Abstract
Background
The efficacy of bevacizumab (BEV) in elderly patients with glioblastoma remains unclear. We evaluated the effect of BEV on survival in this patient population using the Survival, Epidemiology, and End Results (SEER)-Medicare database.
Methods
This retrospective, cohort study analyzed SEER-Medicare data for patients (aged ≥66 years) diagnosed with glioblastoma from 2006 to 2011. Two cohorts were constructed: one comprised patients who had received BEV (BEV cohort); the other comprised patients who had received any anticancer treatment other than BEV (NBEV cohort). The primary analysis used a multivariate Cox proportional hazards model to compare overall survival in the BEV and NBEV cohorts with initiation of BEV as a time-dependent variable, adjusting for potential confounders (age, gender, Charlson comorbidity index, region, race, radiotherapy after initial surgery, and diagnosis of coronary artery disease). Sensitivity analyses were conducted using landmark survival, propensity score modeling, and the impact of poor Karnofsky Performance Status.
Results
We identified 2603 patients (BEV, n = 597; NBEV, n = 2006). In the BEV cohort, most patients were Caucasian males and were younger with fewer comorbidities and more initial resections. In the primary analysis, the BEV cohort showed a lower risk of death compared with the NBEV cohort (hazard ratio, 0.80; 95% confidence interval, 0.72–0.89; P < .01). The survival benefit of BEV appeared independent of the number of temozolomide cycles or frontline treatment with radiotherapy and temozolomide.
Conclusion
BEV exposure was associated with a lower risk of death, providing evidence that there might be a potential benefit of BEV in elderly patients with glioblastoma.
Keywords: bevacizumab, elderly, glioblastoma, SEER-Medicare, survival
Glioblastoma accounts for the majority of adult malignant brain tumors, with an overall incidence of 3.2 per 100,000 individuals.1 The median age at diagnosis is 64 years, and the incidence in patients aged ≥65 years is likely to increase due to an ageing population.1,2 Despite standard-of-care treatment (surgical resection, followed by radiotherapy and temozolomide [Stupp protocol]),3,4 the prognosis for elderly patients with glioblastoma remains poor.2 Median survival for patients who receive treatment is 6 to 9 months5–7 compared with <6 months for untreated patients, reflecting less favorable tumor biology, less aggressive treatment, and more comorbidities.2,8
Since receiving US Food and Drug Administration (FDA) approval in 2009 for the treatment of adults with recurrent glioblastoma,9–11 bevacizumab (BEV) has been widely used for this indication in the US (in two US cohorts, 80% to 86% of patients with recurrent glioblastoma had received BEV12,13). In phase III trials, BEV prolonged progression-free survival (PFS) in adults with newly diagnosed glioblastoma, but did not confer an overall survival (OS) benefit in the overall population.14–17 The lack of OS benefit may partially be attributed to crossover between arms, with patients in the placebo arm receiving BEV in later therapy lines (in the AVAglio and RTOG 0825 trials, 31% and 48% of patients in the placebo arms received BEV, respectively).
There is variability in vascular endothelial growth factor (VEGF) expression between glioblastoma subtypes, and BEV may have greater beneficial effect in elderly patients given the evidence from uncontrolled studies of the increased activity of VEGF in elderly patients.11,18,19 However, information on outcomes in this patient population is limited; these patients are often excluded from randomized controlled trials (RCTs), and guidelines do not exist for patients aged >70 years.20–22 A recent randomized, open-label, phase II trial (ARTE) evaluated the efficacy of the addition of BEV to radiotherapy in the elderly (≥65 years), but was limited to newly diagnosed patients (n = 75), which does not reflect the current BEV label, and was not adequately powered to evaluate survival among the patients studied.17
Registry data provide an alternative avenue to evaluate the role of BEV in elderly patients with glioblastoma. The Survival, Epidemiology, and End Results (SEER) Program is a population-based, national network of cancer registries that covers approximately 26% of the US population. Medicare is a publicly funded US health insurance system, open to people who are ≥65 years of age or diagnosed with end-stage renal disease or some other disability, which was estimated to have covered 14% of the total US population and the entire elderly population (41 million elderly individuals) in 2012.23 The SEER-Medicare database links these two databases, and has been shown to be a feasible method of evaluating survival outcomes in elderly patients with incident cancer.24–27
There have been no previous descriptions of BEV use, and subsequent outcomes, in elderly patients with glioblastoma from a population-based setting. The primary objective of this study was to estimate the risk of death of elderly patients with glioblastoma who had received BEV, compared with those who had never received BEV, using SEER-Medicare data. A secondary objective was to describe the pattern of BEV treatment in this patient population.
Methods
Study Design and Data Source
This was a retrospective, cohort study using the 2015 SEER-Medicare database, the latest data set available. The 2015 SEER-Medicare release data include SEER diagnoses between January 1, 1993 and December 31, 2012 and Medicare claims data through December 31, 2014. Data on tumor characteristics, demographics, and date of death were extracted from the SEER database and individual treatment data were extracted from the Medicare claims.
Cohort Selection
From the SEER database, we identified a cohort of patients ≥66 years of age, newly diagnosed with a glioblastoma tumor between 2006 and 2011, who received an initial resection or biopsy (15 days prior to, or 60 days after, diagnosis)24 and anticancer treatment any time after surgery. Patients were extracted based on International Classification Disease for Oncology (ICD-O) histology (9440/3, 9441/3, and 9442/3)1 and topology codes (ICD-O: C70.0 and C71.0–C71.9). The period from 2006 to 2011 was chosen to coincide with the first approval of bevacizumab in 2006, when patients could have received treatment for glioblastoma off-label. Patients diagnosed in 2012 were excluded to allow a minimum 1-year follow-up after diagnosis. In order to have a full year of data prior to diagnosis, all patients <65 years of age were excluded as their Medicare claims would not be systematically available. Patients who did not undergo surgery, whose surgery data were missing, or who only received best supportive care after surgery were excluded.
