Abstract
Background
Rhabdomyosarcoma (RMS) is a highly malignant tumor typically affecting children and adolescents. Central nervous system (CNS) dissemination is rare in RMS patients, but seems to have a particularly negative impact. The aim of this study was to analyze treatment and outcome of patients with RMS and evidence of CNS disease who were registered in the protocols coordinated by the Italian Soft Tissue Sarcoma Committee from March 1979 to December 2016.
Methods
We analyzed 39 patients with CNS disease. Depending on when their CNS disease was identified, we grouped patients as: Group A, at diagnosis; Group B, progression during treatment; Group C, at first relapse.
Results
Six patients were in Group A (2.7% of metastatic RMS patients at diagnosis); 24 were in Group B and 9 in Group C (6.5% of patients with tumor progression/relapse included in the protocols). Only 5 patients (4 in Group A, 1 in Group B) survived the event and are alive in complete remission with a median follow-up of 17.5 years. These 5 patients received systemic chemotherapy and craniospinal radiotherapy, and 2 of them also received intrathecal therapy with topotecan.
Conclusions
CNS involvement at diagnosis is a rare and prognostically negative event in RMS patients, but not always fatal when it is found at diagnosis. It is more frequent during or shortly after treatment, and the more dismal prognosis in these cases underscores the need to improve our ability to identify patients at risk of CNS dissemination in order to attempt more effective treatments that can sterilize the meninges.
Keywords: central nervous system involvement, central nervous system metastasis, meningeal dissemination, meningeal spread, rhabdomyosarcoma
Rhabdomyosarcoma (RMS) is a highly malignant tumor that typically affects children and adolescents, with an annual incidence of 4.3 cases per million population aged <20 years. It can occur anywhere in the body, but most commonly arises in the head and neck region, genitourinary tract, and extremities. In 20% to 25% of patients, the diagnostic workup demonstrates metastatic disease to the lungs, bone, bone marrow, and, less frequently, to other sites.1
Approximately 70% of patients with localized RMS can now be cured thanks to improved multimodality treatments. The results remain unsatisfactory, however, for patients with metastatic RMS or those experiencing tumor relapse. In both these groups, the prognosis is not uniformly negative, but depends on a series of factors, including primary tumor site, histology, type and number of metastases, and—in the case of relapse—the characteristics of this event.2–4
RMS spreading to the central nervous system (CNS) is rarely seen, either at diagnosis or at relapse, but it is regarded as a particularly negative event.5 There are few reports on the impact of CNS disease in RMS patients, and little is known about their treatment, especially for patients with CNS relapse. The aim of this study was to analyze the treatment and outcome of patients with RMS and evidence of CNS disease registered in protocols coordinated by the AIEOP (Associazione Italiana Ematologia e Oncologia Pediatrica) and STSC (Soft Tissue Sarcoma Committee).
Patients and Methods
From March 1979 to December 2016, there were 1192 patients under 21 years old with a diagnosis of RMS, who were registered in seven different national and international protocols for localized (RMS79, RMS88, RMS96, EpSSG RMS2005) or metastatic patients (MMTIV89-91, RMS4.99, EpSSG MET2008). All the protocols recommended testing for CNS disease at diagnosis with MRI and/or CT scans, and cerebrospinal fluid examination in cases of RMS localized in parameningeal sites or when patients exhibited neurological symptoms when the primary tumor involved other body sites. The data of each protocol were prospectively collected and they were reviewed for the purposes of this study. The present analysis only concerned children with evidence of CNS disease, with or without meningeal dissemination, at diagnosis or relapse, as demonstrated by radiological and/or cytological investigations. Patients with parameningeal RMS extending intracranially, or with clinical signs of nerve involvement but without any radiological or cytological evidence of distant CNS lesions or meningeal spread, were not considered.
