Abstract
Background
There is recognition that breast cancer is a collection of heterogeneous diseases divided in subtypes based on combined molecular features such as hormonal receptors (HR) and human epidermal growth factor receptor 2 (HER2) status. We aimed to study clinical differences among biological subtypes in brain metastasis from breast cancer after targeted therapy introduction.
Methods
This was a retrospective study with 406 consecutive patients with brain metastasis from breast cancer treated at MD Anderson Cancer Center from 1998 to 2013. Overall, 315 of these patients met the study criteria and were analyzed. Subtypes were classified as HER2-/HR+ (96 patients), HER2+/HR+ (57 patients), HER2+/HR- (63 patients), and triple negative (HER2-/HR-) (99 patients). End points were time to development of brain metastasis (TDBM), brain metastasis-free survival (BMFS), and overall survival from start of treatment of brain metastasis (OSBM). Univariate and multivariate Cox proportional hazard regression models were used to analyze the data.
Results
TDBM was 41 months for HER2-/HR+; 58 months for HER2+/HR+; 30 months for HER2+/HR-; and 27 months for triple negative (P < .001). BMFS was 9 months for HER2-/HR+; 24 months for HER2+/HR+; 9 months for HER2+/HR-; and 7 months for triple negative (P = .06). OSBM was 20 months for HER2-/HR+; 22 months for HER2+/HR+; 24 months for HER2+/HR-; and 9 months for triple negative (P < .001). On multivariate analyses, triple negative showed lower OSBM compared with other subtypes, with a hazard ratio of 1.9 (P < .001).
Conclusion
Comparing all breast cancer subgroups we noticed that HR and HER2 are the most significant biomarkers in brain metastasis behavior. Patients who received targeted therapy had better outcomes, but not in the triple negative group. Prospective studies with different treatment modalities for each subgroup are recommended.
Keywords: brain metastasis, breast cancer, HER2, hormonal receptors, molecular subtypes
Breast cancer is typically the most common cause of brain metastasis in females. It accounts for 5% to 30% of all metastatic brain tumors in women.1,2 Approximately 10% to 16% of patients with metastatic breast cancer develop symptoms from brain metastasis during the course of their disease,3 but up to 30% are found to have brain metastasis at autopsy.4 It typically occurs late in the course of metastatic breast cancer. Several risk factors have been implicated in the development of brain metastasis in patients with advanced breast cancer, such as young age,4,5 negative hormone receptor status,5–8 human epidermal growth factor receptor 2 (HER2) amplification,5–13 p53 overexpression, and lung metastasis as the first site of relapse.14
Historically, the prognosis of advanced breast cancer patients with brain metastasis has not been encouraging, with an expected overall survival after brain metastasis was diagnosed (OSBM) of only 20% at year 1 and 2% at year 2.15 However, with recent improvements in systemic and local therapy, the overall prognosis has improved substantially and has become more favorable than brain metastasis from lung cancer,16 which holds true particularly for HER2+ tumors.17
There is a recognition that breast cancer is a collection of heterogeneous diseases that can be divided into subtypes based on combined molecular features that include hormonal receptor status (estrogen receptor [ER] and/or progesterone receptor [PR]) and HER2 amplification status.18,19 Patients with tumors positive for hormonal receptors (HR) have higher risk of bone relapse, but brain relapse is less common.20 HER2 positivity is known to be a risk factor for the development of brain metastasis and has been shown to be a strong prognostic factor for shorter disease-free time and OSBM.21,22 Triple negative tumors have shown different clinical behavior with higher rate of distant relapse and concern for brain metastasis.5,8,19 Regarding exclusively brain metastasis, clinical and prognostic differences have been reported in several aspects such as time to develop brain metastasis, age of onset, response to local and systemic treatment, and OSBM.7,23,24
In 1998 the United States Food and Drug Administration approved the humanized anti-HER2 monoclonal antibody trastuzumab, which has redefined clinical practice for patients with HER2-positive breast cancer.25 Numerous large-scale, randomized studies have shown the benefits of trastuzumab use, including increased survival in early and metastatic breast cancer.26 Patients with HER2-positive breast cancer and brain metastasis who are treated with trastuzumab have better survival outcomes, probably because of better control of extracranial disease achieved by it.27 We aimed to study clinical differences among biological subtypes in patients with brain metastasis from breast cancer after targeted therapy introduction and local treatment of the brain disease.
