Abstract
Background
Optic neuropathy in the context of leukemia or lymphoma has a broad differential diagnosis, including infiltration, infection, inflammation, compression, and medication effects. Confirming the underlying etiology in a timely manner is crucial as, while infiltration carries a poor prognosis, treatment modalities can have serious consequences themselves.
Methods
A review of the literature was conducted for cases of isolated optic neuropathy in the context of leukemia or lymphoma, in which the underlying etiology remained unclear following initial clinical examination and neuroimaging. Clinical, radiological, and pathological characteristics of the cases are summarized.
Results
Ninety-two cases meeting inclusion criteria were identified. Leukemic or lymphomatous infiltration was the presumed diagnosis in 72% of the reports, indicating this is the most likely etiology in such cases. The remaining reports were attributed to inflammation, infection, or drug toxicity. For illustrative purposes, the previously unpublished case of an 11-year-old girl with remitted T lymphoblastic lymphoma is presented. She suffered recurrence in the form of isolated left optic nerve infiltration that required transconjunctival biopsy to confirm diagnosis.
Conclusions
Optic nerve infiltration by leukemia or lymphoma requires both diagnostic certainty and urgent management. Recommendations are made for a step-wise, yet rapid investigative approach that may ultimately require biopsy of the optic nerve.
Keywords: B-Cell, lymphoma, optic neuropathy, optic nerve neoplasms, T-cell lymphoma
Leukemic or lymphomatous infiltration of the optic nerve is a neuro-oncologic emergency, the management of which typically involves urgent initiation of therapy including corticosteroids and chemotherapy and/or radiation. Involvement of the optic nerve in hematologic malignancy is not uncommon, with autopsy studies demonstrating optic nerve infiltration in 18% and 16% of acute and chronic leukemias, respectively.1 Conversely, leukemic or lymphomatous infiltration comprises an estimated 5% of secondary optic nerve tumors.2 The optic nerve may be particularly susceptible to malignancy recurrence, as the posterior pole is believed to be a pharmacologic sanctuary, relatively unaffected by chemotherapy.3,4
Optic nerve infiltration usually occurs in concert with other neurologic or systemic signs and symptoms, and is only rarely the sole manifestation of a leukemic or lymphomatous process. In such cases, making a definitive diagnosis is challenging as the clinical presentation can mimic inflammatory, ischemic, toxic-metabolic, and compressive mechanisms of optic nerve injury. Confirming the diagnosis of optic nerve infiltration in a timely manner is often crucial to ensuring optimal patient outcomes. Delaying treatment may have severe consequences; however, the treatment modalities themselves may also carry serious risk.
In this paper we review the literature surrounding optic neuropathy in the context of leukemia or lymphoma with the aims of assessing the spectrum of possible etiologies of optic nerve dysfunction in the context of hematologic malignancy, and identifying any consistent patterns of clinical presentation, response to treatment, and outcome. Based on the results of the review, an approach to diagnosis of such presentations is given. We also present the case of a girl with T lymphoblastic lymphoma relapse taking the form of isolated optic neuropathy, in order to illustrate the potential diagnostic challenges and pitfalls.
Illustrative Case
An 11-year-old girl presented with 2 days of progressive vision loss in her left eye. She described blurry vision, pain with eye movement, and mild headache. On examination, she was afebrile. Best corrected visual acuity measured 20/20 (Snellen equivalent) in the right eye and count fingers at 2 feet in the left eye. Pupils were both reactive to light, and there was a large left relative afferent pupillary defect. Bedside visual field testing in the right eye was normal, whereas there was a dense central scotoma in the left eye. Funduscopic examination revealed a normal optic disc in the right eye and optic disc edema with venous tortuosity in the left eye.
Her past medical history was significant for a diagnosis of T lymphoblastic lymphoma initially presenting as an anterior mediastinal mass, 16 months previously. She had been in complete remission and on maintenance chemotherapy (oral methotrexate, mercaptopurine, vincristine and prednisone) for 12 months until 2 weeks prior to the onset of her visual symptoms. At that time, her chemotherapy had been discontinued due to an episode of prolonged neutropenia.
