Abstract
Background
In patients with recurrent glioblastoma, the benefit of bevacizumab beyond progression remains uncertain. We prospectively evaluated continuing or ceasing bevacizumab in patients who progressed while on bevacizumab.
Methods
CABARET, a phase II study, initially randomized patients to bevacizumab with or without carboplatin (Part 1). At progression, eligible patients underwent a second randomization to continue or cease bevacizumab (Part 2). They could also receive additional chemotherapy regimens (carboplatin, temozolomide, or etoposide) or supportive care.
Results
Of 120 patients treated in Part 1, 48 (80% of the anticipated 60-patient sample size) continued to Part 2. Despite randomization, there were some imbalances in patient characteristics. The best response was stable disease in 7 (30%) patients who continued bevacizumab and 2 (8%) patients who stopped receiving bevacizumab. There were no radiological responses. Median progression-free survival was 1.8 vs 2.0 months (bevacizumab vs no bevacizumab; hazard ratio [HR], 1.08; 95% CI, .59–1.96; P = .81). Median overall survival was 3.4 vs 3.0 months (HR, .84; 95% CI, .47–1.50; P = .56 and HR .70; 95% CI .38–1.29; P = .25 after adjustment for baseline factors). Quality-of-life scores did not significantly differ between arms. While the maximum daily steroid dose was lower in the continuation arm, the difference was not statistically significant.
Conclusions
Patients who continued bevacizumab beyond disease progression did not have clear survival improvements, although the study was not powered to detect other than very large differences. While these data provide the only randomized evidence related to continuing bevacizumab beyond progression in recurrent glioblastoma, the small sample size precludes definitive conclusions and suggests this remains an open question.
Keywords: bevacizumab, carboplatin, glioblastoma, phase II trial
Glioblastoma has a universally poor prognosis, a high morbidity and mortality burden, and the highest average years lost for any tumor type.1–3 Management approaches for recurrent glioblastoma are limited. Bevacizumab, a humanized monoclonal antibody against vascular endothelial growth factor (VEGF) is commonly used, having received accelerated approval from the Food and Drug Administration (FDA) in 2009 4 on the basis of radiological response rates (up to 50%) and progression-free survival (PFS) (up to 9 months) in phase 2 trials.5–7 However, no phase 3 study has demonstrated an overall survival (OS) benefit. Most recently, the phase 3 EORTC 26101 study did not find an OS benefit when using bevacizumab in combination with lomustine over lomustine monotherapy, despite initial enthusiasm and a reported survival benefit from the phase 2 BELOB study.8,9 Bevacizumab use in the recurrent glioblastoma setting is not universal: the European Medicines Agency did not approve it, in part due to the absence of chemotherapy-alone comparative data at that time.10,11
The role of continuing bevacizumab beyond initial progression remains controversial. Some clinicians favor continuing bevacizumab on the basis of metastatic colorectal cancer studies indicating benefit beyond progression,12,13 and small retrospective glioblastoma studies have suggested that cessation may result in accelerated disease progression, rapid revascularization, and rebound edema.14–17 Conversely, a retrospective review of 54 glioblastoma patients reported a 6-month PFS of only 2% for patients continuing with a second bevacizumab-containing regimen.18
Despite a relative lack of prospective supportive data, the current National Comprehensive Cancer Network guidelines for recurrent glioblastoma specifically indicate support for continuing bevacizumab beyond progression.19 Reasonable preclinical rationale has existed for this practice. Although the drug’s exact mechanism of action in glioblastoma is poorly understood, its antiangiogenic effects are thought to contribute directly. These effects, as well as possible enhanced delivery of chemotherapy to the tumor site through vascular normalization, should in theory be maintained throughout different lines of chemotherapy.20 The antiedema effect of bevacizumab is also well documented.21 This, together with published benefits in administering the drug beyond progression in colorectal cancer12,13 and the lack of randomized data in the setting of glioblastoma, provided the rationale for this study.
The two-part, stratified, nonblinded, randomized phase 2 study, Carboplatin and Bevacizumab in Recurrent Glioblastoma (CABARET), was conducted in Australia. In Part 1 of CABARET, 120 patients were randomized to bevacizumab plus carboplatin or bevacizumab alone. The primary objective was to determine the effect of bevacizumab plus carboplatin, versus bevacizumab monotherapy on PFS using modified Response Assessment in Neuro-Oncology (RANO) criteria.22 Published outcomes showed no PFS or OS benefit from adding carboplatin to bevacizumab.23
We report here results from Part 2 of CABARET, in which, at progression, eligible patients were randomized to continue or cease bevacizumab in addition to a specified chemotherapy regimen or best supportive care. The objectives were to determine the effect of continuing or stopping bevacizumab after progression.
Methods
Patient Eligibility
Eligibility criteria for CABARET Part 1 have been previously described.23,24 In brief, consenting adults with recurrent glioblastoma after receiving radiotherapy and temozolomide, with no other prior chemotherapy for glioblastoma, and ECOG performance status 0−2, were eligible. Prior recurrences were not exclusionary, provided no chemotherapy other than temozolomide had been used (prior 5-day or metronomic schedules permitted).
Progressive disease during Part 1 was determined by site investigators and could be either clinical or radiological, as per RANO criteria.22 Following progression on Part 1, eligible and consenting patients were randomized. Eligibility criteria for entry onto Part 2 included: patients with RANO-defined (radiological or clinical) progression on Part 1 of CABARET; considered appropriate by the site investigator to undergo further active therapy; had not withdrawn from bevacizumab during Part 1 due to toxicity; and did not have contraindications to the ongoing use of bevacizumab. Reasons for patients not continuing on to Part 2 were documented (Supplementary Table 1).
