Abstract
Background
High-grade gliomas are the most frequently occurring brain tumors and carry unfavorable prognosis. Literature is controversial regarding the effects of surgery on cognitive functions.
Methods
We analyzed a homogenous population of 30 patients with high-grade glioma who underwent complete resection. Patients underwent extensive neuropsychological analysis before surgery, 7 days after surgery, and approximately 40 days after surgery, before adjuvant treatments. Thirty-four neuropsychological tests were administered in the language, memory, attention, executive functions, and praxis domains.
Results
The preoperative percentage of patients with impairment in the considered tests ranged from 0% to 53.3% (mean 20.9%). Despite a general worsening at early follow-up, a significant recovery was observed at late follow-up. Preoperative performances in language and verbal memory tasks depended on the joint effect of tumor volume, volume of surrounding edema, and tumor localization, with major deficits in patients with left lateralized tumor, especially insular and temporal. Preoperative performances in attention and constructive abilities tasks depended on the joint effect of tumor volume, volume of surrounding edema, and patient age, with major deficits in patients ≥ 65 years old. Recovery at late follow-up depended on the volume of resected tumor, edema resorption, and patient age.
Conclusions
Longitudinal neuropsychological performance of patients affected by high-grade glioma depends, among other factors, on the complex interplay of tumor volume, volume of surrounding edema, tumor localization, and patient age. Reported results support the definition of criteria for surgical indication based on the above factors. They may be used to propose more customized surgical, oncological, and rehabilitative strategies.
Keywords: cognitive functions, high-grade gliomas, neuropsychology, outcome, surgery
The annual incidence of high-grade gliomas (ie, World Health Organization [WHO] grade III/IV) is 3 to 4 cases per 100 0001. These are the most frequent malignant brain tumors in adults,2 with an unfavorable prognosis.3 Overall survival depends on different factors, including age, extent of resection, and performance status.4 Neurological and cognitive functions have a dramatic impact on daily living and, given the short overall survival of patients affected by high-grade glioma, limiting neurocognitive deficits is crucial.
The Karnofsky Performance Status5 is a widely accepted scale for clinical assessment of patients with high-grade gliomas. While this scale provides information about the presence of neurological symptoms, ability to work, and need for assistance in basic self-care, it does not provide reliable information on the whole spectrum of cognitive functions. Over the last decade, the role of extensive neuropsychological assessment in patients affected by brain tumors has dramatically increased, in parallel to the widespread increase in surgical treatment of low-grade gliomas.6 However, only a few studies have evaluated the effects of surgical treatment on the cognitive functions of patients diagnosed with high-grade gliomas.7–13 Retrospective design and nonspecific or nonextensive screening tools (eg, Mini Mental State Examination)14 have been commonly used for assessing cognitive functions.15–17 Moreover, most studies include heterogeneous populations of patients affected by different tumors (ie, both low-grade and high-grade gliomas)13,18–22 with no comparable physiopathology, treatment, or natural history of disease. For all these reasons, even if surgery is commonly considered effective in alleviating neurocognitive deficits, the literature is still controversial.
Some authors reported neurocognitive deficits17–19,22 at least up to the first 3 months after surgery,10,18 and others showed no effect of surgical resection.13,19–21,23,24 Even if different studies suggest that patients with tumors in the dominant hemisphere and in language areas have a worse neuropsychological outcome than patients with tumors in the nondominant hemisphere or noneloquent areas,9,17–19,22,24–28 this is not a common finding.20,29 The impact of patient age is also debated.9,13,15,17,20–22 To the best of our knowledge, only one study assessed the impact of mass effect on cognitive function in a homogeneous population of high-grade gliomas,9 demonstrating a positive correlation between mass effect and preoperative impairment in the executive and psychomotor functions.
Our goal is to study the joint effects of tumor location, patient age, and mass effect, as well as the potential effects of the edema surrounding the tumor, providing new insights into the determinants of neurocognitive outcome over time in patients with high-grade glioma who have undergone complete resection.
Materials and Methods
Study Population
Thirty consecutive patients underwent resection of high-grade gliomas at the Neurosurgical Division of Santa Chiara Hospital in Trento, Italy between October 2012 and November 2015. The study was approved by the Ethical Committee of the Azienda Sanitaria per i Servizi Sanitari of Trento. Patients were informed about the details of the study by neurosurgeons (S.S. and F.C.) and a neuropsychologist (M.D.) and they gave their informed consent to an extensive preoperative neuropsychological assessment and follow-up. Exclusion criteria were: history of neurological or psychiatric disorders and insufficient knowledge of the Italian language.
All patients were medicated with 16 mg dexamethasone (8 mg twice a day) before surgery; medication was progressively reduced and stopped within 20 days after surgery. All patients underwent radiotherapy treatment and 27 patients (90%) underwent chemotherapy treatment. The mean time from surgery to adjuvant treatment was 46.5 days (median 43.5 days).
Neuropsychological Assessment
Different cognitive functions, including language, memory, attention, executive functions, praxis, and hemispheric dominance, were evaluated with a battery of neuropsychological tests.30–42 Hand-preference was assessed by the Edinburg Handedness Inventory Test.42 Thirty-one neuropsychological tests (resulting in 34 specific subitems) were administered to all patients the day before surgery, 7 days after surgery (early follow-up), and 28 to 40 days after surgery (median 40 days, mean 35.7 days, late follow-up). The late follow-up was set just before adjuvant treatment in order to exclude possible interferences on the cognitive status. The duration of the neuropsychological evaluation was never shorter than 1.5 hours and never longer than 2 hours. Parallel versions of some tests were used at early and late postoperative follow-up, in order to avoid possible learning effects. Raw scores were adjusted for age, education, and gender; specific cut-off values were adopted to evaluate impairments in each test. Adjusted scores allow, for instance, evaluating the net effect of age on patients’ performances.
Surgical Treatment
All patients underwent resection under general anesthesia. A preoperative MR image for neuronavigation and a diffusion-weighted imaging scan for tractography reconstructions were obtained within 3 days before surgery and merged intraoperatively. Diffusion tensor imaging reconstructions (with deterministic FACT approach) of the bundles bordering the tumors were analyzed by the same surgeon (S.S.), used for preoperative planning, and merged with T1 sequence for the neuronavigation. The resection was performed with subpial and subarachnoid dissection with the Sonopet ultrasonic aspirator. The resection was stopped when healthy brain tissue was reached in all directions, with a variable margin beyond the area of contrast enhancement, taking into account the functional limit imposed by bundle reconstruction.