To ensure data completeness and make sure that detailed claims for all individuals were reported to Medicare and available in the database, all patients included in the study required continuous enrollment in Medicare Part A and Part B for ≥1 month prior to diagnosis until death or the end of the study period (December 2012).28 Any patient enrolled in a Health Maintenance Organization (HMO) 1 month prior to diagnosis until death or the end of the study period (December 2013) was excluded, as treatment information covered by an HMO would not be reported to the Medicare database. Additional exclusion criteria were: cases identified solely at autopsy/by death certificates, or with an unknown month of death; prior diagnosis of another primary cancer within 1 year of glioblastoma diagnosis; enrollment in hospice care before, or at the time of, glioblastoma diagnosis; and patients lacking Part B Medicare coverage, as information regarding outpatient services were not available.
Primary Analysis Cohorts
Two cohorts were constructed based on BEV exposure: one cohort comprised patients who had undergone initial surgery/resection and received BEV at any time after diagnosis (BEV cohort), and another cohort comprised patients who had undergone initial surgery/resection and received any anticancer treatment other than BEV at any time after diagnosis (NBEV cohort).
Covariates
Patient demographics were obtained from the SEER database. The prevalence of comorbidities extracted from Medicare data was assessed with the Klabunde adaptation (a validated method of calculating comorbidity indices from SEER-Medicare dataset)29,30 of the Charlson comorbidity index (CCI). Comorbidities commonly associated with glioblastoma were identified a priori to assess their prevalence at glioblastoma diagnosis; CCI and comorbidities data were calculated 1 year prior to diagnosis. Socioeconomic status was evaluated according to the percentage of people living at or below the federal poverty level in a patient’s census tract of residence (<10% vs ≥10%), using data from the 2008 to 2012 American Community Survey. Surgery, radiation, and chemotherapy claims were obtained from Medicare; an individual was considered to have received a treatment if one or more claim included a code for each specific treatment. ICD-9 diagnosis, procedure, national coverage determinations codes, and revenue cost codes were used to identify initiation of treatment.
Statistical Analysis
The effectiveness on OS of BEV over non-BEV treatment was the primary objective of this study. OS was measured from date of diagnosis until death. All statistical analyses were performed using SAS® (version 9.3; SAS Institute, Cary, NC) and R.
Demographic and Clinical Characteristics by Treatment Group
Comparisons between cohorts were made with chi-square tests for categorical variables and Wilcoxon rank-sum tests for continuous variables (two-sided test; P value threshold ≤ .05); no adjustments for multiple comparisons were made.
Survival Modeling
The primary analysis used a multivariate Cox proportional hazards model to compare OS in the BEV and NBEV cohorts, adjusting for potential confounders (age at diagnosis, gender, CCI, region of treatment, race, radiotherapy, diagnosis of coronary artery disease, marital status, and socioeconomic status), with initiation of BEV as a time-dependent variable (patients were considered “unexposed” prior to their first BEV initiation and “exposed” thereafter). Variables chosen for the final model were based on known prognostic factors and forward stepwise selection, and were confirmed by backward stepwise selection. The proportional hazards assumption was evaluated with the proportionality test and cumulative martingale residuals for all variables; collinearity across the model was evaluated with variation inflation factor and tolerance.
Sensitivity Analyses
Sensitivity analyses were conducted to evaluate the strength of the primary analysis results, including the potential impact of extent of resection, temozolomide exposure, period of diagnosis, baseline Karnofsky Performance Status (KPS), and propensity score modeling. A landmark method was also explored, with and without an intent-to-treat (ITT) approach, to control for immortal time bias.31,32 Additional details on the sensitivity analyses are available in the Supplementary methods.
Results
Patients
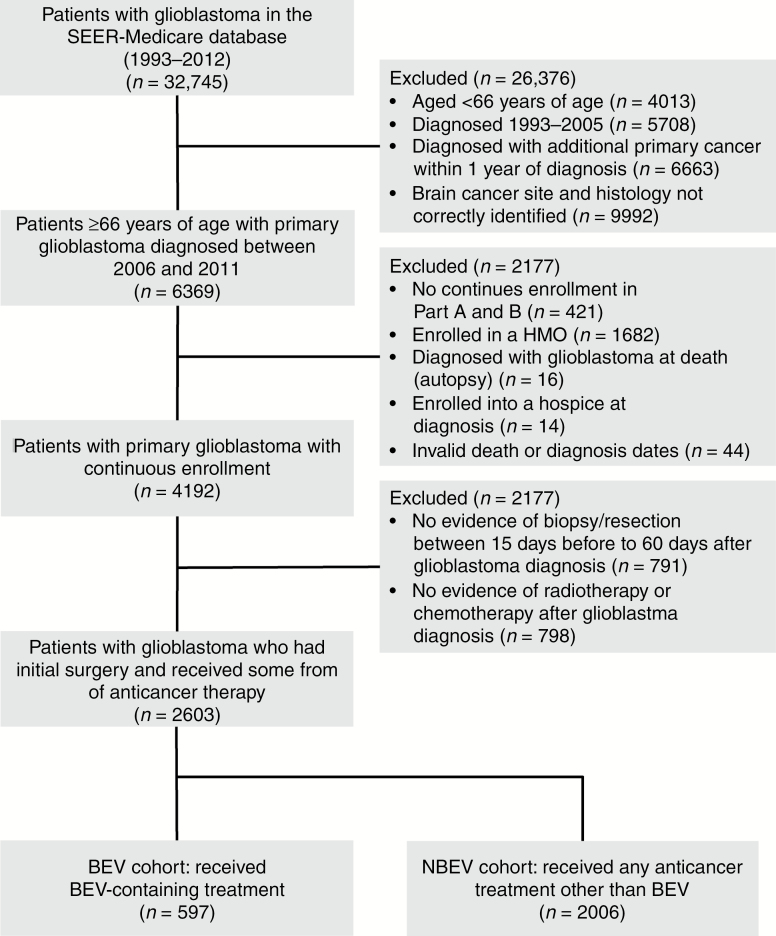
Of the 32,745 patients with glioblastoma who were identified in the SEER-Medicare database, 2603 met the study inclusion/exclusion criteria and were included in the full cohort (BEV, n = 597 [23%]; NBEV, n = 2006 [77%]; Fig. 1). Patients in the BEV cohort were younger, male, Caucasian, married, and from a lower socioeconomic status (census tract poverty level <10%), and had fewer comorbidities, compared with the NBEV cohort. A higher proportion of patients in the BEV cohort underwent an initial resection compared with the NBEV cohort (81% vs 68%, respectively; Table 1).