We classified patients in three groups depending on when their CNS disease was identified: Group A, at diagnosis; Group B, progression during treatment; and Group C, at first RMS relapse. Disease was staged according to the tumor, nodes, metastasis (TNM) and Intergroup Rhabdomyosarcoma Study (IRS) systems. In the TNM system, T1 indicates tumors confined to the organ or tissue of origin, and T2 lesions invade contiguous structures, then T1 and T2 are further classified as A or B according to whether the tumor diameter is ≤5 cm or >5 cm, respectively; N1 indicates regional lymph node involvement; and M1 evidence of distant metastasis (including CNS spread). In the IRS system, group I defines completely excised tumors, group II indicates macroscopically resected tumors with microscopic residual disease and/or regional lymph node involvement, group III indicates macroscopic residual disease after incomplete resection or biopsy, and group IV is used to denote metastatic disease.
After their initial diagnosis, all patients received intensive systemic chemotherapy according to the various protocols, based mainly on alkylating agents (ifosfamide and cyclophosphamide), vincristine, and actinomycin D, with or without doxorubicin. Details of the different regimens have already been published.6–8 If there was evidence of CNS involvement, the RMS79 protocol recommended intrathecal chemotherapy with methotrexate, cytosine arabinoside, and steroids, while all the protocols recommended craniospinal radiotherapy (35 Gy). No protocols existed for patients with relapsing or progressive disease.
Ethics Statement: All the protocols have been approved by the ethics committees of each institution.
Results
We found 39 patients with CNS disease; 7 of them with intraparenchymal lesions and 32 with radiological evidence of meningeal dissemination. In 17 patients, there was also evidence of tumor cells in the cerebrospinal fluid. The patients’ clinical characteristics are summarized in Table 1. Twenty-one of the 39 children had an embryonal histology, but the alveolar subtype was more represented than in the general RMS population. The primary tumor was localized in the parameningeal sites in 72% of cases, the extremities being the second most frequent site (17.9%).
Table 1.
Patients’ Clinical Characteristics
| Characteristic | When CNS involvement was identified | Total | ||
|---|---|---|---|---|
| Group A (at RMS diagnosis) |
Group B
(on RMS progression) |
Group C
(at RMS relapse) |
||
| Sex | ||||
| Male | 3 | 18 | 4 | 25 |
| Female | 3 | 6 | 5 | 14 |
| Age | ||||
| <1 yrs | 0 | 1 | 0 | 1 |
| 1–3 yrs | 1 | 5 | 6 | 12 |
| 3–10 yrs | 3 | 12 | 2 | 17 |
| >10 yrs | 2 | 6 | 1 | 9 |
| Primary site | ||||
| Orbit | 0 | 0 | 0 | 0 |
| Head and neck | 0 | 1 | 0 | 1 |
| Parameningeal | 3 | 17 | 7 | 27 |
| Genitourinary tract | 1 | 1 | 0 | 2 |
| Extremities | 1 | 4 | 2 | 7 |
| Other sites | 0 | 1 | 0 | 1 |
| Unknown | 1 | 0 | 0 | 1 |
| Histology | ||||
| Alveolar | 2 | 10 | 4 | 16 |
| Embryonal | 3 | 14 | 4 | 21 |
| Not otherwise specified | 1 | 0 | 1 | 2 |
| T Classification | ||||
| T1 | 0 | 4 | 3 | 7 |
| T2 | 5 | 20 | 6 | 31 |
| Missing | 1 | 0 | 0 | 1 |
| N Classification | ||||
| N0 | 3 | 11 | 6 | 20 |
| N1 | 3 | 12 | 3 | 18 |
| Missing | 0 | 1 | 0 | 1 |
| Tumor size | ||||
| <5 cm | 2 | 3 | 2 | 7 |
| >5 cm | 3 | 18 | 7 | 28 |
| Missing | 1 | 3 | 0 | 4 |
| IRS Group | ||||
| I | 0 | 0 | 1 | 1 |
| II | 0 | 1 | 0 | 1 |
| III | 1 | 14 | 5 | 20 |
| IV | 5 | 9 | 3 | 17 |
| CNS involvement | ||||
| Metastasis | 2 | 3 | 2 | 7 |
| Meningeal spread | 4 | 21 | 7 | 32 |
| Total | 6 | 24 | 9 | 39 |
Abbreviations: CNS, central nervous system; IRS, intergroup rhabdomyosarcoma study; NOS, not otherwise specified.
