Abstract
Frontotemporal lobar degeneration with τ-pathology (FTLD-tau) is one of a group of neurodegenerative diseases that manifests with cognitive decline. Alzheimer (AD) and cerebrovascular lesions are commonly noted in the brains of most elderly individuals, begging the question as to whether 1. co-existing AD and vascular pathology or age contribute to the development of FTLD-tau disorders and vice versa and 2. FTLD-tau-like pathology can be found in nondiseased individuals. We studied brains of FTLD-tau cases exhibiting 1. argyrophilc grain disease (AGD), 2. progressive supranuclear paralysis (PSP), 3. corticobasal degeneration (CBD), or 4. Picks disease (PiD) for coexisting AD and vascular pathology for comparison to that of non-diseased individuals and AD patients. As we previously reported, we confirmed that AGD lowered the threshold for AD pathology to cause dementia. Such an effect was not seen in PSP, CBD or PiD. In PiD, white matter degeneration and demyelination was observed in the frontal and temporal lobes in association with small vessel disease (SVD)-related changes in white matter arteries. Age at death varied among the four types of FTLD-tau. PiD cases were youngest at death followed by CBD, PSP and finally AGD. In 9.8 % of non-diseased controls, we found grains, coiled bodies, and τ-positive astrocytes mimicking an AGD-like pattern. Moreover, the prevalence of FTLD-tau pathology in non-diseased individuals increased with age. In summary, this study demonstrates that age impacts of the diversity of neuropathological changes in FTLD-tau. The age-related coexistence of AD-related pathology is, thereby, associated with AGD but not with PSP, CBD and PiD. Moreover, severe SVD and white matter demyelination is associated with PiD indicating a role of vascular co-pathology in this type of FTLD-tau. Finally, our finding that FTLD-tau-related pathological lesions occur in non-diseased individuals suggests that preclinical stages of FTLD-tau exist. As such, our results indicate that age, together with vascular and AD-related co-pathology contribute to the morphological appearance of FTLD-tau.
Keywords: FTLD-tau, Alzheimer’s disease, vascular pathology, preclinical stages, tau, age
Introduction
Dementia is a symptom of multiple different neurodegenerative and cerebrovascular disorders. Among the neurodegenerative disorders Alzheimer’s disease (AD), with its intraneuronal aggregates of abnormal phosphorylated τ-protein in neurofibrillary tangles (NFTs) and its extracellular aggregates of amyloid β-protein (Aβ) in amyloid plaques, is the most frequent cause of dementia (Thies et al. 2013). In contrast, frontotemporal lobar degeneration (FTLD) represents a heterogeneous group of dementing disorders that features aggregates of different proteins such as the microtubule-associated protein tau τ, transactive response DNA-binding protein (TDP43) or fused in sarcoma protein (FUS) (Mackenzie et al. 2010). FTLD subtypes characterized by τ pathology (FTLD-tau) include argyrophilic grain disease (AGD), progressive supranuclear paralysis (PSP), corticobasal degeneration (CBD), Picks disease (PiD), NFT-predominant dementia and others (Mackenzie et al. 2010). All types of FTLD-tau may lead to dementia but PSP and CBD cases are more likely to present with motor symptoms before onset of dementia. AGD frequently co-occurs with AD-related pathology (Braak and Braak 1998a).
AD-related pathology, vessel wall pathology, e.g. atherosclerosis (AS), cerebral small vessel disease (SVD), cerebral amyloid angiopathy (CAA), and vascular tissue lesions (brain infarction, microinfarction, and cerebral hemorrhage) are frequently found in elderly brains (Arriagada et al. 1992; Braak et al. 2011; Price et al. 1991; Thal et al. 2012; Thal et al. 2013). It is not clear whether there are differences regarding AD and/or vascular pathology among FTLD-tau subtypes and whether there are respective differences regarding the age range at death.
Moreover, FTLD-tau-like pathological lesions are reported in non-demented individuals exhibiting a pattern similar to AGD (Braak and Braak 1998a; Thal et al. 2005; Tolnay et al. 1997). Recently, NFT-pathology in the absence of amyloid plaques in non-demented individuals has been considered to represent a primary age-related tauopathy (PART) (Crary et al. 2014). It is not clear whether non-diseased cases with argyrophilic grains, coiled bodies and τ-positive astrocytes may represent preclinical stages of specific FTLD-tau subtypes or whether such lesions are age-related as suggested for PART.
To address these questions we studied the four most common types of FTLD-tau (AGD, PSP, CBD, and PiD) for their association with age, AD pathology, SVD, CAA, brain infarction, hemorrhage and white matter lesions as well as for their occurrence in a collection of non-diseased individuals.
Material and Methods
Study cohort
A total sample of 373 post mortem brains of patients who died between 18 and 101 years of age (mean = 73,77 years) covering all stages of AD-related NFT and Aβ pathology was investigated, including 187 non-demented elderly (age range: 18–99 years), 104 AD (age range: 56–101 years), 30 AGD (age range: 62–98 years), 28 PSP (age range: 59–92 years), 15 CBD (age range: 52–86 years), and 9 PiD cases (age range: 53–74 years) (Suppl. Tab. 1d). All autopsy brains were collected in accordance with local ethical committee guidelines and the federal law governing the use of human tissue in Germany, the USA, and the United Kingdom. Dementia was considered when individuals clinically met the DSM-IV criteria for dementia (American Psychiatric Association 1994) as reported in the records.