MRI, Overall Mass Effect
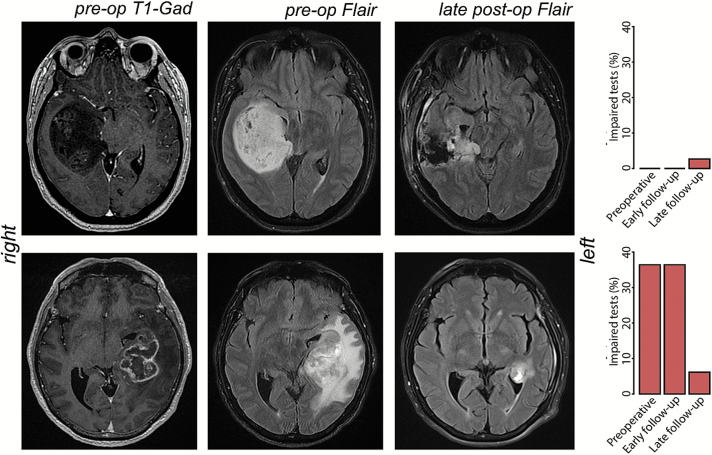
All patients underwent preoperative, early postoperative (24 hours after surgery), and late postoperative (approximately 40 days after surgery, on average) MRI. The tumor volume was computed by manual drawing of multiple regions of interest around the enhancing area on preoperative T1-weighted MR images using Osirix. Similarly, preoperative, early, and late postoperative fluid-attenuated inversion recovery (FLAIR) extents were computed. Preoperative and early postoperative FLAIR extent was available for all patients; late postoperative FLAIR extent was available for 16 patients (Figure 1). We defined “overall mass effect” as the volume of the union of tumor and preoperative FLAIR extent, thus taking into account not only the tumor volume but also the surrounding edema and infiltration areas.
Fig. 1.
Evolution of tumor volume and overall mass effect and the respective neuropsychological outcomes over the follow-up of two large WHO grade IV high-grade gliomas in the right non-dominant (up) and left-dominant hemisphere (down).
Statistical Analysis
Differences in preoperative adjusted scores by patient age (two groups were considered: patients < 65 years old and patients ≥ 65 years old) and tumor location were assessed by Wilcoxon rank sum test. Differences in the percentage of patients with cognitive impairment by patient age and tumor location were assessed by proportion test. Correlations of preoperative adjusted scores with overall mass effect and tumor volume were assessed by Spearman rank correlation. A linear model was employed to evaluate the predictability of preoperative adjusted scores on the basis of patient age, tumor lateralization, and overall mass effect: S = ci + cmM + cAA + cLL, where S is the adjusted score, M is the overall mass effect (in cm3), A is the patient age (in years), and L is a binary variable representing tumor laterality (0: right, 1: left); ci is the intercept and cm, cA, and cL are the regression coefficients.
Longitudinal differences (eg, from preoperative to early follow-up or from early follow-up to late follow-up) of adjusted scores were assessed by paired Wilcoxon test.
We defined the random variable (Sl-Sp), which we refer as to “adjusted scores variation,” to evaluate recovery at late follow-up; it represents the difference between the adjusted score at late follow-up (Sl) and at the preoperative assessment (Sp). The difference between overall mass effect (Mp) and early postoperative FLAIR extent (Fe) may be interpreted as an indicator of the benefit of surgery (Mp-Fe), which we refer as to “decrease of overall mass effect”; it provides a measure of resected tumor and edema resorption. Correlations of adjusted scores variation with tumor volume and with decrease of overall mass effect were assessed by Spearman rank correlation. We performed the same analysis by considering the random variable (Mp-Fl), where Fl represents the late postoperative FLAIR extent. A linear model was employed to evaluate the predictability of the adjusted scores variation on the basis of patient age, tumor lateralization, and decrease of overall mass effect.
Results
The age of the 30 patients ranged from 32 to 83 years (mean ± SD: 59.3 ± 13.9 years), with a preponderance of males (63.3%; 19/30). Twenty-nine patients were right-handed and 1 patient was ambidextrous. Twenty-six patients had glioblastoma (grade IV, WHO), 4 patients had anaplastic astrocytoma (grade III, WHO), and there was a higher prevalence of left-lateralized tumors (60%; 18/30). The tumor volume (mean ± SD) was 28.8 ± 28.3 cm3, surrounded by an edema about 2.5 times larger, resulting in an overall mass effect of 100.9 ± 64.7 cm3. We found that the edema resorbs soon after surgery (from 72.2 ± 53.4 to 46.5 ± 37.1 cm3), and afterwards it remains nearly constant (40.3 ± 31.4 cm3 at late postoperative assessment). Details are reported in Table 1.
Table 1.
Patient demographics and tumor features
| Variable | Patients (N = 30) |
|---|---|
| Age (years) | |
| >65 | 10 (33.3%) |
| ≤65 | 20 (66.6%) |
| Gender | |
| Male | 19 (63.3%) |
| Female | 11 (36.7%) |
| Scholarity (years) | |
| ≤8 | 15 (50%) |
| >8 | 15 (50%) |
| Handedness | |
| Left | 0 (0%) |
| Right | 29 (96.7%) |
| Bilateral | 1 (3.3%) |
| Tumor grade | |
| III Anaplastic Astrocytoma | 4 (13.3%) |
| IV Glioblastoma | 26 (86.7%) |
| Tumor Location # | |
| Frontal | 14 (46.7%) |
| Insular | 6 (20%) |
| Temporal | 11 (36.7%) |
| Parietal | 7 (23.3%) |
| Occipital | 2 (6.7%) |
| Tumor Lateralization | |
| Left | 18 (60%) |
| Right | 12 (40%) |
| Volumes (cm 3) | |
| Preoperative T1 extent (tumor volume) | 28.8 ± 28.3 |
| Preoperative FLAIR extent | 72.2 ± 53.4 |
| Overall mass effect (preoperative T1 + FLAIR) | 100.9 ± 64.7 |
| Early postoperative FLAIR extent | 46.5 ± 37.1 |
| Late postoperative FLAIR extent* | 40.3 ± 31.4 |
# Tumors may be located in more than one lobe.
* Available for 16 out of 30 patients.
Preoperative Assessment
Language: Effect of Tumor Location and Patient Age
The percentage of patients showing impairment was above 20% for auditory and visual comprehension of words and for verbal fluency (auditory comprehension of words [ACW: 21.4%], visual comprehension of words [VCW: 21.4%], visual comprehension of sentences [VCS: 33.3%], verbal phonemic fluency [VPF: 36.6%], and verbal semantic fluency [VSF: 23.3%]) (Table 2). Results describing association between tumor location and neuropsychological adjusted scores are reported in Table 3. Patients with left-lateralized tumors performed worse than patients with right-lateralized tumors in naming of nouns (LCNT: 67 vs 77.4) and verbal fluency tasks (VPF: 16.8 vs 27 and VSF: 27.1 vs 36.5). Of patients with left-lateralized tumors, 55.6% showed impairment in verbal phonemic fluencies and 33.3% in verbal semantic fluencies. More in detail, worse adjusted scores in verbal phonemic fluencies were observed for patients with tumor in the left insula (VPF: 12.1 vs 22.6), with 80% of those patients showing impairment, while worse adjusted scores in naming tasks were observed for patients with tumor in the left temporal lobe (LCNT: 59.7 vs 74.7; naming of nouns [NN]: 8.3 vs 9.8; and naming of verbs [NV]: 8.1 vs 8.9), with 50% of those patients showeing impairment in the naming of nouns tasks (NN and LCNT). Patients with tumor in the left ventral stream had worse adjusted scores in the repetition of numbers task (NRep: 9.2 vs 9.8), especially those with tumor in the left insula (NRep: 8.8 vs 9.8), with 40% of patients showing impairment. We did not find any significant difference of adjusted scores by age. However, 60% of patients ≥ 65 years old had lower-than-normal scores in auditory comprehension of words while no impairments were recorded in younger patients (P = .052). The same results emerged from the analysis of visual comprehension of words.
Table 2.