There were 6 patients in group A, and they accounted for 2.7% of all the RMS patients with metastases at diagnosis enrolled in the protocols. These patients had no tumor spread to other sites apart from the CNS. The 3 patients with parameningeal RMS had meningeal spread without any symptoms of nerve involvement. The patients with tumor involving the extremities or genitourinary tract had intraparenchymal CNS lesions. One patient was diagnosed with RMS on cells obtained by lumbar puncture, and an extensive diagnostic workup revealed no evidence of a primary tumor elsewhere. Thirty-three patients were classified in Groups B or C, and together represented 6.5% of the patients with tumor progression/relapse included in the STSC protocols. Meningeal involvement emerged during the treatment of 24 children (Group B), from 2.2 to 17.6 months (median 6.6 months) after their diagnosis. The primary tumors were large (>5 cm) in 18 patients, and invasive (T2) in 20. Twelve patients had nodal involvement at diagnosis, and 9 were metastatic at diagnosis (but without any evidence of meningeal involvement).
Nine patients were in Group C and their CNS involvement emerged when their RMS recurred from 1 to 88.8 months after the end of their therapy (median 1.4 months). The primary tumors were large (>5 cm) in 7 patients and invasive (T2) in 6. Three patients had nodal involvement at diagnosis and 2 were metastatic at diagnosis (but without any evidence of CNS involvement). All 9 patients in Group C had disease recurring at the primary sites and developing in the CNS at the same time. Seven of them had parameningeal primary, and developed meningeal dissemination.
After their initial diagnosis, all patients received systemic chemotherapy according to the protocol they were enrolled in. Twenty-six patients received radiation therapy to the primary tumor (4 in Group A, 17 in Group B, and 5 in Group C), with a median dose of 45 Gy (range 30 to 60 Gy).
Treatment of CNS Disease
The treatment directed against the CNS disease included intrathecal chemotherapy in 5 children (1 in Group A, 3 in Group B, and 1 in Group C). The drugs employed were topotecan in 3 patients, thiotepa in 1, and unknown in 1. Tumor response was evaluable in 2 patients: the tumor cells disappeared from the meningeal fluid in 1 child after topotecan, whereas the disease progressed after thiotepa in the other.
Only 4 of the 6 patients in Group A received craniospinal irradiation because the disease progressed rapidly in the other 2. Five Group B and 3 Group C patients were irradiated. The reasons for not administering radiotherapy were: rapid disease progression in 9 patients (all in Group B), parameningeal RMS with disease progression/relapse during or soon after radiotherapy in 5 patients, and at the center’s discretion or due to parents’ refusal in 11.
Systemic chemotherapy was administered according to a variety of regimens, and included high doses and stem cell rescue in 4 children.
