Abstract
Background:
Little is known about the behavioral effects of non-nicotine ingredients in electronic cigarette liquids. Propylene glycol (PG) and vegetable glycerin (VG) are the most common humectants used in electronic cigarette liquids. These ingredients may influence stimulus effects (e.g., visibility of exhalant, taste, or smell), which have played a role in the abuse liability of conventional cigarettes. In the current study, the stimulus effects of aerosol from liquids varying only in PG and VG content were assessed.
Methods:
Sixteen electronic cigarette users completed five sessions (one practice and four testing sessions). Following one hour of nicotine deprivation, two sampling puffs from liquid formulations containing 100/0, 75/25, 50/50, 25/75, and 0/100% PG/VG concentrations were administered in random order during five assessments, each separated by 20 min. Measures included self-reported stimulus effects and breakpoint on a multiple-choice procedure with options consisting of sampled puffs or varying amounts of money.
Results:
VG content was associated with greater reports of visibility of the exhalant (i.e., “cloud”). Liquids with only PG or VG engendered lower reports of inhalation sensations (e.g., throat hit) and greater reductions of systolic blood pressure compared to mixtures of PG and VG. There was no effect of liquid formulation on the multiple-choice procedure, but puffs were rarely chosen over even the smallest monetary option ($0.05), suggesting minimal reinforcing efficacy.
Conclusions:
Liquids containing greater concentrations of VG are more capable of producing visible exhalant and mixtures of PG and VG engender greater airway sensory effects than either ingredient alone.
Keywords: Abuse Liability, Nicotine, Smoking, Behavioral Pharmacology, E-cig
1. Introduction
Electronic cigarette (EC) use has been continually increasing in the United States (Bao et al., 2018), and there is debate on the extent ECs reduce harms associated with conventional tobacco smoking relative to causing new public health problems. Most EC liquids comprise of propylene glycol (PG) and vegetable glycerin (VG) (e.g., 80–97%, Han et al., 2016). Stimulus effects from conventional cigarettes (CCs), such as airway sensory effects (e.g., strength of the puffs in the chest, throat), are associated with subjective liking (Westman et al., 1996), and PG/VG may alter stimulus effects of ECs. If PG and VG influence the stimulus effects of ECs, they could be important regulatory targets, and harm-reduction efforts could better tailor ECs to increase smoking cessation efficacy. The current study tested whether EC user perceptions (e.g., Harvanko et al., 2018) of greater VG engendering desirable stimulus effects (i.e., good taste, good smell, large cloud), and greater PG engendering undesirable stimulus effects (i.e., sore throat, headache, dizziness), could be replicated under controlled conditions.
2. Methods
2.1. Participants
Recruitment included online and radio advertisements, and word-of-mouth referrals. Volunteers reporting the use of an EC at least 20 of the past 30 days and answering affirmatively to “do your liquids typically contain nicotine” were invited to participate. Individuals were excluded if they used CCs more frequently than ECs (determined using Timeline Follow-Back Assessments), planned to cut-down or cease their EC usage, or reported any condition that would increase risk for study participation (e.g., recent use of psychoactive drugs other than caffeine or alcohol) as determined by the study physician (CA Martin). Sixteen volunteers provided informed consent and completed the study (see Table 1 for volunteer demographics, nicotine dependence scores, tobacco usage history, and typical device parameter setting use). This study was approved by the University of Kentucky’s Institutional Review Board and accorded with the Declaration of Helsinki.
