Abstract
Background
Bipolar disorder (BD) and schizophrenia (SZ) show substantial overlap. It has been suggested that a subgroup of patients might contribute to these overlapping features. This study employed a cross-diagnostic cluster analysis to identify subgroups of individuals with shared cognitive phenotypes.
Method
143 participants (68 BD patients, 39 SZ patients and 36 healthy controls) completed a battery of EEG and performance assessments on perception, nonsocial cognition and social cognition. A K-means cluster analysis was conducted with all participants across diagnostic groups. Clinical symptoms, functional capacity, and functional outcome were assessed in patients.
Results
A two-cluster solution across 3 groups was the most stable. One cluster including 44 BD patients, 31 controls and 5 SZ patients showed better cognition (High cluster) than the other cluster with 24 BD patients, 35 SZ patients and 5 controls (Low cluster). BD patients in the High cluster performed better than BD patients in the Low cluster across cognitive domains. Within each cluster, participants with different clinical diagnoses showed different profiles across cognitive domains.
Limitations
All patients are in the chronic phase and out of mood episode at the time of assessment and most of the assessment were behavioral measures.
Conclusions
This study identified two clusters with shared cognitive phenotype profiles that were not proxies for clinical diagnoses. The finding of better social cognitive performance of BD patients than SZ patients in the Lowe cluster suggest that relatively preserved social cognition may be important to identify disease process distinct to each disorder.
Keywords: cluster analysis, social cognition, nonsocial cognition, bipolar disorder, schizophrenia
1. INTRODUCTION
A longstanding question in psychiatry is whether bipolar disorder (BD) and schizophrenia (SZ) are independent, distinct disease entities. Since Kraepelin proposed BD and SZ as two different disorders, they have been diagnosed and treated separately. However, a large number of studies have shown that SZ and BD share many features (Bramon and Sham, 2001; Cardno and Owen, 2014; Grozeva et al., 2010; Koreen et al., 1993; Maier, 2008; Maier et al., 2006; Shinn et al., 2012), challenging the idea that they represent two distinct disease processes. Given that both BD and SZ are highly heterogeneous, it has been proposed that subgroups of patients might contribute to overlapping features of the two disorders. A typical approach has been to identify subgroups of patients based on clinical features (e.g., psychotic symptoms) and then to compare their similarities and differences on cognitive phenotypes (Anticevic et al., 2015; Anticevic et al., 2014). Using an alternative, cognitive phenotype-guided approach, this study sought to identify clusters of individuals with shared cognitive phenotype profiles across diagnoses and to examine the relationship between membership in these clusters and clinical diagnoses.
A considerable literature has compared cognitive phenotypes of BD and SZ patients. SZ patients show substantial impairment compared to controls across multiple cognitive assessments. The degree of impairment of BD patients relative to SZ patients and healthy controls varies across types of cognition. BD patients show intermediate performance between SZ patients and controls on nonsocial cognition (Bora and Pantelis, 2015). Findings for social cognition are not as consistent, in that some studies found similar levels of impairment in BD and SZ patients (Daros et al., 2014) whereas others showed more severe impairment of SZ patients compared to BD patients (Ruocco et al., 2014).
To identify subgroups of patients with similar cognitive profiles, several studies have focused on the presence or absence of psychotic symptoms in BD. BD patients with a history of psychosis showed better performance than SZ patients, but profiles of both patient groups were similar (Barch and Sheffield, 2014; Hill et al., 2013), suggesting that common mechanisms may be affected at the neural level in both groups. If the presence of psychosis could explain commonality between BD and SZ, this also suggests that BD patients with a history of psychosis would differ from BD patients without such a history. However, findings of nonsocial cognition comparison between BD patients with and without a history of psychosis are mixed. Some found no difference across multiple nonsocial cognitive assessments (Sanchez-Morla et al., 2009; Selva et al., 2007) whereas others found significant group differences on certain cognitive domains (e.g., working memory, reasoning) but not on other domains (e.g., attention, visual memory) (Bora et al., 2007; Bora et al., 2010; Glahn et al., 2007). Thus, it is not clear whether a categorical approach using a history of psychosis can satisfactorily explain shared features between BD and SZ.
Two studies employed a cluster analysis to identify subgroups of patients according to their cognitive profiles. A cluster analysis is well suited to classify individuals on dimensional measures (e.g., cognitive performance) regardless of any category relying on clinical features (e.g., psychotic symptoms, clinical diagnoses). Using only BD patients, one study identified 3 BD subgroups, global impairment, selective impairment, and intact cognition, and 3 subgroup did not differ on a history of psychotic symptoms (Burdick et al., 2014). Another study focused on the psychosis spectrum between BD and SZ (i.e., BD patients with psychosis, SZ patients and schizoaffective patients) and identified 4 clusters with distinct cognitive profiles: all of the clinical diagnoses were represented in 4 clusters but they were not evenly distributed across the clusters (Lewandowski et al., 2014). These findings suggest that although clinical diagnoses or clinical symptoms alone are not sufficient to identify participants with shared cognitive profiles, there may be a subtle interaction between cognition-driven clusters and clinical diagnoses. However, both of these studies included assessments only on nonsocial cognition and a limited range of clinical groups (e.g., only BD patients, or only patients with psychosis). Thus, it is not clear what would be seen with a wider range of assessments on multiple cognitive domains that have been previously examined in both SZ and BD and with participants who have a large range in cognitive functioning, including normal levels.
Using a cross-diagnostic cluster analysis, this study aimed to determine subgroups of individuals based on cognitive profile across clinical diagnoses and to examine how the cluster membership is related to clinical diagnoses, with an assessment battery that covers perception, cognition and social cognition on both auditory and visual modalities. First, we examined whether there are subgroups with shared cognitive profiles and whether the clusters show similar cognitive profiles or not. Second, we investigated the overlap between clinical diagnosis and cluster membership. Specifically we compared cognitive profiles between diagnoses within a cluster, as well as cluster differences within a diagnosis, to examine whether subgroups defined by cognitive profile could provide similar information across clinical diagnoses. Third, we examined the relationships between cognition and functional outcome within each cluster.
2. METHODS
2.1. Participants
There were 143 participants, 68 with DSM-IV diagnoses of bipolar disorder I or II, 39 with a DSM-IV diagnosis of schizophrenia and 36 healthy controls. Patients were recruited from University of California, Los Angeles (UCLA) and the Veterans Affairs Greater Los Angeles Healthcare System (VAGLAHS) and from local board and care facilities in Los Angeles. Controls were recruited through website postings. The diagnostic eligibility for all participants were confirmed with the Structured Clinical Interview for DSM-IV (SCID) Axis I Disorders (First et al., 1997). Exclusion criteria for all participants were: a lifetime history of loss of consciousness for more than one hour due to head trauma, a significant neurological disorder, or insufficient fluency in English to understand the procedures (based on judgment of the clinical assessor). Additional exclusion criteria for patients were: substance dependence in the last six months, substance abuse in the past month, and a history of low IQ (i.e., < 70) based on review of medical records. Controls were excluded if they had: a history of schizophrenia, other psychotic disorders, bipolar disorder, recurrent major depressive disorder, substance dependence disorder or substance abuse in the past month; family history of psychotic disorder or bipolar disorder among first degree relatives based on self-report; and any of the following Axis II disorders: avoidant, paranoid, schizoid, schizotypal, or borderline based on the SCID for Axis II disorders (First et al., 1996). Demographic data (e.g., age, gender, parental education) were collected for all participants. All participants had normal or corrected to normal vision of at least 20/30. At the time of participation, all patients were clinically stable. All schizophrenia patients were taking antipsychotic medication. Of the 68 BD patients, 41 were taking antipsychotic medication and 13 were taking lithium. All participants were out of mood episode in the past month and did not take any sedative or benzodiazepine within 12 hours of testing.