Neuropsychological tests, acronym and reference, cut-off for normal performance, and results: mean adjusted score (S) and percentage of impaired patients (I) at preoperative assessment (1), early follow-up (2) and late follow-up (3)
| Cognitive tests in different domains | Acronym [Ref] | Cut-off | S1 | S2 | S3 | I1 | I2 | I3 |
|---|---|---|---|---|---|---|---|---|
| Language | ||||||||
| Repetition of words | RepW [9] | ≥8.8 | 9.7 | 9.5 | 9.8 | 3.3 | 6.7 | 0 |
| Repetition of non-words | RepNW [9] | ≥2 | 4.7 | 4.4 | 4.7 | 3.3 | 3.3 | 0 |
| Repetition of sentences | RepS [9] | ≥3 | 2.9 | 2.5 | 2.8 | 6.7 | 23.3 | 13.3 |
| Reading of words | ReaW [9] | ≥6.4 | 8 | 7.9 | 7.9 | 16.7 | 10 | 10 |
| Reading of non-words | ReaNW [9] | ≥4 | 4.3 | 4.1 | 4.4 | 16.7 | 23.3 | 13.3 |
| Reading of sentences | ReaS [9] | ≥1.3 | 1.7 | 1.6 | 1.8 | 13.3 | 16.7 | 10 |
| Writing of sentences | WS [9] | ≥0.6 | 1.3 | 1 | 1 | 6.7 | 23.3 | 16.7 |
| Naming of nouns (Laiacona-Capitani) | LCNT [28] | ≥61 | 71.7 | 66 | 74.5 | 10 | 25 | 5 |
| Naming of nouns | NN [9] | ≥8.2 | 9.5 | 9.1 | 9.7 | 13.3 | 20 | 3.3 |
| Naming of verbs | NV [9] | ≥6.1 | 8.7 | 8.2 | 9.3 | 10 | 16.7 | 0 |
| Auditory comprehension of words | ACW [9] | ≥18.4 | 18.6 | 19.2 | 19.2 | 21.4 | 7.1 | 14.3 |
| Auditory comprehension of sentences | ACS [9] | ≥11.6 | 13.2 | 12.9 | 13.2 | 10 | 13.3 | 10 |
| Visual comprehension of words | VCW [9] | ≥17 | 17.9 | 17.3 | 18.5 | 21.4 | 28.6 | 14.3 |
| Visual comprehension of sentences | VCS [9] | ≥11.3 | 11.6 | 10.8 | 12.5 | 33.3 | 26.7 | 20 |
| Repetition of numbers | NRep [9] | ≥8.8 | 9.6 | 8.8 | 9.6 | 6.7 | 20 | 10 |
| Reading of numbers | NRea [9] | ≥7.6 | 8.9 | 8.4 | 9 | 6.7 | 20 | 6.7 |
| Verbal fluency on phonemic cue | VPF [11] | ≥17.35 | 20.9 | 18.8 | 23.7 | 36.7 | 53.3 | 36.7 |
| Verbal fluency on semantic cue | VSF [31] | ≥25 | 30.9 | 30.1 | 34.6 | 23.3 | 43.3 | 20 |
| Memory | ||||||||
| Digit-span, short term verbal memory | DS [33] | ≥3.75 | 4.7 | 4.2 | 4.9 | 20 | 30 | 16.7 |
| Corsi-span, short term visuospatial memory | CS [33] | ≥3.5 | 3.7 | 3.7 | 4.3 | 40 | 26.7 | 3.3 |
| 15 Rey’s word list: immediate recall | 15RWL-IR [10] | ≥28.53 | 31.9 | 25 | 32.4 | 33.3 | 56.7 | 40 |
| 15 Rey’s word list: delayed recall | 15RWL-DR [10] | ≥4.69 | 4.9 | 4 | 4.7 | 46.7 | 73.3 | 56.7 |
| 15 Rey’s word list: recognition memory | 15RWL-Rec [10] | ≤2 | 3.9 | 10.1 | 6.1 | 33.3 | 63.3 | 46.7 |
| 15 Rey’s word list: accuracy in recognition | 15RWL-Acc [10] | ≥88 | 82.6 | 67.4 | 80.4 | 40 | 56.7 | 43.3 |
| Rey–Osterrieth complex figure delayed copy | ROCF-DC [8] | ≥9.47 | 9.8 | 10.6 | 11.9 | 53.3 | 50 | 40 |
| Attention and executive functions | ||||||||
| Lines cancellation task, visual attention: accuracy | LCA [1] | ≥59 | 57.3 | 56 | 58.7 | 23.3 | 23.3 | 13.3 |
| Lines cancellation task, visual attention: speed | LCS [1] | ≤105 | 37.7 | 44.8 | 33.7 | 0 | 0 | 0 |
| Attentional matrices, visual selective attention | AM [44] | ≥30 | 35.4 | 34.8 | 40.4 | 16.7 | 26.7 | 16.7 |
| Trail making test, visuospatial attention | TMTA [20] | <94 | 52.1 | 90.9 | 40.9 | 13.3 | 30 | 10 |
| Trail making test, cognitive flexibility | TMTB [20] | <283 | 192.9 | 225.5 | 216.9 | 30 | 40 | 40 |
| Trail making test, executive function | TMTB-A [20] | <187 | 158.3 | 189.8 | 182.5 | 33.3 | 43.3 | 40 |
| Praxis | ||||||||
| Orofacial skills | OP [15] | ≥16 | 18.1 | 18 | 19.3 | 6.7 | 13.3 | 0 |
| Ideomotor skills | IP [14] | >16 | 18.6 | 18.8 | 19.5 | 13.3 | 10 | 3.3 |
| Rey–Osterrieth complex figure copy | ROCF-C [8] | ≥28.88 | 26.3 | 25.2 | 29 | 46.7 | 46.7 | 43.3 |
Table 3.
Neuropsychological tests in different domains with observed statistical differences for tumor location (patients in Group G vs patients not in group G, nonG, were tested). Differences in both mean adjusted scores (S-G and S-nonG) and percentage of impaired patients (I-G and I-nonG) were evaluated. Columns pS and pI show the resulting P values
| Test | Group (G) | S-G | S-nonG | pS | I-G (%) | I-nonG (%) | PI |
|---|---|---|---|---|---|---|---|
| Language | |||||||
| LCNT | Left | 67.0 | 77.4 | .008 | 18.2 | 0 | .549 |
| LCNT | Left-temporal | 59.7 | 74.7 | .018 | 50.0 | 0.0 | .040 |
| VPF | Left | 16.8 | 27.0 | .015 | 55.6 | 8.3 | .025 |
| VPF | Left-insular | 12.1 | 22.6 | .040 | 80.0 | 28.0 | .09 |
| VSF | Left | 27.1 | 36.5 | .040 | 33.3 | 8.3 | .252 |
| NN | Left-temporal | 8.3 | 9.8 | .004 | 50.0 | 4.2 | .022 |
| NV | Left-temporal | 8.1 | 8.9 | .032 | 16.7 | 8.3 | 1 |
| NRep | Left-ventral | 9.2 | 9.8 | .033 | 20.0 | 0.0 | .196 |
| NRep | Left-insular | 8.8 | 9.8 | .051 | 40.0 | 0.0 | .022 |
| Memory | |||||||
| 15RWL-IR | Left | 26.7 | 39.7 | .005 | 50.0 | 8.3 | .048 |
| 15RWL-IR | Left-insular | 18.5 | 34.6 | .021 | 80.0 | 24.0 | .057 |
| 15RWL-DR | Left | 3.7 | 6.7 | .005 | 72.2 | 8.3 | .002 |
| 15RWL-DR | Left-ventral | 3.0 | 5.8 | .011 | 80.0 | 30.0 | .028 |
| 15RWL-DR | Left-temporal | 2.1 | 5.6 | .008 | 100.0 | 33.3 | .014 |
| 15RWL-Rec* | Left | 5.8 | 1.1 | .064 | 50.0 | 8.3 | .048 |
| 15RWL-Acc | Left | 75.3 | 93.7 | .007 | 55.6 | 16.7 | .080 |
| 15RWL-Acc | Left-ventral | 74.6 | 86.7 | .052 | 50.0 | 35.0 | .693 |
| DS | Left-insular | 3.5 | 4.9 | .036 | 60.0 | 12.0 | .066 |
| Attention | |||||||
| AM | Right-dorsal | 29.5 | 37.0 | .004 | 25.0 | 17.4 | 1 |
AM = attentional matrices, visual selective attention; DS = digit-span, short term verbal memory; LCNT = naming of nouns (Laiacona-Capitani); NN = naming of nouns; NRep = repetition of numbers; NV = naming of verbs; VPF = verbal fluency on phonemic cue; VSF = verbal fluency on semantic cue; 15RWL-Acc = 15 Rey’s word list: accuracy in recognition; 15RWL-DR = 15 Rey’s word list: delayed recall; 15RWL-IR = 15 Rey’s word list: immediate recall; 15RWL-Rec = 15 Rey’s word list: recognition memory.
