes (n = 58) (48.03 ± 9.80 vs 41.45 ± 6.90 mmol/mol [6.5 ± 0.9 vs 5.9 ± 0.6%], P= 0.003).
Supporting Information: Tables S1–S4 show comparisons of OGTT indices between the lowest and highest tertiles of the C-peptide indices in Figure 1.
3.4 |. The use of the timing of the C-peptide response to account for HbA1c variance
We utilized multivariable regression models to assess whether adding measures of the timing of C-peptide responsiveness would better account for HbA1c variance. Table 3 shows overall and partial R2 values for associations of HbA1c with C-peptide indices as covariates in the multivariable regression models, with and without the inclusion of BMI z-score and age. As indicated for the partial R2 indices, HbA1c was significantly associated with each of the C-peptide indices in all models (range: P < 0.001 to P = 0.013). Within the models, the contribution of the measures in explaining variance tended to be similar.
TABLE 3.
R2 values for associations of HbA1c with C-peptide indices in multivariable models
| Model | Unadjusted |
BMI z-score and age included in model |
||||
|---|---|---|---|---|---|---|
| Overall R2 of model | Partial R2 | P-value for partial R2 | Overall R2 | Partial R2 | P-value for partial R2 | |
| (A) AUC C-peptide; 120/30 C-peptide | 0.20 | 0.07, 0.12* | 0.012, 0.002 | 0.22 | 0.12, 0.10* | 0.001, 0.004 |
| (B) Peak C-peptide; peak C-peptide after 60 minutes | 0.20 | 0.10, 0.10* | 0.003, 0.004 | 0.21 | 0.14, 0.08* | <0.001, 0.013 |
| (C) Early C-peptide; 120/30 C-peptide | 0.25 | 0.14, 0.18** | <0.001, <0.001 | 0.24 | 0.14, 0.17** | <0.001, <0.001 |
| (D) Early C-peptide; late C-peptide | 0.20 | 0.09, 0.12** | 0.005, 0.001 | 0.20 | 0.10, 0.13** | 0.004, 0.001 |
Abbreviations: AUC, area under the curve; BMI, body mass index; HbA1c, hemoglobin A1c.
P ≤ 0.01.
P ≤ 0.001 for contribution to the R2 from the addition of the second term in each model.
When timing variables of C-peptide secretion were added to overall measures in Model A (120/30 C-peptide added to AUC C-peptide) and Model B (peak C-peptide after 60 minutes added to peak C-peptide), the contributions to the R2 were statistically significant in both the unadjusted and adjusted models (P ≤ 0.05 for all). When both variables were measures of timing (ie, absent overall measures) in Model C (early C-peptide and 120/30 C-peptide) and Model D (early C-peptide and late C-peptide), all contributed significantly to the R2 in the unadjusted and adjusted models (P ≤ 0.01 for all).
3.5 |. Associations of the 2-hour glucose with C-peptide measures
As HbA1c values at diagnosis were indicative of glycemia for several months prior to diagnosis, we assessed whether the association of glycemia with the timing of the C-peptide response was also evident within the same OGTT at diagnosis. Associations were therefore examined between the 2-hour glucose (designated as the measure of glycemia post-glucose load) and C-peptide indices. Similar to HbA1c, the 2-hour glucose correlated inversely with both early and late C-peptide responses (r = −0.43 and −0.46, respectively; P < 0.001) for both. There was again an inverse correlation with the 120/30 C-peptide (r = −0.51; P < 0.001). When the early C-peptide response and the 120/30 C-peptide were included as independent variables in a regression model, 43.0% of the 2-hour glucose variance was explained. The AUC C-peptide alone accounted for 16.4% of the 2-hour glucose variance.