An enhanced CT scan showed thickening and enhancement of the left optic nerve (supplementary material). The serum WBC count was still low (2.9 × 109/L) and erythrocyte sedimentation rate was elevated (49 mm/hr). Serum hemoglobin, platelets, C reactive protein, uric acid, and lactate dehydrogenase were all normal. She was admitted to hospital and urgent brain MRI was arranged 12 hours later to rule out CNS disease. The MRI confirmed thickening of the left optic nerve, with increased T2 signal, restricted diffusion, and enhancement with gadolinium (Fig. 1). The only other abnormalities were nonspecific white matter changes in the frontal lobes, which were not enhancing and thought consistent with methotrexate-associated effects. There was no leptomeningeal or dural enhancement with gadolinium injection.
Fig. 1.
MRI brain 36 hours after onset of visual symptoms. Left optic nerve is thickened and enhanced on both axial (A) and coronal (B) images.
A cerebrospinal fluid (CSF) specimen showed protein, cell count, and glucose all within normal limits. The sample was negative for blasts and flow cytometry analysis was unremarkable. Bacterial culture, fungal culture, routine viral studies, and protein electrophoresis were also negative. Despite the absence of circulating blasts, flow cytometry was also performed on peripheral blood and showed a minute fraction (0.11%) of immature T-cells with a similar phenotype to those detected at initial diagnosis. Although concerning for disease recurrence, the low level minimal residual disease coupled with a negative CSF sample and the absence of signs of systemic relapse did not constitute a firm diagnosis of lymphoma relapse.
In that setting, the diagnosis of demyelinating optic neuritis was initially considered. For this reason, and in an effort to hasten visual recovery, the patient was initiated on a three day course of intravenous methylprednisolone (20 mg/kg/day), followed by a two week tapering oral regimen of prednisone during which time her left eye vision improved to 20/50.
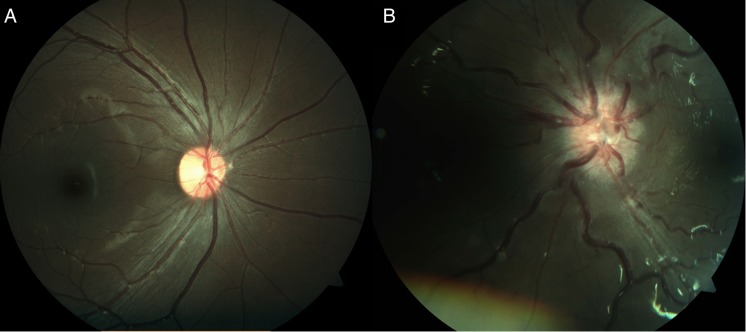
One week following the completion of her steroid course, the girl developed painless, recurrent vision loss in the left eye. Ophthalmologic examination now revealed elevated disc with flame hemorrhages, engorged retinal vessels, and dot and blot hemorrhages at the posterior pole (Fig. 2). There was also subtle choroidal mottling. Repeat MRI showed left optic nerve thickening and enhancement, now extending into the prechiasmal segment.
Fig. 2.
Fundus photographs taken at the time of first recurrence of optic neuropathy. The right eye (A) is normal, while the left (B) shows significant disc edema, engorgement of venous structures and hemorrhages.
Given the apparent disease progression on MRI, absence of pain, and the vision loss observed with cessation of steroids, lymphomatous infiltration causing optic neuropathy was an increasing concern although no sign of systemic relapse was present. CSF analysis for blasts, peripheral blood flow cytometry, and bone marrow biopsy were performed, none of which disclosed any sign of lymphomatous recurrence. Given the significant therapeutic implication of a T-cell lymphoma relapse, the need for a tissue biopsy to confirm or rule out relapse was discussed and biopsy was scheduled for 3 days later. Meanwhile the patient was initiated on a second course of corticosteroids (dexamethasone 0.4 mg/kg/day) to reverse her rapidly progressing visual loss. Within 72 hours, repeated MRI revealed significant reduction of the optic nerve infiltration rendering the probability of diagnostic biopsy unlikely. In addition, the girl's visual acuity recovered to 20/20 within a week of steroid initiation. At this point, a plan was made to stop the dexamethasone, monitor vision and imaging closely, and to undertake biopsy if there was any new sign of recurrence.
Three weeks after the cessation of steroid therapy, the girl again developed painless vision loss in the left eye. Repeated MRI showed findings similar to earlier studies, and the lesion was felt to be amenable to biopsy, which was performed via transconjunctival approach after discussion with both the neurosurgery and ophthalmology services. Microscopic examination confirmed lymphoblastic infiltration of the optic nerve with atypical lymphoid cells with a similar morphology to the original lesion (supplementary material). Most cells were positive for CD3, CD7, and CD8, with smaller numbers also positive for CD10 and CD99. Nuclear staining for TdT was seen in roughly 30% of cells, confirming a T lymphoblastic origin.