* Note that larger scores in these tasks indicate worse performances (see Table 2).
Memory: Effect of Tumor Location and Patient Age
The percentage of patients showing impairment in memory was above 20% in all considered tasks. The highest percentages of patients showing impairment was found with long-term memory tasks, both visuospatial (ROCF-DC: 53.3%) and verbal (15RWL-DR: 46.7%) (Table 2). Results describing association between tumor location and neuropsychological adjusted scores are reported in Table 3. Patients with left-lateralized tumors performed worse than patients with right-sided tumors in the verbal memory tasks, both long-term (15RWL-DR: 3.7 vs 6.7) and short-term (15RWL-IR: 26.7 vs 39.7); the long-term recognition task (15RWL-Rec: 5.8 vs 1.1); and the accuracy in long-term recognition task (15RWL-Acc: 75.3 vs 93.7). The percentages of patients with abnormal scores on these tasks ranged from 50% to 100%. More specifically, worse adjusted scores were observed in patients with tumor in the left ventral stream. Patients with tumor in the left insula had the lowest scores in the short-term verbal memory tasks (15RWL-IR: 18.5 vs 34.6 and DS: 3.5 vs 4.9, with 80% and 60% of patients showing impairment on these tests, respectively). It is worth noticing that the percentage of patients with left-side tumors who showed impairment in the long-term verbal memory task (15RWL-DR) was always larger than 72.2%.
Patients younger than 65 years old performed better than older patients in the long-term verbal memory tasks (15RWL-Acc: 87.1 vs 73.7, P = .008; 15RWL15-Rec: 2.85 vs 6.0, P = .025).
Attention and Executive Functions: Effect of Tumor Location and Patient Age
The percentage of patients showing impairment was above 20% for the accuracy on visual attention task (LCA: 23.3%); the visual divided attention task, in particular the cognitive flexibility task (TMTB: 30%); and the executive function task (TMTB-A: 33.3%) (Table 2). As for association between tumor location and neuropsychological adjusted scores, we found that patients with tumor in the right dorsal stream performed worse in the selective attention task (AM: 29.5 vs 37, Table 3). Patients younger than 65 years old performed better than older patients in both the visual selective attention task (AM: 39.2 vs 27.8, P = .037) and the divided attention task, namely the cognitive flexibility task (TMTB: 151.3 vs 276.2, P = .008) and executive functions task (TMTB-A: 114.2 vs 246.4, P = .008).
Praxis: Effect of Tumor Location and Patient Age
The percentage of patients showing impairment was above 20% only in constructive skills (ROCF-C: 46.6% of impaired patients) (Table 2). We did not find any significant difference of adjusted scores by tumor location. Patients younger than 65 years old performed better than older patients in constructive skills (ROCF-C: 29.0 vs 20.8, P = .015).
Overall Mass Effect
We found a significant impact of the overall mass effect on the preoperative performances of patients in 9 tests (Table 4). In the language domain, we detected statistically significant negative correlations between overall mass effect and adjusted scores in verbal fluencies, both phonemic (VPF: ρ = -0.48) and semantic (VSF: ρ = -0.45). As for the memory domain, we found negative correlation in short-term (15RWL-IR: ρ = -0.63) and long-term (15RWL-DR: ρ = -0.37) verbal memory tasks, and short-term spatial memory (CS: ρ = -0.4). In the praxis domain, we found negative correlation in constructive praxis (ROCF-C: ρ = -0.37). In the attention domain, we found negative correlation in visual attention tasks, namely selective attention (AM: ρ = -0.39), and positive correlations in cognitive flexibility (TMTB: ρ = 0.52) and executive functions (TMTB-A: ρ = 0.49). Moreover, the tumor volume was negatively correlated to the adjusted scores in naming nouns (NN: ρ = -0.36), verbal phonemic fluency (VPF: ρ = -0.39), short-term verbal memory (DS: ρ = -0.36), and constructive abilities (ROCF-C: ρ = -0.35). Tumor volume was positively correlated to the adjusted scores in cognitive flexibility (TMTB: ρ = 0.56) and executive functions (TMTB-A: ρ = 0.54) (Table 4). These results demonstrate the possible impact of both tumor volume and surrounding edema on the neuropsychological outcome in all considered domains.
Table 4.
Spearman rank correlation of neuropsychological adjusted scores with tumor volume (column ρT1) and overall mass effect (column ρM). Columns pT1 and pM show the corresponding P values
| Test | ρT1 | pT1 | ρM | pM |
|---|---|---|---|---|
| Language | ||||
| NN | -0.36 | .048 | – | – |
| VPF | -0.39 | .033 | -0.48 | .007 |
| VSF | – | – | -0.45 | .012 |
| Memory | ||||
| CS | – | – | -0.40 | .029 |
| DS | -0.36 | .047 | – | – |
| 15RWL-IR | – | – | -0.63 | <.001 |
| 15RWL-DR | – | – | -0.37 | .043 |
| Attention | ||||
| AM | – | – | -0.39 | .031 |
| TMTB* | 0.56 | .001 | 0.52 | .003 |
| TMTB-A* | 0.54 | .002 | 0.49 | .005 |
| Praxis | ||||
| ROCF-C | -0.35 | 0.054 | -0.37 | .045 |
AM = attentional matrices, visual selective attention; CS = Corsi-span, short term visuospatial memory; DS = digit-span, short term verbal memory; NN = naming of nouns; ROCF-C = Rey -Osterrieth complex figure copy; TMTB = trail making test, cognitive flexibility; TMTB-A = trail making test, executive function; VPF = verbal fluency on phonemic cue; VSF = verbal fluency on semantic cue; 15RWL-DR = 15 Rey’s word list: delayed recall;15RWL-IR = 15 Rey’s word list: immediate recall.
* Note that larger scores in these tasks indicate worse performances (see Table 2).
