Abstract
Lyme disease is an emerging infectious disease of public health concern in the northeastern United States. The disease’s vector, Ixodes scapularis (Say) (Blacklegged Tick), has increased its range in the past twenty years. In its newly endemic northern range there have been few studies of the Blacklegged Tick’s habitat associations. From 2016–2018, we sampled for nymphal Blacklegged Ticks in the Champlain Valley and Green Mountains of Addison County, Vermont, and tested them for Borrelia burgdorferi, the Lyme disease agent. We found 10 times more ticks in the Champlain Valley than in the Green Mountains. Nymphal infection prevalence was 0.21 and did not vary by year or region. The difference in tick density reported has public health consequences, as Vermont has one of the highest rates of Lyme disease in the United States.
Introduction
Lyme disease is an important emerging infectious disease found in many parts of the temperate northern hemisphere and is now the most prevalent vector-borne disease in both the United States and western Europe (Kilpatrick et al. 2017, Rosenberg et al. 2018). It is caused by the spirochete bacterium Borrelia burgdorferi and transmitted by ticks in the genus Ixodes (Latreille).
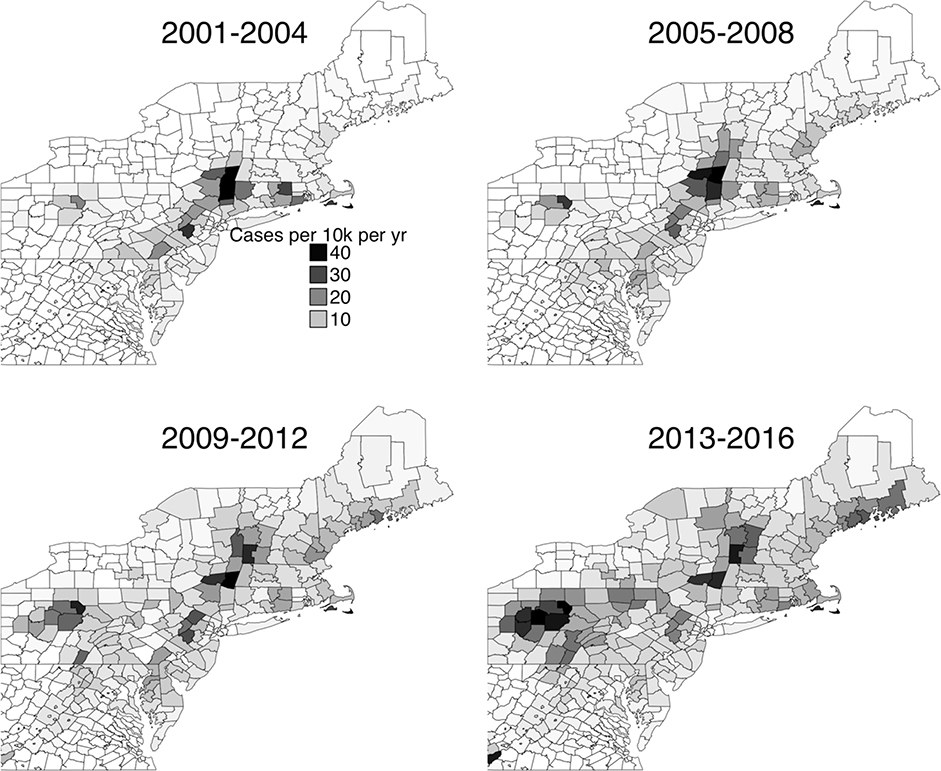
In the last 20 years, the number of confirmed cases in the United States has more than doubled from 12,801 in 1997 to 29,513 in 2017 (CDC 2019). There has also been a northward shift in disease incidence in the eastern North America, with more cases in northern New England and southern Canada (Kugeler et al. 2015, Ogden et al. 2009, Fig. 1). In particular, Vermont has seen a dramatic rise with 100 or fewer cases reported every year prior to 2008 and more than 1000 cases in 2017 (Vermont Department of Health 2018). A similar northward shift in the incidence of the disease has been seen in Europe (Jore et al. 2011, Mysterud et al. 2017). There has been a parallel northward shift in the distribution of the disease’s vectors, Ixodes scapularis (Say) (Blacklegged Tick) in eastern North America and Ixodes ricinus (L.) (Castor Bean Tick) in Europe (Eisen et al. 2016, Jore et al. 2011). It is thought that this northward movement of Lyme disease vectors is due to climate change (Simon et al. 2014).
Figure 1:
County level incidence map of Lyme diseases cases reported to the CDC. Each subgraph gives the average annual number of cases per 10,0000 people over the four-year period. Lyme disease case numbers from the CDC (2018). Annual county population estimates from the US Census Bureau (2018a, 2018b).
Borrelia burdorferi is maintained in an enzootic cycle by tick vectors and a diverse community of vertebrate reservoirs. Tick infection rate depends on the composition of this vertebrate community because each species has a different reservoir competency for B. burgdorferi (LoGiudice et al. 2003). In the northeastern United States Peromyscus leucopus (Rafinesque) (White-footed Mouse) is thought to be the most important reservoir, although other small mammal species also contribute (Bouchard et al. 2011, Brisson et al. 2008). Tick survival and behavior are sensitive to temperature and leaf-litter relative humidity (Berger et al. 2014b, Ogden et al. 2004, Perret et al. 2004). This collection of biotic and abiotic factors makes it difficult to predict and understand small-scale temporal and spatial variation in B. burgdorferi-infected tick populations (Eisen et al. 2015). On the other hand, broad-scale patterns of Blacklegged Tick distribution are successfully explained by climate, terrain, and land cover variables (Diuk-Wasser et al. 2010, Hahn et al. 2016). Specifically, Diuk-Wasser et al. (2010) and Hahn et al. (2016) both found decreasing tick presence with elevation.
Within their original distribution in southern New England and the Mid-Atlantic the ecology of the Blacklegged Tick and B. burgdorferi is well studied. But within the newly expanded northern range there are fewer studies (some of the few examples are Bouchard et al. 2011, Lubelczyk et al. 2004, Serra et al. 2013, Simon et al. 2014). There is reason to believe that Blacklegged Tick population and infection dynamics are different in this northern emergent region (Burtis et al. 2016). There is a gap in understanding of what controls Blacklegged Tick population numbers and B. burgdorferi-infection rate in this region.