Table 1:
Demographic and Clinical Variables
| N=16 | |
|---|---|
| Age, mean (SD) | 24.8 (3.65) |
| Sex, N (%) | |
| Male | 12 (75.0) |
| Female | 4 (25.0) |
| PSCDI Score,* mean (SD) | 14.8 (3.8) |
| Typical electronic cigarette nicotine concentration (mg/ml), mean (SD) | 7.13 (5.2) |
| Typical electronic cigarette nicotine wattage, mean (SD) | 64.5 (33.4) |
| Milligrams of electronic cigarette liquid per day, mean (SD) | 6.6 (7.5) |
| Years of electronic cigarette use, mean (SD) | 2.7 (1.8) |
| Use of other tobacco products in the past month | |
| Conventional Tobacco Cigarettes, N (%) | 2 (12.5) |
| Chewing Tobacco | 1 (6.3) |
| Hookah | 1 (6.3) |
PSCDI= Penn State Cigarette Dependence Index (Foulds et al., 2015)
2.2. Procedures
An EC with a refillable tank (Nautilus, Aspire, Shenzhen, China) and adjustable power supply (iStick TC100W, Eleaf, Shenzhen, China) were used for aerosol administration. Liquids were provided by a commercial vendor (Nude Nicotine, San Diego) who also provided gas chromatography and mass spectrometry verification of formulation compositions. All liquids were devoid of flavorants. Parameter settings were 10 watts (the setting most often used by regular EC users [Harvanko et al., 2018]) and 12 mg/ml nicotine concentration (the most popular concentration by the vendor) for the administration of all study aerosols. Participants completed one practice day to acclimate them to study procedures, followed by four identical test days (to increase statistical sensitivity and reliability of estimates) each approximately 3h long. Each day five liquid formulations (100/0, 75/25, 50/50, 25/75, 0/100 PG/VG) were assessed in a random order that was balanced between the 5-days and participants to control for order effects. Data from the practice day were collected but not analyzed.
To standardize nicotine conditions and EC use prior to testing each day, 10 three-second puffs with 30-second inter-puff intervals were administered from an EC containing liquid with 50/50 PG/VG and 12 mg/ml nicotine concentration, followed by a one-hour deprivation period. In order to focus on effects of PG and VG under controlled puffing conditions, while engendering minimal acute nicotine effects (puff topography and nicotine effects were assessed by the control measures described below), puffing bouts consisted of two three-second sample-puffs with 30 second inter-puff intervals, controlled by computer prompts to “inhale” and “exhale.” Twenty minutes separated each assessment, with a different liquid formulation tested during each assessment.
2.3. Measures
2.3.1. Control Measures.
Cardiovascular Measures (Dinamap Pro 200, General Electric). Heart rate and blood pressure were recorded using an automated blood pressure monitor.
2.3.2. Puff Topography (Spa-D; Sodim, France).
Puff topography (volume and duration) was recorded with a volumetric transducer.
2.3.3. Questionnaire of Smoking Urges-Brief (QSU-B)
(Cox et al., 2001). This 10-item questionnaire focused on nicotine withdrawal and was used to monitor nicotine withdrawal effects on a 100-unit visual analog scale (VAS).
2.3.4. Visual Analog Scale - Smoking Effects (VAS-SE) (Blank et al., 2008).
This ten-item measure was used to monitor acute nicotine effects on a 100-unit VAS scale.
2.4. Outcome Measures
2.4.1. Duke Sensory Questionnaire (DSQ) (Westman et al., 1996).
This seven-item questionnaire asks participants to rate sensory effects on a 7-point Likert scale. Items include: “how much nicotine do you estimate was in the last puffs,” “how similar were those puffs to your typical electronic cigarette,” “what was the strength of the puffs on your tongue,” “what was the strength of the puffs in your nose,” “what was the strength of the puffs in the back of your mouth and throat,” “what was the strength of the puffs in your windpipe,” and “what was the strength of the puffs in your chest?”
2.4.2. Visual Analog Scale - Post Sampling (VAS-PS).
This 12-item questionnaire assesses stimulus effects on a 100-unit VAS scale. Items included: “feel stimulated,” “like the effects,” “want to use the electronic cigarette again,” “enjoy the electronic cigarette,” “crave the electronic cigarette,” “get pleasure from the electronic cigarette,” “produce a large vapor cloud,” “experience a throat hit,” “feel dry-mouth,” “feel sore-throat,” “enjoy the smell,” and “enjoy the taste?”