Clinical characteristics for patients were assessed with the Hamilton Depression Rating Scale (HAM-D)(Hamilton, 1960), the Young Mania Rating Scale (YMRS)(Young et al., 1978) and the expanded 24-item version of the Brief Psychiatric Rating Scale (BPRS)(Ventura et al., 1993). All interviewers were trained through the Treatment Unit of the Department of Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center. SCID interviewers were trained to a minimum kappa of 0.75 for key psychotic and mood items, and symptom raters were trained to a minimum intraclass correlation of 0.80. All participants were evaluated for the capacity to give informed consent and provided written informed consents after all procedures were fully explained, according to procedures approved by the Institutional Review Boards at CLA and VAGLAHS.
2.2. Cognitive Phenotype Assessments
This study administered a range of electrophysiological and performance-based assessments that measured perception, nonsocial cognition and social cognition on both auditory and visual modalities (collectively referred to as cognition hereinafter). Using a 64-channel Biosemi ActiveTwo amplifier (Biosemi B.V., The Netherlands), two electrophysiological phenotypes were collected: mismatch negativity (MMN) for early auditory perception, and N170/N250 for facial affect processing. Using behavioral measures, we also assessed early visual perception, nonsocial cognition and social cognition. The diagnostic group differences have been previously published with detailed descriptions of assessments (Jahshan et al., 2012; Jahshan et al., 2014; Lee et al., 2013; Wynn et al., 2013) and therefore we only included brief descriptions of each assessment below.
2.2.1. MMN for early auditory perception
In a duration-deviant auditory oddball task, participants listened to tones (standard [90% probability] or deviant [10% probability]) using ear inserts while watching a silent movie. The main dependent measure was the average mean amplitude of MMN across a time window (i.e., 135–205 msec) that was obtained by subtracting standard from deviant average waveforms derived from electrodes at frontocentral sites (Jahshan et al., 2012).
2.2.2. N170 and N250 for facial affect processing
In an affect differentiation task with 3 conditions, participants were asked to identify the height of a building (building condition), the gender of a face (gender condition), or the emotional expression of faces (emotion condition) (Wynn et al., 2013). The main dependent measures were the mean amplitude for N170 and N250 across time windows that were obtained by subtracting ERPs in the building condition from the emotion condition.
2.2.3. Early visual perception
Two visual masking tasks, a location masking task and a 4-dot masking task, were used (Jahshan et al., 2014). In the location masking task, a single target was either preceded (referred to as forward) or followed (referred to as backward) by a mask with six stimulus-onset asynchronies (SOA). Participants were asked to detect the location of the target. The main dependent measure was the average accuracy of backward condition across SOAs. In the 4-Dot masking, 4 potential targets with a notch at the top, bottom, or left side of the square appeared followed by a mask surrounding one of the 4 potential targets at 8 SOAs. Participants were asked to indicate the location of the notch of the target. The main dependent measure was the average accuracy for the first 4 SOAs.
2.2.4. Nonsocial Cognition
To assess nonsocial cognitive function, the MATRICS Consensus Cognitive Battery (MCCB: Nuechterlein and Green, 2006) was used. The MCCB assesses 6 nonsocial cognitive domains: speed of processing, attention/vigilance, working memory, verbal learning, visual learning, and reasoning/problem solving. The main dependent measures were the summary scores for each domain.
2.2.5. Social cognition
Five social cognitive assessments were administered (Lee et al., 2013): a Facial Affect Recognition Task, Part 3 of the Awareness of Social Inference Test (TASIT), an Empathic Accuracy Task, a Self-referential Memory Task and the Managing Emotion Branch of the Mayer-Salovey-Caruso Emotion Intelligence Test (MSCEIT). The Facial Affect Recognition Task assessing facial affect recognition asked participants to identify which emotional expression (happy, sad, angry, afraid, surprised, disgusted, and neutral) best represents an Ekman face photo (Ekman, 2004; Horan et al., 2009). The main dependent measure was accuracy. The TASIT assesses mental state attribution (McDonald et al., 2002) and the dependent measures are two sub-scores: one each for lies and sarcasm. (Kern et al., 2009; Mancuso et al., 2011). The Empathic Accuracy Task assesses the accuracy of empathic judgment using 12 video clips in which an individual (called “target”) discusses a positive or negative autobiographical event. The dependent measure was the correlation between participant’s ratings of the target’s emotion and the target’s ratings of their own emotion (Lee et al., 2013). The Self-referential Memory Task assesses self-referential memory bias using trait adjectives (Harvey et al., 2011). The dependent measure was a measure of sensitivity (A’) for the self condition in which participants were asked to remember adjectives that, earlier, they had rated as to whether it described themselves or not. As part of the MCCB, the Managing Emotion branch of the MSCEIT (Nuechterlein and Green, 2006) assesses the participants’ understanding of ways of regulating emotion in oneself and others. The main dependent measure was the T score.
2.3. Assessments for functional capacity and community functioning
The UCSD-Performance-Based Skills Assessment (UPSA) (Patterson et al., 2001) and the Maryland Assessment of Social Competence (MASC) (Bellack et al., 1994) were employed to assess functional capacity. The UPSA involves role-play tasks in 5 skill areas considered essential for daily functioning in the community: general organization, finance, social/communication, transportation, and household chores. The main dependent measure was the total UPSA summary score across the 5 areas. The MASC involves four 3-minute role-play scenarios to assess an ability to solve common problems in an interpersonal context (e.g., interaction with a casual acquaintance, negotiation). The interactions were videotaped and scored by one of two independent raters who achieved ICC’s exceeding .85 for all the MASC variables on a set of 10 videos that were not from the current study. The main dependent measure was the total MASC summary score. To assess community functioning, the Role Functioning Scale (RFS) was employed (Brekke et al., 2005; McPheeters, 1984). The RFS was rated on a 7-point rating from a semi-structured interview on 4 areas (work, independent living, family relations, and social functioning) and captures both the quantity and quality of functioning in each domain. The main dependent measure was the average rating across the 4 areas.