Fig. 1.
Patient flow. Abbreviations: BEV, bevacizumab; HMO, Health Maintenance Organization; SEER, Surveillance, Epidemiology, and End Results.
Table 1.
Patient demographics and clinical characteristics
| Characteristic | BEV Cohort (n = 597) |
NBEV Cohort (n = 2006) |
Total Cohort (n = 2603) |
|---|---|---|---|
| Age, years | |||
| Mean (SD)a,b | 72.3 (5.0) | 74.8 (5.8) | 74.2 (5.7) |
| Median (range) | 71 (66–90) | 74 (66–97) | 74 (66–97) |
| Age group, n (%)a,c | |||
| 66 to <70 years | 220 (37) | 444 (22) | 664 (26) |
| 70 to <75 years | 196 (33) | 576 (29) | 772 (30) |
| 75 to <80 years | 120 (20) | 546 (27) | 666 (26) |
| 80 to <85 years | 49 (8) | 333 (17) | 382 (15) |
| ≥85 years | 12 (2) | 107 (5) | 119 (5) |
| Female, n (%)a,c | 247 (41) | 926 (46) | 1173 (45) |
| Race, n (%)a,c | |||
| Caucasian | 569 (95) | 1827 (91) | 2396 (92) |
| Other | 28 (5) | 179 (9) | 207 (8) |
| Regiona,c | |||
| Midwest | 63 (11) | 293 (15) | 356 (14) |
| Northeast | 162 (27) | 399 (20) | 561 (22) |
| South | 101 (17) | 477 (24) | 578 (22) |
| West | 271 (45) | 837 (42) | 1108 (43) |
| Marital status, n (%)a,c | |||
| Single | 27 (5) | 130 (7) | 157 (6) |
| Married | 450 (75) | 1306 (65) | 1756 (67) |
| Divorced/separated | 31 (5) | 113 (6) | 144 (6) |
| Widowed | 76 (13) | 395 (20) | 471 (18) |
| Unknown | 13 (2) | 62 (3) | 75 (3) |
| Census tract poverty level, n (%)a,c,d | |||
| <10% poverty | 407 (68) | 1118 (56) | 1525 (59) |
| ≥10% poverty | 187 (31) | 856 (43) | 1043 (40) |
| Patients with a date of diagnosis post-2009, n (%)a,c | 391 (66) | 955 (48) | 1346 (52) |
| Adaptation of CCI, median (range) a,b | 0 (0–6) | 0 (0–10) | 0 (0–10) |
| Type of initial surgery, n (%)a,c | |||
| Biopsy | 112 (19) | 633 (32) | 745 (29) |
| Resection | 485 (81) | 1373 (68) | 1858 (71) |
| Received radiotherapy after initial surgery, n (%) | 586 (98) | 1987 (99) | 2573 (99) |
| Radiotherapy/temozolomide as front-line treatment, n (%)a,c,e | 394 (66) | 942 (47) | 1336 (51) |
| Comorbidities associated with glioblastoma, n (%) | |||
| Seizures | 17 (3) | 68 (3) | 85 (3) |
| Coronary artery diseasea,c | 58 (10) | 316 (16) | 374 (14) |
| Cerebrovascular accidenta,c | 13 (2) | 84 (4) | 97 (4) |
aSignificant difference between treatment cohorts (P < .05).
bWilcoxon rank-sum test.
cChi-square test.
dSocioeconomic status was evaluated according to the percentage of people living at or below the federal poverty level in a patient’s census tract of residence (<10% vs ≥10%) according to the 2008–2012 American Community Survey (data were not available for all patients).
eStandard of care (Stupp protocol).3 Abbreviations: BEV, bevacizumab; BEV cohort, patients who had received BEV-containing treatment; CCI, Charlson comorbidity index; NBEV cohort, patients who had received any anticancer treatment other than BEV; SD, standard deviation.
Treatment Exposures
In the full cohort, 71% and 99% of patients underwent surgical resection and radiotherapy within 90 days of diagnosis, respectively; exposure to radiotherapy did not differ (98% and 99% of patients in the BEV and NBEV cohorts, respectively). Temozolomide was the most frequent treatment across the full cohort, and was more common in the BEV than NBEV cohort (75% vs 51%). The median time from diagnosis to temozolomide initiation was 1.5 months (Table 2).
Table 2.
Temozolomide and BEV treatment exposure
| Treatment Exposure | BEV Cohort (n = 597) |
NBEV Cohort (n = 2006) |
Total Cohort (n = 2603) |
|---|---|---|---|
| Temozolomide | |||
| Patients who received temozolomide, n (%) | 447 (75) | 1029 (51) | 1476 (57) |
| Number of cycles, mean (SD) | 7.5 (6.7) | 4.1 (5.0) | 5.1 (5.8) |
| Number of cycles, median | 6 | 2 | 3 |
| Median time to initiation of treatment from diagnosis, months | 1.4 | 1.5 | 1.5 |
| Mean time to initiation of treatment from diagnosis, months (SD) | 2.0 (88.4) | 1.9 (90.6) | 1.9 (89.9) |
| BEV | |||
| Number of cycles, mean (SD) | 9.5 (11) | ||
| Number of cycles, median (IQR) | 6 (2–13) | ||
| Median time to initiation of treatment from diagnosis, months (IQR) | 8 (5–13) | ||
| Mean time to initiation of treatment from diagnosis, months (SD) | 10 (7.7) | ||
Abbreviations: BEV, bevacizumab; BEV cohort, patients who had received BEV-containing treatment; IQR, interquartile range; NBEV cohort, patients who had received any anticancer treatment other than BEV; SD, standard deviation.
A total of 597 patients (23%) had received BEV. In patients who had been diagnosed with glioblastoma after 2009, 391 of 1346 (29%) received BEV. The median time to initiation of BEV from diagnosis was 8 months (interquartile range [IQR], 5–13), and patients received a median of 6 cycles (IQR, 2–13; Table 2).