The Joint Effect of Overall Mass Effect, Age and Tumor Lateralization
The overall mass effect was not statistically different between the patients with left-sided and right-sided tumors (P = .47). Moreover, the overall mass effect was not correlated with age (P = .27). Since the variables were uncorrelated, we calibrated a linear model for estimating the preoperative adjusted scores on the basis of overall mass effect, tumor lateralization, and age. Results of this analysis demonstrated that preoperative adjusted scores in naming tasks (LCNT) and verbal fluency tasks, both phonemic (VPF) and semantic (VSF), were worse in patients with left-lateralized tumor and large overall mass effect (Table 5). Preoperative adjusted scores in comprehension tasks, both auditory (ACW) and visual (VCW), were worse in patients ≥ 65 years old with large overall mass effect (Table 5).
Table 5.
Results of linear models predicting pre-operative neuropsychological scores. The values of the coefficients (cI: intercept, cL: tumor lateralization, cM: overall mass effect, cA: age) and the respective P values (pI, pL, pM, and pA) are shown. Column R2 reports the coefficient of determination. Note that negative values for lateralization indicate low scores in patients with left-lateralized tumor
| Test | cI | pI | cL | pL | cM | pM | cA | pA | R2 |
|---|---|---|---|---|---|---|---|---|---|
| Language | |||||||||
| LCNT | 81.05 | <.001 | -9.35 | .011 | -0.05 | .111 | – | – | 0.44 |
| VSF | 44.9 | <.001 | -7.41 | .115 | -0.09 | .012 | – | – | 0.30 |
| VPF | 34.67 | <.001 | -8.34 | .010 | -0.09 | <.001 | – | – | 0.48 |
| NN | 10.1 | <.001 | – | – | -0.01 | .047 | – | – | 0.13 |
| VCW | 30.88 | <.001 | – | – | -0.04 | .001 | -0.15 | .003 | 0.69 |
| ACW | 25.95 | <.001 | – | – | -0.02 | .015 | -0.09 | .010 | 0.55 |
| Memory | |||||||||
| 15RWL-Rec* | -7.99 | .219 | – | – | 0.05 | .014 | – | – | 0.17 |
| 15RWL-IR | 50.4 | <.001 | -10.57 | .003 | -0.12 | <.001 | – | – | 0.59 |
| 15RWL-DR | 8.01 | <.01 | -2.66 | .007 | -0.02 | .041 | – | – | 0.36 |
| 15RWL-Acc | 108.27 | <.001 | -15.03 | .078 | -0.16 | .014 | – | – | 0.31 |
| CS | 4.26 | <.001 | – | – | -0.01 | .021 | – | – | 0.18 |
| Attention | |||||||||
| TMTB-A* | -193.1 | .025 | – | – | 0.90 | .001 | 4.38 | .001 | 0.45 |
| TMTB* | -169.9 | .072 | – | – | 1.02 | .001 | 4.37 | .003 | 0.43 |
| TMTA* | -120.4 | .010 | – | – | 0.42 | .005 | 2.19 | .002 | 0.40 |
| AM | 60.40 | <.001 | – | – | -0.09 | .007 | -0.31 | .038 | 0.30 |
| Praxis | |||||||||
| ROCF-C | 55.57 | <.001 | – | – | -0.07 | .003 | -0.38 | <.001 | 0.46 |
ACW = auditory comprehension of words; AM = attentional matrices, visual selective attention; CS = Corsi-span, short term visuospatial memory; LCNT = naming of nouns (Laiacona-Capitani); NN = naming of nouns; ROCF-C = Rey–Osterrieth complex figure copy; TMTA = trail making test, visuospatial attention; TMTB = trail making test, cognitive flexibility; TMTB-A = trail making test, executive function; VCW = visual comprehension of words; VPF = verbal fluency on phonemic cue; VSF = verbal fluency on semantic cue; 15RWL-Acc = 15 Rey’s word list: accuracy in recognition; 15RWL-DR = 15 Rey’s word list: delayed recall; 15RWL-IR = 5 Rey’s word list: immediate recall;15RWL-Rec = 15 Rey’s word list: recognition memory.
* Note that larger scores in these tasks indicate worse performances (see Table 2).
In the memory domain, preoperative adjusted scores in verbal memory tasks, both short-term (15RWL-IR) and long-term (15RWL-DR), and accuracy in recognition memory (15RWL-Acc) were worse in patients with left-lateralized tumor and large overall mass effect (Table 5). Worse adjusted scores in short-term visuospatial memory (CS) were found in patients with large overall mass effect (Table 5).
Patients ≥ 65 years old with large overall mass effect had worse preoperative adjusted scores in visual selective attention (AM) and divided attention, namely visuospatial attention (TMTA), cognitive flexibility (TMTB), and executive functions (TMTA-B) (Table 5). Similar results were obtained in the praxis domain, and in particular in the constructive skills task (ROCF-C) (Table 5).
Short-term Effect of Surgery
The early postoperative MRI (24 hours after surgery) showed the complete resection of the enhancing areas in all the patients. By comparing preoperative mean scores with early and late follow-up mean scores we observed a typical pattern, characterized by a worsening at early follow-up (with respect to preoperative assessment) and a recovery at late follow-up (Table 2). Specifically, a significant worsening from preoperative assessment to early follow-up was observed in 6 tasks associated with repetition of sentences (RepS: p = 0.049) and numbers (NRep: p = 0.044), short-term and long-term verbal memory (15RWL-Acc: p = 0.009; 15RWL-IR: p = 0.037; 15RWL-Rec: p = 0.006), and visual attention (TMTA: p = 0.009). Remarkably, a significant decrease of the proportion of low test scores from preoperative to early follow-up assessment was observed only in 1 patient (preoperative deficits: 70%; early follow-up deficits: 33%, P = .007), while we observed a significant increase in 6 patients. A recovery from early to late follow-up was observed in 17 tasks (including the 6 tasks for which a worsening from preoperative to early follow-up assessment was observed): visual comprehension of sentences (VCS: P = .007); naming verbs (NV: P = .005) and nouns (LCNT: P = .003); verbal phonemic fluency (VPF: P = .009); repetition of sentences (RepS: P = .049), non-words (RepNW: P = .031), and numbers (NRep: P = .014); visual attention (AM: P = .01; TMTA: P = .001; LCS: P = .005); short-tem and long-term verbal memory (DS: P = .016; 15RWL-Acc: P = .049; 15RWL-IR: P = .003; 15RWL-Rec: P = .026); visuospatial memory (CS: P = .018); orofacial praxis (OP: P = .041); and constructive praxis (ROCF-DC: P = .017). We did not report any significant improvement from preoperative assessment to early follow-up or significant worsening from early to late follow-up.