2.4. Statistical Analysis
To identify subgroups of participants across diagnoses that had similar cognitive phenotype profiles, we implemented the following data analyses. First, the mean amplitude of MMN and mean amplitudes of N170 / N250 were reverse-coded to make higher values of all the cognitive assessments indicate better performance or performance similar to one observed in healthy controls. Second, we standardized the scores for the main dependent variable of each phenotype assessment to improve the accuracy of the clustering algorithm (Mohamad and Usman, 2013). Third, missing data were imputed. A total of 10% of the data were missing, ranging from 5% to 25% depending on the phenotype assessment. Imputation of the missing data was performed using the expectation-maximization with bootstrapping algorithm Amelia in R (Honaker et al., 2011). The Amelia algorithm assumes that the complete data are multivariate normal and that the missing data are missing at random. The fit of the imputation model was assessed with overimputation (Blakewell et al., 2015). Overimputation involves generating hundreds of imputed values of each observed value as if it had been missing, constructing a confidence interval with the imputed values and then assessing whether the observed data falls within the region where it would have been imputed had it been missing. When running this procedure through all the observed values, we found that for all variables the estimates of each observed values agree with the true values, indicating the overall good fit of the imputation model.
Fourth, eight phenotype composite scores were created using the imputed standardized scores: MMN, N170+N250, Visual Perception, Speed of Processing, Working Memory, Learning, Low-level Social Cognition, and High-level Social Cognition (see below). Visual Perception included both location masking and 4-Dot masking. For nonsocial cognition, 3 subdomains were created based on a published factor structure of the MCCB (Burton et al., 2013): Speed of Processing (speed of processing and reasoning/problem solving), Working Memory (attention/vigilance and working memory), and Learning (verbal learning and visual learning). Low-level Social Cognition involves an ability to perceive and recognize social cues and included the Facial Affect Recognition Task and TASIT lie subscale. High-level Social Cognition involves a higher-order inferential process on others and included the Empathic Accuracy Task, TASIT sarcasm subscale, Self-referential Memory Task and Managing emotion of MSCEIT. Fifth, composite scores were re-standardized using means and standard deviations of the complete data, similar to what was done for the individual phenotype assessment.
A K-means cluster analysis was conducted to identify subgroups using phenotype composites across clinical diagnoses. We selected the standard global partitioning method, K-means, over other clustering methods because with K-means cluster the solution for more clusters is not constrained by solutions with less clusters. Because K-means clustering algorithm produces round clusters, it is critical to standardize data to improve good quality clusters and improve the accuracy of clustering algorithm (Mohamad and Usman, 2013). To identify an optimal number of clusters, we used a combination of: visual inspection of the scatterplots, a measure of cluster stability (Hubert and Arabie, 1985), prediction strength (Tibshirani and Walther, 2005), and clinical interpretability. Because the K-means clustering could depend on the initial clusters, we examined the stability of each cluster solution using the Adjusted Rand Index (ARI). The ARI examines the agreement among the solutions and it lies between 0 and 1, where 1 indicates that the two cluster partitions agree perfectly. Specifically we ran 10,000 K-means cluster analyses with this data set and examined the agreement among the solutions. The prediction strength method divides the data into a training set and a test set and then, computes the proportion of pairs of observation in the test cluster that are also assigned to the same cluster by the training set. Prediction strength starts at value 1 with one cluster and becomes smaller as more clusters are added. The optimal number of clusters “K” is considered as the largest K for which the prediction strength is around 0.8. Finally, we tested the significance of the cluster solution using the sigclust test (Liu et al., 2008). The null hypothesis of this test is that the data can be modeled as coming from one multivariate Gaussian distribution. Small p-values reject the null hypothesis and indicate that the cluster partition is significant such that the partition of the data is not an artifact of the sampling variation.
Subsequent analyses were conducted on the identified cognitive phenotype clusters. First, we examined cognitive profiles of clusters. Then, we examined the overlap between cognitive clusters and clinical diagnoses by examining cognitive profiles between diagnostic groups within clusters and cluster differences within a diagnosis. One-way ANOVAs were used to compare demographic and clinical features between subgroups. Repeated measures ANOVAs with cognitive phenotype as within-subject variable and subgroup as between-subject variable were employed to compare the phenotype profiles among subgroups. Finally, we examined whether phenotype composite scores are associated with indices of community functioning and, if so, whether this relationship differs across subgroups. Specifically, using a series of linear regression analyses, we entered phenotype composite scores in Step 1, entered dummy-coded subgroups in Step 2 and interaction between phenotype scores and subgroup in Step 3. For each phenotype composite score, any regression coefficient with p value less than 0.16 (i.e., 0.05/3) was considered statistically significance after controlling for multiple comparisons.
3. RESULTS
3.1. Cognitive phenotype clusters
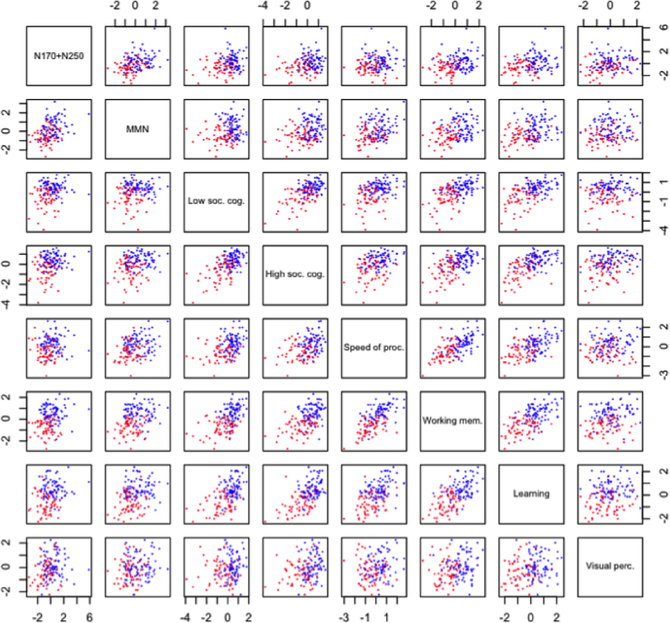
The K-means cluster analysis found a 2-cluster solution to provide the best separation between clusters and to be the most stable. See Figure 3 for the scatter plots. Regarding stability, the ARI of the 2-cluster solution was 1.0 compared with only 0.42 for a 3-cluster solution. Prediction strength of the 2-cluster solution was 0.76 compared with 0.47 or less for solution of 3 or more clusters. The 2-cluster solution was significance according to the sigclust test (p <0.001). To examine potential effects of missing data, we compared this 2-cluster solution with the cluster solutions with samples with compete data set. The ARI of these two solutions was 0.95, indicating high agreement between the solutions. Thus, it is unlikely that the 2-cluster solution was driven by imputation for missing data.
Figure 3.
Scatter plots of 2 cluster solutions from a K-means cluster analysis. The x-axis and y-axis of each graph indicates z scores. The High cluster is indicated in blue and the Low cluster is indicated in red. Phenotype composite scores are shown along the diagonal line.