Patients and Methods
Objectives and Patient Selection
The study end point was to analyze survival outcomes among biological subtypes in brain metastasis from breast cancer patients. After FDA approval trastuzumab therapy was readily incorporated in routine practice at MD Anderson Cancer Center. We therefore reviewed the Neurosurgery Department database at MD Anderson Cancer Center from September 1998 to December 2013 to identify patients with breast cancer who were treated for newly diagnosed brain metastasis. Demographic and clinical data were extracted from electronic individual medical records. Image data (MRI) were reviewed by a neurosurgeon and a neuroradiologist. Patients with incomplete clinical and/or molecular information, leptomeningeal disease, lack of follow-up information, male gender, and ongoing treatment for disease recurrence in the brain were excluded from the study. The study was approved by the Institutional Review Board at our institution. Informed consent was waived by the IRB for all patients enrolled in the study.
Data Collection and Statistical Analyses
Clinical and demographic data were acquired at the time of brain metastasis diagnosis, including age, KPS, presence of extracranial disease, control of primary tumor, histology, and molecular features of the primary tumor. Systemic treatment modalities (targeted therapy, hormonal therapy, conventional therapy, alone or combined) were acquired at the time of diagnosis of brain metastasis and at the subsequent follow-ups. MRI scans were reviewed at diagnosis of brain metastasis for number of brain lesions, size, and anatomic and functional location (according to Sawaya et al28), and at each follow-up looking for local and distance recurrence. Data on local treatment (surgery alone, surgery plus stereotactic radiosurgery [SRS], SRS alone), use of adjuvant whole brain radiation therapy (WBRT), and resection technique were also collected.
Univariate and multivariate Cox proportional hazard regression models were used. We defined time to development of brain metastasis (TDBM) as the interval from breast cancer diagnosis to the development of brain metastasis, excluding patients that had initially discovered breast cancer simultaneously with brain metastasis. We further analyzed the brain metastasis-free survival (BMFS) as the interval from treatment of brain metastasis to local or distant recurrence. The OSBM was defined as the length of time from the first treatment of brain metastasis until the date of death or last contact on follow-up. TDBM, BMFS, and OSBM were estimated by the Kaplan-Meier product-limit method.
Patient characteristics were compared between groups using the χ2 test. Univariate and multivariate Cox proportional hazard regressions models were used to assess the effects of treatment (local and systemic) and other predictive and prognostic factors. The analyses were performed using SPSS ver. 21 (SPSS Inc., Chicago, IL, USA). P values of less than .05 were considered statistically significant.
Classification of Groups
Patients were categorized based on the receptor status of their primary tumor as follows: HER2-/HR+ (ER+ or PR+ and HER2-), HER2+/HR+ (ER+ or PR+ and HER2+), HER2+/HR- (ER- and PR- and HER2+), and triple negative (ER- and PR- and HER2-). ER and PR status were determined on the basis of immunohistochemistry (IHC) staining. Tumors were only considered HER2+ if they were either scored 3+ by IHC or if they were scored 2+ by IHC and also HER2 amplified (ratio > 2.0) on the basis of fluorescence in situ hybridization (FISH). In the absence of positive FISH data, tumors scored 2+ by IHC were considered negative for HER2.
Treatment Plan and Follow-up
The screening for brain metastasis in patients with initial or metastatic disease was done according to local protocol, with brain imaging with MRI most frequently performed when symptoms of CNS metastasis occurred or when the patient presented with signs of CNS metastasis.
Metastatic breast cancer patients with brain metastasis were treated according to the current evidence-based standard of care within MD Anderson’s Breast Cancer Group protocols, which included targeted therapy for HER2+ patients, endocrine therapy for HR+ patients, as well as the current protocol for systemic therapy in place during treatment. They were evaluated for neurosurgical procedures by the neurosurgeons from the Neurosurgery Department. Systemic therapy included chemotherapy, endocrine treatment, and targeted therapy (anti-HER2), combined or alone. Therapy for brain metastasis included surgery, SRS, and WBRT, combined or alone. Follow-up was conducted every 3 months with MRI scans, or whenever judged necessary by the oncology or neurosurgical teams. If a patient was found to have brain metastasis recurrence, she would be evaluated by a neurosurgeon who would make a decision on which treatment option was more appropriate for the case.
Results
Overall, we identified 406 consecutive patients diagnosed with brain metastasis from breast cancer in the Department of Neurosurgery. Ninety-one patients had no follow-up available and therefore were excluded from this study, resulting 315 patients who met the inclusion criteria. Patients with HER2+ tumors comprised 38% of the cohort (n = 120), whereas patients presenting with ER+ tumors comprised 44% (n = 139). When analyzing molecular subtypes, HER2-/HR+ and triple negative subtypes were the most frequent (30% and 31%, respectively), followed by HER2+/HR- (20%) and HER2+/HR+ (18%). Table 1 summarizes clinicopathologic characteristics at the time of diagnosis of brain metastasis. Table 2 summarizes demographic and clinical information stratified by each subtype. At the time of diagnosis of brain metastasis, the majority of patients were 50 years old or older (57%), had a KPS equal or higher than 70 (93%), and had a single brain lesion (50%). Table 3 shows local and systemic therapy stratified by each subtype. For localized therapy, 42% had only SRS as the first treatment to their local metastasis, 17% had surgery alone, 30% had surgery plus WBRT, 5% had surgery plus SRS, and 55% had SRS plus WBRT.