Survival
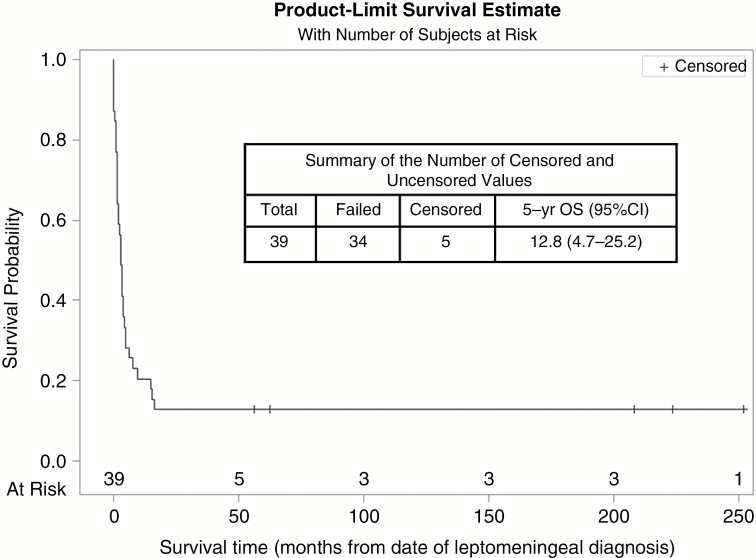
Overall, 5 of the 39 patients (4 in Group A and 1 in Group B) are alive in complete remission (Fig. 1), with a median follow-up of 17.5 years (range, 5–31 years). All 5 patients received systemic chemotherapy and craniospinal radiotherapy, and 2 of them also had intrathecal therapy with topotecan. The other 34 patients died of their disease a median of 2.6 months (range, 0–10.6 months) after evidence of CNS dissemination came to light.
Fig. 1.
Survival of patients.
Discussion
The results of this study confirm that CNS involvement at diagnosis is a rare finding in patients with RMS. Among the patients included in the protocols for metastatic RMS, only 2.7% revealed CNS involvement at diagnosis. Surprisingly, meningeal dissemination at diagnosis is not always incurable, and 4 of our 6 patients are long-term survivors. Our sample is limited, but confirms the findings in another small series of 4 patients with parameningeal tumors and tumor cells in the cerebrospinal fluid, 3 of whom were alive 6.5 to 16.9 years after their diagnosis.9
It is also relatively rare for CNS involvement to emerge when RMS relapses, but meningeal dissemination during treatment is an important cause of treatment failure, especially for parameningeal tumors. We found that CNS dissemination was involved in 6.5% of all relapses, and this event may occur early during treatment. Despite second-line chemotherapy, sometimes including the intrathecal administration of a cytostatic drug and whole-brain or craniospinal radiotherapy, the survival rate is poor and patients usually die rapidly (in fact, only palliative treatment was administered to a substantial proportion of our patients).
This pattern of early relapse during treatment and a very short survival after CNS dissemination reflects other published experiences. In a series of children treated at the St. Jude Children’s Research Hospital, the incidence of brain metastasis was 2.4% in a population of 419 RMS patients. The median survival after this event was just 2.7 months, and the estimated 1-year survival was 23.8%.10 In a series of 611 patients with parameningeal RMS treated according to the Intergroup Rhabdomyosarcoma Study Group protocols IRS-II through IRS-IV, the incidence of first failure in the CNS was 7%, and it was significantly related to meningeal involvement at diagnosis. The median time from initial RMS diagnosis to CNS dissemination was 0.88 years, confirming that this is an early event. Only 2 of 35 children were alive after relapse, despite a short follow-up (36 and 42 weeks).5 A recent report described the treatment and outcome of 23 patients with CNS relapse, 9 of whom relapsed before completing their first-line treatment. Second-line chemotherapy and new whole-brain or craniospinal radiotherapy were administered in 15 patients, and there was radiological evidence of an improvement in 8. Twenty-one patients nonetheless died of their disease after a median of 5 months. Interestingly, the authors report that the 3 children given at least 1 dose of intra-Ommaya monoclonal antibody 131I-8H9 survived for longer.11 In our study, only 1 patient survived after tumor progression, and none after relapse. This 1 surviving patient received craniospinal irradiation, intrathecal topotecan, and systemic chemotherapy with vincristine and irinotecan. He is alive 4 years after tumor progression was demonstrated.
Conclusion
CNS involvement at diagnosis is rare in patients with RMS, and not always fatal. It is more likely to emerge during or soon after treatment, in which case the prognosis is more dismal. Hence the need to improve our ability to identify patients at risk of CNS dissemination at diagnosis in order to attempt more effective treatments that can sterilize the meninges.