AD was diagnosed when dementia was observed and when the degree of AD-related neuropathology indicated at least a moderate likelihood for AD according to acknowledged criteria (Hyman et al. 2012). PSP, CBD, and PiD were diagnosed neuropathologically in the event that clinical recordings displayed respective deficits (Dickson and Litvan 2003; Hauw and Agid 2003; Mackenzie et al. 2010). AGD was diagnosed when clinical records reported cognitive deficits, the presence of argyrophilic grains could be confirmed neuropathologically, and the amount of AD-related pathology, vascular pathology or any other kind of pathology failed to explain these cognitive deficits (Josephs et al. 2008; Thal et al. 2005). Cases without neurological or psychiatric symptoms in their history were considered as control cases for the symptomatic diseases. Non-diseased cases exhibiting AD-related τ-lesions as well as amyloid plaques and vascular pathology were classified as controls with preclinical AD pathology (p-preAD) (Thal et al. 2013). Control cases exhibiting NFTs in the absence of amyloid plaques were considered to represent PART (Crary et al. 2014). Likewise, FTLD-tau-like lesions such as argyrophilic grains, coiled bodies, τ-containing astrocytes of different morphologies and ballooned neurons in non-diseased cases were noted as FTLD-tau-related pathology.
Neuropathology and immunohistochemistry
The brains of all individuals were fixed in a 4% aqueous formaldehyde solution for at least three weeks before undergoing neuropathological evaluation. Tissue blocks covering the regions listed in table 1 were excised and embedded in paraffin or polyethylene glycol (PEG; Merck-Schuchardt, Hohenbrunn, Germany). The paraffin sections were cut at 10 μm, whereas the PEG-blocks were sectioned at 100 μm. Paraffin sections were stained with hematoxylin & eosin (H&E) for general pathology assessment, PEG sections with aldehydefuchsin – Darrow red, Campbell-Switzer silver staining to detect amyloid plaques and white matter demyelination, and Gallyas silver staining to visualize NFTs and neuritic plaques (NPs) (Braak and Braak 1991a). The elastica van Gieson staining technique was used to document SVD-related lesions (Tab. 2).
Table 1:
Brain regions studied for FTLD-tau, AD-and vascular pathology (H&E = hematoxilin and eosin; AF-DR = aldehydefuchsin Darrow red)
| region | Staining | ||
|---|---|---|---|
| H&E or AF-DR | anti-Aβ | anti-abnormal T-protein | |
| Frontal cortex | + | FTLD-tau: PSP, PiD, CBD | |
| occipital cortex (Brodman area 17) | + | + | + |
| Hippocampus, entorhinal cortex | + | + | + |
| Basal ganglia | + | − | FTLD-tau: PSP, PiD, CBD |
| Midbrain | + | − | − |
| Pons | + | − | − |
| medulla oblongata | + | − | − |
| cerebrellum | + | − | − |
Table 2:
List of stainings and antibodies
| Senile Plaques + CAA |
NFT/ NT/ NP |
Grains | Coiles bodies | Astroglial τ inclusions | Vessel wall pathology (Atherosclerosis, SVD) | Vascular tissue lesions (infarts hemorrhages, white matter demeylination (WML)) | Reference/ Clone, Distributor, dilution, pretreatment | |
|---|---|---|---|---|---|---|---|---|
| H&E | + | + | ||||||
| Elastica van Gieson (EVG) | + | |||||||
| Gallyas | (+) | + | + | + | + | Braak & Braak 1991 | ||
| Campbell-Switzer | + | (+) | WML | Braak & Braak 1991 | ||||
| anti-abnormal τ protein | + | + | + | + | AT-8, Pierce-Endogen, USA, 1/1000 | |||
| anti-amyloid β protein | + | 4G8, Covance, Dedham, USA, 1/5000, formic acid pretreatment |
Abbreviations: CAA = cerebral amyloid angiopathy; NFT = neurofibrillary tangle; NT = neuropilthread; NP = neuritic plaque.
The presence or absence of brain infarcts was recorded dichotomously for each case. Gross infarcts, lacunar infarcts and microinfarcts were considered as infarcts being present. Likewise, intracerebral hemorrhages of all sizes were considered and rated dichotomously as presence of hemorrhage. Microbleeds, i.e. bleedings restricted to the perivascular space, were not considered. Immunohistochemistry was performed with antibodies listed in table 2 to stain amyloid plaques, NFT, NPs, and non-AD τ pathology as recommended by Brain Net Europe (Alafuzoff et al. 2008a; Alafuzoff et al. 2006; Alafuzoff et al. 2008b). Primary antibodies were detected with biotinylated secondary antibodies and the ABC complex (Vectastain: Vector Laboratories, Burlingame, CA, USA). This reaction was subsequently visualized with 3,3-diaminobenzidine (DAB). Immunolabeled paraffin sections were counterstained with hematoxylin. Positive and negative controls were included to confirm immunostaining results.
Aβ phases as determined in the medial temporal lobe (Thal et al. 2000), NFT-stages (Braak et al. 2006; Braak and Braak 1991b), CERAD scores for NP pathology (Mirra et al. 1991) were used to determine the level of AD pathology according to recently published guidelines (Hyman et al. 2012). FTLD-tau pathology was determined and classified as previously described (Braak and Braak 1998a; Dickson and Litvan 2003; Hauw and Agid 2003; Mackenzie et al. 2010). Since reliable neuropathological staging systems for CBD, PSP and PiD are not available we determined only presence or absence of AGD to compare the data for all four FTLD-tau diseases in a similar dichotomous manner. Therefore, stages of AGD (Saito et al. 2004) were not determined for this study. SVD was classified morphologically based on H&E-stained sections. The expansion of SVD was staged according to previously published criteria (Thal et al. 2003). CAA was detected by anti-Aβ immunohistochemistry. The severity of CAA was determined according to the Vonsattel criteria (Vonsattel et al. 1991). The type of CAA based on the capillary involvement was determined as previously described (Thal et al. 2002). The presence or absence of infarcts and microinfarcts as well as hemorrhages was assessed. Severe white matter lesions seen in H&E stained sections and /or in Campbell-Switzer stained sections were documented.