Table 1.
Patients’ clinicopathologic characteristics at the time of diagnosis of brain metastasis
| Type | Number | % |
|---|---|---|
| HER2 | ||
| Positive | 120 | 38 |
| Negative | 195 | 62 |
| Estrogen Receptor | ||
| Positive | 138 | 44 |
| Negative | 177 | 56 |
| Progesterone Receptor | ||
| Positive | 111 | 35 |
| Negative | 204 | 65 |
| Biological Subtype | ||
| Triple Negative | 101 | 32 |
| HER2-/HR+ | 100 | 32 |
| HER2+/HR+ | 51 | 16 |
| HER2+/HR- | 63 | 20 |
| Inflammatory Subtype | ||
| Yes | 38 | 12 |
| No | 277 | 88 |
| Diameter of larger brain metastasis | ||
| <1 cm | 64 | 21 |
| 1–2 cm | 68 | 22 |
| 2–3 cm | 74 | 24 |
| >3 cm | 104 | 33 |
| Tumor Location | ||
| Supratentorial | 184 | 58 |
| Infratentorial | 56 | 18 |
| Infra- and Supratentorial | 75 | 24 |
| Functional Location | ||
| Eloquent | 103 | 33 |
| Non-Eloquent | 143 | 45 |
| Near-Eloquent | 69 | 22 |
| Extracranial Metastasis Location | ||
| Lungs | 31 | 12 |
| Bones | 15 | 6 |
| Lymph nodes | 27 | 10 |
| Others | 15 | 7 |
| Liver + Lungs | 11 | 4 |
| Liver + Other | 29 | 12 |
| Lungs + Other | 45 | 17 |
| 2 Other Locations | 21 | 8 |
| Lungs + Liver + Other | 19 | 7 |
| 3 or More Other Locations | 45 | 17 |
Table 2.
Patient’s demographic, clinical, systemic, and local therapy information at diagnosis of brain metastasis stratified by subtype
| Type | HER2-/HR+ (%) | HER2+/HR+ (%) | HER2+/HR- (%) | Triple Negative (%) |
|---|---|---|---|---|
| Age | ||||
| <40 years | 9 (9) | 5 (10) | 10 (16) | 12 (12) |
| 40–49 years | 32 (32) | 19 (37) | 17 (27) | 30 (29) |
| 50–59 years | 33 (33) | 15 (29) | 22 (35) | 35 (35) |
| 60–69 years | 19 (19) | 10 (20) | 10 (16) | 17 (17) |
| >70 years | 7 (7) | 2 (4) | 4 (6) | 7 (7) |
| Local Control | ||||
| Yes | 87 (87) | 44 (86) | 54 (86) | 83 (82) |
| No | 13 (13) | 7 (14) | 9 (14) | 18 (18) |
| KPS | ||||
| <70 | 3 (3) | 6 (12) | 4 (6) | 9 (9) |
| >70 | 97 (97) | 45 (88) | 59 (94) | 92 (91) |
| Number of Brain Metastasis | ||||
| 1 | 53 (53) | 22 (43) | 30 (48) | 54 (54) |
| 2–3 | 38 (38) | 22 (43) | 24 (38) | 35 (35) |
| >3 | 9 (9) | 7 (14) | 9 (14) | 12 (12) |
| Number of Extracranial Metastasis | ||||
| 0 | 19 (19) | 5 (10) | 15 (24) | 15 (15) |
| 1 | 23 (23) | 10 (20) | 16 (25) | 42 (41) |
| 2 | 32 (32) | 22 (43) | 23 (37) | 28 (28) |
| >3 | 26 (26) | 14 (27) | 9 (14) | 16 (16) |
| Brain Metastasis Treatment | ||||
| Surgery | 19 (19) | 5 (10) | 12 (19) | 19 (19) |
| SRS | 43 (43) | 23 (45) | 21 (33) | 45 (45) |
| WBRT + Surgery | 26 (26) | 16 (31) | 25 (40) | 29 (28) |
| SRS + Surgery | 7 (7) | 3 (6) | 1 (2) | 6 (6) |
| SRS + WBRT | 5 (5) | 4 (8) | 4 (6) | 2 (2) |
| Anti-HER2 Treatment | ||||
| Yes | 2 (2) | 45 (88) | 54 (86) | 2 (2) |
| No | 98 (98) | 6 (12) | 9 (14) | 99 (98) |
| Hormonal Therapy | ||||
| Yes | 84 (84) | 47 (92) | 2 (3) | 2 (2) |
| No | 16 (16) | 4 (8) | 61 (97) | 99 (98) |
Table 3.