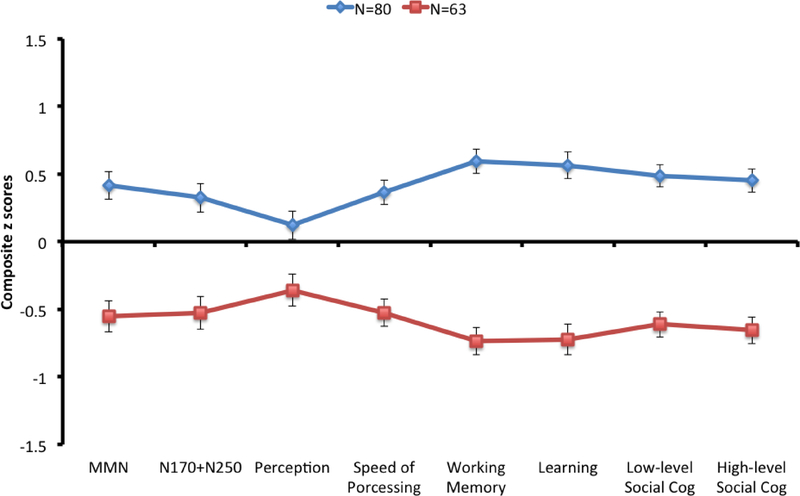
Figure 1 shows the phenotype profiles of the two clusters. There was a significant cluster effect (F1,141=213.16, p<.001, η2partial = .602, 95% CI=.501 - .674) and a significant cognition by cluster interaction (F7,987=4.02, p<.001, η2partial = .028, 95% CI=.006 - .044). A significant interaction indicates that two clusters did not show similar cognitive profile. There were clear group differences across all types of composite scores, with the smallest group differences on Visual Perception, which accounted for cognition by cluster interaction. We refer to the two phenotype clusters as High (N=80) and Low (N=63) based on the phenotype composite scores hereinafter.
Figure 1.
Phenotype profiles of two clusters. The y-axis indicates z scores and values are shown as means (standard error).
3.2. Phenotype clusters and clinical diagnosis
Next, we examined the distribution of cluster membership across clinical diagnoses. The High cluster consisted of 44 BD patients (65%), 5 SZ patients (13%) and 31 healthy controls (86%); the Low included 24 BD patients (35%), 34 SZ patients (87%) and 5 healthy controls (14%). A large majority of SZ patients and healthy controls were in the Low and High clusters, respectively, but BD patients were represented in both clusters. To further examine the relationship between clusters and clinical diagnoses, we focused on the following 4 subgroups: BD patients and healthy controls in the High cluster (BD-High and HC-High, respectively) and BD patients and SZ patients in the Low cluster (BD-Low and SZ-Low, respectively). We did not include the small number of SZ patients in the High cluster and small number of healthy controls in Low cluster in the subsequent analyses.
3.2.1. Demographic and clinical features
Demographic and clinical features and functioning measures of the subgroups can be seen in Table 1. For demographics, significant group differences were found for personal education (F3, 131=10.26, p<.001, η2partial = .073, 95% CI = .011 - .169) and parental education (F3, 128=2.98, p<.05, η2partial = .065, 95% CI = .000 −.144) but not for age or gender. The BD-High subgroup had higher parental education than the other 3 groups. The SZ-Low subgroup had lower personal education than the other 3 groups, which is not uncommon given that the onset of illness often interrupts formal education.
Table 1.
Demographic and clinical characteristics of diagnostic groups within clusters
| BD-High (N=44) | BD-Low (N=24) | SZ-Low (N=34) | HC-High (N=31) | Post-hoc comparisons | |
|---|---|---|---|---|---|
| Age | 43.2 (10.9) | 45.3 (10.1) | 44.3 (9.2) | 41.8 (9.9) | |
| Sex (% female) | 50 | 37.5 | 45.5 | 45.2 | |
| Personal Edu | 14.6 (2.4) | 13.4 (1.3) | 12.3 (2.4) | 13.4 (1.3) | BD-High = BD-Low = HC-High < SZ-Low |
| Parental Edu | 15.5 (2.9) | 13.4 (3.1) | 13.6 (3.5) | 13.4 (3.1) | BD-High > BD-Low = SZ-Low = HC-High |
| BPRS | 33.3 (7.1) | 33.5 (6.8) | 41.9 (9.0) | BD-High = BD-Low < SZ-Low | |
| YMRS | 3.1 (3.8) | 3.9 (4.2) | 6.0 (6.1) | BD-High = BD-Low < SZ-Low | |
| HAMD | 7.8 (6.2) | 8.4 (6.7) | 7.2 (4.8) | ||
| Age of onset | 17.4 (7.1) | 19.9 (5.5) | 20.2 (5.5) | ||
| UPSA | .87 (.06) | .84 (.08) | .73 (.11) | BD-High = BD-Low > SZ-Low | |
| MASC | 3.9 (.5) | 3.7 (.5) | 3.2 (.55) | BD-High = BD-Low > SZ-Low | |
| RFS Total | 19.7 (4.5) | 18.0 (4.6) | 13.3 (4.3) | BD-High = BD-Low > SZ-Low | |
| % BD I | 72.7 | 58.3 | χ2=1.47, p = .22 | ||
| % BD I with a history of psychosis | 28.1 | 42.9 | χ2=.96, p = .32 | ||
| % Antipsychotic Medication | 50 | 79.2 | χ2=5.51, p < .05 | ||
| % Lithium | 25 | 8.3 | χ2=2.79, p =.09 | ||
| % Euthymic | 84.1 | 62.5 | χ2=4.02, p < .05 | ||
| % Alcohol/Substance dependence | 40.5 | 62.5 | χ2=1.83, p =.60 | ||
| Duration of past manic episodes (months) | 22.1 (51.6) | 19.0 (24.8) | BD-High = BD-Low | ||
| Duration of past depressive episodes (months) | 39.1 (48.0) | 41.8 (90.0) | BD-High = BD-Low |
When comparing clinical features and functioning measures of the BD-High, BD-Low and SZ-Low subgroups, significant subgroup differences were found for YMRS (F2, 98=3.47, p<.05, η2partial = .066, 95% CI = .000 - .166), BPRS (F2, 96=13.16, p<.001, η2partial = .215, 95% CI = .078 - .339), MASC (F2, 89=15.36, p<.001, η2partial = .257, 95% CI = .105 - .384), UPSA (F2, 92=25.03, p<.001, η2partial = .352, 95% CI = .193 - .472), and RFS (F2, 95=18.65, p<.001, η2partial = .282, 95% CI = .131 - .405). Compared to the SZ-Low subgroup, the two BD subgroups showed lower scores on YMRS and BPRS, mainly due to ratings of psychotic symptoms, and lower scores on MASC, UPSA and RFS. The 3 subgroups did not differ on HAMD or age of onset.