Following this diagnosis, reinduction chemotherapy was initiated with a protocol involving prednisone, vincristine, daunorubicin, PEG/asparaginase and aracytine, in addition to intrathecal therapy, with the plan to consolidate remission with allogeneic bone marrow transplantation. However 2 weeks into the reinduction protocol, the patient developed fulminant septic shock secondary to Klebsiella infection and died.
Search Strategy and Selection Criteria
Reports of isolated optic neuropathy in the context of leukemia or lymphoma in which initial examination and neuroimaging did not yield an obvious diagnosis were reviewed. A detailed explanation of inclusion and exclusion criteria is in supplementary material. References were identified via a search of Pubmed from 1966 to May 2015, for the term combinations ‘optic nerve and leukemia,’ ‘optic nerve and lymphoma,’ ‘optic neuropathy and leukemia,’ and ‘optic neuropathy and lymphoma,’ with additional papers identified from the reference lists of papers. Only English language papers were included.
Results
Ninety-two reports meeting inclusion/exclusion criteria were identified and included in the analysis, including 20 pediatric cases. Clinical signs, symptoms, and imaging findings for all cases are summarized in supplementary material with a complete reference list, grouped by the provisional diagnosis made by the authors of the paper. Clinical features of cases attributed to infiltration are summarized in Table 1.
Table 1.
Clinical features of infiltrative cases
| Etiology | Subtype | Percent of cases with Malignant Cells in CSF | Percent of cases with Optic Neuropathy as Initial Presentation | Mean number of Months between Malignancy Diagnosis and Optic Neuropathy (number of cases) | Mean number of Months between Optic Neuropathy and Death (number of cases) |
|---|---|---|---|---|---|
| Lymphoma Infiltration | Hodgkin's | 0 (0/2) | 100 (2/2) | 0 (2) | 5 (2) |
| B cell NHL | 43 (6/14) | 55 (11/20) | 7.0 (20) | 13 (7) | |
| T cell NHL | 100 (1/1) | 25 (1/4) | 11 (3) | 2.5 (2) | |
| Other | 50 (1/2) | 25 (1/4) | 1 (2) | 1 (1) | |
| Leukemia Infiltration | ALL | 57 (8/14) | 11 (2/19) | 18 (16) | 11 (7) |
| AML | 75 (3/4) | 0 (0/5) | 12 (4) | 1 ( 1) | |
| CLL | 83 (5/6) | 25 (2/8) | 59 (8) | 14 (3) | |
| CML | 0 (0/1) | 100 (4/4) | 0 (4) | 81 (2) | |
| Overall | 55 (24/44) | 35 (23/66) | 12 (79) | 16 (25) | |
Fractions given are based on the number of reports in which the information in question was available and reported. See supplementary material for complete reference list.
Abbreviations: NHL, non-Hodgkin's lymphoma; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia.
Leukemic Infiltration of the Optic Nerve
Thirty-six of the cases were attributed to leukemic infiltration. The majority of these (53%) were secondary to acute lymphoblastic leukemia (ALL), with the remainder associated with acute myeloid leukemia (AML; 14%), chronic lymphocytic leukemia (CLL; 22%), or chronic myelogenous leukemia (CML; 11%). Nine of the cases were pediatric, all of which were attributed to ALL. Malignant cells were identified in the CSF 64% of the time. Only 2 of the cases were proven with biopsy,5,6 and 1 case was diagnosed at autopsy.7 Neuroimaging (CT or MRI) was normal in 43% of cases. The remainder showed optic nerve thickening, with or without enhancement or T2 signal change.
Lymphomatous Infiltration of the Optic Nerve
Thirty cases were attributed to lymphomatous optic nerve infiltration. The underlying process was B cell non-Hodgkin's lymphoma (NHL) in the majority (67%), with T-cell NHL (13%), Hodgkin's lymphoma (7%), histiocytic lymphoma (7%), and undifferentiated/intermediary (7%) making up the remainder. There were only 2 pediatric cases, both classified as B cell lymphoma. Diagnosis was made by optic nerve biopsy in 12 cases and at autopsy in 4 more. Notably, 1 biopsy report noted that the optic nerve sheath was not infiltrated,8 demonstrating that biopsy of the nerve itself is required to confidently rule out lymphomatous infiltration.