To address this gap, this study compares tick density and B. burgdorferi infection across two ecoregions in Vermont, the Champlain Valley and the Green Mountains. This provides a measure of how tick density changes in two geographically close but ecologically and climatically different regions. Importantly, it also provides one of the few thorough measurements of tick density in Vermont (along with Serra et al. 2013), a state with one of the highest incidences of Lyme disease in the country (CDC 2019, Fig. 1).
Field Site Description
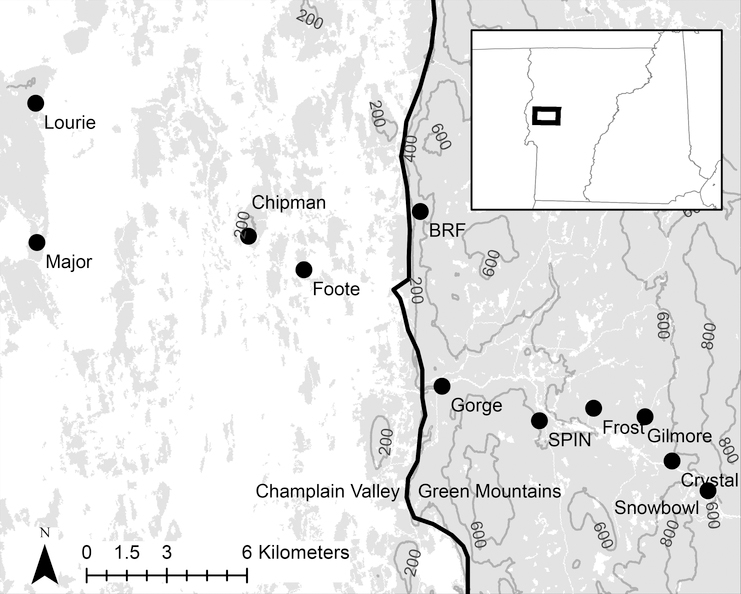
We used EPA ecoregions level III classifications to delineate the two regions in this study (Omernik and Griffith 2014). The Champlain Valley is part of the Eastern Great Lakes Lowlands ecoregion and the Green Mountains are part of the Northeastern Highlands ecoregions. We used a shapefile with ecoregion boundaries to delineate the regions (EPA 2018). We chose sites based on the following criteria: closed-canopy forest; predominantly deciduous trees at sites; leaf litter layer on the forest floor; having sites within both of the ecoregions; and along an elevation gradient from the Champlain Valley to the spine of the Green Mountains. We preferentially selected sites on Middlebury College owned land. Based on these criteria we selected the 11 sites used in this study (Table 1). The elevation of sites ranged between 126 m to 693 m above sea level: Champlain Valley sites had a mean elevation of 156 m (126–210 m) and Green Mountains sites a mean of 471 m (254–693 m). The ruggedness and steepness did not differ greatly between sites in the two ecoregions because some Champlain Valley sites were located on hills in the valley (Chipman Hill and Snake Mountain).
Table 1:
Density of questing nymphal ticks from May 15 to July 15 at each of 11 sampling sites across the three years of the study. Three sites were added in 2017, so do not have values for 2016. Ecoregion is either CV for Champlain Valley (i.e., Eastern Great Lakes Lowlands) or GM for Green Mountains (Northeastern Highlands), as determined from (EPA 2018). Mean annual temperature and total precipitation are 30-year normal, 1981–2010 (PRISM Climate Group 2019).
| Site name | Ecore gion | Elev. (m) | Mean temp. (C) | Mean annual precipitation (mm) | 2016 nymphs per 200 m2 (± SE) | 2017 nymphs per 200 m2 (± SE) | 2018 nymphs per 200 m2 (± SE) |
|---|---|---|---|---|---|---|---|
| Major | CV | 126 | 7.7 | 968 | NA | 15.75 (± 3.22) | 1.33 (± 0.86) |
| Lourie | CV | 134 | 7.6 | 938 | NA | 22.38 (± 3.60) | 4.00 (± 2.20) |
| Foote | CV | 152 | 7.5 | 977 | 12.21 (± 4.12) | 26.25 (± 7.67) | 6.27 (± 2.34) |
| Chipman | CV | 210 | 7.2 | 966 | 4.38 (± 2.15) | 16.27 (± 4.93) | 3.27 (± 2.52) |
| Gorge | GM | 254 | 6.3 | 1095 | 3.63 (± 0.65) | 9.50 (± 1.46) | 0.75 (± 0.63) |
| BRF | GM | 382 | 6.2 | 1113 | 0.62 (± 0.44) | 1.13 (± 0.60) | 0.38 (± 0.53) |
| SPIN | GM | 405 | 5.7 | 1209 | 0.14 (± 0.27) | 1.38 (± 0.83) | 0.00 (± 0.00) |
| Frost | GM | 462 | 5.1 | 1216 | 0.21 (± 0.25) | 0.58 (± 0.46) | 0.33 (± 0.37) |
| Gilmore | GM | 532 | 5 | 1236 | 1.13 (± 0.88) | 0.88 (± 0.59) | 0.00 (± 0.00) |
| Crystal | GM | 569 | 4.8 | 1326 | 0.36 (± 0.43) | 0.27 (± 0.37) | 0.14 (± 0.22) |
| Snowbowl | GM | 693 | 4.3 | 1408 | NA | 0.00 (± 0.00) | 0.13 (± 0.25) |
All sites were in northern hardwood forests dominated by Acer saccharum (Marshall) (Sugar Maple), Acer rubrum (L.) (Red Maple), Fagus grandifolia (Ehrh.) (American Beech), and Betula alleghaniensis (Britt.) (Yellow Birch). There were smaller fractions of Fraxinus americana (L.) (White Ash), Tsuga canadensis (L.) (Eastern Hemlock), and Pinus strobus (L.) (White Pine). Low elevation sites had more Quercus rubra (L.) (Red Oak), while high elevation sites had more Picea rubens (Sarg.) (Red Spruce). All sites had a thick leaf litter layer on the forest floor. The amount of herbaceous ground cover or shrub layer varied between the sites, although without consistent patterns of denser cover in either ecoregion. Shrub layer species composition did differ between regions. Champlain Valley sites had Lonicera spp. (L.) (Honeysuckle) and Berberis thunbergii (DC.) (Japanese Barberry). Lubelczyk et al. (2004) found higher tick abundance in sites with these invasive shrubs. The shrub layer at the Green Mountain sites was dominated by Viburnum lantanoides (Michx.) (Hobblebush). All samples were taken in forest interiors at least 100 m away from the nearest forest edge.