Late Follow-up Assessment
A significant improvement from preoperative assessment to late follow-up was observed in 7 tasks (Table 2) associated with visual comprehension of sentences (VCS: P = .028), naming verbs (NV: P = .036) and nouns (LCNT: P = .007), short-term spatial memory (CS: P = .01), recognition memory (15RWL-Ric: P = .049), long-term spatial memory (ROCF-DC: P = .049), and orofacial praxis (DP: P = .015). We did not observe any significant worsening from preoperative assessment to late follow-up. We found that the adjusted scores variation is correlated with the decrease of overall mass effect in 10 tasks in all examined domains (Table 6). Specifically, naming of nouns (LCNT: ρ = 0.76), verbal semantic fluency (VSF: ρ = 0.39), reading of words (ReaW: ρ = 0.37), auditory comprehension of words (ACW: ρ = 0.57), visuospatial memory, both short-term (CS: ρ = 0.34) and long-term (ROCF-DC: ρ = 0.44), short-term verbal memory (15RWL-IR: ρ = 0.53), visuospatial attention, namely cognitive flexibility (TMTB: ρ = -0.52), executive functions (TMTB-A: ρ = -0.52), and constructive praxis (ROCF-C: ρ = 0.39). Despite the smaller sample size, comparable results were obtained by considering the random variable (Mp-Fl) (Table 6). We found a significant correlation with the tumor volume in 5 tests (Table 6).
Table 6.
Spearman rank correlation of adjusted scores variation (Sl-Sp) with decrease of overall mass effect (Mp-Fe) (column ρMp-Fe), random variable (Mp-Fl) (column ρMp-Fl) and tumor volume (column ρT1). Columns PMp-Fe, PMp-Fl and PT1 show the respective P values. Results of linear models estimating adjusted scores variation (Sl-Sp) are also shown. The values of the coefficients (cI: intercept, cL: tumor lateralization, cMp-Fe: decrease of overall mass effect, cA: age) and the respective P values (pI, pL, pMp-Fe, and pA) are shown. Column R2 reports the coefficient of determination. Note that negative values for lateralization indicate low scores in patients with left-lateralized tumor
| Test | ρMp-Fe | PMp-Fe | ρMp-Fl# | PMp-Fl# | ρT1 | PT1 | cI | pI | cL | pL | cMp-Fe | pMp-Fe | cA | pA | R2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Language | |||||||||||||||
| LCNT | 0.76 | <.001 | 0.77 | .005 | – | – | -0.52 | .745 | – | – | 0.06 | .015 | – | – | 0.29 |
| VSF | 0.39 | .031 | 0.63 | .017 | – | – | – | – | – | – | – | – | – | – | – |
| VPF | – | – | 0.52 | .045 | 0.37 | .04 | – | – | – | – | – | – | – | – | – |
| ReaW | 0.37 | .041 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| VCW | – | – | – | – | – | – | -12.59 | .014 | – | – | 0.07 | .007 | 0.16 | .022 | 0.52 |
| ACW | 0.57 | .032 | – | – | 0.36 | .047 | -8.10 | .019 | – | – | 0.03 | .062 | 0.12 | .014 | 0.45 |
| Memory | |||||||||||||||
| CS | 0.34 | .044 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 15RWL- Rec* | – | – | – | – | – | – | -13.53 | .082 | – | – | – | – | 0.26 | .040 | 0.14 |
| 15RWL-IR | 0.53 | .003 | 0.62 | .017 | – | – | -7.33 | .037 | – | – | 0.14 | .007 | – | – | 0.25 |
| ROCF-DC | 0.44 | .014 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Attention | |||||||||||||||
| TMTB-A* | -0.52 | .003 | -0.58 | .028 | -0.37 | .046 | 115.30 | .006 | – | – | -1.63 | .005 | – | – | 0.24 |
| TMTB* | -0.52 | .003 | -0.62 | .018 | -0.38 | .038 | 113.70 | .015 | – | – | -1.60 | .014 | – | – | 0.20 |
| TMTA* | – | – | – | – | – | – | 143.02 | .035 | – | – | -0.75 | .015 | -1.89 | .06 | 0.24 |
| AM | – | – | – | – | – | – | -21.51 | .111 | -16.64 | .008 | 0.18 | .007 | 0.44 | .040 | 0.34 |
| Praxis | |||||||||||||||
| ROCF-C | 0.39 | .033 | – | – | 0.37 | .043 | -20.53 | .015 | – | – | 0.11 | .005 | 0.29 | .020 | 0.32 |
ACW = auditory comprehension of words; AM = attentional matrices, visual selective attention; CS = Corsi-span, short term visuospatial memory; LCNT = naming of nouns (Laiacona-Capitani); ReaW = reading of words; ROCF-DC = Rey -Osterrieth complex figure delayed copy; ROCF-C = Rey -Osterrieth complex figure copy; TMTA = trail making test, visuospatial attention; TMTB = trail making test, cognitive flexibility; TMTB-A = trail making test, executive function; VCW = visual comprehension of words; VSF = verbal fluency on semantic cue; VPF = verbal fluency on phonemic cue; 15RWL-IR = 15 Rey’s word list: immediate recall; 15RWL-Rec = 15 Rey’s word list: recognition memory.
* Note that larger scores in these tasks indicate worse performances (see Table 2).
# Data available for 16 out of 30 patients.
We calibrated a linear model for estimating the adjusted scores variation on the basis of decrease of overall mass effect, tumor lateralization, and age. Results of this analysis showed that recovery at late follow-up is mainly attributable to the decrease of overall mass effect and age (in particular for tests where preoperative assessment is affected by age, namely VCW, ACW, AM, TMTA, and ROCF-C; see Table 5), and that tumor lateralization has minimal impact (Table 6).
Discussion
Considering the crucial role of extent of resection in prolonging survival, surgery plays an important role in the whole treatment of patients affected by high-grade gliomas.43 As a consequence, the growing efforts of neuro-oncological surgeons and the technical advancements in this field aim to push resection to the limit as much as possible. Nonetheless, the real impact of sole radical surgery (ie, complete resection) on cognitive outcome of patients affected by high-grade gliomas is still controversial. Our results suggest that, despite a worsening of neuropsychological performances at early follow-up, surgery might be effective also for improving the cognitive performances of patients and thus their quality of life. The worsening immediately after surgery, observed in spite of the mass effect reduction and edema resorption, is possibly due to the concurrent short-term effects of surgery, anesthesia, and/or postsurgical pain treatment. Hereafter we discuss the role of the main determinants, namely overall mass effect, tumor localization and age, of the neuropsychological outcome.
Mass Effect
A negative correlation between tumor volume and preoperative performances in the executive and psychomotor functioning was demonstrated in a population of high-grade gliomas.9 Moreover, a negative correlation between tumor volume and preoperative memory performances was demonstrated in a mixed population of low-grade and high-grade gliomas.18 Remarkably, our results suggest that the preoperative neuropsychological outcome might depend on both tumor volume and volume of the edema surrounding the tumor itself. Moreover, we demonstrated that the overall mass effect might affect performances in a much wider spectrum of neurocognitive abilities, covering all examined domains, namely comprehension, naming, verbal fluency, verbal and spatial memory, attention, and executive functions and constructive abilities.
We demonstrated that recovery at late follow-up might depend on both tumor resection and decrease of hyperintense areas surrounding the tumor lesion. Indeed, the decrease of overall mass effect is proportional to the resected tumor mass and to the surrounding edema, and inversely proportional to eventual damages caused by surgery (microvascular injuries, manipulation, etc.). Interestingly, recovery was observed exclusively in the tasks with direct and positive correlation between impairment and overall mass effect. This evidence supports a possible effect of surgery (ie, resection in contact with eloquent bundles or cortices, postoperative edema, subclinical seizures, partial damage to eloquent connectivity) in the early postoperative impairment. However, this negative effect was demonstrated to be temporary probably because of the respect of subcortical eloquent limits and/or because of the occurrence of early plasticity phenomena in the early postoperative period.19,25 Reported results suggest that the tumor volume or the overall mass effect should not impact the surgical indication. Rather, they support the crucial principle of resecting the tumor as much as possible, respecting the eloquent white matter (WM) bordering the tumor (ie, avoiding or limiting surgical damage to the perilesional functional structures).