Part 2 Study Design
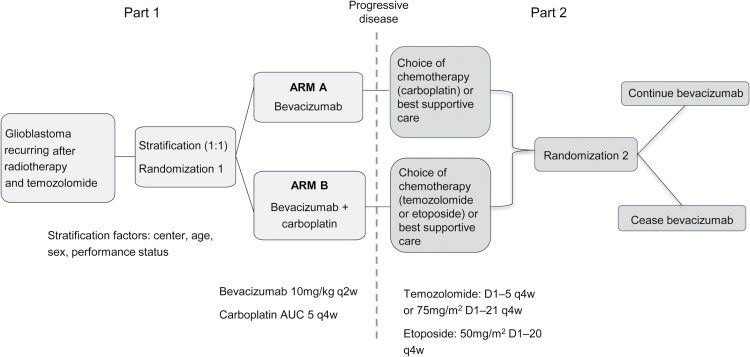
The study design is outlined in Fig. 1. Following progression on Part 1 and prior to Part 2 randomization, patients continuing to Part 2 could elect to receive specified chemotherapy or best supportive care without chemotherapy at clinician discretion in consultation with the patient. After this decision had been recorded, eligible patients were then randomized 1:1 to continue bevacizumab 10 mg/kg intravenously 2-weekly, or to cease bevacizumab. Part 2 randomization was stratified by center, age, sex, performance status, and Part 1 treatment allocation. The randomization occurred after the decision about additional therapy in order to avoid bias from the possibility of patients subsequently choosing not to remain on trial if they chose no chemotherapy and were randomized to receive no bevacizumab.
Fig. 1.
Study design (Parts 1 and 2)
Patients who had received bevacizumab monotherapy in Part 1 were treated with either carboplatin (AUC 5) 4-weekly, or best supportive care without chemotherapy. Patients who had received bevacizumab plus carboplatin in Part 1 could receive: etoposide (50 mg/m2 daily for 20 days every 28 days); temozolomide (150−200 mg/m2 daily for 5 days every 28 days, or 75 mg/m2 daily for 20 days every 28 days), or best supportive care without chemotherapy. These drug choices were limited by safety recommendations from Roche: availability of safety data was required for use of these agents in combination with bevacizumab, and at the time data existed to support the use of temozolomide rechallenge or etoposide in this setting.25,26 Additional limitations included unavailability of lomustine and irinotecan on the Australian Pharmaceutical Benefits Scheme for this indication at the time of study design, and the safety and efficacy of bevacizumab in combination with lomustine had not been reported at the time of study design.
Supportive care was permitted throughout for all patients including concomitant antibiotics, analgesics, corticosteroids, transfusions, and other necessary symptomatic therapy, except other investigational antitumor agents, chemotherapy, hormonal therapy, or immunotherapy.
Part 2 Outcomes
The primary outcome was median PFS. Response rate, OS, health-related quality of life (QOL), cognitive function, corticosteroid dose, and toxicities were also assessed.
Dose Modification
The toxicity criteria for discontinuing or suspending treatment have been previously reported.23 To summarize: no bevacizumab dose reductions were permitted, but bevacizumab was discontinued for clinically relevant central nervous system hemorrhage, nephrotic syndrome, or grade 4 hypertension. The causative drug was discontinued for any grade 3 or 4 hypersensitivity reaction. Any drug could be delayed for up to 8 weeks for lesser adverse events. In Part 2, modifications to the specified chemotherapy regimen were at clinician discretion.
Response Evaluation
Response evaluation was determined by magnetic resonance imaging (MRI), clinical status, and steroid dosing, according to RANO criteria22 and incorporated a modification using a 5-point scale to define the extent of T2/FLAIR signal abnormality (modified RANO criteria).23 PFS for Part 2 was defined as the time between date of progression on Part 1 treatment determined at the trial site (using RANO criteria) and the date of disease progression on Part 2 treatment as determined by central radiology review, or death from any cause. Patients were censored at commencement of any other anticancer therapy or if alive and progression-free at last assessment. Overall survival for Part 2 was calculated from the date of Part 1 site-determined progression to date of death from any cause. The date of progression on Part 1 of the study served as the baseline for patients who participated in Part 2 as in clinical practice this is also likely to be the date at which decisions about further treatment are made, and thus most relevant to comparing survival between alternative treatments. Response rates and progression were determined by central radiology review according to modified RANO criteria.23
Precontrast and postcontrast T1 and T2/FLAIR MRI were performed 8-weekly during Part 2, the baseline MRI being defined as the MRI that confirmed Part 1 progression. MRIs were reviewed both by site investigators and centrally. The radiological and clinical assessment of progression at the trial site was used to make decisions about study treatment continuation or cessation. Central radiology review was used for reporting trial endpoints.
Clinical assessments, including physical examination and neurocognitive and QOL assessments, were performed 4-weekly. Following Part 2 progression, monthly follow-up assessments were conducted until the patient died or withdrew from the trial.
Safety
Adverse events were classified and graded using National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0,27 and safety data were collected until at least 30 days after the last study treatment. The protocol was approved by all relevant human research ethics committees. Written informed consent was required before any study procedure commencement. Cooperative Trials Group for Neuro-Oncology (COGNO), coordinated at the National Health and Medical Research Council (NHMRC) Clinical Trials Centre, University of Sydney, was responsible for collection, maintenance, integrity, and confidentiality of all data. The trial management committee was responsible for study conduct. An independent data safety monitoring committee monitored safety aspects. Roche Products Pty Limited (Australia) provided trial funding and bevacizumab, but was not involved in data monitoring, analysis, or manuscript preparation.
Statistical Design and Analysis
We compared two arms: patients randomized to continue bevacizumab versus patients randomized to cease bevacizumab, irrespective of additional treatment received. It was anticipated that approximately 60 patients (50 percent of the Part 1 cohort) would be randomized to Part 2, and as such, Part 2 was powered to detect only a large difference between arms. A sample of 30 patients per group would have provided 80% power to detect a large difference in survival (hazard ratio [HR] of 2, median survival 2 versus 4 months) and a one-sided alpha of 5%. At the time of study design in early 2010, retrospective data reported a median PFS of 2 months for 19 patients with recurrent glioblastoma who discontinued bevacizumab and received other salvage therapies.28
For safety assessments, all patients, except one who withdrew prior to receiving any Part 2 treatment, were included. Efficacy assessments comparing continuing versus ceasing bevacizumab included the intention-to-treat (ITT) population of all randomized patients, using the Kaplan-Meier method to estimate median survival and 6-month survival percentage and proportional hazards regression to calculate the HR. The best responses in each patient were compared between treatments using a chi-square test. In addition, a Cox regression analysis of treatment effect on survival was undertaken with adjustment for key baseline factors: age, sex, performance status, prior recurrence, and planned chemotherapy regimen, as a sensitivity analysis.