Statistical analysis
To identify differences in the extent of AD-related pathology, vessel pathology and that of vascular lesions among AGD, PSP, CBD, PiD, AD, and control brains we used analysis of variances (ANOVA) corrected for multiple testing with the Games-Howell post-hoc test and multinominal logistic regression analysis controlled for age and gender. The detailed statistical analysis is shown in Suppl. Tab. 1.
Results
Cerebral vessel pathology and vascular lesions in FTLD-tau and AD
SVD was found in all forms of FTLD-tau as well as in AD and healthy control cases (Fig. 1). PiD usually exhibited widely distributed SVD fulfilling the criteria of SVD stage 3 (Thal et al. 2003). When controlled for age and gender, PiD cases had higher SVD stages than non-diseased controls whereas AGD, PSP, CBD, and AD cases did not show significantly higher stages of SVD than non-disease controls (Fig. 1c; Statistical analysis: Suppl. Tab. 1)
Fig. 1:
Medial temporal lobe atrophy and cerebral SVD in FTLD-tau cases. a: Medial temporal lobe atrophy was evident in PiD but not in AGD, PSP and CBD when comparing to a healthy control. The atrophy seen in PiD was even more severe than in AD. b: SVD showing the pattern of arteriolosclerosis with concentric hyalinization of the vessel wall or lipohyalinosis with focal fibrosis was observed in cases with AGD, PSP, CBD, PiD and AD. Healthy vessels were frequently seen in control cases. c: Distribution of SVD stages in AGD, PSP, CBD, PiD, AD, and control cases. PiD showed increased stages of SVD. d: Distribution of CAA severity grades (Vonsattel et al. 1991) in AGD, PSP, CBD, PiD, AD, and control cases. CAA was significantly more advanced in AD. e: Distribution of CAA types in AGD, PSP, CBD, PiD, AD, and control cases. CAA of both types was most frequently seen in AD cases. There were no differences among FTLD-tau subtypes. f: No difference in the prevalence of cerebral infarction among the FTLD-tau types. g: No difference in the prevalence of cerebral hemorrhage among the FTLD-tau types. Stainings in a and b: AGD, PSP, CBD, PiD – hematoxylin & eosin; AD, control – elastica von Gieson. Calibration bar in a corresponds to: a: 2.5 mm; b: 40 μm.
* p < 0.05; Statistical analysis see Suppl. Tab. 1.
The severity of CAA distribution in AD cases was higher than that in healthy controls, AGD, PSP, CBD, or PiD cases (Fig. 1d; Statistical analysis: Suppl. Tab. 1). In contrast, no significant differences in the severity of CAA were observed between healthy controls and AGD, PSP, CBD, and PiD. Capillary CAA indicating the presence of CAA-type 1 as previously published (Thal et al. 2002) was strongly associated with AD whereas only few healthy controls exhibited CAA type 1. FTLD-tau cases did not differ significantly in the prevalence of CAA-type 1 and 2 cases from non-diseased controls (Fig. 1e; Statistical analysis: Suppl. Tab. 1)
Brain infarctions were present in 22.3 % of healthy controls, in 26.7 % of AGD, 23.1 % of PSP, 33.3 % of CBD, 33.3 % of PiD, and 23.3 % of AD cases without significant differences among the groups (Fig. 1f; Statistical analysis: Suppl. Tab. 1). Hemorrhage was seen in 10.1 % of the controls, in 16.7 % of AGD, 3.8 % of PSP, 20 % of CBD, 0 % of PiD, and 7.8 % of AD cases. Although none of our 9 PiD cases showed signs of hemorrhage (Fig. 1g; Statistical analysis: Suppl. Tab. 1) the sample of PiD cases may not be representative in this matter as the frequency of hemorrhage in control cases was 10.1 % indicating that the statistical power of the PiD group (0.085) was too small to draw conclusions regarding the prevalence of hemorrhage in the PiD-cases in our sample. White matter lesions were found in relation to SVD in control, FTLD-tau and AD cases. In PiD white matter degeneration and demyelination was mainly located in the frontal and temporal lobes and associated to distal white matter areas in gyri with severe cortical and white matter τ-pathology (Fig. 2: arrows). Areas with intact white matter had less severe cortical and white matter τ-pathology (Fig. 2: arrowheads). Abnormal τ-containing axons with a spiral shaped pattern prevailed in the white matter. The white matter arteries in these areas often exhibited slightly enlarged perivascular spaces and a thickening of the lamina adventitia as signs of SVD (Fig. 3). The SVD-altered vessels were predominantly found in those parts of the cerebral white matter that showed white matter degeneration and severe cortical τ-pathology (Fig. 3).
Fig. 2:
Local association of τ and white matter pathology in PiD. a, b. Campbell-Switzer stained sections from the autopsy brain of a 74 year-old woman with PiD, at level of the anterior basal ganglia (a), thalamus (b). Severe white matter myelin losses were observed in the frontal and temporal subcortex (arrows), whereas other parts of the subcortical white matter exhibit a regular myelin sheet staining (arrowheads). c, d. Sections adjacent to those in a and b stained with an antibody against hyperphosphorylated τ protein (clone AT-8) showed most severe τ pathology in cortical areas neighboring subcortical areas with myelin loss (arrows), whereas less τ pathology was seen in cortical areas close-by subcortical areas with unaltered myelination (arrowheads). The inset in c shows a high power Z-project reconstruction of 8 scans taken from the frontal white matter (box in d) in ~10 μm distance in the Z-axis. Axons contain abnormal τ (arrow) and exhibit a spiral-like staining pattern. Calibration bar in d valid for a, c: 10 mm; b, d: 8.5 mm; inset c: 20 μm.