The described clinical presentation was quite variable overall. Although disc edema with or without associated hemorrhage was commonly described, there were 4 reports describing normal fundi at the time of visual symptom onset. CSF contained malignant cells in only 42% of cases. CT scans showed optic nerve thickening in most, but were reported normal in 2 cases. On MRI, thickening and enhancement of the optic nerve(s) were the most common findings, though some reports commented on thickening in the absence of enhancement while others noted enhancement without thickening. Restricted diffusion was mentioned in only 1 report, likely due in part to the relative novelty of this technique.9 A single report included a positron emission tomography scan that showed increased uptake at the affected optic nerve.10
Medication and Radiation Effects on the Optic Nerve
Fifteen cases were attributed primarily to effects of medication and/or radiation. Of these, 14 were thought secondary to cancer treatments themselves, with 1 case attributed to an antibiotic (linezolid).11 Of the cases thought secondary to cancer treatments, 4 were attributed to vincristine toxicity12–15 and 1 attributed to each of arsenic,16 cyclosporin A,17 cytarabine,18 deferoxamine,19 imatinib,20 dasatinib,21 interferon alpha-2A,22 and methotrexate.23 The remaining 2 cases were more generally attributed to the combined effects of chemotherapy and radiation.24,25 Both the imatinib and methotrexate cases presented with associated disc edema. The only report with abnormal imaging was the case of presumed methotrexate-induced optic neuropathy in which MRI showed optic nerve thickening, T1 hypointensity, T2 hyperintensity, and no gadolinium enhancement.
Inflammatory
Optic neuritis was the provisional diagnosis in 6 cases. Only one case occurred following bone marrow transplant, however the authors specifically mentioned that they did not believe the presentation represented graft versus host disease.26 CT was normal in all 3 cases in which results were reported. MRI was reported in 3 cases; normal in 1 and showing thickening and enhancement in the other 2. None of the cases reported biopsy results, with 2 describing findings at autopsy; the first notes scattered lymphocytes and edema along the optic nerves27 and the second atrophy, myelin breakdown, and perivascular cuffing with mature lymphocytes.28
In most of the reported cases, ruling out infiltration or drug effects was difficult; however, 1 presentation in particular was suggestive of autoimmune inflammation as an independent mechanism.29 That report described a woman initially presenting with Sjogren's syndrome and found to be seropositive for anti-Ro and anti-La. Lymphoma was not diagnosed until 2 months later, at which time she declined treatment. Unilateral optic neuropathy developed 7 months later and showed clinical improvement with pulse methylprednisolone. In general, antibody panels were rarely sent in the reported cases, with the only other case being anti-Hu positive.30
Infection
Infection was the provisional diagnosis in 4 cases. Two of these were attributed to cytomegalovirus (CMV),31,32 and 1 each to syphilis33 and progressive multifocal leukoencephalopathy.34 Imaging studies were normal in all 4. Notably, the patient described by Bhatt et al31 initially had left eye optic neuropathy secondary to leukemic infiltration, a diagnosis supported by coincident development of blasts in the CSF, which resolved after radiation and chemotherapy. Nine months later the patient presented with almost identical symptoms and ophthalmologic examination, but the CSF had no abnormal cells and MRI did not show optic nerve thickening. The patient later developed retinitis, had CSF PCR positive for CMV, and showed clinical response to ganciclovir.
Discussion
Leukemic or lymphomatous optic nerve infiltration is a neuro-oncologic emergency, which often presents a clinical dilemma as other etiologies may be difficult to rule out. Autoimmune inflammation, infection and medication or radiation effects can manifest similar features at presentation, and may be clinically indistinguishable based on standard diagnostic techniques alone. The results of this review indicate that isolated infiltrative optic neuropathy most commonly occurs with ALL and B cell NHL, though the presentation has also been reported with AML, CML, CLL, T-cell NHL, Hodgkin's lymphoma, and histiocytic lymphoma. With respect to pediatric cases, ALL is by far the most common cause of isolated infiltration (75% of reports) with the few remaining reports associated with B cell NHL. Our illustrative case is the first pediatric report of isolated optic nerve infiltration with T-cell lymphoma. These proportions roughly match with known incidences of these hematologic malignancies in children,35 suggesting ALL and NHL have roughly equivalent risks of developing isolated optic nerve involvement in this age group.