The EORTC QLQ-C30 social functioning, overall QOL, role functioning, physical functioning, cognitive functioning, and fatigue, drowsiness, communication deficit and motor dysfunction symptom scales were compared between arms. QOL is reported as time to deterioration, measured as time between the end of Part 1 and first recorded deterioration on the global QOL scale. Deterioration was defined as a decline of ≥10 points on a 0−100 scale persisting for 2 consecutive assessments; or a single decline of ≥10 points on a 0−100 scale where further measurements were not obtained because of progression or death; disease progression on central review without QOL decline or completion; or inability to complete assessments because of neurological deterioration or death. The median and interquartile range (IQR) of time to deterioration was calculated using the Kaplan-Meier method, and the HR for deterioration was calculated using proportional hazards regression.
Mean and maximum daily steroid doses in the first 30 days of treatment were calculated for each patient; the mean calculated by summing total steroid intake divided by 30 or the number of days the patient was on Part 2 treatment, whichever was lower. The medians of these values were compared between arms using the Wilcoxon rank-sum test.
Results
Baseline Characteristics
Characteristics of patients who continued to Part 2 are shown in Table 1. In total, 48 patients (80% of the anticipated n = 60 from Part 1) were recruited to Part 2. There were some differences between the two arms with respect to number of relapses at the time of Part 1 recruitment, with 48% (n = 11) of patients randomized to continue bevacizumab having more than one recurrence, compared with 20% (n = 5) of those randomized to cease bevacizumab (P = .05). Patients with ECOG performance status of 2 and 3 were equally balanced between arms, but with a higher proportion of patients with undocumented status in the bevacizumab continuation arm.
Table 1.
Baseline characteristics of patients participating in Part 2 of the CABARET trial
| Characteristic | Value | Continued bevacizumab (n = 23) | Ceased bevacizumab (n = 25) | Did not continue to Part 2 (n = 74) |
|---|---|---|---|---|
| Age (years) at Part 2 randomization | 50 (30–70) | 54 (34–74) | 57 (25–82) | |
| Sex | Female | 9 (39%) | 12 (48%) | 34 (46%) |
| Male | 14 (61%) | 13 (52%) | 40 (54%) | |
| ECOG performance status at Part 2 baseline/end Part 1 | ||||
| 0/1 | 8 (35%) | 12 (48%) | 13 (18%) | |
| 2 | 7 (30%) | 8 (32%) | 12 (16%) | |
| 3 | 3 (13%) | 3 (12%) | 10 (14%) | |
| 4 | 0 | 0 | 5 (7%) | |
| Not recorded | 5 (22%) | 2 (8%) | 34 (46%) | |
| Time on CABARET Part 1 (median months, range) | 3.6 (1.3–12.9) | 4.1 (1.7–20.3) | ||
| Reasons for Part 1 progression | T1 MRI changes | 4 (17%) | 2 (8%) | NA |
| T2 MRI changes | 1 (4%) | 3 (12%) | ||
| New lesion on MRI | 2 (9%) | 1 (4%) | ||
| Clinical only | 6 (26%) | 4 (16%) | ||
| Combination of above | 10 (43%) | 15 (60%) | ||
| Prior diagnosis of grade I−III astrocytoma, oligoastrocytoma, or oligodendroglioma | No | 19 (83%) | 21 (84%) | 66 (89%) |
| Yes | 4 (17%) | 4 (16%) | 8 (11%) | |
| Relapse at time of Part 1 recruitment | First | 12 (52%) | 19 (76%) | 49 (66%) |
| Second or more | 11 (48%) | 5 (20%) | 24 (32%) | |
| Unknown | 0 (0%) | 1 (4%) | 1 (1%) | |
| Initial surgery | Biopsy | 2 (9%) | 3 (12%) | 10 (14%) |
| Debulking | 6 (26%) | 8 (32%) | 23 (31%) | |
| Resection | 15 (65%) | 14 (56%) | 41 (55%) | |
| Surgery for recurrent disease | Unknown | 0 (0%) | 1 (4%) | 1 (1%) |
| No | 11 (48%) | 13 (52%) | 42 (57%) | |
| Yes | 12 (52%) | 11 (44%) | 31 (42%) | |
| Corticosteroid use at Part 2 randomization | No | 3 (13%) | 4 (16%) | NA |
| Yes | 20 (87%) | 21 (84%) | NA | |
| Cytotoxic drug selected for Part 2 | Carboplatin | 13 (57%) | 13 (52%) | NA |
| Temozolomide | 2 (9%) | 6 (24%) | ||
| Etoposide | 7 (30%) | 5 (20%) | ||
| No chemotherapy | 1 (4%) | 1 (4%) |
Characteristics of patients who did not continue to Part 2 are shown in Table 1 for comparison. These patients tended to be older, with a poorer performance status and a shorter OS (median 2.0 versus 3.3 months for those randomized). The most common reasons for decisions not to continue onto Part 2 were that the patient was not considered appropriate to receive further chemotherapy or bevacizumab (n = 34); and patient preference (n = 14). Other reasons included death while on Part 1 (n = 5); surgery for recurrence (n = 4); and withdrawal from bevacizumab during Part 1 (n = 4) (Supplementary Table 1). Supplementary Table 2 shows the treatment received after Part 1 for patients who did not continue to Part 2, and for those who had treatment beyond Part 2.