Effect of Tumor Location
We found that tumor lateralization affected preoperative performances in verbal fluency, both phonemic and semantic, naming of words, and verbal memory, both short-term and long-term. Similar results in the language domain were previously reported by other groups13,18,20 and are not surprising. These results depend, in fact, on the intrinsic specialization of the left hemisphere in performing language tasks, since language is a distributed, multimodal, and plastic but strongly lateralized network, especially in right-handers.18–20,22,25,27,28,44 Remarkably, however, we obtained more detailed results. In particular, we demonstrated that performances in verbal fluency and naming of words were worse in patients with tumor in the left insula and left temporal lobe, producing mass effect on both dorsal and ventral streams. This may be explained by considering the course of the ventral semantic stream (ie, inferior fronto-occipital fascicle, inferior longitudinal fascicle, uncinate fascicle), connecting the occipital and frontal lobes through the temporal lobe, and the dorsal phonological stream (arcuate fascicle), connecting the temporal and frontal lobes running around the perisilvian sulcus.44
Results in the memory domain can be explained by considering that the language network is essential to performing verbal memory tasks. Patients with left-ventral tumors, in fact, had a worse performances in all the verbal memory tasks. This is expected considering that cognitive functions depend on large, distributed, and highly integrated networks, including several specialized brain regions and bundles.45 It is well known, for instance, that language networks are much more distributed and plastic than previously assumed (even because of potential reorganization induced by the tumor growth). Moreover, associative, connection, and projection fibers subserving this network could also support other functions in a more integrated connectomic view of brain processing.46 It is worth noticing that while preoperative performances clearly depend on the dominance of the affected hemisphere, the final outcomes do not (Figure 1).
Effect of Patient Age
We demonstrated that age might affect preoperative performances in auditory and visual comprehension, selective and divided attention, and constructive abilities, with worse performances in patients ≥ 65 years old. We report here that these results go beyond the well-known evidence that the neurocognitive performance of individuals ≥ 65 years old is worse with respect to that of younger individuals. Indeed, the use of adjusted scores allows evaluating the real effect of age on patients’ performances. In other words, when we state, for instance, that patients ≥ 65 years old perform worse than younger individuals, we also mean that these patients perform worse than healthy individuals of same age.
Results in the attention and executive functions domain can be explained by considering that mental flexibility and attention resources are more limited under cognitive deterioration,47 thus the outcome of patients ≥ 65 years old could be affected by lower baseline cognitive performance. Younger patients, on the contrary, could have more functional resources for a better recovery of attentional abilities.
Results in language and praxis domains can be explained by considering the high cognitive demand required by comprehension and constructive abilities tasks. Indeed, comprehension requires immediate and critical concentration, shifting attention continuously between auditory and visual stimuli. This hypothesis is partially supported by the analysis of attention tasks in patients ≥ 65 years old. Specifically, performance in the cognitive flexibility task (TMTB) was worse in these patients than in to younger patients. Moreover, constructive abilities require visuospatial planning. Therefore, worse performances of patients ≥ 65 years old in this task can be induced by worse performances in visuospatial (TMTA) and selective attention (AM).
We demonstrated that age might affect recovery at early follow-up. These results can be explained by considering that an improvement in visuospatial and selective attention might result in better performances in those tasks requiring the availability of high cognitive demand, such as comprehension and constructive abilities tasks.
Another striking pattern is the general worsening observed at the early follow-up assessment of patients ≥ 65 years old. It could be related to less-effective short-term mechanisms of brain plasticity in these patients or it could be also due to different effects of surgery, anesthesia, and/or postoperative pain treatment, which are less tolerated by the elderly and may complicate immediate recovery.15,21 However, at the late follow-up patients recovered to preoperative levels, suggesting that age alone should not be considered as an absolute exclusion criterion.
Conclusions
We demonstrated that longitudinal neuropsychological performances of patients affected by high-grade glioma depend, among other factors, on the complex interplay of tumor volume, volume of surrounding edema, tumor localization, and patient age.
Our analysis demonstrated that postoperative cognitive performances can be predicted, at least to some extent, by combining information on tumor lateralization, age and overall mass effect; recovery at late follow-up is associated with patient age and decrease of overall mass effect; and the final outcome does not depend on the dominance of the affected hemisphere.
Our results provide simple models for predicting the preoperative and postoperative cognitive outcome in several functional domains, giving new insights into the main determinants of surgical recovery before adjuvant treatments.
Funding Statement
The extensive analysis of neuropsychological outcome included in this paper is supported by CARITRO Foundation (Trento, Italy) within the NePsi Project (Division of Neurosurgery, Santa Chiara Hospital, Trento, Italy).
Conflict of interest statement. None of the authors have a conflict of interest related to this research.
References
- 1. DeAngelis LM. Brain tumours. N Engl J Med. 2001;344(2):114–123. [DOI] [PubMed] [Google Scholar]
- 2. Polednak AP, Flannery JT. Brain, other central nervous system and eye cancer. Cancer. 1995;75:330–337. [DOI] [PubMed] [Google Scholar]
- 3. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 4. Li J, Wang M, Won M, et al. Validation and simplification of the radiation therapy oncology group recursive partitioning analysis classification for glioblastoma. Int J Radiat Oncol Biol Phys. 2011;81(3):623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol. 1984;2(3):187–193. [DOI] [PubMed] [Google Scholar]
- 6. Duffau H, ed. Brain Mapping from Neural Basis of Cognition to Surgical Applications. New York, NY: Springer Wien; 2012. [Google Scholar]
- 7. Archibald YM, Lunn D, Ruttan LA, et al. Cognitive functioning in long-term survivors of high-grade glioma. J Neurosurg. 1994;80:247–253. [DOI] [PubMed] [Google Scholar]
- 8. Meyers CA, Hess KR. Multifaceted end points in brain tumor clinical trials: Cognitive deterioration precedes MRI progression. Neuro Oncol. 2003;5:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Esther Habets JJ, Kloet A, Walchenbach R, Vecht CJ, Klein M, Taphoorn MJB. Tumour and surgery effects on cognitive functioning in high-grade glioma patients. Acta Neurochir. 2014;156:1451–1459. [DOI] [PubMed] [Google Scholar]