Though leukemic or lymphomatous infiltration was the predominant presumed etiology of isolated optic neuropathy in this review (72% of cases), the presumptive diagnosis was not definitive in many of the published reports, and only a minority of cases included biopsy results. Our illustrative case demonstrates the diagnostic challenges of this presentation, as well as the importance of obtaining a definitive diagnosis. Aggressive chemotherapy with or without radiation carries significant risk, much greater than optic nerve biopsy, and should not be undertaken without clinical confidence that the infiltration diagnosis is correct.
With these considerations in mind, we propose a diagnostic approach for patients with a history of leukemia or lymphoma, presenting with optic neuropathy (supplementary material). An urgent ophthalmology consult is essential, in part because abnormalities in the globe can influence the differential diagnosis and change the order of subsequent investigations. Patients may also rapidly progress to a number of ophthalmologic complications, including central retinal artery occlusion, central retinal vein occlusion, neovascular glaucoma, and retinal detachment.36–38 If infiltration is obvious, treatment can be immediately initiated, potentially involving orbital radiation and/or induction chemotherapy with the goal of preserving vision.
MRI of the brain and orbits should be done as soon as possible, in order to rule out a compressive lesion and assess for disseminated disease. Clarifying whether the pathology is limited to the optic nerve sheath, versus the optic nerve proper, is also helpful in determining etiology and guiding further investigations, such as biopsy. Investigations to rule out CNS or systemic leukemic relapse should be undertaken as soon as possible as well.
For cases involving severe vision loss, optic nerve biopsy should be considered as soon as possible, in the event that preliminary testing has not yielded a clear diagnosis. Some may have reservations about what is perceived as an invasive procedure with significant potential morbidity; however, the consequences of missed or delayed diagnosis outweigh the procedural risks. Nevertheless, the decision to undertake this procedure should only be made after a multidisciplinary discussion involving at least neurology, neurosurgery, ophthalmology, hematology/oncology, and neuroradiology. For cases in which the intra-orbital portion of the nerve is involved, a transconjunctival approach has less risk than craniotomy,39 but the ultimate surgical decision should be made based on imaging in discussion with both ophthalmology and neurosurgery, as the intracranial portion of the nerve cannot be reached via a transconjunctival orbitotomy.
Appropriate timing of corticosteroid therapy is a challenging decision and will depend on the individual clinical scenario. This therapy should ideally not be initiated until all investigations, including a biopsy if necessary, have been completed, since corticosteroids frequently mask the clinical manifestations of hematologic malignancies, as demonstrated in our illustrative case. In addition, steroids are known to alter the pathological findings, risking false negative results that further delay a definitive diagnosis.40 The need for diagnostic precision must be balanced against the importance of long-term preservation of vision, and the timing of corticosteroid therapy decided based on clinical suspicion for specific etiologies, as well as the patient's degree of visual impairment.
Funding
This study did not receive any funding support.
Conflict of interest statement
None declared.
Supplementary Material
References
- 1. Kincaid MC, Green WR. Ocular and orbital involvement in leukemia. Surv Ophthalmol. 1983;27(4):211–232. [DOI] [PubMed] [Google Scholar]
- 2. Christmas NJ, Mead MD, Richardson EP, Albert DM. Secondary optic nerve tumors. Surv Ophthalmol. 1991;36(3):196–206. [DOI] [PubMed] [Google Scholar]