Champlain Valley sites were generally in small forest fragments separated by agricultural land: hay fields, corn fields, cow pasture, or apple orchards. Green Mountain sites were largely contiguous with a section of the Green Mountain National Forest. We delineated the boundary of the forest fragment at each site by using leaf-on satellite imagery (Vermont Center for Geographic Information 2018). We estimated the area of this fragment using ArcGIS 10.3 (ESRI 2014). Forest fragment area for Champlain Valley sites had a mean area of 721 ha (range 26 – 1378 ha). For Green Mountain sites it was 4348 ha (18 – 17960 ha). PRISM modeled 30-year climate normals showed differences between the ecoregions (PRISM Climate Group 2019, Table 1). With elevation there was a decline in mean annual temperature and an increase in total annual precipitation. The Champlain Valley sites had similar climates, while the Green Mountain sites had larger, elevation-based climate differences (Table 1).
Methods
Tick sampling
At each site we established two or three 50 m by 50 m square drag sampling plots depending on the size of the forest sampled. To sample for ticks, we dragged a 1 m2 white flannel cloth over the 200 m perimeter of the sampling plot. We stopped every 10 m to check both sides of the drag cloth for ticks. Drag cloth sampling is a standard method to measure the density of questing ticks (Daniels et al. 2000). In the field we put ticks on ice in a cooler and then upon returning to the lab stored them at −80° C until identification and DNA extraction.
We drag sampled for ticks from April to October in 2016, 2017, and 2018. We sampled monthly in early spring and late summer to fall, and then sampled every other week during the period of peak nymphal activity, mid-May to early August. It is important to sample regularly during peak activity period to make comparisons across sites (Dobson 2013). We focused on nymphal activity because nymphs are responsible for the majority of human Lyme disease cases (Barbour and Fish 1993).
B. burgdorferi testing
We identified nymphs to species by dichotomous key (Durden and Keirans 1996, Lindquist et al. 2016). We followed the DNA extraction protocol of Wang et al. (2014), except that for increased tissue lysis we initially bead beat the ticks (Ammazzalorso et al. 2015). We placed ticks in 180 μL of ATL buffer (Qiagen, Hilden Germany) and homogenized the animals with a FastPrep-24 bead beater (MP Biomedicals, Santa Ana, CA, USA) in Lysis Matrix H (MP Biomedicals). We then added 20 μL of proteinase K solution (600 mAU/ml) and incubated the ticks overnight at 56° C. After that we followed the standard DNeasy Blood and Tissue protocol (Qiagen).
We assessed whether each tick carried B. burgdorferi by amplifying a 600 bp region of the ospC gene (Wang et al. 1999). This region was amplified using primers OC6+ 5’-AAAGAATACATTAAGTGCGATATT-3’ and OC602− 5’-GGGCTTGTAAGCTCTTTAACTG-3’. The 20 μL PCR reactions contained 10 μL Phusion High-Fidelity Master Mix (New England BioLabs, Ipswich, MA, USA), 0.7 μL 10 mM OC6+, 0.7 μL 10 mM OC602−, 3 μL of sample, and 5.6 μL of nuclease-free water. We used the following thermocycler conditions: an initial denaturing step at 98° C for 2 m; then 32 cycles of 98° C for 10 s, 52° C for 30 s, and 72 ° C for 45 s; and then 72 ° C for 7 m for final elongation. We ran negative controls with DNA extracted from uninfected ticks from the Oklahoma State University Tick Rearing Facility and positive controls with B. burgdorferi DNA strain B31 provided by Professor Bob Cluss. The PCR product was visualized on a 1% agarose gel. We sequenced a subset of PCR products to confirm we were amplifying B. burgdorferi sensu stricto.
Analysis
Tick density with ecoregion.
We sampled for ticks from April to October. However, to compare nymphal density, we looked at only the period of peak nymphal activity, May 15 to July 15. We compared questing tick count per 200 m2 sample during that time period using a generalized mixed effects model. Tick count was assumed to be negative binomially distributed and we took ecoregion and year as fixed effects while sampling site was a random effect. We included site as a random effect because tick collections were nested within site, with each site having two or three sampling plots. We used a log link function (Bolker et al. 2009). The model was fit in the lme4 package in the statistical computing language R (Bates et al. 2015, R Core Team 2017).
Tick density with elevation.
We also compared nymphal tick density with elevation, focusing only on nymphal density from May 15 to July 15. We compared the average tick density at each site across this time period to elevation. We performed a simple linear regression between log tick density plus one and elevation. The analysis was conducted separately on each year’s data. The log transformation was performed because of the wide range of tick density observed between sites. We added one to density because some sites had a density of zero, thus could not be log transformed.
Tick infection.
We compared B. burgdorferi-infection rate in nymphs with a generalized mixed effects model. Here infection was taken as a binomial variable, ecoregion and year were fixed effects, and sampling site was a random effect. We used a logit link function (Bolker et al. 2009). We calculated the 95% binomial confidence interval around estimated infection rate for each year-region combination using the binom package (Dorai-Raj 2014).