2.4.3. Multiple Choice Procedure (MCP) (Griffiths et al., 1993).
This computerized form of the MCP was comprised of 21-choices between: two more three-second puffs of the sampled liquid formulation or an amount of money beginning at $.00 and increasing in increments of $.05 until $1.00 was reached. The computer randomly selected one of the choices, and if two more puffs were selected, two more puffs from the same formulation were administered following control and outcome measures. If money was selected, that amount was added to the compensation provided at the end of the session. The dependent variable on this measure was the breakpoint at which participants chose money over two more puffs.
2.5. Data Analysis
Mixed models (PROC MIXED) examined differences among liquid formulations with PC-SAS, version 9.3 (SAS Institute Inc., Cary, NC) and alpha set at p ≤ 0.05. All models used participant as the repeated variable, liquid formulation the fixed independent variable, and compound symmetry as the covariance structure. For control measures, change scores were calculated for each formulation (post-minus pre-sampling values). Since heart rate and blood pressure were taken at the beginning of each session and following each puff bout, change relative to that day’s baseline was calculated to account for daily fluctuations in resting heart rate and blood pressure. Scores on all measures were averaged across the four test sessions. Contrasts for linear and quadratic “dose-response” relationships across the formulations were used to examine main effects.
3. Results
3.1. Smoking Puff Topography
The average volume of each puff was 168.13 (SE = 20.30) ml and lasted 4.10 (SE = .30) seconds. Expectedly, there were no significant effects of liquid formulation on volume or duration of the puffs.
3.2. Control Measures
Heart rate significantly decreased an average of 6.07 (SE=1.90) beats per minute from session baseline for all of the liquid formulations (ps<.05) and, as expected, there was no significant effect of formulation. There was a significant change in systolic blood pressure (SBP) (F[55]=3.67, p=.010) with a significant quadratic relationship among formulations (F[55]=12.66, p<.001), such that SBP decreased significantly more following 100/0 PG/VG (m=−6.55, SE=1.60) and 0/100 PG/VG (m=−4.47, SE=1.47) compared to 50/50 PG/VG (m=−6.5, SE=1.60).
Expectedly, mixed models for QSU-B or VAS-SE items did not indicate significant effects of liquid formulation.
3.3. Outcome Measures
For DSQ items, there was a significant effect of liquid formulation on “how much nicotine do you estimate was in the puffs” (F[60]=3.05, p=.023), “what was the strength of the puffs on your tongue” (F[60]=3.98, p=.006), and “what was the strength of the puffs in your chest” (F[60]=3.52, p=.012). There were significant quadratic relationships between liquid formulation and these items, such that mixtures of PG/VG were rated more highly than PG or VG alone (e.g., right panel, Figure 1). Magnitudes of these effects, however, were small (Cohen’s f2’s <.15).
Figure 1.
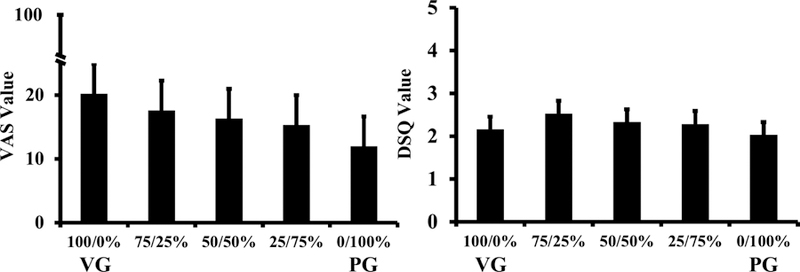
Ratings of “did the electronic cigarette produce a large cloud?” and “what was the strength of the puffs on your tongue?”