Cox model for OSBM stratified for each biological subtype
| Variable | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| HER2+/HR- | 1 | ||
| Triple Negative | 1.9 | 1.3–2.7 | .001 |
| HER2-/HR+ | 1.1 | 0.78–1.6 | .486 |
| HER2+/HR+ | 1.0 | 0.65–1.53 | .998 |
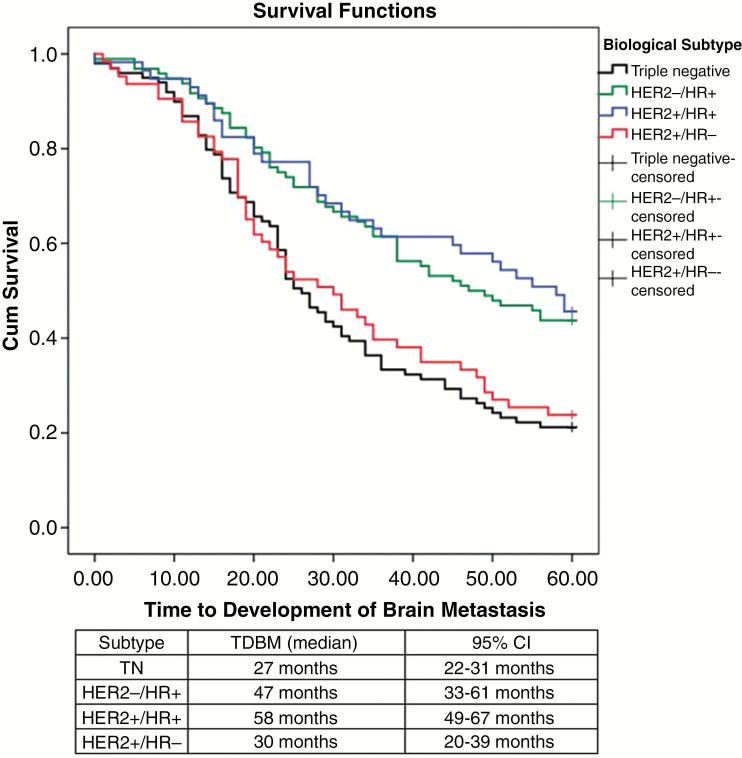
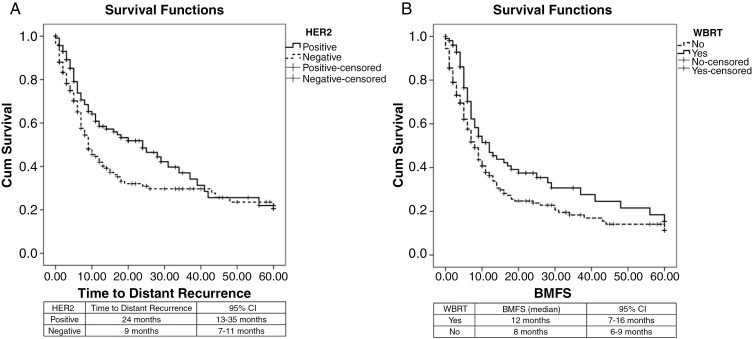
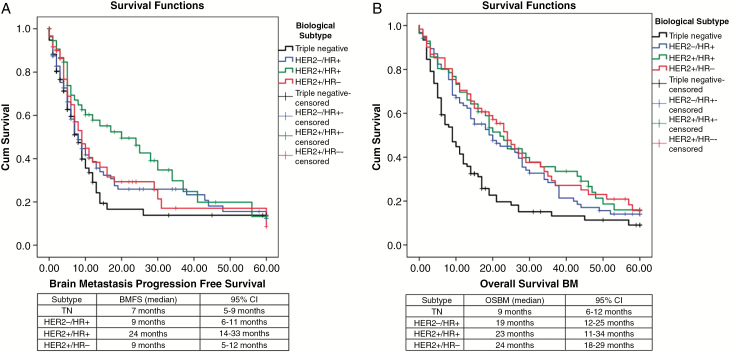
The median TDBM was 35 months (95% CI, 29–40). Patients with HER2+/HR+ tumors had the longest TDBM (58 months), followed by those with HER2-/HR+ (47 months), HER2+/HR- (30 months), and triple negative (27 months) tumors (P < .01) (Figure. 1). The local recurrence rate was 25% and the median time to local recurrence was 40 months; no statistical difference was found regarding biological subtype, local treatment, or use of WBRT upfront in univariate and multivariate analyses. The distant recurrence rate was 54.1% and the median time to local recurrence rate was 12 months (95% CI, 9–15). No differences were found between biological subtypes or local treatment modalities when analyzing distant recurrence, but interestingly, patients with HER2-positive receptors had a longer time for distant recurrence (median 24 months; 95% CI, 12–35) compared to patients with HER2-negative receptors (median 9 months; 95% CI, 7–11) (P = .025) (Figure. 2A). The presence of HR did not show any statistical significance for distant recurrence. Use of adjuvant WBRT increased the BMFS (Figure 2B), and local treatment without adjuvant WBRT showed a hazard ratio of 1.5 (95% CI, 1.1–2.1) for brain metastasis recurrence, but no difference in OSBM was found. The median overall BMFS was 9 months (95% CI, 7–10). HER2+/HR+ was associated with the longest BMFS (median 24 months), followed by HER-/HR+ and HER2+/HR- (both with median of 9 months); triple negative was associated with the worst BMFS (7 months) (P = .018) (Figure. 3A). Patients with HER2-amplified tumors presented with a longer BMFS (median 12 months; 95% CI, 7–17) when compared to those with tumors with no HER2 amplification (median 7 months; 95% CI, 5–8) (P = .027) (Figure 3A).