4 |. DISCUSSION
This study is the first to examine the relationship of HbA1c at diagnosis with measures of the timing of C-peptide responses. HbA1c was at least as associated with C-peptide measures of timing and relative timing at diagnosis as with the AUC and peak C-peptide. When C-peptide measures of timing and relative timing were added to the overall measures, appreciably more HbA1c variance was explained. The lower HbA1c values associated with a greater late C-peptide response, a higher 120/30 C-peptide, and a peak C-peptide occurring after 60 minutes suggest the importance of maintaining insulin secretion during the latter part of the OGTT, and the need for its quantitative assessment. The AUC C-peptide and the peak C-peptide do not specifically capture this important aspect of β-cell function. We also observed appreciable associations between the 2-hour glucose and the C-peptide timing measures. These findings are relevant, since the 2-hour glucose is a major diagnostic criterion for T1D.
Our findings also showed that besides the specific effect of adding C-peptide timing indices to overall measures, timing indices can provide appreciable information with regard to glycemia even in the absence of overall measures (ie, AUC and peak C-peptide levels). The combination of the early C-peptide response and the 120/30 C-peptide in a model resulted in an appreciable R2 value, as seen in Table 3. The contributions in explaining glycemia by the additions of the C-peptide timing indices should be considered in the context of the low range of C-peptide values at the diagnosis of T1D. Those indices help to discern even subtle changes of C-peptide in that range. Although such changes might be small in overall magnitude, they could have a substantial influence on glycemia.
Other studies such as The Environmental Determinants of Diabetes in the Young (TEDDY) and the Diabetes Autoimmunity Study in the Young (DAISY) studies have assessed β-cell function in children with T1D near diagnosis. In both studies,5,6 researchers assessed residual β-cell function post-diagnosis using serial mixed meal tolerance tests (MMTTs). However, neither study assessed measures of timing of C-peptide during a diagnostic OGTT.
The results further suggest that the timing and relative timing of the C-peptide response should be considered in developing end-points for β-cell preservation trials. Because measures assessing the timing of C-peptide secretion have been used as predictor(s) of islet allograft dysfunction in islet transplant studies,7 such measures could possibly also be useful for assessing residual β-cell function in new onset clinical trials.
Clinically, an understanding of the pattern of insulin responsiveness to a glucose challenge could provide insights into the heterogeneity of new onset T1D, and potentially provide more physiologic approaches for individualized treatment paradigms.
The range of HbA1c values and the differing patterns of C-peptide responsiveness could be reflective of the variability of β-cell function at the diagnosis of T1D, even among those diagnosed by OGTT. However, it is unclear to what extent the differences were a function of the timing of the OGTT during progression or because of the heterogeneity of T1D. It is important to determine whether more preserved C-peptide secretion at diagnosis persists, as those with even modest residual β-cell function have a lower risk of microvascular complications and hypoglycemia.8–10
The study has limitations. HbA1c levels might not have reflected actual concurrent associations between glucose and C-peptide at diagnosis, as HbA1c is indicative of glycemia over several months. However, associations were similar for the 2-hour glucose of the diagnostic OGTT. MMTTs have been used in insulin preservation trials of new onset patients, and OGTT timing measures are not necessarily applicable to MMTTs. Still, the evidence for the importance of sustaining later insulin secretion would appear likely to translate to MMTTs.
Future investigations of the timing of C-peptide responses appear warranted. Associations of glycemia with such measures should be performed in other cohorts. C-peptide timing measures could be assessed as predictors of T1D in natural history and prevention trials. Those measures could also be assessed as end-points indicative of C-peptide loss in the peri-diagnostic period. Although MMTTs are not performed at diagnosis, the timing of C-peptide responsiveness might be examined in MMTTs near diagnosis. Finally, it would be of interest to study associations of continuous glucose monitoring (CGM) indices with the timing of C-peptide responses in OGTTs or MMTTs performed in close proximity to the CGM.
In conclusion, overall measures of C-peptide secretion, the AUC C-peptide and the peak C-peptide, are not fully indicative of β-cell secretory function at diagnosis. The consideration of the timing of insulin secretion following a glucose challenge would allow for a better characterization of β-cell dynamic function at the diagnosis of T1D.