The BD-High and BD-Low subgroups showed comparable levels of clinical symptoms and performance on functioning measures. We next examined more closely the two BD subgroups (see Table 1). Compared to the BD-Low subgroup, the BD-High subgroup had a higher proportion of euthymic patients than the BD-Low subgroup. The BD-High subgroup also had a smaller percentage of patients who were taking antipsychotic medication than the BD-Low subgroup, but BD patients in both subgroups were taking equivalent dosage of antipsychotic medication (Chlorpromazine equivalent, BD-High subgroup, 211.3 ± 170.5; BD-Low subgroup, 292.1 ± 216.6).
3.2.2. Phenotype profiles
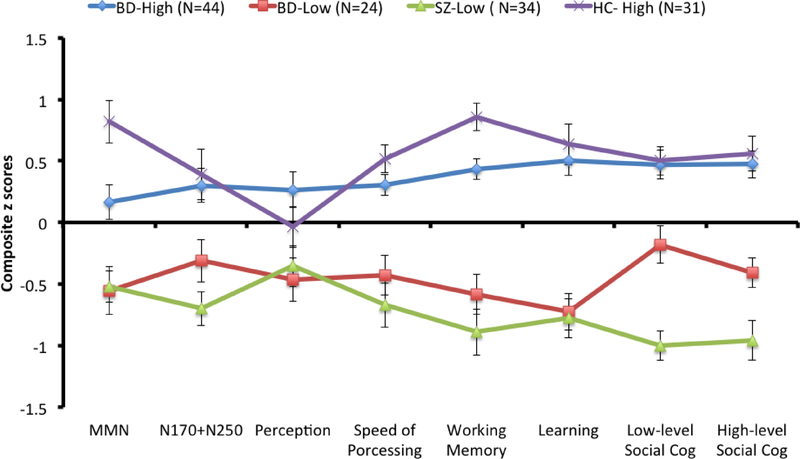
Figure 2 shows phenotype profiles of the 4 subgroups. Analyses showed a significant phenotype by subgroup interaction (F21, 903=2.80, p<.001, η2partial =.061, 95% CI = .016 - .071) and a significant subgroup effect (F3, 129=79.97, p<.001, η2partial = .650, 95% CI = .547 - .712). A significant phenotype by subgroup interaction indicates that the subgroups did not have similar cognitive profiles. To further determine which subgroup(s) had dissimilar cognitive profiles, we examined whether the two subgroups within each cluster (defined by diagnosis) differed from each other or whether the two subgroups across clusters (with the same diagnoses) differed from each other, using the following pairs of subgroups: BD-High with HC-High; SZ-Low and BD-Low; and BD-High with BD-Low.
Figure 2.
Phenotype profiles of diagnostic groups in the High and Low clusters. The y-axis indicates z scores. Values are shown as means (standard error).
For BD-High versus HC-High, there was a significant effect of phenotype (F7, 511=3.21, p<.01, η2partial = .042, 95% CI = .006 - .068) and a significant phenotype by subgroup interaction (F7, 511=2.4, p<.05, η2partial = .032, 95% CI = .001 - .054) that was largely due to significant subgroup differences on MMN and Working Memory. Both subgroups in the High cluster showed a different pattern of phenotype profiles, suggesting an influence of clinical diagnosis. Similarly, for BD-Low and SZ-Low subgroups, a subgroup effect (F1, 56=6.16, p<.05, η2partial = .099, 95% CI = .003 - .257) and a phenotype by subgroup interaction (F7, 392=2.09, p<.05, η2partial = .036, 95% CI = .000 - .062) were significant. The BD-Low subgroup performed significantly better on Low-level Social Cognition and High-level Social Cognition compared to SZ-Low subgroup. Finally, when comparing BD-High and BD-Low subgroups, only the subgroup effect was significant (F1, 66=96.92, p<.001, η2partial = .595, 95% CI = .436 - .693). BD-High subgroup performed better than BD-Low subgroup across all phenotypes but there was no significant interaction between phenotypes and subgroups. We also added parental education as a covariate to examine whether this subgroup effect could be explained by different levels of parental education and observed that findings did not change. Overall, the comparison of phenotype profiles using 4 subgroups showed that clinical diagnoses relate to distinct phenotype profiles, even when they are part of the same cluster. Further, patients with same diagnosis (i.e., BD) showed similar phenotype profiles.
3.2.3. Association between phenotype and functioning measures
Table 2 shows findings from linear regression analyses (see Supplement for results of correlation analyses between phenotype assessments and functioning measures and clinical symptoms). The relationship between Working Memory and UPSA was significantly different across subgroups such that Working Memory positively predicted UPSA only in the SZ-Low subgroup. Other phenotype scores did not predict any indices of community functioning differentially across subgroups.
Table 2.
Linear multiple regression analyses to examine associations between indices of community functioning and phenotype composite scores
| Step 1 | Step 2 a | Step 3 b | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictor | R2 | R2 | Δ R2 | R2 | Δ R2 | Unstandardized coefficient c | |||
| SZ-Low | BD-High | SZ-Low vs. BD-Low | |||||||
| MMN | UPSA | .01 | .35 | .33** | .36 | .01 | |||
| MASC | .02 | .26 | .23** | .27 | .01 | ||||
| RFS | .03 | .28 | .25** | .29 | .01 | ||||
| N170+N250 | UPSA | .03 | .36 | .32** | .42 | .05 | |||
| MASC | .01 | .28 | .27** | .30 | .01 | ||||
| RFS | .08** | .28 | .20** | .30 | .13 | ||||
| Percept | UPSA | .01 | .35 | .34** | .35 | .00 | |||
| MASC | .01 | .26 | .24** | .27 | .01 | ||||
| RFS | .03 | .28 | .25** | .29 | .01 | ||||
| Speed of Processing | UPSA | .13** | .37 | .24** | .42 | .04 | |||
| MASC | .06* | .26 | .20** | .26 | .00 | ||||
| RFS | .07** | .29 | .21** | .33 | .05 | ||||
| Working Memory | UPSA | .12** | .37 | .16** | .46 | .09** | .08** | .01** | −.03** |
| MASC | .10** | .26 | .16** | .27 | .01 | ||||
| RFS | .12** | .28 | .16** | .29 | .01 | ||||
| Learning | UPSA | .17** | .38 | .21** | .41 | .03 | |||
| MASC | .10** | .27 | .17** | .28 | .01 | ||||
| RFS | .04* | .29 | .24** | .29 | .00 | ||||
| Low-level Social Cognition | UPSA | .23** | .37 | .14** | .38 | .01 | |||
| MASC | .09** | .26 | .17** | .27 | .01 | ||||
| RFS | .14** | .29 | .14** | .29 | .00 | ||||
| High-level Social Cognition | UPSA | .28** | .40 | .12** | .42 | .02 | |||
| MASC | .09** | .51 | .16** | .52 | .01 | ||||
| RFS | .18** | .30 | .12** | .31 | .01 | ||||
Step2 included diagnostic groups that were represented as 2 dummy variables. The SZ-Low group was served as a reference group.
Step 3 included interaction between diagnostic groups and predictors and we present unstandardized coefficients for the interaction between each diagnostic group and a predictor.