Fig. 1.
Kaplan-Meier Time to Development of Brain Metastasis (TDBM) curves for biological subtypes (P < .001).
Fig. 2.
Kaplan-Meier curves for: A) Distant recurrence in tumors with and without HER2 amplification (P = .025); B) Use of adjuvant whole brain radiation therapy (WBRT) (P = .013).
Fig. 3.
Kaplan-Meier curves for: A) Brain metastasis-free survival (BMFS) by biological subtype (P = .027); B) Overall survival from start of treatment of brain metastasis (OSBM) stratified by molecular subtype (P < .001).
At 60 months follow-up, the median OSBM was 17 months (CI 95%, 14–19). At 12 months the OSBM was 58%, at 24 months 39%, at 36 months 26%, at 48 months 17%, and at 60 months 13%. HER2+/HR- subtype was associated with the longest OSBM (median 24 months), followed by HER2+/HR+ (median 23 months) and HER2-/HR+ (19 months). The triple negative subtype was associated with the worst OSBM (median 9 months) (P = .013) (Figure 3B). To assess for confounding factors, we ran a multivariate analysis among the 4 subtypes regarding OSBM. It showed a hazard ratio of 1.9 attributable to triple negative tumors (CI 95%, 1.3–2.7, P < .001), highlighting the importance of HR and HER2 status.
Regarding multimodality local treatment, univariate and multivariate analyses were performed, assessing OSBM, time to local recurrence, and BMFS. No difference was found between SRS, surgery, or a combination of both, regardless of single or multiple metastases.
Discussion
Breast cancer subtypes differ not only in tumor characteristics, but also in metastatic behavior.29 The use of anti-HER2 regimens during the last 15 years has achieved remarkable progress in managing HER2+ breast cancer and has changed the natural history of metastatic disease, although central nervous system (CNS) disease is still a major concern with continued risk of disease progression.10 Recently the American Society of Clinical Oncology released an expert-consensus recommendation on disease management for patients with advanced HER2+ breast cancer and brain metastasis, recognizing the importance of this subtype, its adverse clinical behavior, and targeted therapy.17
The presence of HER2+ amplification occurs in 20% of primary breast carcinomas and has been associated in the past with a decreased overall survival22 and more recently with a higher propensity for development of brain metastasis.30 The use of the anti-HER2 monoclonal antibody trastuzumab has also been identified as a potential risk factor for CNS metastasis in a series of retrospective studies, with CNS recurrence rates of 25% to 40%.11,12 Four meta-analyses have evaluated the incidence of CNS metastasis in adjuvant trastuzumab trials, reporting rates that vary from a 1.3- to 1.8-fold risk increase.31,32 A retrospective analysis of prospectively collected cancer registry data from 1458 patients with stage I to III invasive breast cancer showed that the administration of trastuzumab either as adjuvant therapy or for metastatic disease was associated with an increased risk in CNS involvement. The authors concluded that HER2-positive breast cancers have a significantly higher incidence of CNS metastasis after being treated with trastuzumab.33 A recent multicenter retrospective study with 588 patients with HER-positive early-stage breast cancer receiving adjuvant trastuzumab showed an increase risk of brain metastasis as the first site of disease recurrence.34 One possible explanation is that the use of trastuzumab improves the systemic control and overall survival, but has a limited penetration on CNS creating the opportunity for brain metastasis to become a major clinical problem.35
However, there is increasing recognition that breast cancer tumors with HER2 positivity are not a homogeneous group. Studies have shown that the ER status defines 2 distinct subtypes within HER2+ breast cancer, including patterns of recurrence and OSBM.21,36 Preclinical studies suggest a cross-talk between HER2 and ER signaling pathways. Breast cancer patients with ER-positive status (ER+) and resistance to endocrine therapy, showed a restoration in endocrine sensitivity after HER2 targeted therapy.37,38 Similarly, with sustained HER2 inhibition, the ER functions as a key escape/survival pathway in ER+/HER2+ cells,39,40 suggesting interdependence of the 2 signaling pathways when co-expressed in breast cancer cells. In the present study, the HER2+/HR- subtype had BMFS similar to the triple negative subtype, and the best OSBM among the 4 subtypes (mean of 24 months). This is in agreement with the most updated literature that shows patients with metastatic HER2+ breast cancer treated with targeted therapy directed to HER2 have a median survival time that exceeds 3 years.9,10 HER2-positive patients presented with a longer time for distant