Fig. 3:
Regional differences in Pick’s disease-related white matter demyelination. a: Leukoencephalopathy in a PiD case with matter demyelination (arrows) in the frontal cortex. b: In the medial temporal lobe of the same case there was no demyelination. c: At a higher magnification level the frontal cortex (boxed area in a) showed severe damage near areas of demyelination (arrows). d: The entorhinal cortex (boxed area in b) of the same case showed mainly intact lamination and no areas of demyelination. e: High power view on an artery in the demyelination area (boxed area in c) exhibited thickening of the adventitia and its basement membrane as well as focal fibrosis of the lamina media (arrows). f: White matter vessels in the entorhinal white matter did not exhibit such changes in the vessel wall.
a-f: Case: PiD, 65 years of age, male. Aβ phase 0, Braak-NFT stage II, CERAD score 0, no infarcts, no hemorrhage.
Calibration bar in b valid for a, b: 2.6 mm; c, d: 500 μm; e, f: 35 μm.
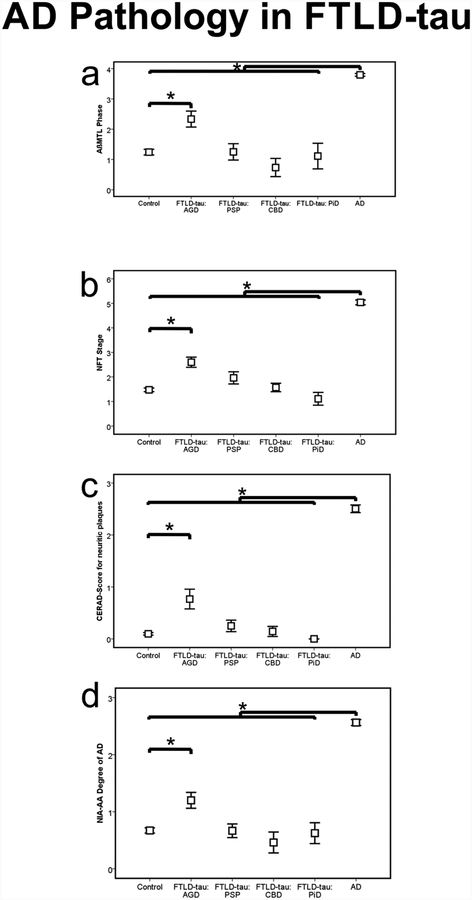
Coexisting AD pathology in FTLD-tau
Cases with AGD showed higher phases of Aβ plaque pathology, NFT-stages, CERAD-scores for neuritic plaque frequency and higher degrees of AD pathology according to the NIA-AA guidelines for neuropathological diagnosis of AD than control cases but lower levels in comparison to pure AD cases (Fig. 4, Suppl. Tab. 1b, c). The phases of Aβ plaque pathology, NFT-stages, CERAD-scores and the NIA-AA degrees of AD pathology did not differ between PSP, CBD, PiD and healthy controls but were lower than those of pure AD cases (Fig. 4, Suppl. Tab. 1b).
Fig. 4.
Distribution of AD pathology (Aβ phase, Braak-NFT-stage, CERAD score for neuritic plaque pathology and NIA-AA degree of AD pathology) in AGD, PSP, CBD, PiD, AD, and control cases.
* p < 0.05; Statistical analysis see Suppl. Tab. 1.
Age spectrum of FTLD-tau cases
The four different forms of FTLD-tau differ in their distribution of age at autopsy (Fig. 5). PiD cases were youngest in mean age followed by CBD, PSP and finally AGD (Suppl. Tab. 1e). PiD cases were most frequently younger than 70 years of age at death (mean 63.11 years, range: 53 – 74 years). Only one PiD case in our cohort (11.1 %) was older than 70 years of age (74 years). CBD cases were 52 – 86 years at death with most cases being aged between 60 and 80 years (mean 69.53 years). PSP cases were distributed most frequently between 60 and 90 years at death (mean 75.61 years, range: 59 – 92 years) whereas AGD had the highest age at death spectrum of these four types of FTLD-tau (mean 81.2 years, range: 62 – 98 years). The age spectrum of AGD was similar to that found in AD cases (mean 81.58 years, range: 56 – 101 years). Non-diseased controls were 18 – 99 years of age (mean 68.88 years). ANOVA revealed significant differences between the four types of FTLD among age confirming a ranking of the FTLD-tau types by increasing age as given by the respective mean age: 1 PiD, 2. CBD, 3. PSP, and 4. AGD (Statistical analysis: Suppl. Tab. 1e).
Fig. 5.
Age-related distribution of FTLD-tau cases. In this figure only clinically diseased cases were considered. All AGD, PSP, CBD, or PiD are considered considered as 100 % each and the percentages of AGD, PSP, CBD, or PiD cases in each age group are provided, respectively.
FTLD-tau pathology in non-diseased control cases
In our non-diseased control cases we observed FTLD-tau lesions (argyrophilic grains restricted to the medial temporal lobe, coiled bodies in oligodendrocytes and τ-positive astrocytes) (Fig. 6a–d) in addition to AD-related plaque-and NFT-pathology, vascular lesions and vessel pathology. The prevalence of these FTLD-tau lesions in non-diseased individuals increased with age in parallel with that of AD pathology as defined according to Hyman et al. (2012) (Fig. 6e). Morphologically the pattern exhibiting argyrophilic grains, coiled bodies and τ-positive astrocytes fitted best with the pattern described for AGD (Botez et al. 1999; Braak and Braak 1987; Tolnay et al. 2003) not exceeding the medial temporal lobe corresponding to AGD-stage 1 according to Saito et al. (Saito et al. 2004) and did not fulfill the criteria for PART (Crary et al. 2014). To distinguish non-diseased cases exhibiting argyrophilic grains, coiled bodies and τ-positive astrocytes from pure PART and p-preAD cases we will refer to them here as cases with FTLD-tau-related pathology. FTLD-tau-related pathology was found in 10.9 % of p-preAD, 9.6 % of PART cases, and in 4 % of the non-p-preAD, non-PART controls (Tab. 3). 27.8 % of all non-diseased controls in our sample matched the criteria for definite PART 58.8 % that of p-preAD.