Unstandardized coefficients are presented with p values from t-tests.
4. Discussion
With participants across 3 diagnostic groups, we identified two phenotype clusters that differed from each other both quantitatively by showing different levels of performance and qualitatively by having dissimilar profiles. The High cluster included 44 BD patients and a large majority of controls, and the Low cluster included 24 BD patients and a large majority of SZ patients. Notably, the High-cluster included 5 SZ patients, consistent with previous findings of a small number of SZ patients with preserved cognitive ability (Heinrichs et al., 2008; MacCabe et al., 2012). In both the High and Low clusters, participants with different clinical diagnoses showed dissimilar cognitive profiles. In contrast, BD patients from two phenotype clusters showed similar cognitive profile with different levels of performance. Thus, this study identified two phenotype clusters that are distinct from clinical diagnosis, but still found a subtle effect of diagnoses on phenotype profiles within clusters.
In this study, BD patients and SZ patients in the Low cluster had different cognitive profiles. Even though both subgroups showed similar levels of impairment on nonsocial cognitive assessments, the BD-Low subgroup showed better social cognitive performance than the SZ-Low subgroup, suggesting that social cognition is relatively preserved in this BD subgroup. This is consistent with previous studies reporting better social cognitive performance relative to nonsocial cognition in BD (Burdick et al., 2011; Burdick et al., 2014) and relatively more impaired social cognition than nonsocial cognition in SZ (Fanning et al., 2012). It is possible that brain regions related to social cognitive processes may function more aberrantly in the SZ-Low subgroup than in the BD-Low subgroup. Determining the underlying causes of impaired social cognition could guide us to disease process that may be unique to SZ versus BD (Lee and Green, 2016). Finally, although the BD-Low subgroup showed better social cognitive performance and better functioning than the SZ-Low subgroups, this study did not find any association between social cognitive performance and social functioning in the BD-Low subgroup. It is possible that a relatively small sample size in the BD-Low subgroup may contribute to non-significant association, and it remains to be determined the extent to which relatively preserved social cognition plays a role in everyday functioning in this subgroup.
This study identified two BD subgroups, one with higher phenotype scores (BD-High) and one with low phenotype scores (BD-Low). The BD-High subgroup showed phenotype composite scores comparable to those of controls on several phenotype measures. The presence of a subset of BD patients with intact cognition is consistent with previous findings (Altshuler et al., 2004; Burdick et al., 2014; Lewandowski et al., 2014). However, the BD-High subgroup also showed poorer performance than healthy controls on two phenotype composites: MMN and Working Memory. It is possible that neural regions that are associated with both MMN and Working Memory, such as the prefrontal cortex, could be more susceptible in BD than other brain regions (Chakalov et al., 2014; Gaebler et al., 2015; Ivleva et al., 2013; Wager and Smith, 2003).
When comparing the two BD subgroups, poorer performance of the BD-Low subgroup was not explained by a history of psychotic symptoms. However, the proportion of patients taking antipsychotic medication was significantly higher in the BD-Low subgroup. One possibility is that antipsychotic medication could have had a negative impact on cognition in BD. Consistently, several studies have reported that BD patients taking antipsychotic medication performed worse on cognitive tasks than BD patients not taking antipsychotics (Altshuler et al., 2004; Frangou et al., 2005; Palsson et al., 2013; Savitz et al., 2008; Torres et al., 2014). To further explore this question, we looked only within the High cluster and compared BD patients taking antipsychotic medications to those not taking the medications (50:50 split), and did not find any significant difference on their cognitive profiles (see supplemental table 1), though a small sample size could have affected the lack of group difference. Another possibility is that sicker patients with worse performance could have required treatments with antipsychotics. While the BD-Low and BD-High subgroups did not differ on duration of past manic or depressive episode, the BD-High subgroup had a higher percent of euthymic patients that the BD-Low subgroup. Further studies are needed to determine the mechanism through which antipsychotic medication affects cognition in BD remains to be determined.
This study has a few limitations. Both SZ and BD patients were in the chronic phase and it is not clear whether similar patterns of phenotype profiles could be observed in recent-onset patients. All patients were taking various types of psychotropic medication and it remains to be determined the potential effects of pharmacological treatments on phenotype profiles. Similarly, all patients were out of mood episode at the time of testing and it remains to be determined the extent to which current mood symptoms affect phenotype profiles of BD and SZ patients. Although we included two electrophysiological phenotypes, most of the phenotype assessments in this study were performance measures. It remains to be determined whether cognitive profiles observed in this study could have corresponding neural activation patterns. We did not exclude all the psychiatric comorbidities of SZ and BD (e.g., anxiety disorder) and this study cannot rule out the potential effects of psychiatric comorbidities on phenotype profiles. With smaller number of SZ patients compared to BD patients, this study may not be well suited to detect any subgroups within the SZ patients. Finally this study included both electrophysiological measures and performance measures. While this is one of unique features of this study, it should also be noted that higher amplitude of electrophysiological measures do not necessarily indicate better performance.
To summarize, using a wide range of cognitive assessments and a trans-diagnostic cluster analysis, this study identified two cognitive phenotype clusters across clinical diagnoses, a High cluster and a Low cluster. The two cognitive clusters were not likely to be a proxy for psychiatric diagnoses, consistent with the NIMH Research Domain Criteria (RDoC) project that focuses on dimensions cutting across traditional diagnostic categories (Cuthbert, 2014). A presence of cognitive profile across diagnoses could also guide us to examine underlying neural mechanisms that may overlap between clinical diagnoses. Finally, although phenotype clusters were identified regardless of clinical diagnoses, this study observed a subtle effect of clinical diagnoses on cognitive profiles within cluster.
Supplementary Material
Highlights.
This study aimed to identify subgroups of individuals with shared cognitive phenotype profiles using a cross-diagnostic cluster analysis.
Sixty-eight patients with bipolar disorder, 39 patients with schizophrenia and 36 healthy controls completed a battery of EEG and performance assessments on perception, nonsocial cognition and social cognition.
This study identified two clusters with shared cognitive phenotype profiles that were not proxies for clinical diagnoses.
The findings of this study suggest that relatively preserved social cognition may be important to identify pathophysiology distinct to each disorder.
Acknowledgement
The authors wish to thank Crystal Gibson, Christen Waldon, Amanda Bender, Mark R. McGee and Cory Tripp for assistance in data collection.
Financial support:
Support for this study came from National Institute of Mental Health Grant MH043292 and MH089634 (PI: Michael F. Green, PhD).