recurrence. Also, patients with the HER2+/HR+ subtype had the longest BMFS. But HER2+/HR- and HER2-/HR+ patients had BMFS rates comparable to triple negative patients, which further suggests some interdependence of the 2 signaling pathways when co-expressed in breast cancer cells. Fifty percent of all HER2-positive breast tumors also express ER.36 With sustained HER2 inhibition, ER might function as a key survival pathway in tumor cells.39 On the other hand HER2 overexpression has been associated with endocrine therapy resistance.37,40 Clinically, the ER has a major impact on breast cancer characteristics, retaining its impact when co-expressed with HER2. Also there is evidence that HER2 modulates ER+ breast cancer making it more aggressive.36 Based on growing evidence of cross-talk between HER2 and ER, Vaz-Luis et al, proposed that the ER status defines two distinct subtypes within HER2-positive breast cancer.41 Thus the combined blocking of both receptors might contribute to the current study findings.
Breast cancer is an example of a hormone-dependent cancer. Estrogen plays a major role in development and progression of the disease. Seventy percent of breast tumors express the ER and/or PR. In the current study, 44% of the tumors were ER positive. Comprising 32% of the molecular subtypes, the HER2-/HR+ subtype had a TDBM comparable to but shorter than the HER2+/HR+ subtype. OSBM was also shorter than HER2+/HR- and HER2+/HR+ subtypes. In the last 30 years, endocrine therapy has been the cornerstone therapy for breast cancer with tamoxifen responsible for improved survival in early breast cancer, as well as in metastatic disease.42 However predictive biomarkers to guide clinicians through the wide variety of different treatment options are still needed. In the setting of CNS metastasis, the brain is capable of endogenous biosynthesis of estrogen and progesterone43 and the extragonadal production tends to increases with age.44 This highlights the importance of endocrine therapy in patients with brain metastasis even after menopause.
Among the 4 subtypes, triple negative had the shortest time to development of brain metastasis after the diagnosis of the primary tumor. Several studies have demonstrated that triple negative patients have a higher rate of brain metastasis than other patients and the prognosis after CNS relapse is particularly poor for those patients.13,23 The peak risk of recurrence of this subtype is greater between the first and third year following initial diagnosis.45 Even after adjustment for age, stage, race, adjuvant chemotherapy, tumor size, grade, and lymph node status, this subtype was still associated with worse OSBM.13 Our study confirmed that the triple negative subytpe was associated with the worst OSBM, with a median of only 9 months, and was an independent risk factor for short OSBM, with a hazard ratio of 1.9 on multivariate analyses.
The present study has numerous limitations. It is a retrospective study, with patients who underwent some form of treatment in the Neurosurgery Department, namely surgery and/or radiosurgery, therefore comprises a more selected group, with overall higher KPS and, in a number of cases, more limited disease. Also, due to the time span of this case series, several systemic treatments were used, which increases the heterogeneity of the group of patients analyzed. Nonetheless, our results demonstrated that the presence of molecular targets such as HER and HR poses as important predictive factors contributes expressively to a longer OSBM. In the case of HER2+/HR- patients, despite the fact that these patients have a shorter TDBM and BMFS, the OSBM was not negatively impacted. This should bring awareness to the fact that these patients are more prone to CNS recurrence, which requires a close surveillance. These findings should also encourage aggressive treatment of the brain metastasis since longer OSBM is expected. Unfortunately, little advance has been achieved in systemic control for triple negative tumors, which still remains the subtype with the worst prognosis despite advances in systemic therapy. These patients should have a low threshold for cranial imaging, especially if symptoms develop, noting their relatively poor prognosis.
In conclusion future prospective trials should differentiate between the molecular subtypes in order to address the true clinical importance among them. HR and HER2 are the most significant biomarkers that drive breast cancer behavior, including in brain metastasis. Patients who received targeted therapy had better outcomes, but not in the triple negative group. Prospective studies with different treatment modalities for each subgroup are recommended.
Funding
No funding to disclousure.
Conflict of Interest. No conflict of interest.