Treatment
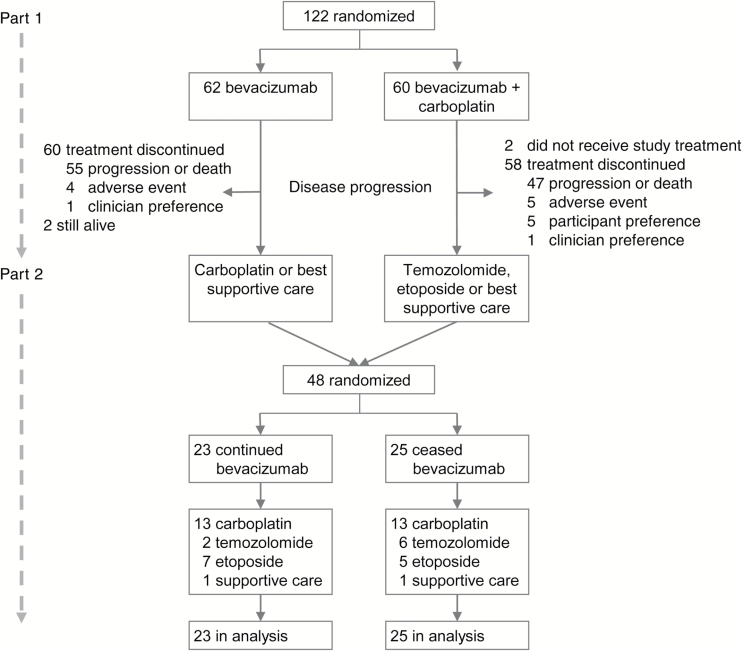
Forty-eight of the 120 patients who received at least one dose of study treatment in Part 1 were randomized to Part 2 between July 2011 and September 2013, from 15 of the 18 participating sites (Fig. 2). Of these, 48% were randomized to continuing bevacizumab and 52% to ceasing bevacizumab. Two patients were still receiving treatment on Part 1 at the time of study closure, so were not eligible to participate in Part 2, and received ongoing compassionate access to bevacizumab. The median time from discontinuing Part 1 to randomization to Part 2 was 2.5 days. Chemotherapy choices for both arms were similar (Table 1). One patient randomized to cease bevacizumab chose to discontinue participation, dying 24 days after Part 2 randomization, and is included in the ITT analysis.
Fig. 2.
CONSORT diagram
Median time on study was 1.5 months for bevacizumab continuation and 1.1 months for bevacizumab cessation. The mean number of bevacizumab doses was 3 (range, 1−14). Reasons for ceasing Part 2 treatment (site-determined) were progression (65%, n = 31), patient choice (23%, n = 11), clinician preference (6%, n = 3), death from cancer (4%, n = 2), and adverse event (2%, n = 1; grade 2 elevated alanine transaminase).
Efficacy
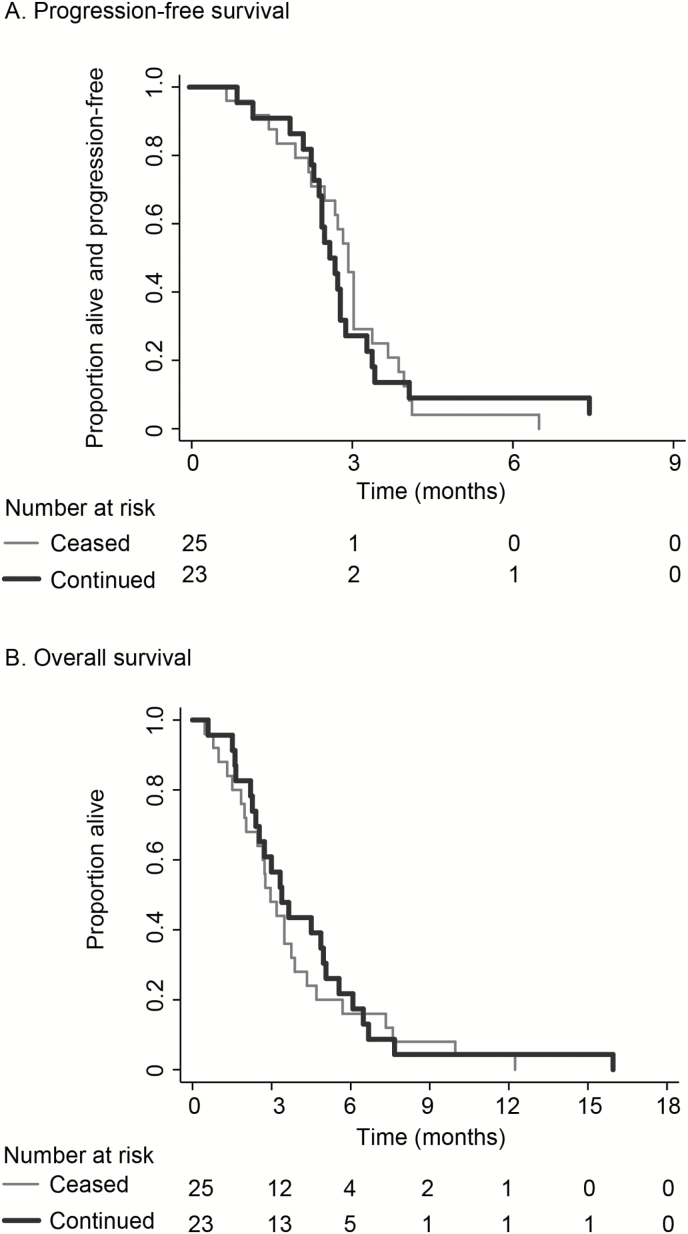
No patients had an objective response, as determined by central radiology review. The best response by site review was stable disease in 30% (n = 7) of those continuing bevacizumab and 8% (n = 2) of those ceasing (P = .047). Only 30 of 48 patients (63%) underwent MRI on Part 2; thus progression was determined clinically for a substantial proportion. There was no difference in median PFS between arms: 1.8 months in the continuation arm and 2.0 months in the cessation arm (HR, 1.08; 95% CI, .59−1.96; P = .81) (Fig. 3a). Six-month PFS was 5% (n = 1) for bevacizumab continuation and 0% for cessation.
Fig. 3.