- 3. Killer HE, Jaggi GP, Flammer J, Miller NR, Huber AR, Mironov A. Cerebrospinal fluid dynamics between the intracranial and the subarachnoid space of the optic nerve. Is it always bidirectional? Brain. 2007;130(Pt 2):514–520. [DOI] [PubMed] [Google Scholar]
- 4. Ridgway EW, Jaffe N, Walton DS. Leukemic ophthalmopathy in children. Cancer. 1976;38(4):1744–1749. [DOI] [PubMed] [Google Scholar]
- 5. Townsend JH, Dubovy SR, Pasol J, Lam BL. Transient optic perineuritis as the initial presentation of central nervous system involvement by pre-B cell lymphocytic leukemia. J Neuroophthalmol. 2013;33(2):162–164. [DOI] [PubMed] [Google Scholar]
- 6. Howard RS, Duncombe AS, Owens C, Graham E. Compression of the optic chiasm due to a lymphoreticular malignancy. Postgrad Med J. 1987;63(746):1091–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Currie JN, Lessell S, Lessell IM, Weiss JS, Albert DM, Benson EM. Optic neuropathy in chronic lymphocytic leukemia. Arch Ophthalmol. 1988;106(5):654–660. [DOI] [PubMed] [Google Scholar]
- 8. Mavrikakis I, Heran MK, Rootman J. MR findings in a patient with isolated intrinsic optic nerve lymphoma. Ophthal Plast Reconstr Surg. 2006;22(6):482–484. [DOI] [PubMed] [Google Scholar]
- 9. Sudhakar P, Rodriguez FR, Trobe JD. MRI restricted diffusion in lymphomatous optic neuropathy. J Neuroophthalmol. 2011;31(4):306–309. [DOI] [PubMed] [Google Scholar]
- 10. Morita K, Yoshimi A, Masuda A, Ichikawa M, Yatomi Y, Kurokawa M. Unique association of Waldenstrom macroglobulinemia with optic neuritis and monoclonal T cell expansion. Int J Hematol. 2013;98(2):247–249. [DOI] [PubMed] [Google Scholar]
- 11. Joshi L, Taylor SR, Large O, Yacoub S, Lightman S. A case of optic neuropathy after short-term linezolid use in a patient with acute lymphocytic leukemia. Clin Infect Dis. 2009;48(7):e73–e74. [DOI] [PubMed] [Google Scholar]
- 12. Sanderson PA, Kuwabara T, Cogan DG. Optic neuropathy presumably caused by vincristine therapy. Am J Ophthalmol. 1976;81(2):146–150. [DOI] [PubMed] [Google Scholar]
- 13. Norton SW, Stockman JA 3rd. Unilateral optic neuropathy following vincristine chemotherapy. J Pediatr Ophthalmol Strabismus. 1979;16(3):190–193. [DOI] [PubMed] [Google Scholar]
- 14. Awidi AS. Blindness and vincristine. Ann Intern Med. 1980;93(5):781. [DOI] [PubMed] [Google Scholar]
- 15. Vecchi V, Maccolini E, Oddo Bravetti G, Guidelli Guidi S, Serra L. Transient optic neuropathy secondary to MOPP chemotherapy in Hodgkin's disease: report of a case. Tumori. 1984;70(6):571–573. [DOI] [PubMed] [Google Scholar]
- 16. Thery JC, Jardin F, Massy N, Massy J, Stamatoullas A, Tilly H. Optical neuropathy possibly related to arsenic during acute promyelocytic leukemia treatment. Leuk Lymphoma. 2008;49(1):168–170. [DOI] [PubMed] [Google Scholar]
- 17. Akagi T, Manabe S, Ishigooka H. A case of cyclosporine-induced optic neuropathy with a normal therapeutic level of cyclosporine. Jpn J Ophthalmol. 2010;54(1):102–104. [DOI] [PubMed] [Google Scholar]
- 18. Hoffman DL, Howard JR Jr., Sarma R, Riggs JE. Encephalopathy, myelopathy, optic neuropathy, and anosmia associated with intravenous cytosine arabinoside. Clin Neuropharmacol. 1993;16(3):258–262. [DOI] [PubMed] [Google Scholar]
- 19. Kaplinsky C, Stark B, Goshen Y, Yaniv I, Bashara S, Zaizov R. Deferoxamine (Desferal)-induced ocular toxicity. Pediatr Hematol Oncol. 1988;5(4):293–297. [DOI] [PubMed] [Google Scholar]
- 20. Govind Babu K, Attili VS, Bapsy PP, Anupama G. Imatinib-induced optic neuritis in a patient of chronic myeloid leukemia. Int Ophthalmol. 2007;27(1):43–44. [DOI] [PubMed] [Google Scholar]