Acknowledgements
We thank Professor Maria do Rosario Dias de Oliveira Latorre for statistical assistance. We thank Dr. Daniel J. Booser for editing assistance.
References
- 1. Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. . Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865–2872. [DOI] [PubMed] [Google Scholar]
- 2. Tabouret E, Chinot O, Metellus P, et al. . Recent trends in epidemiology of brain metastases: an overview. Anticancer Res. 2012;32(11):4655–4662. [PubMed] [Google Scholar]
- 3. DiStefano A, Yong Yap Y, Hortobagyi GN, et al. . The natural history of breast cancer patients with brain metastases. Cancer. 1979;44(5):1913–1918. [DOI] [PubMed] [Google Scholar]
- 4. Tsukada Y, Fouad A, Pickren JW, et al. . Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer. 1983;52(12):2349–2354. [DOI] [PubMed] [Google Scholar]
- 5. Tham YL, Sexton K, Kramer R, et al. . Primary breast cancer phenotypes associated with propensity for central nervous system metastases. Cancer. 2006;107(4):696–704. [DOI] [PubMed] [Google Scholar]
- 6. Pestalozzi BC, Zahrieh D, Price KN, et al. ; International Breast Cancer Study Group (IBCSG) Identifying breast cancer patients at risk for Central Nervous System (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG). Ann Oncol. 2006;17(6):935–944. [DOI] [PubMed] [Google Scholar]
- 7. Niwińska A, Murawska M, Pogoda K. Breast cancer brain metastases: differences in survival depending on biological subtype, RPA RTOG prognostic class and systemic treatment after whole-brain radiotherapy (WBRT). Ann Oncol. 2010;21(5):942–948. [DOI] [PubMed] [Google Scholar]
- 8. Kennecke H, Yerushalmi R, Woods R, et al. . Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28(20):3271–3277. [DOI] [PubMed] [Google Scholar]
- 9. Brufsky AM, Mayer M, Rugo HS, et al. . Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17(14):4834–4843. [DOI] [PubMed] [Google Scholar]
- 10. Olson EM, Najita JS, Sohl J, et al. . Clinical outcomes and treatment practice patterns of patients with HER2-positive metastatic breast cancer in the post-trastuzumab era. Breast. 2013;22(4):525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burstein HJ, Lieberman G, Slamon DJ, et al. . Isolated central nervous system metastases in patients with HER2-overexpressing advanced breast cancer treated with first-line trastuzumab-based therapy. Ann Oncol. 2005;16(11):1772–1777. [DOI] [PubMed] [Google Scholar]
- 12. Bendell JC, Domchek SM, Burstein HJ, et al. . Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97(12):2972–2977. [DOI] [PubMed] [Google Scholar]
- 13. Lin NU, Vanderplas A, Hughes ME, et al. . Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer. 2012;118(22):5463–5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sezgin C, Gokmen E, Esassolak M, et al. . Risk factors for central nervous system metastasis in patients with metastatic breast cancer. Med Oncol. 2007;24(2):155–161. [DOI] [PubMed] [Google Scholar]
- 15. Engel J, Eckel R, Aydemir U, et al. . Determinants and prognoses of locoregional and distant progression in breast cancer. Int J Radiat Oncol Biol Phys. 2003;55(5):1186–1195. [DOI] [PubMed] [Google Scholar]
- 16. Rades D, Lohynska R, Veninga T, et al. . Evaluation of 2 whole-brain radiotherapy schedules and prognostic factors for brain metastases in breast cancer patients. Cancer. 2007;110(11):2587–2592. [DOI] [PubMed] [Google Scholar]
- 17. Ramakrishna N, Temin S, Chandarlapaty S, et al. . Recommendations on disease management for patients with advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(19):2100–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sorlie T, Perou CM, Tibshirani R, et al. . Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869–10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smid M, Wang Y, Zhang Y, et al. . Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68(9):3108–3114. [DOI] [PubMed] [Google Scholar]
- 21. Vaz-Luis I, Ottesen RA, Hughes ME, et al. . Impact of hormone receptor status on patterns of recurrence and clinical outcomes among patients with human epidermal growth factor-2-positive breast cancer in the National Comprehensive Cancer Network: a prospective cohort study. Breast Cancer Res. 2012;14(5):R129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Slamon DJ, Clark GM, Wong SG, et al. . Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. [DOI] [PubMed] [Google Scholar]
- 23. Nam BH, Kim SY, Han HS, et al. . Breast cancer subtypes and survival in patients with brain metastases. Breast Cancer Res. 2008;10(1):R20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sperduto PW, Kased N, Roberge D, et al. . The effect of tumor subtype on the time from primary diagnosis to development of brain metastases and survival in patients with breast cancer. J Neurooncol. 2013;112(3):467–472. [DOI] [PubMed] [Google Scholar]