Note: Figure 1. Ratings of “did the electronic cigarette produce a large cloud” on the Visual Analog Scale (left) and “what was the strength of the puffs on your tongue” rated on the Duke Sensory Questionnaire (right). Values represent least-squares mean estimates and standard error bars derived from the mixed model. A significant main effect of liquid condition was indicated on “large cloud” (F[60]=2.77, p=.035) and post-hoc tests indicated a significant linear relationship (F[60]=10.59, p=.002). A significant main effect of liquid condition was indicated on “strength on the tongue” (F[60]=3.98, p=.006) and post-hoc tests indicated a significant quadratic relationship (F[60]=9.78, p=.003).
For VAS-PS items, significant effects of liquid formulation were indicated on “did the e-cig produce a large cloud” (F[60]=2.77, p=.035) and “did you experience a throat hit” (F[60]=3.43, p=.014). A linear relationship was indicated on large cloud (F[60]=10.59, p=.002) (left panel, Figure 1) and a quadratic relationship on throat hit (F[60]=7.66, p=.008), such that throat hit was rated slightly lower on formulations containing 100% VG (36.4, SE=5.7) or 100% PG (32.4, SE=5.7) compared to formulations with 50/50% PG/VG (38.0 SE=5.7). No effects of formulation were observed on the MCP. Notably, the monetary value of the breakpoint for these formulations was low ($0.04 + .02, mean + SE).
4. Discussion
Consistent with our hypotheses, greater VG was associated with greater visibility of exhalant. Unexpectedly, liquids with equal concentrations of PG and VG mixtures were associated with greater strength on the tongue and other inhalation sensations, and smaller reductions in systolic blood pressure compared to liquids with solely PG or VG, although the magnitude of these effects was small.
Visibility of exhalant has been noted as an important factor for mimicking the experience of using a conventional cigarette (Barbeau et al., 2013). Results show that greater concentration of VG is associated with this effect, confirming anecdotal reports by EC users (Harvanko et al., 2018) and replicating recent laboratory research (Spindle et al., 2018). Past research has shown that EC users associate throat hit with smoking cessation efficacy of the EC (Etter, 2016) and similarity to CC smoking (Barbeau et al., 2013). Results of this study indicate throat hit and other inhalation sensations are associated with PG/VG mixtures (e.g., 50/50 PG/VG), which is consistent with anecdotal reports by EC users (Harvanko et al., 2018). These results, however, differ with other laboratory research (Spindle et al., 2018) and anecdotal reports (Chen and Zeng, 2016) suggesting PG concentration and throat hit are positively correlated. Disparity among results could be due to differences in device and liquid parameters across studies, or use of controlled puffing bouts or exclusion of flavorants in the current study.
This study was designed to assess stimulus and reinforcing effects of EC liquid formulations under controlled flavorant, puffing, nicotine concentration, and nicotine withdrawal conditions. Plasma nicotine was not measured, but there was no influence of formulation on control self-report measures (i.e., VAS-SE, and QSU-B). Also, the average heart-rate following puffing did not change as a function of liquid formulation, suggesting that nicotine delivery was not significantly different between formulations under these conditions. There was, however, an interesting quadratic relationship between formulations and SBP. Previous research has indicated that PG and VG can influence nicotine delivery (Spindle et al., 2018), although nicotine generally produces more potent effects on heart rate rather than blood pressure (e.g., Benowitz et al., 1982). Further research on the potential sympathomimetic effects of PG/VG mixtures is warranted.
Breakpoints on the MCP were low for each of the liquid formulations, indicating a floor effect that limits interpretation of these data. The modest monetary value of these formulations may be due to the omission of flavorants, which has been previously associated with decreased reports of liking EC aerosol (Audrian-McGovern et al., 2016; Goldenson et al., 2016). Indeed, reports of ‘good taste’ were low following puffing (<15 on a 100-unit scale), and spontaneous reports by participants indicated dissatisfaction with the flavor. Although EC parameters for this study were carefully chosen, they were not the same as those typically used by these participants (e.g., 10 versus typical m=64.5 watts or 12 versus typical m=7.12 mg/ml nicotine concentration), which could explain the low subjective and reinforcing effects observed in this study.