Survival Analysis
By the end of the study period, 2446 (94%) patients had died (BEV cohort: 512 [86%]; NBEV cohort: 1934 [96%]), and 157 (6%) patients were censored (BEV cohort: 85 [14%]; NBEV cohort: 72 [4%]). The median OS for the full cohort was 7.2 months (95% confidence interval [CI]: 6.9–7.6); the median OS was 16.8 months (95% CI: 16.0–17.8) in the BEV cohort and 5.7 months (95% CI: 5.5–5.9) in the NBEV cohort. The 6-, 12-, 18-, and 24-month survival probability was consistently higher in the BEV cohort than in the NBEV cohort (92.8% vs 47.1%, 72.7% vs 19.7%, 45.2% vs 10.3%, and 26.8% vs 5.4%, respectively; Supplementary Table S1).
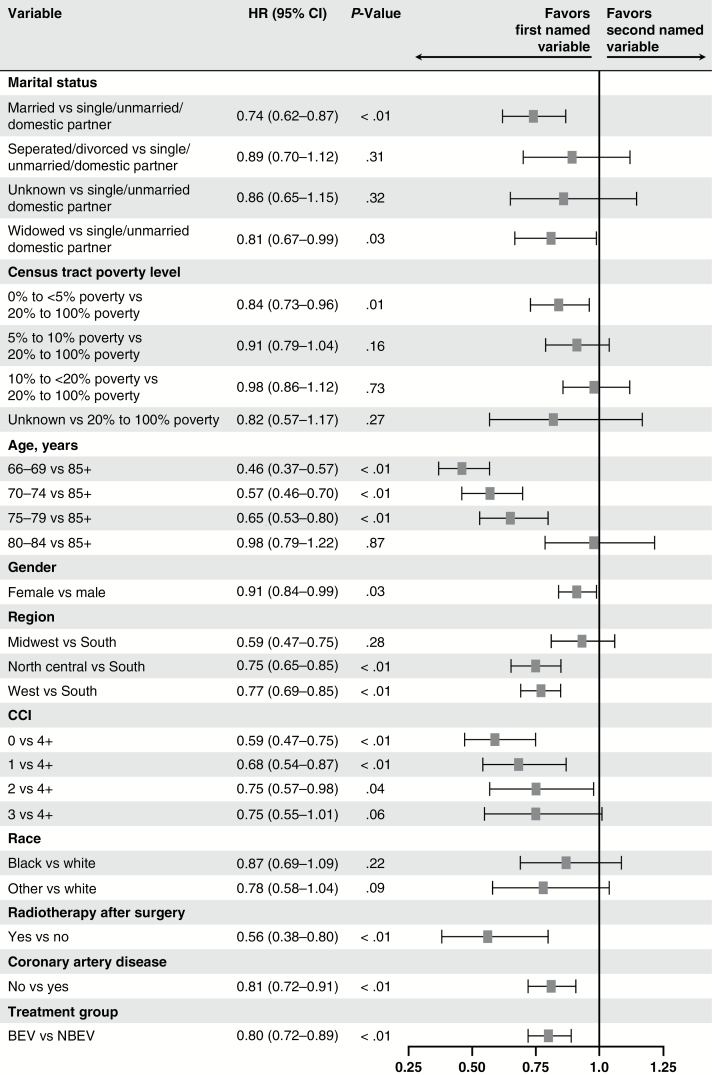
The multivariate Cox model with BEV exposure as a time-dependent variable showed that the BEV cohort was associated with a statistically significant lower risk of death, compared with the NBEV cohort (hazard ratio [HR]: 0.80; 95% CI: 0.72–0.89; P < .01; Fig. 2). Younger age, radiotherapy, fewer comorbidities at baseline, marital status, lower poverty level, and no coronary artery disease at baseline were also associated with longer survival (Fig. 2).
Fig. 2.
Multivariate Cox model of treatment effect on OS with bevacizumab exposure as a time-dependent variable. Abbreviations: BEV, bevacizumab; BEV cohort, patients who had received BEV-containing treatment; CCI, Charlson comorbidity index; CI, confidence interval; HR, hazard ratio; NBEV cohort, patients who had received any anticancer treatment other than BEV; OS, overall survival.
Sensitivity Analyses
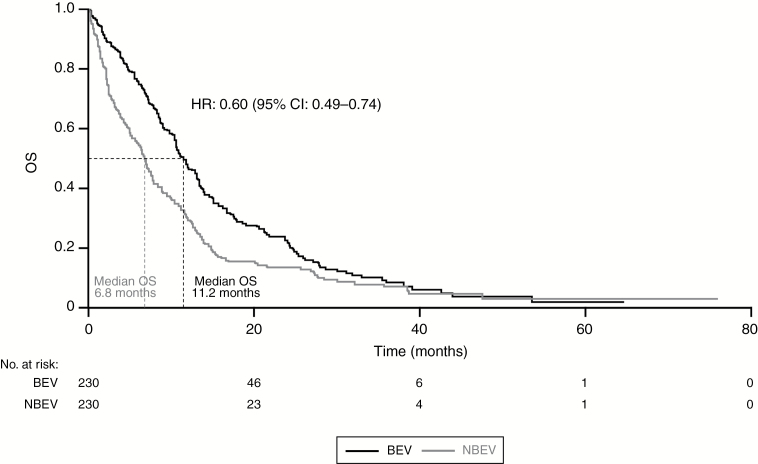
The primary analysis results were consistent after adjusting for additional therapies and KPS in separate models. The effect of BEV on OS appeared independent of the extent of resection, number of temozolomide cycles or front-line treatment with radiotherapy and temozolomide (Stupp protocol), and diagnosis period (post-2009) (Table 3). A propensity score matched (PSM) cohort (416 patients in each cohort) showed a significantly lower risk of death and an observed longer survival in the BEV cohort (HR: 0.54; 95% CI: 0.46–0.63; P < .01). The longer survival associated with the BEV cohort remained when using the landmark analysis without the ITT approach (Table 3). In the comparison of a matched cohort of 460 patients who had lived for ≥8 months, the BEV cohort had longer OS (median, 11.2 months [95% CI: 10.3–13.1]) compared with the NBEV cohort (median, 6.8 months [95% CI: 5.6–7.6]; HR: 0.60; 95% CI: 0.49–0.74; P < .01); OS was measured from the landmark date (Fig. 3). Similar results were observed in a matched cohort of 372 patients who had lived for ≥10 months (Table 3). However, when using the ITT approach (allowing for crossover), no improvement in survival was observed with BEV (Table 3).