Conflict of interest
Dr. Green has consulted for AbbVie, Amgen, Forum, Lundbeck, and Takeda. He is on the scientific board for Mnemosyne and has received research grants from Amgen and Forum. Dr. Miklowitz receives book royalties from Guilford Press and John Wiley and Sons.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altshuler LL, Ventura J, van Gorp WG, Green MF, Theberge DC, Mintz J, 2004. Neurocognitive function in clinically stable men with bipolar I disorder or schizophrenia and normal control subjects. Biol. Psychiatry 56, 560–569. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Savic A, Repovs G, Yang G, McKay DR, Sprooten E, Knowles EE, Krystal JH, Pearlson GD, Glahn DC, 2015. Ventral anterior cingulate connectivity distinguished nonpsychotic bipolar illness from psychotic bipolar disorder and schizophrenia. Schizophr. Bull 41, 133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Yang G, Savic A, Murray JD, Cole MW, Repovs G, Pearlson GD, Glahn DC, 2014. Mediodorsal and visual thalamic connectivity differ in schizophrenia and bipolar disorder with and without psychosis history. Schizophr. Bull 40, 1227–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Sheffield JM, 2014. Cognitive impairments in psychotic disorders: common mechanisms and measurement. World Psychiatry 13, 224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellack AS, Sayers M, Mueser K, Bennett M, 1994. Evaluation of social problem solving in schizophrenia. J. Abnorm. Psychol 103, 371–378. [DOI] [PubMed] [Google Scholar]
- Blakewell M, Honaker J, King J, 2015. A unified approach to measurement error and missing data: overview and applications. Sociological Methods and Research, 1–39. [Google Scholar]
- Bora E, Pantelis C, 2015. Meta-analysis of Cognitive Impairment in First-episode Bipolar Disorder: Comparison with First-episode Schizophrenia and Healthy Controls. Schizophr. Bull. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Vahip S, Akdeniz F, Gonul AS, Eryavuz A, Ogut M, Alkan M, 2007. The effect of previous psychotic mood episodes on cognitive impairment in euthymic bipolar patients. Bipolar disorders 9, 468–477. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C, 2010. Neurocognitive markers of psychosis in bipolar disorder: a meta-analytic study. J. Affect. Disord 127, 1–9. [DOI] [PubMed] [Google Scholar]
- Bramon E, Sham PC, 2001. The common genetic liability between schizophrenia and bipolar disorder: a review. Current psychiatry reports 3, 332–337. [DOI] [PubMed] [Google Scholar]
- Brekke JS, Kay DD, Kee KS, Green MF, 2005. Biosocial pathways to functional outcome in schizophrenia. Schizophr. Res 80, 213–225. [DOI] [PubMed] [Google Scholar]
- Burdick KE, Goldberg TE, Cornblatt BA, Keefe RS, Gopin CB, Derosse P, Braga RJ, Malhotra AK, 2011. The MATRICS consensus cognitive battery in patients with bipolar I disorder. Neuropsychopharmacology 36, 1587–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick KE, Russo M, Frangou S, Mahon K, Braga RJ, Shanahan M, Malhotra AK, 2014. Empirical evidence for discrete neurocognitive subgroups in bipolar disorder: clinical implications. Psychol. Med 44, 3083–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton CZ, Vella L, Harvey PD, Patterson TL, Heaton RK, Twamley EW, 2013. Factor structure of the MATRICS Consensus Cognitive Battery (MCCB) in schizophrenia. Schizophr. Res 146, 244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardno AG, Owen MJ, 2014. Genetic relationships between schizophrenia, bipolar disorder, and schizoaffective disorder. Schizophr. Bull 40, 504–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakalov I, Paraskevopoulos E, Wollbrink A, Pantev C, 2014. Mismatch negativity to acoustical illusion of beat: how and where the change detection takes place? Neuroimage 100, 337–346. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, 2014. The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry 13, 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daros AR, Ruocco AC, Reilly JL, Harris MS, Sweeney JA, 2014. Facial emotion recognition in first-episode schizophrenia and bipolar disorder with psychosis. Schizophr. Res 153, 32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, 2004. Subtle Expression Training Tool (SETT) & Micro Expression Training Tool (METT), Paul Ekman ed. http://www.paulekman.com.
- Fanning JR, Bell MD, Fiszdon JM, 2012. Is it possible to have impaired neurocognition but good social cognition in schizophrenia? Schizophr. Res. 135, 68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin L, 1996. Structured Clinical Interview for DSM-IV Avis II Personality Disorders. Biometrics Research Department. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 1997. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition. Biometrics Research Department. [Google Scholar]
- Frangou S, Donaldson S, Hadjulis M, Landau S, Goldstein LH, 2005. The Maudsley Bipolar Disorder Project: executive dysfunction in bipolar disorder I and its clinical correlates. Biol. Psychiatry 58, 859–864. [DOI] [PubMed] [Google Scholar]
- Gaebler AJ, Mathiak K, Koten JW Jr., Konig AA, Koush Y, Weyer D, Depner C, Matentzoglu S, Edgar JC, Willmes K, Zvyagintsev M, 2015. Auditory mismatch impairments are characterized by core neural dysfunctions in schizophrenia. Brain 138, 1410–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Bearden CE, Barguil M, Barrett J, Reichenberg A, Bowden CL, Soares JC, Velligan DI, 2007. The neurocognitive signature of psychotic bipolar disorder. Biol. Psychiatry 62, 910–916. [DOI] [PubMed] [Google Scholar]
- Grozeva D, Kirov G, Ivanov D, Jones IR, Jones L, Green EK, St Clair DM, Young AH, Ferrier N, Farmer AE, McGuffin P, Holmans PA, Owen MJ, O’Donovan MC, Craddock N, 2010. Rare copy number variants: a point of rarity in genetic risk for bipolar disorder and schizophrenia. Arch. Gen. Psychiatry 67, 318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. Journal of Neurology Neurosurgery and Psychiatry 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PO, Lee J, Horan WP, Ochsner K, Green MF, 2011. Do patients with schizophrenia benefit from a self-referential memory bias? Schizophr. Res 127, 171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs RW, Miles AA, Smith D, Zargarian T, Vaz SM, Goldberg JO, Ammari N, 2008. Cognitive, clinical, and functional characteristics of verbally superior schizophrenia patients. Neuropsychology 22, 321–328. [DOI] [PubMed] [Google Scholar]
- Hill SK, Reilly JL, Keefe RS, Gold JM, Bishop JR, Gershon ES, Tamminga CA, Pearlson GD, Keshavan MS, Sweeney JA, 2013. Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Am. J. Psychiatry 170, 1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honaker J, King G, Blackwell M, 2011. Amelia II: A program for missing data. Journal of Statistical Software 45, 1–47. [Google Scholar]