Results
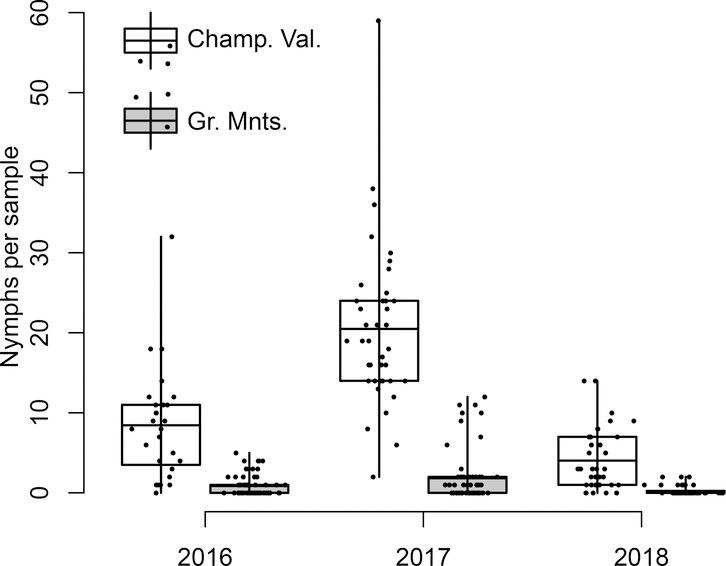
We found wide variation in the density of questing ticks across the 11 sites, from as low as zero ticks per 200 m2 sample for some sites in some years to over 25 ticks per sample (Table 1). Relative rankings of tick density among sites remained largely consistent across the three years of study; sites with a high density of ticks in one year tended to have a high density of ticks in other years (Table 1, Figure 4). Across all sites within a region we found significantly more ticks per sample in the Champlain Valley than in the Green Mountains (Table 2, Fig. 3). On average there were about 10 times more ticks in Champlain Valley sites than in Green Mountain sites.
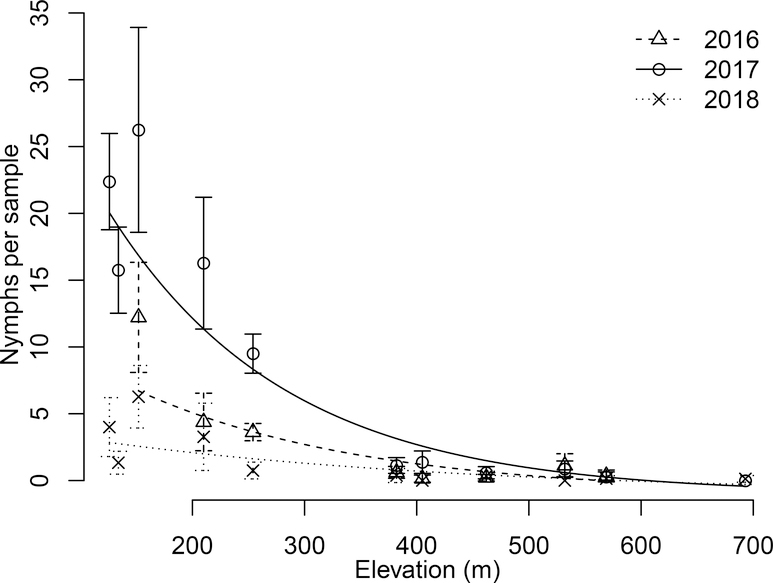
Figure 4:
Density of questing nymphal ticks per 200 m2 drag sample between May 15 and July 15 as a function of site elevation. The error bars indicate the standard error around each mean. For each year we performed a simple linear regression between log tick density plus one and elevation. We took the inverse of the transformation to plot untransformed tick density, so these lines became negative exponential curves. Each linear relationship was significant: 2016 F1,6 = 16.4, p = 0.007; 2017 F1,8 = 92.0, p = 0.000005; and 2018 F1,9 = 17.9 p = 0.002.
Table 2:
Generalized linear mixed model of questing nymph density per 200m2 sample between May 15 and July 15. Ecoregion (Champlain Valley or Green Mountains) and year were fixed effects, while sampling site was a random effect. Estimates compare samples to 2016 Champlain Valley values. Green Mountain sites had fewer ticks than Champlain Valley sites, 2017 had more than 2016, and 2018 had fewer than 2016.
| Estimate | Std. error | Z value | P value | |
|---|---|---|---|---|
| Intercept | 1.762 | 0.383 | 4.601 | 4.2 × 10−6* |
| Region = GM | −2.433 | 0.512 | −4.756 | 2.0 × 10−6* |
| Year = 2017 | 1.032 | 0.138 | 7.469 | 8.1 × 10−14* |
| Year = 2018 | −0.665 | 0.155 | −4.280 | 1.9 × 10−5* |
| GM × 2017 inter. | −0.329 | 0.241 | −1.365 | 0.1722 |
| GM × 2018 inter. | −0.658 | 0.366 | −1.797 | 0.0723 |
indicate significant effects.
Figure 3:
Density of questing nymphal ticks per 200 m2 drag sample between May 15 and July 15. The graph compares the Champlain Valley (white rectangles) to the Green Mountains (gray rectangles) across the three years of the study. The box and whiskers show the mean (horizontal line), two interquartiles (boxes) and full data range (whiskers). Plotted over the box and whiskers are the raw data with an x-axis jitter for clarity.
We also found considerable year-to-year variation in tick densities at our sites. This inter-annual variation was similar across sites, with most having the highest density of ticks in 2017 and the lowest in 2018 with 2016 in the middle (Table 2, Fig. 3). Overall there was a significant effect of year on tick density (Table 2, Fig. 3).
There was a negative relationship between tick density and elevation. For each year the linear regression between log density and elevation was significant with a negative slope (Fig. 4). Taking the inverse of this transformation results in a negative exponential relationship between tick density and elevation, as seen in Figure 4.
Our assay of B. burgdorferi-infection rate successfully identified positive controls while giving negative results for our negative controls. All samples that were sequenced matched the ospC gene for B. burgdorferi sensu stricto in GenBank.
Nymphal B. burgdorferi-infection rates are given in Table 3. Although the Champlain Valley had a slightly higher rate than the Green Mountains, this difference was not statistically significant (Table 4). There was also no difference in infection rate across the three years of the study. Across all years and regions average infection rate was 0.210 (95% binomial confidence interval: 0.180–0.242, n = 704).