5. Conclusion
These data confirm anecdotal reports that VG is associated with greater cloud compared to PG. PG and VG mixtures are associated with greater inhalation sensations than either ingredient alone. Neither ingredient was related to reinforcing effects (e.g., liking), but formulations used in the current study, devoid of flavorants, had little monetary value.
Highlights.
Vegetable glycerin is associated with visible electronic cigarette exhalant (i.e., cloud)
Propylene glycol and vegetable glycerin mixtures enhance reported smoking sensations
Neither propylene glycol or vegetable glycerin influenced measures of abuse liability
Acknowledgements
The authors would like to acknowledge Nude Nicotine for providing electronic cigarette liquids used in this study
Role of Funding Source
The funding source had no role in the conduct of this study. This work was supported by the National Institute on Drug Abuse [T32DA035200]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
No conflict declared.
References
- Baassiri M, Talih S, Salman R, Karaoghlanian N, Saleh R, Hage RE, Saliba N Shihadeh A, 2017. Clouds and “throat hit”: Effects of liquid composition on nicotine emissions and physical characteristics of electronic cigarette aerosols. Aerosol Sci. Technol 51, 1231–1239. 10.1080/02786826.2017.1341040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao W, Xu G, Lu J, Snetselaar LG, Wallace RB, 2018. Changes in electronic cigarette use among adults in the united states, 2014–2016. JAMA, 319, 2039–2041. 10.1001/jama.2018.4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeau AM, Burda J, Siegel M, 2013. Perceived efficacy of e-cigarettes versus nicotine replacement therapy among successful e-cigarette users: A qualitative approach. Addict. Sci. Clin. Pract, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, Jones RT, Rosenberg J, 1982. Interindividual variability in the metabolism and cardiovascular effects of nicotine in man. J. Pharmacol. Exp. Ther, 221, 368–372. [PubMed] [Google Scholar]
- Blank MD, Sams C, Weaver MF, Eissenberg T, 2008. Nicotine delivery, cardiovascular profile, and subjective effects of an oral tobacco product for smokers. Nicotine Tob. Res 10, 417–421. 10.1080/14622200801901880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG, 2001. Evaluation of the brief questionnaire of smoking urges (qsu-brief) in laboratory and clinical settings. Nicotine Tob. Res, 3, 7–16. [DOI] [PubMed] [Google Scholar]
- Etter JF, 2016. Throat hit in users of the electronic cigarette: An exploratory study. Psychol. Addict. Behav 30, 93–100. 10.1037/adb0000137 [DOI] [PubMed] [Google Scholar]
- Foulds J, Veldheer S, Yingst J, Hrabovsky S, Wilson SJ, Nichols TT, Eissenberg T, 2015. Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking e-cigarette users. Nicotine Tob. Res 17, 186–192. 10.1093/ntr/ntu204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Troisi II, Silvermian K, Miumford GK, 1993. Multiple-choice procedure: An efficient approach for investigating drug reinforcement in humans. Behav. Pharmacol 4, 3–14. [PubMed] [Google Scholar]
- Han S, Chen H, Zhang X, Liu T, Fu Y, 2016. Levels of selected groups of compounds in refill solutions for electronic cigarettes. Nicotine Tob. Res 18, 708–714. 10.1093/ntr/ntv189 [DOI] [PubMed] [Google Scholar]
- Harvanko AM, McCubbin AK, Ashford KB, Kelly TH, 2017. Electronic cigarette liquid and device parameters and aerosol characteristics: A survey of regular users. Addict. Behav 84, 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westman EC, Behm FM, Rose JE, 1996. Dissociating the nicotine and airway sensory effects of smoking. Pharmacol. Biochem. Behav 53, 309–15. [DOI] [PubMed] [Google Scholar]
- Yan XS, D’Ruiz C, 2015. Effects of using electronic cigarettes on nicotine delivery and cardiovascular function in comparison with regular cigarettes. Regul. Toxicol. Pharmacol 71, 24–34. 10.1016/j.yrtph.2014.11.004 [DOI] [PubMed] [Google Scholar]