Table 3.
Summary of sensitivity analyses examining OS with BEV versus NBEV
| Sensitivity Analysis | BEV Cohort (n = 597) |
NBEV Cohort (n = 2006) |
|---|---|---|
| Extent of resection | n = 597 | n = 2006 |
| HR for BEV vs NBEV (95% CI) | 0.83 (0.74–0.92) | |
| P value | <.01 | |
| Number of temozolomide cycles (categorical variable)a | n = 597 | n = 2006 |
| HR for BEV vs NBEV (95% CI) | 0.81 (0.73–0.90) | |
| P value | <.01 | |
| Number of temozolomide cycles (continuous variable) | n = 597 | n = 2006 |
| HR for BEV vs NBEV (95% CI) | 0.82 (0.73–0.91) | |
| P value | <.01 | |
| Stratified by Stupp protocol3 | n = 597 | n = 2006 |
| HR for BEV vs NBEV (95% CI) | 0.79 (0.71–0.88) | |
| P value | <.01 | |
| Diagnosed post-2009 | n = 391 | n = 955 |
| HR for BEV vs NBEV (95% CI) | 0.79 (0.69–0.92) | |
| P value | <.01 | |
| PSM cohort | n = 416 | n = 416 |
| Median OS, months (95% CI) | 16.97 (16.10–18.43) | 6.70 (6.37–7.43) |
| HR for BEV vs NBEV (95% CI) | 0.54 (0.46–0.63) | |
| P value | <.01 | |
| PSM landmark analyses (non-ITT approach) | ||
| ≥8 months | n = 230 | n = 230 |
| Median OS, monthsb (95% CI) | 11.23 (10.30–13.10) | 6.77 (5.60–7.60) |
| HR for BEV vs NBEV (95% CI) | 0.60 (0.49–0.74) | |
| P value | <.01 | |
| ≥10 months | n = 186 | n = 186 |
| Median OS, monthsb (95% CI) | 10.83 (8.93–12.03) | 6.17 (5.00–7.73) |
| HR for BEV vs NBEV (95% CI) | 0.66 (0.52–0.83) | |
| P value | <.01 | |
| Landmark analyses (non-ITT approach) | ||
| ≥8 months | n = 519 | n = 688 |
| Median OS, monthsb (95% CI) | 10.2 (9.30–11.2) | 5.17 (4.53–5.77) |
| HR for BEV vs NBEV (95% CI) | 0.72 (0.63–0.82) | |
| P value | <.01 | |
| ≥10 months | n = 474 | n = 515 |
| Median OS, monthsb (95% CI) | 9.17 (8.20–10.0) | 5.60 (4.83–6.67) |
| HR for BEV vs NBEV (95% CI) | 0.67 (0.58–0.77) | |
| P value | <.01 | |
| Landmark analyses (ITT approach) | ||
| ≥8 months | n = 218 | n = 989c |
| Median OS, monthsb (95% CI) | 7.17 (5.77–8.13) | 7.50 (6.77–8.30) |
| HR for BEV vs NBEV (95% CI) | 1.13 (0.96–1.32) | |
| P value | .15 | |
| ≥10 months | n = 239 | n = 750d |
| Median OS, monthsb (95% CI) | 6.53 (5.63–7.53) | 8.03 (7.17–9.07) |
| HR for BEV vs NBEV (95% CI) | 1.28 (1.09–1.51) | |
| P value | <.01 | |
aTemozolomide cycles were considered using the following categories: 0, 1–4, and ≥5 cycles.
bOS from landmark date.
c31% of patients received BEV.
d30% of patients received BEV. Abbreviations: BEV, bevacizumab; BEV cohort, patients who had received BEV-containing treatment; CI, confidence interval; HR, hazard ratio; ITT, intent-to-treat; NBEV cohort, patients who had received any anticancer treatment other than BEV; OS, overall survival; PSM, propensity score matched.
Fig. 3.
Kaplan–Meier estimates of OS in PSM cohort of patients who had lived for ≥8 months. Figure shows survival from 8 months. Abbreviations: BEV, bevacizumab; CI, confidence interval; HR, hazard ratio; OS, overall survival; PSM, propensity score matched.
Baseline KPS scores were not available in the SEER database, and so to evaluate the potential impact of this confounder, a simulation was performed using published prevalence estimates of patients with high (≥70) and low KPS scores (<70).33,34 After assessing the impact of unmeasured KPS on the primary model, the advantage of BEV appeared robust to the impact of performance status across the cohorts. For example, if it were assumed that high KPS (≥70) had a survival advantage with a HR of 0.73, and that 90% of patients in the BEV cohort had high KPS and 50% of the patients in the NBEV cohort had low KPS (<70), the treatment effect of BEV would not be diminished (Supplementary Table S2).
Discussion
Elderly patients (aged ≥66 years) constitute the highest proportion of glioblastoma cases in the US. However, few RCTs have been conducted in this patient population, and their optimal treatment remains unclear. Although not a substitute for RCTs, population-based registries can address research areas that have not been studied in a trial setting. Our analysis provides an insight into the postsurgical treatments used and evidence for the comparative survival benefit of BEV among elderly patients with glioblastoma.
The most common postsurgical treatments in this cohort were radiotherapy (99%) and temozolomide (57%), which is in line with previous studies and National Comprehensive Cancer Network guidelines.8,22,35 BEV was received by 23% of patients; among those diagnosed after 2009 (the year in which BEV received FDA approval), this proportion increased to 29%. This is in the range of previously reported rates of BEV exposure among elderly patients (19% and 39%).35,36 The median time to initiation of BEV treatment from diagnosis was 8 months, suggesting that BEV is primarily used in the recurrent setting.