Table 3:
Nymphal Borrelia burgdorferi-infection rate compared between the two ecoregions and the three years of the study. The table also gives the 95% binomial confidence interval and sample size for each year-region combination. See Table 4 for statistical comparison.
| Champlain Valley | Green Mountains | Total | |
|---|---|---|---|
| 2016 | 0.23 (0.17–0.31, n = 159) | 0.20 (0.11–0.23, n = 60) | 0.22 (0.17–0.29, n = 219) |
| 2017 | 0.21 (0.17–0.26, n = 348) | 0.15 (0.08–0.24, n = 81) | 0.20 (0.16–0.24, n = 429) |
| 2018 | 0.26 (0.14–0.40, n = 47) | 0.22 (0.03–0.60, n = 9) | 0.25 (0.14–0.38, n = 56) |
| Total | 0.22 (0.19–0.26, n = 554) | 0.17 (0.12–0.24, n = 150) | 0.21 (0.18–0.24, n = 704) |
Table 4:
Generalized linear mixed model of nymph infection rate. Ecoregion (Champlain Valley or Green Mountains) and year were fixed effects, while sampling site was a random effect. Estimates compare samples to 2016 Champlain Valley values. There was no significant effect of region or year on infection rate.
| Estimate | Std. error | Z value | P value | |
|---|---|---|---|---|
| Intercept | −1.164 | 0.1723 | −6.756 | 1.4 * 10−11 * |
| Region = GM | −1.311 | 0.240 | −1.295 | 0.195 |
| Year = 2017 | −0.180 | 0.204 | −0.882 | 0.378 |
| Year = 2018 | 0.112 | 0.350 | 0.321 | 0.749 |
Discussion
We found a dramatic difference in nymphal tick density between the Champlain Valley and Green Mountains. This difference holds up in the face of considerable variation in tick density among sites within the two ecoregions. We also found large inter-annual variation in tick density across the three years of this study. This inter-annual variation was roughly consistent across all of our sampling sites. Therefore, although tick density remains very different from site to site, it might be responding to consistent region-wide annual changes, such as weather or host populations. The high rate of spatiotemporal variation in Blacklegged Tick populations has been seen in other studies (Ostfeld et al. 2006, Serra et al. 2013).
We found a negative relationship between tick density and elevation, and this may be the driver for differences in tick density between the Champlain Valley and Green Mountains. Diuk-Wasser et al. (2010) and Hahn et al. (2016) both found negative correlations between Blacklegged Tick presence and elevation in large-scale studies of tick habitat suitability. In Europe, studies along elevation gradients found negative relationships between Castor Bean Tick density and elevation (Gilbert 2010, Jouda et al. 2004, Materna et al. 2005, Qviller et al. 2014). Thus it is very possible that elevation could be responsible for part of the difference we see. This study was conducted near the northern range limit for the Blacklegged Tick, which is understood to be determined by cold temperature as populations of the tick require a minimum total degree days to persist (Ogden et al. 2005). Thus the cold temperatures at higher elevation sites might limit tick population growth.
Another factor that could be responsible for the pattern is the difference in forest fragment area between the two regions. Forest fragment area has a considerable effect on vertebrate communities, and thus, potentially, tick host communities (Andrén 1994). Ostfeld (2011) suggests that small forest fragments support species-poor vertebrate communities dominated by the White-footed Mouse, which is a highly permissive Blacklegged Tick host. There is evidence that smaller forest fragments do, in fact, have higher densities of Blacklegged Ticks (Allan et al. 2003). However, that study took place in a more fragmented landscape with much smaller forest fragment sizes (less than 10 ha) than our Champlain Valley sites (average 721 ha). Still it is possible that the smaller Champlain Valley forest fragments support vertebrate communities more suitable for the Blacklegged Tick than the larger Green Mountain fragments, and that this is the driver of tick density differences. On the other hand, other studies of Ixodes ticks find positive relationships between fragment area and tick density (Ehrmann et al. 2017, Lawrence et al. 2018). The data presented here cannot tease apart the roles of fragment area versus temperature in determining tick density, because fragment size increases with elevation in our study area (Fig. 2). Further we do not have information on the host communities at our sites.
Figure 2:
Map of the sampling sites in this study. The locations of the 11 sites are indicated by the black dots, at each site there were 2–3 plots. The gray area in the map is forest (either deciduous forest, evergreen forest or mixed forest) and the white area is any other land cover type. Land cover classification from the National Land Cover Database 2011 (Homer et al. 2015). The thick black line represents the boundary between the Champlain Valley and Green Mountains (EPA 2018). Contour lines show elevation above sea level in meters. Inset map shows study area in regional context.
We found large variation in tick densities across the three years of the study. Inter-annual variation in Ixodes density is common (Ostfeld et al. 2006, Perret et al. 2000). This variation could result from annual differences in host populations or weather. Ostfeld et al. (2006, 2018) found that nymphal Blacklegged Tick density was best explained by mouse and chipmunk densities in the previous year which in turn was explained by acorn density in the year previous to that. Other studies found that dry spring conditions decrease the number of questing ticks in that year (Berger et al. 2014a, Burtis et al. 2016, Rodgers et al. 2007). After just three years of study, we do not have enough data to say what is responsible for the inter-annual variation in tick density we found.
Overall, we found a nymphal infection rate of about 21%. This is considerably higher than the 10% adult infection rate Serra et al. (2013) reported previously in Vermont. Adult Blacklegged Ticks are expected to have twice the infection rate of nymphs, since they have fed twice on potentially infected hosts versus once for nymphs. That makes the lower adult infection rate reported by Serra et al. (2013) than the nymphal infection rate reported in this study even more striking. This could reflect differences in the reservoir competency of the host communities between the east and west sides of Vermont. The difference could also be temporal, as Serra et al. (2013)’s tick collection was in 2011–2012, four to seven years earlier than this study. Lyme disease incidence has increased dramatically in Vermont, and this increase might reflect an increase in tick infection rate. Finally, the difference between the two studies could also reflect differences in the sensitivities of our B. burgdorferi detection assays. On the other hand, our nymphal infection rate is in line with other estimates (Diuk-Wasser et al. 2012, Feldman et al. 2015, States et al. 2014, Wang et al. 2003). Diuk–Wasser et al. (2012)’s sample of over 5000 nymphal Blacklegged Ticks from across the eastern US had a 20% infection rate—very close to our rate. Some studies have found other values. Horobik et al. (2006) found an infection rate of 32% for nymphs collected in the Hudson Valley, New York. This is an area with a longer history of B. burgdorferi than Vermont. While Simon et al. (2014) found an infection rate of 10% in nymphs in southern Quebec, this is an area with a more recent introduction of the disease than Vermont.