Fig. 6.
a-d: FTLD-tau lesions in non-diseased individuals. Grains (arrows in a, d), τ-positive astrocytes (+ in b, d) and oligodendroglial coiled bodies (c) were found in non-diseased individuals. e: The prevalence of FTLD-tau lesions in non-diseased individuals started in few cases between 50 and 60 years of age and, then, increased with age in parallel with the more prominent increase of AD pathology (as defined according to (Hyman et al. 2012)) in non-diseased individuals. f: In parallel the prevalence of symptomatic AD-cases in our entire sample increased with age whereas that of symptomatic FTLD-tau slightly varied between ~16% and ~24% without a major age-related increase. Calibration bar in a valid for a: 20 μm; b, c: 13 μm; d: 30 μm.
Table 3:
Prevalence of FTLD-like pathology (argyrophilic grains, coiled bodies in oligodendrocytes, τ-positive astrocytes) in % of non-p-preAD + non-PART, p-preAD and PART cases
| Braak-NFT stage | non-p-preAD + non-PART | p-preAD | PART |
|---|---|---|---|
| 0 | 4 | 0.00 | n.a. |
| 1 | n.a. | 11.11 | 8.57 |
| 2 | n.a. | 7.50 | 7.69 |
| 3 | n.a. | 12.50 | 33.33 |
| 4 | n.a. | 66.67 | 0.00 |
| 5 | n.a. | n.d. | n.d. |
| 6 | n.a. | n.d. | n.d. |
n.a. = not assessable by definition; n.d. = not determined (no cases available)
n = 25 (non-p-preAD + non-PART); 110 (p-preAD)*; 52 (definite PART).
p-preAD cases may contain probable PART cases.
Discussion
The major findings of this study are: 1. the types of FTLD-tau have different age spectra with PiD occurring usually earlier than other types of FTLD-tau followed by CBD, PSP, and finally AGD; 2. AGD is associated with AD-related lesions but not PSP, CBD and PiD; 3. FTLD-tau-related pathology can occur in neurologically unimpaired elderly individuals exhibiting argyrophilic grains, oligodendroglial coiled bodies and τ-positive astrocytes; and 4. PiD-related leukoencephalopathy (Dickson 1998) is associated with a SVD-like thickening of the adventitia of local arteries in regions of the brain with severe τ-pathology. These findings give rise to the speculation that age as well as vascular pathology and AD-related lesions may have influence on the pathological manifestation type of non-AD tauopathies.
In detail, our findings showed that age at death differed among the FTLD-tau types PiD, PSP, CBD, and AGD with 50 to 70 years in PiD, 60 to 80 years in CBD, 60 to 90 years in PSP and 70 to 90 years in AGD. This sequence fits with that of the reported ages of onset (PiD: 57 years; CBD: 63 years; PSP: 65–69 years; AGD: 80 years) (Dickson and Litvan 2003; Kovacs et al. 2013; Shiarli et al. 2006).
Since AD pathology, vessel pathology and vascular lesions increase with advancing age (Braak et al. 2011; Thal et al. 2012) these pathologies may influence or modulate non-AD tauopathies in its pathological pattern. In PiD we observed severe SVD-like lesions in association with areas of demyelination in the frontal and temporal cortex whereas parietal and occipital cortex areas were spared. Cases with PiD had higher SVD stages than age-and gender-matched control cases. However, similar degrees of SVD-related vessel wall thickening in older individuals were not necessarily associated with PiD-like severe white matter demyelination in control brains. Accordingly, it is tempting to speculate that white matter degeneration and demyelination in PiD is triggered by severe cortical τ pathology which leads to axonal degeneration (Englund 1998; Leys et al. 1991). This hypothesis is supported by the close association of white matter demyelination with local τ-pathology. SVD in PiD cases, thereby, may be discussed either as a prerequisite required for the clinical manifestation of PiD or as a result of τ- or PiD-derived white matter pathology. AGD, PSP and CBD cases did not show such an association between leukoencephalopathy and τ-pathology in our study. Since PiD cases exhibit a significant axonal τ-pathology (Braak and Braak 1998b; Probst et al. 1996) in contrast to AGD, PSP, or CBD cases the PiD-related axonal tauopathy appears to be critical for the severe leukoencephalopathy seen in PiD. Although SVD was found in all four types of FTLD-tau and in AD, no PiD-like leukoencephalopathy was seen either in AGD, PSP, CBD, or AD. Less severe leukoencephalopathic changes are well known in AD cases (Brun and Englund 1986; McAleese et al. 2013), but until today could not clearly be related to tauopathic lesions. Other vascular lesions were not associated with FTLD-tau in our sample confirming the findings of other authors (Kovacs et al. 2013; Toledo et al. 2013).