- 10. Brown PD, Jensen AW, Felten SJ, et al. Detrimental effects of tumor progression on cognitive function of patients with high-grade glioma. J Clin Oncol. 2006;24(34):5427–5433. [DOI] [PubMed] [Google Scholar]
- 11. Bosma I, Vos MJ, Heimans JJ, et al. The course of neurocognitive functioning in high-grade glioma patients. Neuro Oncol. 2007;9(1):53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown PD, Ballman KV, Rummans TA, et al. Prospective study of quality of life in adults with newly diagnosed high-grade gliomas. J Neurooncol. 2006;76(3):283–291. [DOI] [PubMed] [Google Scholar]
- 13. Santini B, Talacchi A, Squintani G, Casagrande F, Capasso R, Miceli G. Cognitive outcome after awake surgery for tumors in language areas. J Neurooncol. 2012;108(2):319–326. [DOI] [PubMed] [Google Scholar]
- 14. Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state for the clinician. J Psychiat Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 15. Kayl AE, Meyers CA. Does brain tumor histology influence cognitive function? Neuro Oncol. 2003;5(4):255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taylor BV, Buckner JC, Cascino TL, et al. Effects of radiation and chemotherapy on cognitive function in patients with high-grade glioma. Clin Oncol. 1998;16(6):2195–2201. [DOI] [PubMed] [Google Scholar]
- 17. Yoshii Y, Tominaga D, Sugimoto K, et al. Cognitive function of patients with brain tumor in pre- and postoperative stage. Surg Neurol. 2008;69:51–61. [DOI] [PubMed] [Google Scholar]
- 18. Satoer D, Visch-Brink E, Smits M, et al. Long-term evaluation of cognition after glioma surgery in eloquent areas. J Neurooncol. 2014;116:153–160. [DOI] [PubMed] [Google Scholar]
- 19. Satoer D, Vork J, Visch-Brink E, Smits M, Dirven C, Vincent A. Cognitive functioning early after surgery of gliomas in eloquent areas. J Neurosurg. 2012;117:831–838. [DOI] [PubMed] [Google Scholar]
- 20. Talacchi A, Santini B, Savazzi S, Gerosa M. Cognitive effects of tumour and surgical treatment in glioma patients. J Neurooncol. 2010;103(3):541–549. doi:10.1007/s11060-010-0417-0. [DOI] [PubMed] [Google Scholar]
- 21. Kaleita TA, Wellisch DK, Cloughesy TF, et al. Prediction of neurocognitive outcome in adult brain tumor patients. J Neurooncol. 2004;67(1–2): 245–253. [DOI] [PubMed] [Google Scholar]
- 22. Horn J, Reitan RM. Neuropsychological correlates of rapidly or slowly growing intrinsic cerebral neoplasms. J Clin Neuropsychol. 1984;6:309–324. [DOI] [PubMed] [Google Scholar]
- 23. Taphoorn MJ, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159–168. [DOI] [PubMed] [Google Scholar]
- 24. Satoer D, Vincent A, Smits M, Dirven C, Visch-Brink E. Spontaneous speech of patients with gliomas in eloquent areas before and early after surgery. Acta Neurochir. 2013;155:685–692. [DOI] [PubMed] [Google Scholar]
- 25. Scheibel RS, Meyers CA, Levin VA. Cognitive dysfunction following surgery for intracerebral glioma: influence of histopathology, lesion location, and treatment. J Neurooncol. 1996;30:61–69. [DOI] [PubMed] [Google Scholar]
- 26. Klein M, Taphoorn MJ, Heimans JJ, et al. Neurobehavioral status and health-related quality of life in newly diagnosed high-grade glioma patients. J Clin Oncol. 2001;19:4037–4047. [DOI] [PubMed] [Google Scholar]
- 27. Scheibel RS, Hannay HJ, Meyers CA. The category test in patients with lateralized frontal and nonfrontal glioma. J Clin Exp Neuropsychol. 1993;15:69. [Google Scholar]
- 28. Hahn CA, Dunn RH, Logue PE, King JH, Edwards CL, Halperin EC. Prospective study of neuropsychologic testing and quality-of-life assessment of adults with primary malignant brain tumors. Int J Radiat Oncol Biol Phys. 2003;55:992–999. [DOI] [PubMed] [Google Scholar]
- 29. Johnson DR, Sawyer AM, Meyers CA, O’Neill BP, Wefel JS. Early measures of cognitive function predict survival in patients with newly diagnosed glioblastoma. Neuro Oncol. 2012;14(6):808–816. doi:10.1093/neuonc/nos082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carlesimo G, Caltagirone C, Gainotti G, Nocentini U. Batteria per la valutazione del deterioramento mentale (parte II): standardizzazione e affidabilità diagnostica nell’identificazione di pazienti affetti da sindrome demenziale. Arch Psicol Neurol Psichiatr. 1995;56(4):471–488. [Google Scholar]
- 31. Novelli G, Papagno C, Capitani E, Laiacona M, Vallar G, Cappa SF. Tre test clinici di ricerca e produzione lessicale. Taratura su soggetti normali. Arch Psicol Neurol Psichiatr. 1986;47(4):477–506. [Google Scholar]
- 32. Laiacona M, Barbarotti R, Trivelli C, Capitani E. Dissociazioni semantiche intecategoriali: descrizione di una batteria standardizzata e dati normativi. Arch Psicol Neurol Psichiatr. 1993;2:209–249 [Google Scholar]
- 33. Capasso R, Miceli G, eds. Esame Neuropsicologico per l’Afasia E.N.P.A. Milano, Italy: Springer; 2001. [Google Scholar]
- 34. Orsini A, Grossi D, Capitani E, Laiacona M, Papagno C, Vallar G. Verbal and spatial immediate memory span: normative data from 1355 adults and 1112 children. Ital J Neurol Sci. 1987;8:539–548. [DOI] [PubMed] [Google Scholar]
- 35. Carlesimo GA, Caltagirone C, Gainotti G, et al. The mental deterioration battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. Eur Neurol. 1996;36:378–384. [DOI] [PubMed] [Google Scholar]
- 36. Caffarra P, Vezzadini G, Dieci F, Zonato F, Venneri A. Rey–Osterrieth complex figure: normative values in an Italian population sample. Neurol Sci. 2002;22:443–447. [DOI] [PubMed] [Google Scholar]
- 37. Spinnler H, Tognoni G. Standardizzazione e taratura italiana di test neuropsicologici [Italian standardization of neuropsychological tests]. Ital J Neurol Sci. 1987;8(suppl.):1–120. [PubMed] [Google Scholar]
- 38. Giovagnoli AR, Del Pesce M, Mascheroni S, Simoncelli M, Laiacona M, Capitani E. Trail making test: normative values from 287 normal adult controls. Ital J Neurol Sci. 1996;17:305–309. [DOI] [PubMed] [Google Scholar]
- 39. Albert ML. A simple test of visual neglect. Neurology. 1973;23:658–664. [DOI] [PubMed] [Google Scholar]
- 40. De Renzi E, Piezcuro A, Vignolo LA. Oral apraxia and aphasia. Cortex. 1996;2:50–73. [Google Scholar]
- 41. De Renzi E, Piezcuro A, Vignolo LA. Ideational apraxia. Neuropsychologia. 1968;6:41–52. [Google Scholar]
- 42. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. [DOI] [PubMed] [Google Scholar]
- 43. Hervey-Jumper SL, Berger MS. Role of surgical resection in low- and high-grade gliomas. Curr Treat Options Neurol. 2014;16(4):284. [DOI] [PubMed] [Google Scholar]
- 44. Sarubbo S, De Benedictis A, Merler S, et al. Towards a functional atlas of human white matter. Hum Brain Mapp. 2015;36(8):3117–3136. doi:10.1002/hbm.22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bosma I, Reijneveld JC, Klein M, et al. Disturbed functional brain networks and neurocognitive function in low-grade glioma patients: a graph theoretical analysis of resting-state MEG. Nonlinear Biomed Phys. 2009;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sporns O. Contributions and challenges for network models in cognitive neuroscience. Nat Neurosci. 2014;17:652–660. [DOI] [PubMed] [Google Scholar]
- 47. Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–1992. [DOI] [PubMed] [Google Scholar]