The BEV cohort had a lower risk of death than the NBEV cohort, after adjusting for potential confounding variables. The treatment effect of BEV was consistent in a PSM cohort who had lived for ≥8 months. This may represent a patient population with a glioblastoma subtype that is sensitive to BEV, and research has suggested that certain subcohorts of patients with recurrent glioblastoma may derive particular benefit from BEV.37 Previous studies from clinical practice that have included elderly patients have shown an increased survival with BEV in patients with glioblastoma.2,8,35,38 A population-based analysis of the SEER program reported a significant improvement in median OS for patients who died in 2010 (post-BEV era) of 9 months, compared with 7 months among patients who died in 2008 (pre-BEV era). In that study, 587 (31%) patients in the 2008 cohort and 571 (29%) patients in the 2010 cohort were ≥70 years old. The significant increase in survival (P < .0001) was attributed to FDA approval of BEV.39 A US-based, single-center study of 120 elderly patients with glioblastoma reported a significantly lower risk of death associated with BEV treatment in a multivariate stepwise analysis (HR: 0.51; 95% CI: 0.31–0.83; P < .01).35 The lower risk of death associated with BEV in this previous study, compared with our study, may be due to the fact that immortal time bias may not have been fully adjusted for. Furthermore, results from a French cohort of 117 elderly patients with glioblastoma reported a HR of 0.83 in favor of BEV at recurrence, similar to our estimate.38 In addition to patient selection, a survival benefit for the BEV group may also be a result of a general increase in survival over the years, including advances in neurosurgery, improved planning in radiotherapy, as well as advances in structured standardized patient care.
Risk of death increased with age and pretreatment morbidity as expected. Unmarried patients were less likely to receive BEV and had an increased risk of death, which has been shown previously; further research is needed to understand the impact of marital status on outcomes.40 Radiotherapy after surgery also significantly reduced the risk of death, which is aligned with previous clinical studies and routine clinical practice setting suggesting an improvement of survival associated with radiotherapy.5 The magnitude of the effect we observed (HR: 0.56) was similar to a recent cohort of elderly patients with glioblastoma in the US that reported an increased risk of death among patients only receiving postsurgical chemotherapy compared with patients receiving combined chemotherapy and radiotherapy (HR: 1.5, P < .001).8
The survival benefit with BEV observed in our study differs from RCTs of BEV, which did not show an OS benefit.9,10,17 This may be due to the fact that elderly patients only comprise a small proportion of patients in RCTs, despite representing a substantial proportion of patients in clinical practice. The OS observed in RCTs may have been negatively impacted by crossover, in which patients in the control arm received poststudy BEV.41,42 In the landmark analyses with an ITT approach, which mimics a phase III RCT, crossover was 31% and 30% in each of the cohorts; in these cohorts, no significant improvement in survival with BEV was shown. Available trial-based evidence in the elderly can be extracted from the ARTE trial and subgroup analysis of AVAglio. In the ARTE trial (n = 75), which evaluated the combination of BEV with radiotherapy (BEVR) versus radiotherapy alone in elderly patients (≥65 years of age) with newly diagnosed glioblastoma, a longer median PFS in the BEVR arm of 7.6 months versus 4.8 months in the radiotherapy arm was observed, but no difference in median OS of 12.1 and 12.2 months, respectively, was detected. The trial was not adequately powered to evaluate survival among the patients studied and OS findings were inconclusive. Although ARTE was comprised of elderly patients, the treatment setting is not comparable to the patient population in our analysis, as the majority of patients in our analysis were not receiving BEV at diagnosis, which could contribute to conflicting results.17 Additionally, in the phase III AVAglio study, evaluating the effect of the addition of BEV to radiotherapy with concurrent temozolomide, a subgroup analysis of patients ≥70 years of age failed to show an improvement in survival (HR: 0.88, 95% CI: 0.83–1.18).14,15 While the point estimate remains in the same direction as our study, the lack of significance could reflect an issue of statistical power and small groups and the treatment setting in our cohort was not restricted to newly diagnosed patients.21
There are limitations to our analysis. While multivariate and propensity-score based methods can minimize measured confounding, these approaches cannot address sources of unmeasured confounding (KPS and O6-methylguanine-DNA-methyltransferase [MGMT] promotor status). KPS is not captured in the SEER-Medicare database; however, given the patient selection criterion that all patients received adjuvant treatment, the distribution of KPS at diagnosis between the two cohorts may have been similar. Simulations were conducted in which we assumed that there was a bias for better KPS in the BEV cohort, and this did not change the direction of treatment effect. Data for MGMT promotor status, which has been shown to be predictive of treatment response, were also not included in this analysis.43–45 Further evidence is needed on the genetic signatures in elderly patients to assess any potential confounding impact. Although our results are not generalizable to all patients with glioblastoma, the incidence of glioblastoma peaks in patients >65 years of age, and these results describe an important group of patients who are often not included in RCTs. Selection bias may have been introduced due to the exclusion of a small number of patients with incomplete claims and HMO coverage and exclusion of patients who received only best supportive care after surgical biopsy or resection. Elderly patients with no treatment are known to have the worse prognosis and were excluded to enable a fair comparison with patients receiving BEV.8,24
Retrospective observational studies are prone to immortal time bias, where patients who live longer may have a better KPS and other prognostic features and have a higher probability of receiving a certain treatment. Since BEV is the recommended standard-of-care treatment for patients with recurrent glioblastoma in the US,22 patients in the BEV cohort had a higher probability of living long enough to have received BEV, compared with patients in the NBEV cohort. To account for this, we performed modeling that controlled for time-independent factors and initiation of BEV as a time-dependent factor. Time-dependent modeling is a common method to control for immortality bias when assessing treatment effect, but it does not allow any difference in median survival to be assessed without bias due to reverse causality and interpretation of the risk of death represents the risk at initiation of BEV. Given the limitations, the landmark method was also explored in our analysis, which is an additional method to offset immortal time bias in observational research and enables the comparison of OS from a landmark date. However, landmark analyses can be difficult to interpret because of a lack of standardization, and adjusting for survival to a predefined time point may mask the potential short-term survival benefit by the removal of clinically important early deaths due to treatment in aggressive forms of cancer.46
Cancer recurrence and treatment lines are not directly captured in the SEER-Medicare database. Treatment algorithms have been used to estimate treatment lines for different population-based cohorts.47–49 This approach was explored in this study, but is not presented here due to the heterogeneity of treatment gaps observed and the misclassification bias that can occur when determining second-line treatment (a proxy for tumor recurrence) based on a treatment algorithm alone.