- 25. Dawood S, Broglio K, Buzdar AU, et al. . Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2010;28(1):92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith I, Procter M, Gelber RD, et al. ; HERA study team 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369(9555):29–36. [DOI] [PubMed] [Google Scholar]
- 27. Gori S, Rimondini S, De Angelis V, et al. . Central nervous system metastases in HER-2 positive metastatic breast cancer patients treated with trastuzumab: incidence, survival, and risk factors. Oncologist. 2007;12(7):766–773. [DOI] [PubMed] [Google Scholar]
- 28. Sawaya R, Hammoud M, Schoppa D, et al. . Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42(5):1044–1055; discussion 1055. [DOI] [PubMed] [Google Scholar]
- 29. Soni A, Ren Z, Hameed O, et al. . Breast cancer subtypes predispose the site of distant metastases. Am J Clin Pathol. 2015;143(4):471–478. [DOI] [PubMed] [Google Scholar]
- 30. Gabos Z, Sinha R, Hanson J, et al. . Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J Clin Oncol. 2006;24(36):5658–5663. [DOI] [PubMed] [Google Scholar]
- 31. Dahabreh IJ, Linardou H, Siannis F, et al. . Trastuzumab in the adjuvant treatment of early-stage breast cancer: a systematic review and meta-analysis of randomized controlled trials. Oncologist. 2008;13(6):620–630. [DOI] [PubMed] [Google Scholar]
- 32. Olson EM, Abdel-Rasoul M, Maly J, et al. . Incidence and risk of central nervous system metastases as site of first recurrence in patients with HER2-positive breast cancer treated with adjuvant trastuzumab. Ann Oncol. 2013;24(6):1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Musolino A, Ciccolallo L, Panebianco M, et al. . Multifactorial central nervous system recurrence susceptibility in patients with HER2-positive breast cancer: epidemiological and clinical data from a population-based cancer registry study. Cancer. 2011;117(9):1837–1846. [DOI] [PubMed] [Google Scholar]
- 34. Tonyali O, Coskun U, Yuksel S, et al. ; Anatolian Society of Medical Oncology (ASMO) Risk factors for brain metastasis as a first site of disease recurrence in patients with HER2 positive early stage breast cancer treated with adjuvant trastuzumab. Breast. 2016;25:22–26. [DOI] [PubMed] [Google Scholar]
- 35. Le Scodan R, Jouanneau L, Massard C, et al. . Brain metastases from breast cancer: prognostic significance of HER-2 overexpression, effect of trastuzumab and cause of death. BMC Cancer. 2011;11:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alqaisi A, Chen L, Romond E, et al. . Impact of estrogen receptor (ER) and human epidermal growth factor receptor-2 (HER2) co-expression on breast cancer disease characteristics: implications for tumor biology and research. Breast Cancer Res Treat. 2014;148(2):437–444. [DOI] [PubMed] [Google Scholar]
- 37. Shou J, Massarweh S, Osborne CK, et al. . Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96(12):926–935. [DOI] [PubMed] [Google Scholar]
- 38. Arpino G, Wiechmann L, Osborne CK, et al. . Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocr Rev. 2008;29(2):217–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang YC, Morrison G, Gillihan R, et al. . Different mechanisms for resistance to trastuzumab versus lapatinib in HER2-positive breast cancers–role of estrogen receptor and HER2 reactivation. Breast Cancer Res. 2011;13(6):R121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Massarweh S, Schiff R. Resistance to endocrine therapy in breast cancer: exploiting estrogen receptor/growth factor signaling crosstalk. Endocr Relat Cancer. 2006;13(Suppl 1):S15–S24. [DOI] [PubMed] [Google Scholar]
- 41. Vaz-Luis I, Winer EP, Lin NU. Human epidermal growth factor receptor-2-positive breast cancer: does estrogen receptor status define two distinct subtypes?Ann Oncol. 2013;24(2):283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jaiyesimi IA, Buzdar AU, Decker DA, et al. . Use of tamoxifen for breast cancer: twenty-eight years later. J Clin Oncol. 1995;13(2):513–529. [DOI] [PubMed] [Google Scholar]
- 43. Anuka E, Gal M, Stocco DM, et al. . Expression and roles of steroidogenic acute regulatory (StAR) protein in ‘non-classical’, extra-adrenal and extra-gonadal cells and tissues. Mol Cell Endocrinol. 2013;371(1-2):47–61. [DOI] [PubMed] [Google Scholar]
- 44. Cui J, Shen Y, Li R. Estrogen synthesis and signaling pathways during aging: from periphery to brain. Trends Mol Med. 2013;19(3):197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Anders CK, Carey LA. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin Breast Cancer. 2009;9(Suppl 2):S73–S81. [DOI] [PMC free article] [PubMed] [Google Scholar]