Kaplan-Meier estimates of A) progression-free survival and B) overall survival in patients continuing versus ceasing bevacizumab treatment after disease progression
Progression was determined clinically or as death without documented radiological progression in 22 of 48 participants: 9 (39%) in the continuation arm and 13 (52%) in the cessation arm. In the remainder, a combination of radiological and clinical progression was seen, with no clear differences between arms, and specifically no abundance of nonenhancing T2 progression in the bevacizumab cessation arm (Table 2).
Table 2.
Reason for progression
| Reason(s) | Continued bevacizumab (n = 23) | Ceased bevacizumab (n = 25) |
|---|---|---|
| T1 + T2 + clinical | 2 (9%) | 4 (16%) |
| clinical only; MRI completed but not showing progression | 3 (13%) | 1 (4 %) |
| T1 + T2 + new lesion + clinical | 1 (4%) | 1 (4%) |
| T1 + T2 + new lesion | 0 | 2 (8%) |
| T1 + T2 | 2 (9%) | 0 |
| T2 + new lesion + clinical | 1 (4%) | 1 (4%) |
| T2 + clinical | 1 (4%) | 1 (4%) |
| T1 + new lesion + clinical | 1 (4%) | 0 |
| T1 + new lesion | 1 (4%) | 0 |
| T1 + clinical | 1 (4%) | 0 |
| T2 only | 0 | 1 (4%) |
| Clinical deterioration or death without MRI completed to confirm progression | 9 (39%) | 13 (52%) |
| Censored* | 1 (4%) | 1 (4%) |
* Commenced other anticancer treatment
Median OS was 3.4 months (bevacizumab continuation) versus 3.0 months (bevacizumab cessation) (HR, .84; 95% CI, .47−1.50; P = .56) (Fig. 3b). After adjustment for baseline factors, HR, .70; 95% CI .38–1.29; P = .25. Four patients who participated in Part 2 died within 1 month of completing Part 1; 3 in the cessation arm and 1 in the continuation arm. All 4 patients were reported as dying from cancer but none were able to undergo MRI showing RANO progression. These patients are included in all analyses.
Exploratory sensitivity analyses using date of randomization as the baseline date instead of date of progression on Part 1 to calculate PFS and OS also did not result in differences in survival times between arms (data not shown).
Median survival for the cohort of 48 patients who continued to Part 2 was 3.3 months (95% CI, 2.5–3.9) and median survival for the 64 patients who did not go on to Part 2 was 2.0 months (95% CI, 1.4–2.9).
Safety
Treatment-related adverse events are shown in Table 3. There were no unexpected toxicities and no grade 5 toxicities. Ten patients (43%) who continued and 8 (33%) who ceased bevacizumab experienced grade 3 or 4 toxicities. The most common toxicities in both arms were hypertension and fatigue. Specific bevacizumab-related toxicities, including bleeding, deep-vein thrombosis, proteinuria, and hypertension, occurred more frequently in the continuation arm and were uncommon, with the exception of low-grade hypertension, in both arms.
Table 3.
Toxicity
| Adverse event | Grade | Continued bevacizumab (n = 23) | Ceased bevacizumab (n = 24*) |
|---|---|---|---|
| Anemia | All grades | 1 (4%) | 3 (13%) |
| Grade ≥3 | 0 (0%) | 0 (0%) | |
| Febrile neutropenia | All grades | 0 (0%) | 0 (0%) |
| Grade ≥3 | 0 (0%) | 0 (0%) | |
| Neutropenia (without fever) | All grades | 3 (13%) | 2 (8%) |
| Grade ≥3 | 1 (4%) | 1 (4%) | |
| Thrombocytopenia | All grades | 7 (30%) | 2 (8%) |
| Grade ≥3 | 2 (9%) | 1 (4%) | |
| Nausea and vomiting | All grades | 7 (30%) | 8 (33%) |
| Grade ≥3 | 1 (4%) | 0 (0%) | |
| Diarrhoea | All grades | 3 (13%) | 0 (0%) |
| Grade ≥3 | 0 (0%) | 0 (0%) | |
| Constipation | All grades | 6 (26%) | 4 (17%) |
| Grade ≥3 | 0 (0%) | 0 (0%) | |
| Fatigue | All grades | 20 (87%) | 13 (54%) |
| Grade ≥3 | 2 (9%) | 3 (13%) | |
| Central nervous system hemorrhage | All grades | 1 (4%) | 0 (0%) |
| Grade ≥3 | 0 (0%) | 0 (0%) | |
| Bleeding | All grades | 3 (13%) | 1 (4%) |
| Grade ≥3 | 0 (0%) | 0 (0%) | |
| Deep-vein thrombosis | All grades | 1 (4%) | 0 (0%) |
| Grade ≥3 | 0 (0%) | 0 (0%) | |
| Proteinuria | All grades | 1 (4%) | 0 (0%) |
| Grade ≥3 | 0 | 0 | |
| Hypertension | All grades | 19 (83%) | 17 (71%) |
| Grade ≥3 | 1 (4%) | 0 | |
| Abscess | All grades | 1 (4%) | 0 |
| Grade >=3 | 0 | 0 |
* One patient declined to participate after randomization and no further toxicity data are available.
Quality of Life
Some patients in Part 2 did not complete QOL questionnaires beyond baseline, largely because they were too unwell. Completion rates in both arms were similar, with 37 (77%) completing QOL assessment at Part 2 baseline, 24/28 (86%) at 4 weeks, 8/8 (100%) at 8 weeks, and 4/4 (100%) at 12 weeks. The median (IQR) time to deterioration in overall QOL for patients who continued bevacizumab was 1.15 (.89–1.64) months, and 1.64 (.85–2.04) months for those who ceased bevacizumab (HR = 1.25 for the continuation arm relative to the cessation arm, 95% CI, .70–2.24, P = .45). This takes into consideration all patients on study including those who did not complete questionnaires beyond baseline (which was classified as a deterioration for the purposes of the trial). Median times were similar, and there were no clinically or statistically significant differences between arms in any of the subscales tested. For most patients, QOL deterioration was attributed to not completing QOL tools because of neurological deterioration, progression, or death (as specified as a determinant of QOL deterioration) rather than to completed QOL questionnaires having a decrease of 10 points or more.