AD-related Aβ plaques, NFTs, CAA as well as the NIA-AA degrees of AD pathology were as expected highest in AD cases and differed significantly from those in AGD, PSP, CBD, and PiD. AGD exhibited higher levels of AD pathology than healthy controls whereas that of PSP, CBD, and PiD did not differ from that of non-demented controls. As such, our results confirm previous reports that AGD-related dementia is linked to increased levels of AD pathology and in this way AGD appeared to lower the threshold for AD pathology to cause dementia (Josephs et al. 2008; Thal et al. 2005). However, single cases with severe or mutation-triggered AGD are reported to develop dementia in the absence of AD-related changes (Kovacs et al. 2008; Saito et al. 2004). No additive role for the development of dementia was found between AD pathology and PSP, CBD, and PiD. Since AGD and PSP are both FTLD-tau forms of advanced age, and since the extent of AD pathology increases with age (Braak et al. 2011) it is tempting to speculate that a certain degree of AD pathology contributes to the development of symptoms in AGD as previously shown (Josephs et al. 2008; Thal et al. 2005) while this is not the case for the FTLD-tau diseases with an earlier onset, i.e. PSP, CBD and PiD.
Finally, our study showed FTLD-tau-related pathology in non-diseased individuals. For AD, AD-related pathology in non-demented individuals indicates preclinical AD (Dubois et al. 2007; Sperling et al. 2011; Thal et al. 2013). Accordingly, it is very likely that FTLD-tau-like lesions suchas argyrophilic grains, oligodendroglial coiled bodies, and τ-positive astrocytes comprise changes of preclinical FTLD-tau. As it is well-known that these lesions can occur in all types of FTLD-tau including rare forms of tauopathies (Braak and Braak 1998a; Dickson and Litvan 2003; Dickson et al. 2011; Hauw and Agid 2003; Kovacs et al. 2011; Mackenzie et al. 2010) it is difficult to distinguish which type of FTLD-tau is represented by these lesions. However, the age-related increase of FTLD-tau lesions in non-diseased individuals would fit on the basis of its distribution pattern best with AGD, which is frequently seen in advanced age (Braak and Braak 1998a; Thal et al. 2005; Tolnay et al. 1997) as well as with the report on non-AD tauopathic lesions in the occipital and temporal cortex (Pikkarainen et al. 2009). A further argument for FTLD-tau-related pathology in non-diseased elderly is our descriptive finding that the distribution of these lesions is restricted to the medial temporal lobe with a pattern that has been described as an early stage of AGD pathology (Saito et al. 2004). In doing so, this FTLD-tau-related pathology in non-diseased elderly represents an initial stage of the respective pathology. Non-AD NFT-τ-lesions in non-demented individuals have recently been termed PART (= primary age-related tauopathy), were related to NFT-predominant dementia but were different from preclinical AGD-,CBD-, PiD-, and PSP-FTLD-tau changes (Crary et al. 2014).
In summary, this study shows that FTLD-tau disorders vary in their association with AD and vascular lesions and that age may play a role in the association of AGD with AD lesions. The association of PiD with SVD-like vessel pathology and white matter demyelination may point to a possible link between vessel pathology, white matter demyelination and τ-pathology in PiD. As such, our results suggest that age, vascular and AD-related co-pathology may impact the morphological appearance of FTLD-tau.
Supplementary Material
Supplementary Table 1: Statistical analysis.
Acknowledgments
The brain material studied was received from FTLDc-Net Brain Banks in Munich and Ulm (Germany) and the Newcastle Brain Tissue Resource (UK), which is funded in part by a grant from the UK Medical Research Council (G0400074) and by Brains for Dementia research, a joint venture between Alzheimer’s Society and Alzheimer’s Research UK. The authors thank Professor Heiko Braak and Dr. Kelly Del Tredici (Ulm/Germany) for providing stained and unstained sections of some cases of their collection for inclusion into this study.
This study was supported by the BMBF- (ministry of science and technology) Grant FTLDc and Alzheimer Forschung Initiative (AFI) grant #13803; and NIA AG12411. Part of the research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre for Ageing and Age-related disease and the Biomedical Research Unit for Lewy body dementia based at Newcastle upon Tyne Hospitals NHS Foundation Trust and Newcastle University (R:CH/ML/0712). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
Disclosures
DRT received consultant honorary from Simon Kucher and Partners, GE-Healthcare, and Covance Laboratories, speaker honorary from GE-Healthcare and collaborated with Novartis Pharma AG. CAFvA received honoraria from serving on the scientific advisory board of Nutricia GmbH and has received funding for travel and speaker honoraria from Nutricia GmbH, Novartis Pharma GmbH, Lilly Deutschland GmbH, Desitin Arzneimittel GmbH and Dr. Willmar Schwabe GmbH &Co. KG and has received research support from Roche Diagnostics GmbH, Biologische Heilmittel Heel GmbH and ViaMed GmbH.