To our knowledge, this is the largest cohort of elderly patients with glioblastoma (75% of our cohort was >70 years of age) used to assess the role of BEV, providing insights into a patient population typically poorly represented by clinical trials. The extensive geographic coverage of the SEER-Medicare database minimizes the discrepancies inherent to single-institution/single-provider studies, and the large sample size is generalizable to the greater elderly population with glioblastoma in the US and represents real-world clinical practice.
Conclusion
In conclusion, 23% of patients aged ≥66 years who were diagnosed with glioblastoma in the US between 2006 and 2011 with initial surgery received BEV in this retrospective cohort study. Exposure to BEV was associated with a lower risk of death in the patient cohort included in this study; the results remained robust after a number of sensitivity analyses, including controlling for previous temozolomide exposure and Stupp protocol, providing evidence that there might be a potential benefit of BEV in elderly patients with glioblastoma.
Supplementary Material
Supplementary data are available at Neuro-Oncology Practice online.
Funding
This study was sponsored by F. Hoffmann-La Roche, Ltd. Medical writing assistance for this manuscript was provided by Gardiner-Cladwell Communications, paid for by F. Hoffmann-La Roche, Ltd.
Conflict of interest statement. Jessica Davies and Thirupathi Pattipaka declare employment with F. Hoffmann-La Roche Ltd. Irmarie Reyes-Rivera, Josep Garcia, and Lauren Abrey declare stock ownership and employment with F. Hoffmann-La Roche Ltd. All other authors declare that they have no conflict of interest.
Supplementary Material
Acknowledgments
The authors would like to thank Larry Leon for contributing to the statistical methods and results interpretation, Arliene Ravelo for contributing to the study design and results interpretation, and Michael Taylor for support with the SEER-Medicare data. Editorial support, in the form of the development of a draft outline based on the study report, preparation of figures and tables, and assembly of drafts in consultation with the authors, was provided by Thomas Burton, BSMS, of Gardiner-Caldwell Communications, Macclesfield, UK, and was funded by F. Hoffmann-La Roche Ltd.
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. This study was previously presented at the Society for Neuro-Oncology Annual Meeting 2016.
References
- 1. Ostrom QT, Gittleman H, Fulop J et al. . CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17(Suppl 4):iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arvold ND, Reardon DA. Treatment options and outcomes for glioblastoma in the elderly patient. Clin Interv Aging. 2014;9:357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stupp R, Mason WP, van den Bent MJ et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 4. Stupp R, Hegi ME, Mason WP et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 5. Keime-Guibert F, Chinot O, Taillandier L et al. ; Association of French-Speaking Neuro-Oncologists Radiotherapy for glioblastoma in the elderly. N Engl J Med. 2007;356(15):1527–1535. [DOI] [PubMed] [Google Scholar]
- 6. Perry JR, Laperriere N, O’Callaghan CJ et al. ; Trial Investigators Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376(11):1027–1037. [DOI] [PubMed] [Google Scholar]
- 7. Rigamonti A, Imbesi F, Silvani A et al. . Pattern of care and outcome in elderly patients with glioblastoma: data in 151 patients from 3 Lombardia Hospitals. J Neurol Sci. 2017;378:3–8. [DOI] [PubMed] [Google Scholar]
- 8. Rusthoven CG, Koshy M, Sher DJ et al. . Combined-modality therapy with radiation and chemotherapy for elderly patients with glioblastoma in the temozolomide era: a national cancer database analysis. JAMA Neurol. 2016;73(7):821–828. [DOI] [PubMed] [Google Scholar]
- 9. Cohen MH, Shen YL, Keegan P et al. . FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009;14(11):1131–1138. [DOI] [PubMed] [Google Scholar]
- 10. Friedman HS, Prados MD, Wen PY et al. . Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 11. Kreisl TN, Kim L, Moore K et al. . Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Girvan AC, Carter GC, Li L et al. . Glioblastoma treatment patterns, survival, and healthcare resource use in real-world clinical practice in the USA. Drugs Context. 2015;4:doi: 10.7573/dic.212274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen C, Ravelo A, Yu E et al. . Clinical outcomes with bevacizumab-containing and non-bevacizumab-containing regimens in patients with recurrent glioblastoma from US community practices. J Neurooncol. 2015;122(3):595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chinot OL, Wick W, Mason W et al. . Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 15. Gilbert MR, Dignam JJ, Armstrong TS et al. . A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wick W, Brandes AA, Gorlia T et al. . Phase III trial exploring the combination of bevacizumab and lomustine in patients with first recurrence of a glioblastoma: the EORTC 26101 trial. Neuro Oncol. 2015;17(Suppl 5):v1. [Google Scholar]
- 17. Wirsching HG, Tabatabai G, Roelcke U et al. . Bevacizumab plus hypofractionated radiotherapy versus radiotherapy alone in elderly patients with glioblastoma: efficacy and imaging analyses of the ARTE trial. J Clin Oncol. 2017;35(Supp 15):doi:10.1200/JCO.2017.35.15_suppl.2014. [DOI] [PubMed] [Google Scholar]
- 18. Nghiemphu PL, Liu W, Lee Y et al. . Bevacizumab and chemotherapy for recurrent glioblastoma: a single-institution experience. Neurology. 2009;72(14):1217–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lai A, Tran A, Nghiemphu PL et al. . Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2011;29(2):142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holdhoff M, Grossman SA. Controversies in the adjuvant therapy of high-grade gliomas. Oncologist. 2011;16(3):351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mason M, Laperriere N, Wick W et al. . Glioblastoma in the elderly: making sense of the evidence. Neurooncol Pract. 2016;3(2):77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: Central nervous system cancers https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed May 03, 2017.