Steroid Dosing
Mean daily steroid doses in the first 30 days of treatment were similar in both arms, ranging from 0 to 16 mg/day for patients in the continuation arm and 0 to 18 mg/day in the cessation arm (median 4 mg/day in both arms, P = .6). Maximum daily dose per patient ranged from 0 to 16 mg/day in the continuation arm (median 4 mg/day) and 0 to 36 mg/day in the cessation arm (median 8 mg/day), although the higher maximum daily dose in the cessation arm was not statistically significantly different from the continuation arm (P = .55).
Discussion
In this first prospective, randomized clinical trial of continuing versus ceasing bevacizumab beyond progression in recurrent glioblastoma, we were not able to show a significant difference in PFS or OS between arms. However, with the small sample size, the confidence intervals on survival benefits are wide, ruling out only very large effects. Further, an analysis adjusted for some imbalances in baseline factors suggests that moderate effects of continuing bevacizumab are possible. Consequently, this remains an open question, and ultimately a phase 3 study would be required to demonstrate smaller but important benefits of continuing bevacizumab. Nevertheless, Part 2 of CABARET has demonstrated that randomizing patients to continue or cease bevacizumab in this context is indeed feasible and in the absence of a clear effect, further study of the question is warranted.
Median OS for both arms on Part 2 of CABARET was short, and poorer than reported in similar study settings.28,29 No radiological responses were seen, and the higher proportion with recorded stable disease in the continuation arm did not translate to any survival benefit, although it is important to note that many patients did not actually receive their first planned Part 2 MRI and many who did, did not have a subsequent one. From the limited results using the MRIs that were performed, ceasing bevacizumab on progression did not appear detrimental, with no overabundance of rebound effects radiologically in the discontinuation arm. Nevertheless, with the large proportion of patients who did not undergo MRI we are unable to conclude that this does not occur.
Two other important study endpoints, QOL and corticosteroid use, have also been reported. There was no evidence that bevacizumab continuation was associated with better QOL; but we cannot exclude the possibility of a difference that was simply not detected, owing to the low statistical power of this study. The maximum recorded steroid dose included up to 36 mg per day in the cessation arm compared with up to 16 mg per day in the continuation arm, which is a clinically relevant difference. While there was no statistically significant difference in median values for maximum or mean daily steroid dose, again this may relate to the small sample. In retrospective studies, conflict surrounds the merits of continuing or ceasing bevacizumab. While rapid rebound is a noted concern in some studies,14,16,17 others have indicated it may be safe to discontinue bevacizumab for some patients. A small retrospective series of 7 patients discontinuing bevacizumab for reasons other than progression demonstrated a median time to recurrence after discontinuation of 4 months (range 1−26) and a 6-month PFS as high as 43%.30 Three of five patients who resumed bevacizumab had a partial response.
Several publications specifically addressing the role of bevacizumab beyond progression in glioma have been reported since CABARET was designed. Reardon et al published a retrospective pooled analysis of 99 participants in single-arm, phase 2 studies of bevacizumab, comparing outcomes after on-trial progression for those who did and did not continue bevacizumab.31 Median OS was longer for those continuing bevacizumab (5.9 versus 4.0 months), and bevacizumab was an independent predictor of survival in multivariable analysis. Other retrospective series indicate at best a modest effect of continuing bevacizumab beyond progression,17,18,32 including a retrospective review of 42 patients receiving ongoing bevacizumab plus nitrosourea chemotherapy after bevacizumab failure, which did not show any benefit in continuation; response rate was 0% and 6-month PFS was 3%, in keeping with our findings.33 Several prospective phase 2, single-arm studies of bevacizumab continuation after progression have similarly shown no responses, and PFS and OS data together demonstrate doubtful clinically meaningful benefit.7,28,29 The TAMIGA study, a prospective randomized phase 2 trial is yet to report results but will be the only other prospective study to compare continuation and cessation of bevacizumab.34
There are a number of important limitations to this study. First, the small sample size and that the lower number of patients recruited than anticipated meant that it is not possible to draw definitive conclusions on the effectiveness of continuing bevacizumab. But Part 2 of CABARET was only intended as an exploratory study of this question to rule out very large effects and to demonstrate the feasibility of a larger trial subsequently. Second, only 80% of the planned 60 patients were able to be enrolled. This partly represents the difficulties of recruiting patients with more than one recurrence of glioblastoma, the disease not uncommonly rendering them too unwell for participation in a clinical trial. Indeed, beyond Part 2, only 10 of 48 patients then went on to receive any further therapy (Supplementary Table 2). We had already anticipated a 50% drop-out from Part 1 to Part 2, and were aware that, given this was a phase 2 study, Part 2 was aimed mainly to provide information on potential signals for efficacy that might warrant further investigation. Regarding reasons why participants did not continue on to Part 2, usually this was because they were considered not well enough for additional therapy; this is reflected in lower ECOG performance status and lower median survival (2 months in this group versus 3.3 months).
A further limitation is related to chance imbalances in baseline factors at the time of randomization to Part 2, with more patients having more than one recurrence among those assigned to the continuing-bevacizumab arm. Survival analysis with adjustment for this and other baseline factors did not show a significant survival effect between treatments, but confidence intervals did suggest that an even larger effect of continuing bevacizumab could have been missed (compared with the unadjusted analysis).
One concern raised has been whether patients randomized are representative, with many patients being considered to be uncomfortable about ceasing bevacizumab; however, bevacizumab was not otherwise available in Australia for such patients other than on compassionate use, and after ceasing Part 1 of CABARET, only 2 went on to receive further bevacizumab (Supplementary Table 2).
An additional limitation is the restricted chemotherapy choices (temozolomide, etoposide, or carboplatin) for patients after recurrence on Part 1, and the unavailability of the nitrosourea lomustine as an option. These choices available to clinicians at that time were pragmatic and represented standard (Australian) second- and third-line approaches at the time of study design, but have not been shown to improve survival, and rechallenge with temozolomide would generally only be considered from a clinical perspective if progression has not occurred during prior therapy with the drug. Lomustine is now used more commonly in this setting, both in Australia and elsewhere, and has been the reference arm for several prominent clinical trials, none of which had been presented or published by the time of study design.9,8,35 The choice of chemotherapy is less critical to the trial question, since chemotherapy choices were made before randomization and were well balanced between the 2 arms of the trial, which examined the additional value of continuing or stopping bevacizumab on the background of whatever other therapy was chosen.