We expected to find a higher B. burgdorferi-infection rate in the Champlain Valley than in the Green Mountains. Jouda et al. (2004) found a negative relationship between tick B. burgdorferi-infection rate and elevation in Europe. Additionally, the density of ticks is higher in the Champlain Valley, and we expect regions with more vectors to have a higher rate of a vector-borne disease. Finally, the small forest fragments in the Champlain Valley may support a less-diverse, more reservoir competent community of hosts (Allan et al. 2003), which would also cause a higher infection rate in the Champlain Valley. Unexpectedly, we found no statistically significant difference in the infection rate between the two ecoregions. However, the non-significant trend we observed was towards a higher rate in the Champlain Valley.
This work has clear public health implications. Lyme disease is a major human health concern with about 30,000 confirmed cases a year in the United States reported to the CDC (CDC 2018). Cases are under-reported to the CDC, so the actual number may be closer to 300,000 (Hinckley et al. 2014, Nelson et al. 2015). Tick control strategies are costly, and as such must be targeted at areas of high tick-borne disease risk (Eisen et al. 2012). This study provides some guidance on areas of high tick density to target for potential tick control strategies or tick preventative messaging. However, the high level of variation in tick density even within the Champlain Valley means that more information is needed before small-scale targeting of the areas of highest disease risk can be carried out.
Finally, it is important to remember that patterns of Blacklegged Tick distribution and density have changed considerably in the past two decades (Eisen et al. 2016). Thus, the patterns of tick density reported here may not represent the equilibrium state. Tick populations in the Green Mountains may be only starting to establish and will increase to Champlain Valley levels in the future. Even if tick populations are in equilibrium with current conditions, future changes in abiotic and biotic factors could further alter tick distribution and density. It is important to continue to monitor tick populations in their newly emergent northern range. Finally, future work should aim to tease apart the role of climate versus the host community in explaining the regional differences in tick density seen here.
Acknowledgments
Thanks to the Middlebury College students who helped sample for ticks and test them for B. burgdorferi: Maisie Anrod, Harper Baldwin, Robert Cassidy, Evan Fedorov, Meaghan Hickey Nina Job, Sebastian Zavoico, and Grace Zhang. Thanks also to Bob Cluss for his helpful discussions and providing B. burgdorferi positive control DNA. Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103449. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIGMS or NIH. This research was also supported by Middlebury College.
Literature Cited
- Allan BF, Keesing F, and Ostfeld RS. 2003. Effect of forest fragmentation on Lyme disease risk. Conservation Biology 17:267–272. [Google Scholar]
- Ammazzalorso AD, Zolnik CP, Daniels TJ, and Kolokotronis S-O. 2015. To beat or not to beat a tick: comparison of DNA extraction methods for ticks (Ixodes scapularis). PeerJ 3:e1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrén H 1994. Effects of habitat fragmentation on birds and mammals with different proportions of suitable habitat: a review. Oikos 71:355–366. [Google Scholar]
- Barbour AG and Fish D. 1993. The biological and social phenomenon of Lyme disease. Science 260:1610–1616. [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, and Walker S. 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67:1–48. [Google Scholar]
- Berger KA, Ginsberg HS, Dugas KD, Hamel LH, and Mather TN. 2014a. Adverse moisture events predict seasonal abundance of Lyme disease vector ticks (Ixodes scapularis). Parasites and Vectors 7:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger KA, Ginsberg HS, Gonzalez L, Mather TN. 2014b. Relative humidity and activity patterns of Ixodes scapularis (Acari: Ixodidae). Journal of Medical Entomology 51:769–776. [DOI] [PubMed] [Google Scholar]
- Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, and White J-SS. 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends in Ecology and Evolution 24: 127–135. [DOI] [PubMed] [Google Scholar]
- Bouchard C, Beauchamp G, Nguon S, Trudel L, Milord F, Lindsay LR, Bélanger D, and Ogden NH. 2011. Associations between Ixodes scapularis ticks and small mammal hosts in a newly endemic zone in southestern Canada: Implications for Borrelia burgdorferi transmission. Ticks and Tick-borne Diseases 2:183–190. [DOI] [PubMed] [Google Scholar]
- Brisson D, Dykhuizen DE, and Ostfeld RS. 2008. Conspicuous impacts of inconspicuous hosts on the Lyme disease epidemic. Proceedings of the Royal Society B 275: 227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtis JC, Sullivan P, Levi T, Oggenfuss K, Fahey TJ, and Ostfeld RS. 2016. The impact of temperature and precipitation on Blacklegged Tick activity and Lyme disease incidence in endemic and emerging regions. Parasites and Vectors 9:606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). 2019. Lyme disease charts and figures: historical data. Available online at https://www.cdc.gov/lyme/stats/graphs.html. Accessed 23 February 2018.
- Daniels TJ, Falco RC, and Fish D. 2000. Estimating population size and drag sampling efficiency for the Blacklegged Tick (Acari: Ixodidae). Journal of Medical Entomology 37:357–363. [DOI] [PubMed] [Google Scholar]
- Diuk-Wasser MA, Vourc’h G, Cislo P, Hoen A, Melton F, Hamer SA, Rowland M, Cortinas R, Hickling GJ. Tsao JI, Barbour AG, Kitron U, Piesman J, and Fish D. 2010. Field and climate-based model for predicting the density of host-seeking nymphal Ixodes scapularis, an important vector of tick-borne disease agents in the eastern United States. Global Ecology and Biogeography 19:504–514. [Google Scholar]
- Diuk-Wasser MA, Gatewood Hoen A, Cislo P, Brinkerhoff R, Hamer SA, Rowland M, Cortinas R, Vourc’h G, Melton F. Hickling GJ. Tsao JI, Bunikis J, Barbour AG, Kitron U, Piesamn J, and Fish D. 2012. Human risk of infection with Borrelia burgdorferi, the Lyme disease agent, in eastern United States. American Journal of Tropical Medicine and Hygiene 86:320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson ADM 2013. Ticks in the wrong boxes: Assessing error in blanket-drag studies due to occasional sampling. Parasites and Vectors 6:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorai-Raj S 2014. binom: binomial confidence intervals for several parameterizations. R package version 1.1–1. Available online at https://CRAN.R-project.org/package=binom. Accessed 29 July 29 2018.