References
- Alafuzoff I, Arzberger T, Al-Sarraj S, Bodi I, Bogdanovic N, Braak H, Bugiani O, Del Tredici K, Ferrer I, Gelpi E, Giaccone G, Graeber MB, Ince P, Kamphorst W, King A, Korkolopoulou P, Kovács GG, Larionov S, Meyronet D, Monoranu C, Parchi P, Patsouris E, Roggendorf W, Seilhean D, Tagliavini F, Stadelmann-Nessler C, Streichenberger N, Thal DR, Wharton S, Kretzschmar H (2008a) Staging of neurofibrillary pathology in Alzheimer’s disease. A study of the BrainNet Europe Consortium. Brain Pathol. 18:484–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alafuzoff I, Pikkarainen M, Al-Sarraj S, Arzberger T, Bell J, Bodi I, Bogdanovic N, Budka H, Bugiani O, Ferrer I, Gelpi E, Giaccone G, Graeber MB, Hauw JJ, Kamphorst W, King A, Kopp N, Korkolopoulou P, Kovacs GG, Meyronet D, Parchi P, Patsouris E, Preusser M, Ravid R, Roggendorf W, Seilhean D, Streichenberger N, Thal DR, Kretzschmar H (2006) Interlaboratory comparison of assessments of Alzheimer disease-related lesions: a study of the BrainNet Europe Consortium. J Neuropathol Exp Neurol 65:740–57. [DOI] [PubMed] [Google Scholar]
- Alafuzoff I, Pikkarainen M, Arzberger T, Thal DR, Al-Sarraj S, Bell J, Bodi I, Budka H, Capetillo-Zarate E, Ferrer I, Gelpi E, Gentleman S, Giaccone G, Kavantzas N, King A, Korkolopoulou P, Kovacs GG, Meyronet D, Monoranu C, Parchi P, Patsouris E, Roggendorf W, Stadelmann C, Streichenberger N, Tagliavini F, Kretzschmar H (2008b) Inter-laboratory comparison of neuropathological assessments of beta-amyloid protein: a study of the BrainNet Europe consortium. Acta Neuropathol 115:533–46. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders. Washington DC. [Google Scholar]
- Arriagada PV, Marzloff K, Hyman BT (1992) Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology 42:1681–8. [DOI] [PubMed] [Google Scholar]
- Botez G, Probst A, Ipsen S, Tolnay M (1999) Astrocytes expressing hyperphosphorylated tau protein without glial fibrillary tangles in argyrophilic grain disease. Acta Neuropathol (Berl) 98:251–6. [DOI] [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E (1987) Argyrophilic grains: characteristic pathology of cerebral cortex in cases of adult onset dementia without Alzheimer changes. Neurosci Lett 76:124–7. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E (1991a) Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathol 1:213–6. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E (1991b) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–59. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E (1998a) Argyrophilic grain disease: frequency of occurrence in different age categories and neuropathological diagnostic criteria. J Neural Transm 105:801–19. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E (1998b) Involvement of precerebellar nuclei in Pick’s disease. Exp Neurol 153:351–65. [DOI] [PubMed] [Google Scholar]
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K (2011) Stages of the pathological process in Alzheimer’s disease: Age categories 1 year to 100 years. J Neuropathol Exp Neurol 70:960–969. [DOI] [PubMed] [Google Scholar]
- Brun A, Englund E (1986) A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Ann Neurol 19:253–62. [DOI] [PubMed] [Google Scholar]
- Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, Arnold SE, Attems J, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Gearing M, Grinberg LT, Hof PR, Hyman BT, Jellinger K, Jicha GA, Kovacs GG, Knopman DS, Kofler J, Kukull WA, Mackenzie IR, Masliah E, McKee A, Montine TJ, Murray ME, Neltner JH, Santa-Maria I, Seeley WW, Serrano-Pozo A, Shelanski ML, Stein T, Takao M, Thal DR, Toledo JB, Troncoso JC, Vonsattel JP, White CL 3rd, Wisniewski T, Woltjer RL, Yamada M, Nelson PT (2014) Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 128:755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson D, Litvan I (2003) Corticobasal degeneration In: Dickson DW (ed) Neurodegeneration: The molecular pathology of dementia and movement disorders, ISN Neuropath Press, Basel, pp 115–123. [Google Scholar]
- Dickson DW (1998) Pick’s disease: a modern approach. Brain Pathol 8:339–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Kouri N, Murray ME, Josephs KA (2011) Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau). J Mol Neurosci 45:384–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O’Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P (2007) Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol 6:734–46. [DOI] [PubMed] [Google Scholar]
- Englund E (1998) Neuropathology of white matter changes in Alzheimer’s disease and vascular dementia. Dement Geriatr Cogn Disord 9 Suppl 1:6–12. [DOI] [PubMed] [Google Scholar]
- Hauw JJ, Agid Y (2003) Progressive supranuclear palsy (PSP) or Steele-Richardson-Olzewski disease In: Dickson DW (ed) Neurodegeneration: The molecular pathology of dementia and movement disorders, ISN Neuropath Press, Basel, pp 103–114. [Google Scholar]
- Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ (2012) National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement 8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Parisi JE, Knopman DS, Boeve BF, Geda YE, Jack CR Jr., Petersen RC, Dickson DW (2008) Argyrophilic grains: a distinct disease or an additive pathology? Neurobiol Aging 29:566–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GG, Milenkovic I, Wohrer A, Hoftberger R, Gelpi E, Haberler C, Honigschnabl S, Reiner-Concin A, Heinzl H, Jungwirth S, Krampla W, Fischer P, Budka H (2013) Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community-based autopsy series. Acta Neuropathol 126:365–84. [DOI] [PubMed] [Google Scholar]
- Kovacs GG, Molnar K, Laszlo L, Strobel T, Botond G, Honigschnabl S, Reiner-Concin A, Palkovits M, Fischer P, Budka H (2011) A peculiar constellation of tau pathology defines a subset of dementia in the elderly. Acta Neuropathol 122:205–22. [DOI] [PubMed] [Google Scholar]