This study represented a real-world patient cohort. Patients with multiple relapses were eligible; and while performance status was stipulated for Part 1, for Part 2, 6 patients with ECOG 3 were eligible to participate on the basis of their clinician’s decision that they were suitable for additional active treatment. The second randomization itself selected for relatively well patients, that is, those who were considered well enough to continue therapy for their disease, which may limit the generalizability of our findings to the broader population of patients with recurrent glioblastoma but may be very applicable to a population considering continuation or cessation of bevacizumab following progression on this agent.
A substantial proportion of patients in each treatment arm died without documented radiological progression, presumably being too unwell to undergo MRI. This highlights the limitations of using PFS as an endpoint compared with the unequivocal endpoint of OS. It is possible that differences in radiological outcomes between continuing and ceasing bevacizumab were not adequately documented, and radiological PFS has not been captured for all patients in this study. While there were no signals suggesting obvious differences between the 2 arms for those completing scheduled imaging, the proportion who did not have follow-up MRI means that we cannot conclusively state that there is no difference in types of radiological progression. However, in patients with glioblastoma, death almost invariably is a consequence of disease progression; PFS data have not been missed because radiological progression could not be confirmed on MRI, as PFS incorporates clinical progression and death. Thus, clinical progression without MRI documentation is still true progression. MRI testing every 4 weeks may have increased the proportion of patients with documented radiological progression, but was not feasible.
In conclusion, Part 2 of the CABARET clinical trial provides the first randomized evidence on the value of continuing or stopping bevacizumab after progression in patients with recurrent glioblastoma. The study has not been able to demonstrate any significant benefits of continuing bevacizumab, but on the basis of its small sample, only very large benefits have been excluded, and the value of such treatment remains a very open question. The trial does suggest that randomizing patients to this question is challenging, but provides evidence that it is feasible, and further pursuit of this question in a future phase 3 trial should be supported.
Funding
This study was funded by Roche Products, Pty Limited (Australia) with additional support from NHMRC Program Grant 1037786 to the NHMRC Clinical Trials Centre, and Cancer Australia Support for Cancer Clinical Trials Grant and CINSW Cooperative Clinical Trials Grant to COGNO.
Dr. Field was funded through the University of Melbourne Stella Mary Langford Scholarship, Watt-Geyer Memorial Research Fund, and the Royal Melbourne Hospital Research Medal
Conflict of interest statement
EJH has had consulting or advisory roles for Bayer, Janssen Oncology, Pfizer, and Roche, and has received travel grants from GlaxoSmithKline and Sanofi. KMF has received travel grants from Roche.
LC has received honoraria from and has had consulting or advisory roles for Roche Pharma AG, and has received institutional research funding from Celldex, Lilly, Merck, and Roche.
AKN has had consulting or advisory roles for Boehringer Ingelheim and Roche and has received research funding from Boehringer Ingelheim.
JS and KS have received institutional research funding from Roche through the Clinical Trials Centre.
Supplementary Material
Acknowledgments
This trial was conducted under the auspices of the Cooperative Trials Group for Neuro-Oncology (COGNO), coordinated at the NHMRC Clinical Trials Centre, University of Sydney, and supported by Roche Products Pty Limited (Australia). Rhana Pike from the Clinical Trials Centre edited the paper.
Trial Management Committee K Field (chair), J Simes, E Hovey, A Nowak, L Cher, H Wheeler, C Brown, E Barnes, K Sawkins, A Livingstone, M Rosenthal
Independent Central Radiological Review Committee P Phal, G Fitt, C Goh
Independent Data Safety Monitoring Committee M Tattersall (Chair), P Kelly, A Hayden
Clinical Trials Centre K Sawkins, E Barnes, D Espinoza, C Brown, A Livingstone, D Winter, B Tomes, R Pike, J Simes
The following study sites participated in the CABARET study and randomized at least one patient to Part 2 (principal investigator and site coordinator):
Royal Melbourne Hospital, Victoria—K Field/M Rosenthal, L Garrett (7); Prince of Wales Hospital, New South Wales—E Hovey, H Kilsby (6); Monash Medical Centre, Victoria—R Freilich, I Arzhintar (5); St Vincent’s Hospital, Victoria—A Dowling, N Ranieri (4); Epworth HealthCare Richmond, Victoria—R Jennens, F Osmond (4); The Queen Elizabeth Hospital, South Australia—WK Patterson, A Phay (4); Royal Prince Alfred Hospital, New South Wales—J Simes, A Byrne (3); Port Macquarie Base Hospital, New South Wales—S Begbie, P Williams (3); Royal North Shore Hospital, New South Wales—H Wheeler, S Kirby-Lewis (2); Calvary Mater Newcastle, New South Wales—F Abell, L Plowman (2); Austin Hospital, Victoria—L Cher, J Flynn (2);
Royal Brisbane and Women’s Hospital, Queensland—P Inglis, A Ives (2); Sir Charles Gairdner Hospital, Western Australia—A Nowak, S Lobb (2); Mater Adult Hospital, Queensland—Z Lwin/N Woodward, G Crosbie (1); Royal Adelaide Hospital, South Australia—N Singhal, S Smith and M Whelan (1).
CABARET Part 1 was reported at the American Society of Clinical Oncology Annual Meeting (2013); the Society for Neuro-Oncology Annual Meeting (2012); the Australian Cooperative Trials Group for Neuro-Oncology annual meetings (2012−2014); the European Association for Neuro-Oncology and European Society of Medical Oncology annual meetings (2012). The results from CABARET Part 2 were presented at the American Society of Clinical Oncology Annual Meeting (2015).
CABARET was prospectively registered with the Australian New Zealand Clinical Trials Registry (ANZCTR), ACTRN12610000915055 (anzctr.org.au).