- Horan WP, Kern RS, Shokat-Fadai K, Sergi MJ, Wynn JK, Green MF, 2009. Social cognitive skills training in schizophrenia: an initial efficacy study of stabilized outpatients. Schizophr. Res 107, 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert L, Arabie P, 1985. Comparing partitions. Journal of Classification 2, 193–218. [Google Scholar]
- Ivleva EI, Bidesi AS, Keshavan MS, Pearlson GD, Meda SA, Dodig D, Moates AF, Lu H, Francis AN, Tandon N, Schretlen DJ, Sweeney JA, Clementz BA, Tamminga CA, 2013. Gray matter volume as an intermediate phenotype for psychosis: Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am. J. Psychiatry 170, 1285–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahshan C, Wynn JK, Mathis KI, Altshuler LL, Glahn DC, Green MF, 2012. Cross-diagnostic comparison of duration mismatch negativity and P3a in bipolar disorder and schizophrenia. Bipolar disorders 14, 239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahshan C, Wynn JK, McCleery A, Glahn DC, Altshuler LL, Green MF, 2014. Cross-diagnostic comparison of visual processing in bipolar disorder and schizophrenia. J. Psychiatr. Res 51, 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern RS, Green MF, Fiske AP, Kee KS, Lee J, Sergi MJ, Horan WP, Subotnik KL, Sugar CA, Nuechterlein KH, 2009. Theory of mind deficits for processing counterfactual information in persons with chronic schizophrenia. Psychological Medicine 39, 645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koreen AR, Siris SG, Chakos M, Alvir J, Mayerhoff D, Lieberman J, 1993. Depression in first-episode schizophrenia. Am. J. Psychiatry 150, 1643–1648. [DOI] [PubMed] [Google Scholar]
- Lee J, Altshuler L, Glahn DC, Miklowitz DJ, Ochsner K, Green MF, 2013. Social and nonsocial cognition in bipolar disorder and schizophrenia: relative levels of impairment. Am. J. Psychiatry 170, 334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Green MF, 2016. Social Preference and Glutamatergic Dysfunction: Underappreciated Prerequisites for Social Dysfunction in Schizophrenia. Trends Neurosci. 39, 587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski KE, Sperry SH, Cohen BM, Ongur D, 2014. Cognitive variability in psychotic disorders: a cross-diagnostic cluster analysis. Psychol. Med 44, 3239–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Hayes DN, Nobel A, Marron JS, 2008. Statistical Significance of Clustering for High-Dimension, Low-Sample Size Data. J Am Stat Assoc 103, 1281–1293. [Google Scholar]
- MacCabe JH, Brebion G, Reichenberg A, Ganguly T, McKenna PJ, Murray RM, David AS, 2012. Superior intellectual ability in schizophrenia: neuropsychological characteristics. Neuropsychology 26, 181–190. [DOI] [PubMed] [Google Scholar]
- Maier W, 2008. Common risk genes for affective and schizophrenic psychoses. Eur. Arch. Psychiatry Clin. Neurosci 258 Suppl 2, 37–40. [DOI] [PubMed] [Google Scholar]
- Maier W, Zobel A, Wagner M, 2006. Schizophrenia and bipolar disorder: differences and overlaps. Curr Opin Psychiatry 19, 165–170. [DOI] [PubMed] [Google Scholar]
- Mancuso F, Horan WP, Kern RS, Green MF, 2011. Social cognition in psychosis: multidimensional structure, clinical correlates, and relationship with functional outcome. Schizophr. Res 125, 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald S, Flanagan S, Rollins J, 2002. The Awareness of Social Inference Test. Thames Valley Test Company Limited, Suffolk, England. [Google Scholar]
- McPheeters HL, 1984. Statewide mental health outcome evaluation: a perspective of two southern states. Community Ment. Health J 20, 44–55. [DOI] [PubMed] [Google Scholar]
- Mohamad IB, Usman D, 2013. Standardization and its effect on K-means clustering algorithm. Research Journal of Applied Science, Engineering and Technology 6, 3299–3303. [Google Scholar]
- Nuechterlein KH, Green MF, 2006. MATRICS Consensus Cognitive Battery. MATRICS Assessment, Inc., Los Angeles. [Google Scholar]
- Palsson E, Figueras C, Johansson AG, Ekman CJ, Hultman B, Ostlind J, Landen M, 2013. Neurocognitive function in bipolar disorder: a comparison between bipolar I and II disorder and matched controls. BMC Psychiatry 13, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson TL, Goldman S, McKibbin CL, Hughs T, Jeste DV, 2001. UCSD performance-based skills assessment: development of a new measure of everyday functioning for severely mentally ill adults. Schizophr. Bull 27, 235–245. [DOI] [PubMed] [Google Scholar]
- Ruocco AC, Reilly JL, Rubin LH, Daros AR, Gershon ES, Tamminga CA, Pearlson GD, Hill SK, Keshavan MS, Gur RC, Sweeney JA, 2014. Emotion recognition deficits in schizophrenia-spectrum disorders and psychotic bipolar disorder: Findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Schizophr. Res 158, 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Morla EM, Barabash A, Martinez-Vizcaino V, Tabares-Seisdedos R, Balanza-Martinez V, Cabranes-Diaz JA, Baca-Baldomero E, Gomez JL, 2009. Comparative study of neurocognitive function in euthymic bipolar patients and stabilized schizophrenic patients. Psychiatry Res. 169, 220–228. [DOI] [PubMed] [Google Scholar]
- Savitz JB, van der Merwe L, Stein DJ, Solms M, Ramesar RS, 2008. Neuropsychological task performance in bipolar spectrum illness: genetics, alcohol abuse, medication and childhood trauma. Bipolar disorders 10, 479–494. [DOI] [PubMed] [Google Scholar]
- Selva G, Salazar J, Balanza-Martinez V, Martinez-Aran A, Rubio C, Daban C, Sanchez-Moreno J, Vieta E, Tabares-Seisdedos R, 2007. Bipolar I patients with and without a history of psychotic symptoms: do they differ in their cognitive functioning? J. Psychiatr. Res 41, 265–272. [DOI] [PubMed] [Google Scholar]
- Shinn AK, Pfaff D, Young S, Lewandowski KE, Cohen BM, Ongur D, 2012. Auditory hallucinations in a cross-diagnostic sample of psychotic disorder patients: a descriptive, cross-sectional study. Compr. Psychiatry 53, 718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibshirani R, Walther G, 2005. Cluster validation by prediction strength. Journal of Computational and Graphical Statistics 14, 511–528. [Google Scholar]
- Torres IJ, Kozicky J, Popuri S, Bond DJ, Honer WG, Lam RW, Yatham LN, 2014. 12-month longitudinal cognitive functioning in patients recently diagnosed with bipolar disorder. Bipolar disorders 16, 159–171. [DOI] [PubMed] [Google Scholar]
- Ventura J, Lukoff D, Nuechterlein KH, Liberman RP, Green MF, Shaner A, 1993. Brief Psychiatric Rating Scale (BPRS) expanded version: scales, anchor points, and administration manual. Int. J. Methods Psychiatr. Res 3, 227–243. [Google Scholar]
- Wager TD, Smith EE, 2003. Neuroimaging studies of working memory: a meta-analysis. Cogn. Affect. Behav. Neurosci 3, 255–274. [DOI] [PubMed] [Google Scholar]
- Wynn JK, Jahshan C, Altshuler LL, Glahn DC, Green MF, 2013. Event-related potential examination of facial affect processing in bipolar disorder and schizophrenia. Psychol. Med 43, 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA, 1978. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry 133, 429–435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.