- 23. Centers for Medicare and Medicaid Services. CMS fast facts: CMS program data—populations https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/CMS-Fast-Facts/index.html. Accessed May 03, 2017.
- 24. Burton E, Ugiliweneza B, Woo S et al. . A surveillance, epidemiology and end results-medicare data analysis of elderly patients with glioblastoma multiforme: treatment patterns, outcomes and cost. Mol Clin Oncol. 2015;3(5):971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bleicher RJ, Ruth K, Sigurdson ER et al. . Time to surgery and breast cancer survival in the United States. JAMA Oncol. 2016;2(3):330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wright JD, Burke WM, Tergas AI et al. . Comparative effectiveness of minimally invasive hysterectomy for endometrial cancer. J Clin Oncol. 2016;34(10):1087–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bekelman JE, Mitra N, Handorf EA et al. . Effectiveness of androgen-deprivation therapy and radiotherapy for older men with locally advanced prostate cancer. J Clin Oncol. 2015;33(7):716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Engels EA, Pfeiffer RM, Ricker W et al. . Use of surveillance, epidemiology, and end results-medicare data to conduct case-control studies of cancer among the US elderly. Am J Epidemiol. 2011;174(7):860–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. [DOI] [PubMed] [Google Scholar]
- 30. Klabunde CN, Potosky AL, Legler JM et al. . Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. [DOI] [PubMed] [Google Scholar]
- 31. Dafni U. Landmark analysis at the 25-year landmark point. Circ Cardiovasc Qual Outcomes. 2011;4(3):363–371. [DOI] [PubMed] [Google Scholar]
- 32. Massarweh NN, Li LT, Sansgiry S et al. . Primary tumor resection and multimodality treatment for patients with metastatic colon cancer. Ann Surg Oncol. 2016;23(6):1815–1823. [DOI] [PubMed] [Google Scholar]
- 33. Lin DY, Psaty BM, Kronmal RA. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics. 1998;54(3):948–963. [PubMed] [Google Scholar]
- 34. Galsky MD, Stensland KD, Moshier E et al. . Effectiveness of adjuvant chemotherapy for locally advanced bladder cancer. J Clin Oncol. 2016;34(8):825–832. [DOI] [PubMed] [Google Scholar]
- 35. Babu R, Komisarow JM, Agarwal VJ et al. . Glioblastoma in the elderly: the effect of aggressive and modern therapies on survival. J Neurosurg. 2016;124(4):998–1007. [DOI] [PubMed] [Google Scholar]
- 36. Chang-Halpenny CN, Yeh J, Lien WW. Elderly patients with glioblastoma multiforme treated with concurrent temozolomide and standard- versus abbreviated-course radiotherapy. Perm J. 2015;19(1):15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Erdem-Eraslan L, van den Bent MJ, Hoogstrate Y et al. . Identification of patients with recurrent glioblastoma who may benefit from combined bevacizumab and CCNU therapy: a report from the BELOB trial. Cancer Res. 2016;76(3):525–534. [DOI] [PubMed] [Google Scholar]
- 38. Zanello M, Roux A, Ursu R et al. . Recurrent glioblastomas in the elderly after maximal first-line treatment: does preserved overall condition warrant a maximal second-line treatment?J Neurooncol. 2017;135(2):285–297. [DOI] [PubMed] [Google Scholar]
- 39. Johnson DR, Leeper HE, Uhm JH. Glioblastoma survival in the United States improved after Food and Drug Administration approval of bevacizumab: a population-based analysis. Cancer. 2013;119(19):3489–3495. [DOI] [PubMed] [Google Scholar]
- 40. Iwamoto FM, Reiner AS, Panageas KS et al. . Patterns of care in elderly glioblastoma patients. Ann Neurol. 2008;64(6):628–634. [DOI] [PubMed] [Google Scholar]
- 41. Jönsson L, Sandin R, Ekman M et al. . Analyzing overall survival in randomized controlled trials with crossover and implications for economic evaluation. Value Health. 2014;17(6):707–713. [DOI] [PubMed] [Google Scholar]
- 42. Demetri GD, Garrett CR, Schöffski P et al. . Complete longitudinal analyses of the randomized, placebo-controlled, phase III trial of sunitinib in patients with gastrointestinal stromal tumor following imatinib failure. Clin Cancer Res. 2012;18(11):3170–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Malmström A, Grønberg BH, Marosi C et al. ; Nordic Clinical Brain Tumour Study Group (NCBTSG) Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. [DOI] [PubMed] [Google Scholar]
- 44. Reifenberger G, Hentschel B, Felsberg J et al. ; German Glioma Network Predictive impact of MGMT promoter methylation in glioblastoma of the elderly. Int J Cancer. 2012;131(6):1342–1350. [DOI] [PubMed] [Google Scholar]
- 45. Perry JR, O’Callaghan CJ, Ding K et al. . A phase III randomized controlled trial of short-course radiotherapy with or without concomitant and adjuvant temozolomide in elderly patients with glioblastoma (NCIC CTG CE. 6, EORTC 26062-22061, TROG 08.02, NCT00482677). Neuro Oncol. 2014;16(Suppl 3):iii46. [Google Scholar]
- 46. Park HS, Gross CP, Makarov DV et al. . Immortal time bias: a frequently unrecognized threat to validity in the evaluation of postoperative radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83(5):1365–1373. [DOI] [PubMed] [Google Scholar]
- 47. Bikov KA, Mullins CD, Seal B et al. . Algorithm for identifying chemotherapy/biological regimens for metastatic colon cancer in SEER-Medicare. Med Care. 2015;53(8):e58–e64. [DOI] [PubMed] [Google Scholar]
- 48. Deshpande AD, Schootman M, Mayer A. Development of a claims-based algorithm to identify colorectal cancer recurrence. Ann Epidemiol. 2015;25(4):297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Danese M, Griffiths R, Gleeson M et al. . Patterns of care, survival, and costs of second-line treatment in Medicare beneficiaries with diffuse large B-cell lymphoma (DLBCL). Blood. 2013;122:2936. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.