References
- 1. Al-Khatib SM, Granger CB, Huang Y, et al. Sustained ventricular arrhythmias among patients with acute coronary syndromes with no ST-segment elevation: incidence, predictors, and outcomes. Circulation. 2002;106(3):309–312. [DOI] [PubMed] [Google Scholar]
- 2. Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. [DOI] [PubMed] [Google Scholar]
- 3. Schwartzbaum JA, Fisher JL, Aldape KD, et al. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006;2(9):494–503; quiz 1 p following 516. [DOI] [PubMed] [Google Scholar]
- 4. Cohen MH, Shen YL, Keegan P, et al. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009;14(11):1131–1138. [DOI] [PubMed] [Google Scholar]
- 5. Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13(4):1253–1259. [DOI] [PubMed] [Google Scholar]
- 6. Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 7. Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wick W, Brandes AA, Gorlia T, et al. EORTC 26101 phase III trial exploring the combination of bevacizumab and lomustine in patients with first progression of a glioblastoma. J Clin Oncol. 2016;34 (suppl; abstr 2001).
- 9. Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. [DOI] [PubMed] [Google Scholar]
- 10. Balañá C, Etxaniz O, Bugés C, et al. Approval denied by the European Medicines Agency (EMA) for bevacizumab in the treatment of high-grade glioma recurrence: a good idea or a grave error? Clin Transl Oncol. 2011;13(3):209–210. [DOI] [PubMed] [Google Scholar]
- 11. Rahmathulla G, Hovey EJ, Hashemi-Sadraei N, et al. Bevacizumab in high-grade gliomas: a review of its uses, toxicity assessment, and future treatment challenges. Onco Targets Ther. 2013;6:371–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grothey A, Sugrue MM, Purdie DM, et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol. 2008;26(33):5326–5334. [DOI] [PubMed] [Google Scholar]
- 13. Bennouna J, Sastre J, Arnold D, et al. ; ML18147 Study Investigators. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14(1):29–37. [DOI] [PubMed] [Google Scholar]
- 14. Zuniga RM, Torcuator R, Jain R, et al. Rebound tumour progression after the cessation of bevacizumab therapy in patients with recurrent high-grade glioma. J Neurooncol. 2010;99(2):237–242. [DOI] [PubMed] [Google Scholar]
- 15. Mancuso MR, Davis R, Norberg SM, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116(10):2610–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pope WB, Xia Q, Paton VE, et al. Patterns of progression in patients with recurrent glioblastoma treated with bevacizumab. Neurology. 2011;76(5):432–437. [DOI] [PubMed] [Google Scholar]
- 17. Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70(10):779–787. [DOI] [PubMed] [Google Scholar]
- 18. Quant EC, Norden AD, Drappatz J, et al. Role of a second chemotherapy in recurrent malignant glioma patients who progress on bevacizumab. Neuro Oncol. 2009;11(5):550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. NCCN. Central nervous system cancers. NCCN Guidelines 2016; https://www.nccn.org/professionals/physician_gls/f_guidelines.asp Accessed 3 January 2017.
- 20. Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. [DOI] [PubMed] [Google Scholar]
- 21. Vredenburgh JJ, Cloughesy T, Samant M, et al. Corticosteroid use in patients with glioblastoma at first or second relapse treated with bevacizumab in the BRAIN study. Oncologist. 2010;15(12):1329–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 23. Field KM, Simes J, Nowak AK, et al. ; CABARET/COGNO investigators. Randomized phase 2 study of carboplatin and bevacizumab in recurrent glioblastoma. Neuro Oncol. 2015;17(11):1504–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Field K, Simes J, Wheeler H, et al. A randomised phase II study of carboplatin and bevacizumab in recurrent glioblastoma multiforme (CABARET). J Clin Oncol. 2013;31 (suppl; abstr 2017). [Google Scholar]
- 25. Reardon DA, Desjardins A, Vredenburgh JJ, et al. Metronomic chemotherapy with daily, oral etoposide plus bevacizumab for recurrent malignant glioma: a phase II study. Br J Cancer. 2009;101(12):1986–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kong DS, Lee JI, Kim JH, et al. Phase II trial of low-dose continuous (metronomic) treatment of temozolomide for recurrent glioblastoma. Neuro Oncol. 2010;12(3):289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lord SJ, Irwig L, Simes RJ. When is measuring sensitivity and specificity sufficient to evaluate a diagnostic test, and when do we need randomized trials? Ann Intern Med. 2006;144(11):850–855. [DOI] [PubMed] [Google Scholar]
- 28. Reardon DA, Desjardins A, Peters K, et al. Phase II study of metronomic chemotherapy with bevacizumab for recurrent glioblastoma after progression on bevacizumab therapy. J Neurooncol. 2011;103(2):371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reardon DA, Desjardins A, Peters KB, et al. Phase II study of carboplatin, irinotecan, and bevacizumab for bevacizumab naïve, recurrent glioblastoma. J Neurooncol. 2012;107(1):155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sherman WJ, Raizer JJ, Grimm SA. Outcome of discontinuing bevacizumab prior to malignant glioma progression. J Neurooncol. 2013;111(1):87–89. [DOI] [PubMed] [Google Scholar]
- 31. Reardon DA, Herndon JE, 2nd, Peters KB, et al. Bevacizumab continuation beyond initial bevacizumab progression among recurrent glioblastoma patients. Br J Cancer. 2012;107(9):1481–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Iwamoto FM, Abrey LE, Beal K, et al. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology. 2009;73(15):1200–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rahman R, Hempfling K, Norden AD, et al. Retrospective study of carmustine or lomustine with bevacizumab in recurrent glioblastoma patients who have failed prior bevacizumab. Neuro Oncol. 2014;16(11):1523–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brandes AA, Mason W, Pichler J, et al. Can bevacizumab prolong survival for glioblastoma patients through multiple lines of therapy? Future Oncol. 2014;10(7):1137–1145. [DOI] [PubMed] [Google Scholar]
- 35. Batchelor TT, Mulholland P, Neyns B, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31(26):3212–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.