Supplementary Material
ACKNOWLEDGEMENTS
Members of the Type 1 Diabetes TrialNet Study Group and TrialNet Affiliate Centers are listed in the online only (electronic Supporting Information). The Type 1 Diabetes TrialNet Study Group is a clinical trials network currently funded by the National Institutes of Health (NIH) through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, through the cooperative agreements U01 DK061010, U01 DK061034, U01 DK061042, U01 DK061058, U01 DK085461, U01 DK085465, U01 DK085466, U01 DK085476, U01 DK085499, U01 DK085509, U01 DK103180, U01 DK103153, U01 DK103266, U01 DK103282, U01 DK106984, U01 DK106994, U01 DK107013, U01 DK107014, UC4 DK106993, and the Juvenile Diabetes Research Foundation International (JDRF). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the JDRF.
Funding information
National Institute of Allergy and Infectious Diseases; National Institute of Diabetes and Digestive and Kidney Diseases; Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/ Award Numbers: UC4 DK106993, U01 DK107014, U01 DK107013, U01 DK106994, U01 DK106984, U01 DK103282, U01 DK103266, U01 DK103153, U01 DK103180, U01 DK085509, U01 DK085499, U01 DK085476, U01 DK085466, U01 DK085465, U01 DK085461, U01 DK061058, U01 DK061042, U01 DK061034, U01 DK061010
ABBREVIATIONS:
- 120/30 C-peptide
120/30 minutes C-peptide ratio
- CGM
continuous glucose monitor
- MMTT
mixed meal tolerance test
- OGTT
oral glucose tolerance test
- T1D
type 1 diabetes
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no duality of interest associated with this manuscript.
Data availability
The data were analyzed or generated during the study and is available on request from the authors.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Sosenko JM, Skyler JS, Herold KC, Palmer JP. The metabolic progression to type 1 diabetes as indicated by serial oral glucose tolerance testing in the Diabetes Prevention Trial-Type 1. Diabetes 2012;61(6): 1331–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diabetes Prevention Trial-Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med 2002;346:1685–1691. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care 2015;38(suppl 1):S8–S16. [DOI] [PubMed] [Google Scholar]
- 4.Sosenko JM, Geyer S, Skyler JS, et al. The influence of body mass index and age on C-peptide at the diagnosis of type 1 diabetes in children who participated in the diabetes prevention trial-type 1. Pediatr Diabetes 2018;19(3):403–409. 10.1111/pedi.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steck AK, Larsson HE, Liu X, et al. Residual beta-cell function in diabetes children followed and diagnosed in the TEDDY study compared to community controls. Pediatr Diabetes 2017;18(8):794–802. 10.1111/pedi.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan CL, Taki I, Dong F, et al. Comparison of metabolic outcomes in children diagnosed with type 1 diabetes through research screening (Diabetes Autoimmunity Study in the Young [DAISY]) versus in the Community. Diabetes Technol Ther 2015;17(9):649–656. 10.1089/dia.2015.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baidal DA, Faradji RN, Messinger S, et al. Early metabolic markers of islet allograft dysfunction. Transplantation 2009;87(5):689–697. 10.1097/TP.0b013e318195c249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the Diabetes Control and Complications Trial. Diabetes Care 2003;26:832–836. [DOI] [PubMed] [Google Scholar]
- 9.Sjoberg S, Gunnarsson R, Gjotterberg M, Lefvert AK, Persson A, Ostman J. Residual insulin production, glycaemic control and prevalence of microvascular lesions and polyneuropathy in long-term type 1 (insulin-dependent) diabetes mellitus. Diabetologia 1987;30: 208–213. [DOI] [PubMed] [Google Scholar]
- 10.Panero F, Novelli G, Zucco C, et al. Fasting plasma C-peptide and micro- and macrovascular complications in a large clinic-based cohort of type 1 diabetic patients. Diabetes Care 2009;32:301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