Funding
None.
Conflict of interest statement
None.
Acknowledgment
We thank Ilaria Zanetti for data management. The AIEOP Soft Tissue Sarcoma Committee is partially supported by Fondazione “Città della Speranza”, Padova, Italy
References
- 1. Bisogno G, Ferrari A. Soft tissue sarcoma. In: Stevens MCG, Caron HN, Biondi A, eds. Cancer in Children. 6th ed. Oxford, UK: Oxford University Press; 2012:276–292. [Google Scholar]
- 2. Oberlin O, Rey A, Lyden E, et al. . Prognostic factors in metastatic rhabdomyosarcomas: results of a pooled analysis from United States and European cooperative groups. J Clin Oncol. 2008;26(14):2384–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mazzoleni S, Bisogno G, Garaventa A, et al. ; Associazione Italiana di Ematologia e Oncologia Pediatrica Soft Tissue Sarcoma Committee Outcomes and prognostic factors after recurrence in children and adolescents with nonmetastatic rhabdomyosarcoma. Cancer. 2005;104(1):183–190. [DOI] [PubMed] [Google Scholar]
- 4. Chisholm JC, Marandet J, Rey A, et al. . Prognostic factors after relapse in nonmetastatic rhabdomyosarcoma: a nomogram to better define patients who can be salvaged with further therapy. J Clin Oncol. 2011;29(10):1319–1325. [DOI] [PubMed] [Google Scholar]
- 5. Raney RB, Meza J, Anderson JR, et al. . Treatment of children and adolescents with localized parameningeal sarcoma: experience of the Intergroup Rhabdomyosarcoma Study Group protocols IRS-II through -IV, 1978-1997. Med Pediatr Oncol. 2002;38(1):22–32. [DOI] [PubMed] [Google Scholar]
- 6. Bisogno G, Pastore G, Perilongo G, et al. . Long-term results in childhood rhabdomyosarcoma: a report from the Italian Cooperative Study RMS 79. Pediatr Blood Cancer. 2012;58(6):872–876. [DOI] [PubMed] [Google Scholar]
- 7. Bisogno G, De Rossi C, Gamboa Y, et al. . Improved survival for children with parameningeal rhabdomyosarcoma: results from the AIEOP soft tissue sarcoma committee. Pediatr Blood Cancer. 2008;50(6):1154–1158. [DOI] [PubMed] [Google Scholar]
- 8. Bisogno G, Ferrari A, Bergeron C, et al. . The IVADo regimen–a pilot study with ifosfamide, vincristine, actinomycin D, and doxorubicin in children with metastatic soft tissue sarcoma: a pilot study of behalf of the European pediatric Soft tissue sarcoma Study Group. Cancer. 2005;103(8):1719–1724. [DOI] [PubMed] [Google Scholar]
- 9. Raney B, Anderson J, Breneman J, et al. ; Soft-Tissue Sarcoma Committee of the Children’s Oncology Group, Arcadia, California, USA Results in patients with cranial parameningeal sarcoma and metastases (Stage 4) treated on Intergroup Rhabdomyosarcoma Study Group (IRSG) Protocols II-IV, 1978-1997: report from the Children’s Oncology Group. Pediatr Blood Cancer. 2008;51(1):17–22. [DOI] [PubMed] [Google Scholar]
- 10. Parasuraman S, Langston J, Rao BN, et al. . Brain metastases in pediatric Ewing sarcoma and rhabdomyosarcoma: the St. Jude Children’s Research Hospital experience. J Pediatr Hematol Oncol. 1999;21(5):370–377. [DOI] [PubMed] [Google Scholar]
- 11. De B, Kinnaman MD, Wexler LH, et al. . Central nervous system relapse of rhabdomyosarcoma. Pediatr Blood Cancer. 2017;64(11):doi: 10.1002/pbc.26710. [DOI] [PMC free article] [PubMed] [Google Scholar]