- Kovacs GG, Pittman A, Revesz T, Luk C, Lees A, Kiss E, Tariska P, Laszlo L, Molnar K, Molnar MJ, Tolnay M, de Silva R (2008) MAPT S305I mutation: implications for argyrophilic grain disease. Acta Neuropathol 116:103–18. [DOI] [PubMed] [Google Scholar]
- Kovacs GG, Rozemuller AJ, van Swieten JC, Gelpi E, Majtenyi K, Al-Sarraj S, Troakes C, Bodi I, King A, Hortobagyi T, Esiri MM, Ansorge O, Giaccone G, Ferrer I, Arzberger T, Bogdanovic N, Nilsson T, Leisser I, Alafuzoff I, Ironside JW, Kretzschmar H, Budka H (2013) Neuropathology of the hippocampus in FTLD-Tau with Pick bodies: A study of the BrainNet Europe Consortium. Neuropathol Appl Neurobiol. 39:166–178 [DOI] [PubMed] [Google Scholar]
- Leys D, Pruvo JP, Parent M, Vermersch P, Soetaert G, Steinling M, Delacourte A, Defossez A, Rapoport A, Clarisse J, et al. (1991) Could Wallerian degeneration contribute to “leuko-araiosis” in subjects free of any vascular disorder? J Neurol Neurosurg Psychiatry 54:46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, Kovacs GG, Ghetti B, Halliday G, Holm IE, Ince PG, Kamphorst W, Revesz T, Rozemuller AJ, Kumar-Singh S, Akiyama H, Baborie A, Spina S, Dickson DW, Trojanowski JQ, Mann DM (2010) Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol 119:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAleese KE, Firbank M, Hunter D, Sun L, Hall R, Neal JW, Mann DM, Esiri M, Jellinger KA, O’Brien JT, Attems J (2013) Magnetic resonance imaging of fixed post mortem brains reliably reflects subcortical vascular pathology of frontal, parietal and occipital white matter. Neuropathol Appl Neurobiol 39:485–97. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L (1991) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41:479–86. [DOI] [PubMed] [Google Scholar]
- Pikkarainen M, Kauppinen T, Alafuzoff I (2009) Hyperphosphorylated tau in the occipital cortex in aged nondemented subjects. J Neuropathol Exp Neurol 68:653–60. [DOI] [PubMed] [Google Scholar]
- Price JL, Davis PB, Morris JC, White DL (1991) The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer’s disease. Neurobiol Aging 12:295–312. [DOI] [PubMed] [Google Scholar]
- Probst A, Tolnay M, Langui D, Goedert M, Spillantini MG (1996) Pick’s disease: hyperphosphorylated tau protein segregates to the somatoaxonal compartment. Acta Neuropathol 92:588–96. [DOI] [PubMed] [Google Scholar]
- Saito Y, Ruberu NN, Sawabe M, Arai T, Tanaka N, Kakuta Y, Yamanouchi H, Murayama S (2004) Staging of argyrophilic grains: an age-associated tauopathy. J Neuropathol Exp Neurol 63:911–8. [DOI] [PubMed] [Google Scholar]
- Shiarli AM, Jennings R, Shi J, Bailey K, Davidson Y, Tian J, Bigio EH, Ghetti B, Murrell JR, Delisle MB, Mirra S, Crain B, Zolo P, Arima K, Iseki E, Murayama S, Kretzschmar H, Neumann M, Lippa C, Halliday G, Mackenzie J, Khan N, Ravid R, Dickson D, Wszolek Z, Iwatsubo T, Pickering-Brown SM, Mann DM (2006) Comparison of extent of tau pathology in patients with frontotemporal dementia with Parkinsonism linked to chromosome 17 (FTDP-17), frontotemporal lobar degeneration with Pick bodies and early onset Alzheimer’s disease. Neuropathol Appl Neurobiol 32:374–87. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR Jr., Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH (2011) Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DR, Ghebremedhin E, Orantes M, Wiestler OD (2003) Vascular pathology in Alzheimer’s disease: Correlation of cerebral amyloid angiopathy and arteriosclerosis / lipohyalinosis with cognitive decline. J Neuropathol Exp Neurol 62:1287–1301. [DOI] [PubMed] [Google Scholar]
- Thal DR, Ghebremedhin E, Rüb U, Yamaguchi H, Del Tredici K, Braak H (2002) Two types of sporadic cerebral amyloid angiopathy. J Neuropathol Exp Neurol 61:282–93. [DOI] [PubMed] [Google Scholar]
- Thal DR, Grinberg LT, Attems J (2012) Vascular dementia: Different forms of vessel disorders contribute to the development of dementia in the elderly brain. Exp Gerontol 47:816–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DR, Rüb U, Schultz C, Sassin I, Ghebremedhin E, Del Tredici K, Braak E, Braak H (2000) Sequence of Abeta-protein deposition in the human medial temporal lobe. J Neuropathol Exp Neurol 59:733–48. [DOI] [PubMed] [Google Scholar]
- Thal DR, Schultz C, Botez G, Del Tredici K, Mrak RE, Griffin WS, Wiestler OD, Braak H, Ghebremedhin E (2005) The impact of argyrophilic grain disease on the development of dementia and its relationship to concurrent Alzheimer’s disease-related pathology. Neuropathol Appl Neurobiol 31:270–9. [DOI] [PubMed] [Google Scholar]
- Thal DR, von Arnim C, Griffin WS, Yamaguchi H, Mrak RE, Attems J, Rijal Upadhaya A (2013) Pathology of clinical and preclinical Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci 263 (Suppl 2):S137–S145. [DOI] [PubMed] [Google Scholar]
- Thies W, Bleiler L, Alzheimer’s A (2013) 2013 Alzheimer’s disease facts and figures. Alzheimers Dement 9:208–45. [DOI] [PubMed] [Google Scholar]
- Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, Monsell SE, Kukull WA, Trojanowski JQ (2013) Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain 136:2697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolnay M, Ghebremedhin E, Probst A, Braak H (2003) Argyrophilic grain disease In: Dickson D (ed) Neurodegeneration: The molecular pathology of dementia and movement disorders, ISN Neuropath Press, Basel, pp 132–136. [Google Scholar]
- Tolnay M, Schwietert M, Monsch AU, Staehelin HB, Langui D, Probst A (1997) Argyrophilic grain disease: distribution of grains in patients with and without dementia. Acta Neuropathol (Berl) 94:353–8. [DOI] [PubMed] [Google Scholar]
- Vonsattel JP, Myers RH, Hedley-Whyte ET, Ropper AH, Bird ED, Richardson EP Jr. (1991) Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann Neurol 30:637–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Statistical analysis.