- 21. Monge KS, Galvez-Ruiz A, Alvarez-Carron A, Quijada C, Matheu A. Optic neuropathy secondary to dasatinib in the treatment of a chronic myeloid leukemia case. Saudi J Ophthalmol. 2015;29(3):227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fuzzard DR, Mack HG, Symons RC. Bilateral retrobulbar optic neuropathy in the setting of interferon alpha-2a therapy. Case Rep Ophthalmol. 2014;5(2):270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iqbal Y, Palkar V, Al-Sudairy R, Al-Omari A, Abdullah MF, Al-Debasi T. Papilledema, presenting as reversible loss of vision, in a child with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2005;45(1):72–73. [DOI] [PubMed] [Google Scholar]
- 24. Fishman ML, Bean SC, Cogan DG. Optic atrophy following prophylactic chemotherapy and cranial radiation for acute lymphocytic leukemia. Am J Ophthalmol. 1976;82(4):571–576. [DOI] [PubMed] [Google Scholar]
- 25. Margileth DA, Poplack DG, Pizzo PA, Leventhal BG. Blindness during remission in two patients with acute lymphoblastic leukemia: a possible complication of multimodality therapy. Cancer. 1977;39(1):58–61. [DOI] [PubMed] [Google Scholar]
- 26. Ooi J, Takahashi S, Tajika K, Tojo A, Tani K, Asano S. Immune-mediated optic neuritis after unrelated allogeneic bone marrow transplantation. Blood. 1998;91(7):2619. [PubMed] [Google Scholar]
- 27. Holte H, Saeter G, Dahl IM, Abrahamsen AF. Progressive loss of vision in patients with high-grade non-Hodgkin's lymphoma. Cancer. 1987;60(10):2521–2523. [DOI] [PubMed] [Google Scholar]
- 28. Case records of the Massachusetts General Hospital. Weekly clinocopathological exercises. Case 52-1971. N Engl J Med. 1971;285(27):1526–1532. [DOI] [PubMed] [Google Scholar]
- 29. Soldevilla HF, Molina RM, Navarra SV. Breast lymphoma in Sjogren's syndrome complicated by acute monocular blindness. Int J Rheum Dis. 2010;13(2):164–170. [DOI] [PubMed] [Google Scholar]
- 30. Wirth MG, Bergamin O, Goede JS, Landau K. The value of white blood cell count in patients with swollen discs. Klin Monbl Augenheilkd. 2004;221(5):360–363. [DOI] [PubMed] [Google Scholar]
- 31. Bhatt UK, Gregory ME, Madi MS, Fraser M, Woodruff GH. Sequential leukemic infiltration and human herpesvirus optic neuropathy in acute lymphoblastic leukemia. J AAPOS. 2008;12(2):200–202. [DOI] [PubMed] [Google Scholar]
- 32. Marmor MF, Egbert PR, Egbert BM, Marmor JB. Optic nerve head involvement with cytomegalovirus in an adult with lymphoma. Arch Ophthalmol. 1978;96(7):1252–1254. [DOI] [PubMed] [Google Scholar]
- 33. Pless ML, Kroshinsky D, LaRocque RC, Buchbinder BR, Duncan LM. Case records of the Massachusetts General Hospital. Case 26-2010. A 54-year-old man with loss of vision and a rash. N Engl J Med. 2010;363(9):865–874. [DOI] [PubMed] [Google Scholar]
- 34. Zaman AG, Graham EM, Sanders MD. Anterior visual system involvement in non-Hodgkin's lymphoma. Br J Ophthalmol. 1993;77(3):184–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gurney JG, Severson RK, Davis S, Robison LL. Incidence of cancer in children in the United States. Sex-, race-, and 1-year age-specific rates by histologic type. Cancer. 1995;75(8):2186–2195. [DOI] [PubMed] [Google Scholar]
- 36. Shukla D, Arora A, Hadi KM, Kumar M, Baddela S, Kim R. Combined central retinal artery and vein occlusion secondary to systemic non-Hodgkin's lymphoma. Indian J Ophthalmol. 2006;54(3):204–206. [DOI] [PubMed] [Google Scholar]
- 37. Richards RD. Simulataneous occlusion of the central retinal artery and vein. Trans Am Ophthalmol Soc. 1979;77:191–209. [PMC free article] [PubMed] [Google Scholar]
- 38. Mateo J, Abarzuza R, Nunez E, Cristobal JA. [Bilateral optic nerve infiltration in acute lymphoblastic leukemia in remission]. Arch Soc Esp Oftalmol. 2007;82(3):167–170. [DOI] [PubMed] [Google Scholar]
- 39. Gunduz K, Catak E, Erden E. Optic nerve biopsy via a medial transconjunctival orbitotomy approach in the diagnosis of optic nerve and sheath tumors. Orbit. 2010;29(4):190–193. [DOI] [PubMed] [Google Scholar]
- 40. Borenstein SH, Gerstle T, Malkin D, Thorner P, Filler RM. The effects of prebiopsy corticosteroid treatment on the diagnosis of mediastinal lymphoma. J Pediatr Surg. 2000;35(6):973–976. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.