- Durden LA and Keirans JE. 1996. Nymphs of the Genus Ixodes (Acari: Ixodidae) in the United States: Taxonomy, Identification Key, Distribution, Hosts, and Medical/Veterinary Importance. Entomological Society of America Lanham, MD: 95 pp. [Google Scholar]
- Ehrmann S, Liira J, Gärtner S, Hansen K, Brunet J, Cousins SAO, Deconchat M, Decocq G, De Frenne P, De Smedt P, Diekmann M, Gallet-Moron E, Kolb A, Lenoir J, Lindgren J, Naaf T, Paal T, Valdés A, Verheyen K, Wulf M, and Scherer-Lorenzen M. 2017. Environmental drivers of Ixodes ricinus in forest fragments of rural European landscapes. BMC Ecology 17:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, and Beard CB. 2016. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. Journal of Medical Entomology 53:349–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, Ogden NH, and Beard CB. 2015. Linkages of weather and climate with Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae), enzootic transmission of Borrelia burgdorferi, and Lyme disease in North America. Journal of Medical Entomology 53:250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Piesman J, Zielinski-Gutierrez E, and Eisen L. 2012. What do we need to know about disease ecology to prevent Lyme disease in the northeastern United States? Journal of Medical Entomology 49:11–22. [DOI] [PubMed] [Google Scholar]
- Environmental Systems Research Institute (ESRI). 2014. ArcMap 10.3. Redlands, CA. [Google Scholar]
- Feldman KA, Connally NP, Hojgaard A, Jones EH, White JL, and Hinckley AF. 2015. Abundance and infection rates of Ixodes scapularis nymphs collected from residential properties in Lyme disease-endemic areas of Connecticut, Maryland, and New York. Journal of Vector Ecology 40: 198–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environmental Protection Agency (EPA). 2018. Level III and IV ecoregions of the continental United States. Available online at https://www.epa.gov/eco-research/level-iii-and-iv-ecoregions-continental-united-states. Accessed 14 September 2018.
- Gilbert L 2010. Altitudinal patterns of tick and host abundance: A potential role for climate change in regulating tick-borne diseases? Oecologia 162:217–225. [DOI] [PubMed] [Google Scholar]
- Hahn MB, Jarnevich CS, Monaghan AJ, and Eisen RJ. 2016. Modeling the geographic distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the contiguous United States. Journal of Medical Entomology 53:1176–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinckley AF, Connally NP, Meek JI, Johnson BJ, Kemperman MM, Feldman KA, White JL, and Mead PS. 2014. Lyme disease testing by large commercial laboratories in the United States. Clinical Infectious Diseases 59:676–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homer CG, Dewitz JA, Yang L, Jin S, Danielson P, Xian G, Coulston J, Herold ND, Wickham JD, and Megown K. 2015. Completion of the 2011 National Land Cover Database for the conterminous United States representing a decade of land cover change information. Photogrammetric Engineering and Remote Sensing 81:345–354. [Google Scholar]
- Horobik V, Keesing F, and Ostfeld RS. 2006. Abundance and Borrelia burgdorferi-infection prevalence of nymphal Ixodes scapularis ticks along forest-field edges. EcoHealth 3:262–268. [DOI] [PubMed] [Google Scholar]
- Jouda F, Perret J-L, and Gern L. 2004. Ixodes ricinus density, and distribution and prevalence of Borrelia burgdorferi sensu lato infection along an altitudinal gradient. Journal of Medical Entomology 41:162–169. [DOI] [PubMed] [Google Scholar]
- Kilpatrick AM, Dobson ADM, Levi T, Salkeld DJ, Swei A, Ginsberg HS, Kjemtrup A, Padgett KA, Jensen PM, Fish D, Ogden NH, and Diuk-Wasser MA. 2017. Lyme disease ecology in a changing world: Consensus, uncertainty and critical gaps for improving control. Philosophical Transactions of the Royal Society of London B 372:20160117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugeler KJ, Farley GM, Forrester JD, and Mead PS. 2015. Geographic distribution and expansion of human Lyme disease, United States. Emerging Infectious Diseases 21:1455–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jore S, Viljugrein H, Hofshagen M, Brun-Hansen H, Kristoffersen AB, Nygård K, Brun E, Ottesen P, Saevik BK, and Ytrehus B. 2011. Multi-source analysis reveals latitudinal and altitudinal shifts in range of Ixodes ricinus at its northern distribution limit. Parasites and Vectors 4:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence A, O’Connor K, Haroutounian V, and Swei A. 2018. Patterns of diversity along a habitat size gradient in a biodiversity hotspot. Ecosphere 9:e02183. [Google Scholar]
- Levi T, Keesing F, Holt RD, Barfield M, and Ostfeld RS. 2016. Quantifying dilution and amplification in a community of hosts for tick-borne pathogens. Ecological Applications 26:484–498. [DOI] [PubMed] [Google Scholar]
- Lindquist EE, Galloway TD, Artsob H, Lindsay LR, Drebot M, Wood H, and Robbins RG. 2016. A Handbook to the Ticks of Canada (Ixodida: Ixodidae, Argasidae). Biological Survey of Canada, Ottawa, Canada: 317 pp. [Google Scholar]
- LoGiudice K, Ostfeld RS, Schmidt KA, and Keesing F. 2003. The ecology of infectious disease: Effects of host diversity and community composition on Lyme disease risk. Proceedings of the National Academy of Sciences 100:567–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubelczyk CB, Elias SP, Rand PW, Holman MS, Lacombe EH, and Smith RP Jr. 2004. Habitat associations of Ixodes scapularis (Acari: Ixodidae) in Maine. Journal of Medical Entomology 33:900–906. [Google Scholar]
- Materna J, Daniel M, and Danielová V. 2005. Altitudinal distribution limit of the tick Ixodes ricinus shifted considerably towards higher altitudes in central Europe: Results of three years monitoring in the Krkonoše Mts. (Czech Republic). Central European Journal of Public Health 13:24–28. [PubMed] [Google Scholar]
- Mysterud A, Jore S, Østerås O, and Viljugrein H. 2017. Emergence of tick-borne diseases at northern latitudes in Europe: A comparative approach. Scientific Reports 7:16316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA, Saha S, Kugeler KJ, Delorey MJ, Shankar MB, Hinckley AF, and Mead PS. 2015. Incidence of clinician-diagnosed Lyme disease, United State, 2005–2010. Emerging Infectious Diseases 21:1625–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden NH, Bigras-Poulin M, O’Callaghan CJ, Barker IK, Lindsay LR, Maarouf A, Smoyer-Tomic KE, Waltner-Toews D, and Charron D. 2005. A dynamic population model to investigate effects of climate on geographic range and seasonality of the tick Ixodes scapularis. International Journal for Parasitology 35:375–389. [DOI] [PubMed] [Google Scholar]
- Ogden NH, Lindsay LR, Beauchamp B, Charron D, Maarouf A, O’Callaghan CJ, Waltner-Toes D, and Barker IK. 2004. Investigation of relationships between temperature and development rates of tick Ixodes scapularis (Acari: Ixodidae) in the laboratory and field. Journal of Medical Entomology 41:622–633. [DOI] [PubMed] [Google Scholar]
- Ogden NH, Lindsay LR, Morshed M, Sockett PN, and Artsob H. 2009. The emergence of Lyme disease in Canada. Canadian Medical Association Journal 180:1221–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omernik JM and Griffith GE. 2014. Ecoregions of the conterminous United States: Evolution of a hierarchical spatial framework. Environmental Management 54:1249–1266. [DOI] [PubMed] [Google Scholar]
- Ostfeld RS 2011. Lyme Disease: The Ecology of a Complex System. Oxford University Press, Oxford, United Kingdom: 216 pp. [Google Scholar]
- Ostfeld RS, Canham CD, Oggenfuss K, Winchcombe RJ, and Keesing F. 2006. Climate, deer, rodents, and acorns as determinants of variation in Lyme-disease risk. PLoS Biology 4:e145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostfeld RS, Levi T, Keesing F, Oggenfuss K, and Canham CD. 2018. Tick-borne disease risk in a forest food web. Ecology 99:1562–1573. [DOI] [PubMed] [Google Scholar]
- Perret J-L, Rais O, and Gern L. 2004. Influence of climate on the proportion of Ixodes ricinus nymphs and adults questing in a tick population. Journal of Medical Entomology 41:361–365. [DOI] [PubMed] [Google Scholar]
- PRISM Climate Group. 2019. Oregon State University. Available online at https://prism.oregonstate.edu/. Accessed on 22 February 2019.
- Qviller L, Grøva L, Viljugrein H, Klingen I, and Mysterud A. 2014. Temporal pattern of questing tick Ixodes ricinus density at differing elevations in the coastal region of western Norway. Parasites and Vectors 7:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. 2017. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Rodgers SE, Zolnik CP, and Mather TN. 2007. Duration of exposure to suboptimal atmospheric moisture affects nymphal blacklegged tick survival. Journal of Medical Entomology 44: 372–375. [DOI] [PubMed] [Google Scholar]
- Rosenberg R, Lindsey NP, Fischer M, Gregory CJ, Hinckley AF, Mead PS, Paz-Bailey G, Waterman SH, Drexler NA, Kersh GJ, Hooks H, Partridge SK, Visser SN, Beard CB, and Petersen LR. Vital signs: Trends in reported vectorborne disease cases—United States and territories, 2004–2016. Morbidity and Mortality Weekly Report 67:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra AC, Warden PS, Fricker CR, and Giese AR. 2013. Distribution of ticks and prevalence of Borrelia burgdorferi in the upper Connecticut River valley of Vermont. Northeastern Naturalist 20:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JA, Marrotte RR, Desrosiers N, Fiset J, Gaitan J, Gonzales A, Koffi JK, Lapointe F-J, Leighton PA. Lindsay LR, Logan T, Milord F, Ogden NH, Rogic A, Roy-Dufresne E, Suter D, Tessier N, and Millien V. 2014. Climate change and habitat fragmentation drive the occurrence of Borrelia burgdorferi, the agent of Lyme disease, at the northeastern limit of its distribution. Evolutionary Applications 7:750–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- States SL, Brinkerhoff RJ, Carpi G, Steeves TK, Folsom-O’Keefe C, DeVeaux M, and Diuk-Wasser MA. 2014. Lyme disease risk not amplified in a species-poor vertebrate community: Similar Borrelia burgdorferi tick infection prevalence and OspC genotype frequencies. Infection, Genetics, and Evolution 27:566–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Census Bureau. 2018a. Counter intercensal tables: 2000–2010. Available online at https://www.census.gov/data/tables/time-series/demo/popest/intercensal-2000-2010-counties.html. Accessed 7 October 2018.
- United States Census Bureau. 2018b. County population totals and components of change: 2010–2017. Available online at https://www.census.gov/data/datasets/2017/demo/popest/counties-total.html#par_textimage_739801612. Accessed 7 October 2018.
- Vermont Center for Geographic Information. 2018. Data and imagery. Available online at http://vcgi.vermont.gov/opendata. Accessed on 1 May 2018.
- Vermont Department of Health. 2018. Lyme disease. Available online at http://www.healthvermont.gov/disease-control/tickborne-diseases/lyme-disease. Accessed on 7 October 2018.
- Wang G, Liveris D, Brei B, Wu H, Falco RC, Fish D, and Schwartz I. 2003. Real-time PCR for simultaneous detection and quantification of Borrelia burgdorferi in field-collected Ixodes scapularis ticks from the northeastern United States. Applied and Environmental Microbiology 69:4561–4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Liveris D, Mukherjee P, Junqnick S, Margos G, and Schwartz I. 2014. Molecular typing of Borrelia burgdorferi. Current Protocols in Microbiology 12C.5.1–12.C.5.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Dykhuizen DE, Qiu W, Dunn JJ, Bosler EM, and Luft BJ. 1999. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics 151:15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]